Abstract
The progression of Alzheimer’s disease (AD) correlates poorly with insoluble amyloid-β (Aβ) in the human brain, but correlates more closely with hyperphosphorylated tau. We developed sensitive cellular assays using HEK293T cells to quantify self-propagating conformational forms of Aβ in brain samples from patients with AD or other neurodegenerative diseases. Postmortem brain tissue from patients with AD had measurable amounts of pathological Aβ conformations. Individuals over 80 years of age had the lowest amounts of prion-like Aβ and phosphorylated tau. Unexpectedly, the longevity-dependent decrease in self-propagating tau conformers occurred in spite of increasing amounts of total insoluble tau. When corrected for the abundance of insoluble tau, the ability of postmortem AD brain homogenates to induce misfolded tau in the cellular assays showed an exponential decrease with longevity with a half-time of approximately one decade over the age range 37 to 99 years. Thus, our findings demonstrate an inverse correlation between longevity in AD patients and the abundance of pathological tau conformers. Our cellular assays could be applied to patient selection for clinical studies and the development of new drugs and diagnostics for AD.
One Sentence Summary
Cellular assays reveal a decline in Aβ and tau prion-like conformers in postmortem Alzheimer’s disease brain samples that correlates with increasing age at patient death.
Introduction
The plaques and tangles of Alzheimer’s disease (AD) were first described more than a century ago (1). In 1984, the Aβ peptide was described (2), and tau protein in neurofibrillary tangles (NFTs) was reported soon thereafter (3–6). Both Aβ and tau proteins adopt pathogenic conformations that spread through the brain (7, 8)in a manner that may be similar to that of mutated forms of the prion protein (PrP) that causes Creutzfeldt–Jakob disease, Gerstmann–Sträussler–Scheinker (GSS) syndrome, fatal familial insomnia, and kuru (9). As our knowledge of prions has expanded with the identification of physiological non-pathogenic prions, a more inclusive definition has emerged: Prions are composed of host-encoded proteins that adopt alternative conformations, which are self-propagating (10). Here, we refer to Aβ and tau as “prion-like” proteins.
Although the vast majority of AD cases are sporadic, an important but small number of familial cases have been instructive. In 1989, one of us reported genetic linkage of the P102L mutation in the PrP open reading frame that causes familial GSS (11). Two years later, familial AD was linked to the V717I mutation in the amyloid precursor protein (APP) open reading frame (12). The importance of Aβ was further elucidated through investigations of AD pathogenesis based on genetic linkage studies between inherited AD and mutations in either amyloid precursor protein (APP) or its processing enzymes (13, 14). Sequential cleavage of APP yields Aβ40 and Aβ42 as the major peptide isoforms (14). Aβ42 can polymerize into oligomers or fibrils and ultimately forms amyloid deposits in the brain (14). The role of tau in AD was clarified in 1998 when three different point mutations in the MAPT gene encoding tau protein isoforms were shown to cause familial Pick’s disease (15, 16).
In familial cases of cerebral amyloid angiopathy (CAA) caused by mutations in the Aβ coding region of APP, death generally occurs in the fourth or fifth decade of life and in the absence of substantial accumulation of insoluble tau (17, 18). In contrast to CAA, misfolded tau spreads through many brain regions in both sporadic and familial AD resulting in cognitive decline. Hypotheses about the role of Aβ abound: Aβ deposition is considered inconsequential by some (19, 20) or Aβ is thought to transiently initiate tau misfolding and polymerization into NFTs by others (21–24). Aβ might also have transient toxicity due to the accumulation of Aβ oligomers, which peak in the early, prodromal phase of AD progression (25, 26). Alternatively, an early steady progression of Aβ oligomerization, deposition and spreading has been proposed. The latter hypothesis is supported by the correlation of increasing AD severity with the spread of Aβ throughout the brain (27, 28) and decreased Aβ42 in cerebrospinal fluid (CSF) (29). In this view, early and progressive formation of prion-like Aβ conformers leads to dysfunction in the central nervous system including an inability to clear misfolded tau molecules leading to the accumulations of NFTs.
To address this hypothesis, assays are needed to measure and rapidly compare Aβ and tau prion-like activities in the postmortem brain tissue of deceased AD patients. However, current methods for the measurement of pathogenic Aβ conformations rely on either time-consuming transgenic mouse models or in vitro biophysical methods that are performed at super-physiological peptide concentrations. Earlier studies showed that AD patient-brain derived Aβ and synthetic Aβ injected into a transgenic mouse brain behaved in a prion-like manner (30–34). Current clinical imaging ligands measure insoluble amyloids, the role of which in disease is uncertain. Importantly, these imaging methods fail to measure biologically active neuroinvasive prion-like Aβ and tau proteins. In contrast, highly reproducible and rapid cell-based methods have been devised to measure prion-like tau and α-synuclein proteins expressed as fusions with fluorescent proteins in mammalian cells (35–38). Here, we describe the development of an analogous cellular bioassay for Aβ, allowing Aβ and tau to be compared in postmortem brain tissue samples from patients with AD or other neurodegenerative diseases. Strikingly, prion-like Aβ and tau activity decreased with longevity despite the presence of increasing NFTs. This decrease in tau prion-like activity paralleled similar decreases in tau phosphorylation. Thus, the greatest tau prion-like activities were found in individuals who died at relatively young ages, despite having the lowest abundance of total insoluble tau. Indeed, it is the relative abundance of biologically active, phosphorylated prion-like tau and not the total amount of inert insoluble tau that correlated with longevity.
Results
Development of isoform-specific and sequence-specific Aβ cellular assays.
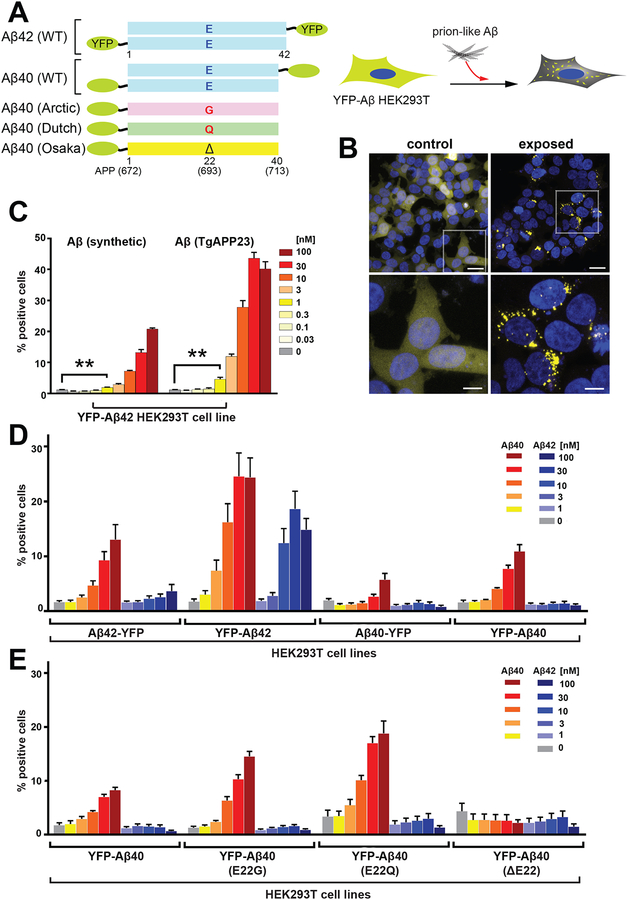
Following earlier studies (39–41), we built constructs in which Aβ40 and Aβ42 peptides were either N-terminally or C-terminally fused to YFP (Fig. 1A). Several structures of Aβ42 demonstrated that the C-terminal residues are buried in an inaccessible core (42–44), whereas the N-terminus is largely disordered. We therefore compared constructs in which YFP was fused to either the N-terminus or the C-terminus of Aβ42. We then examined the ability of synthetic Aβ fibrils to induce aggregation of these constructs in the cytoplasm of mammalian HEK293T cells, as determined from the number of cells showing yellow fluorescent puncta (Fig. 1A–E). For these studies, we used a preparation of synthetic Aβ fibrils (fig. S1A and B) that had previously been characterized biophysically and shown to act as prion-like particles as assessed from their ability to propagate their aberrant conformations after injection into the brains of transgenic mice (33, 34). As expected, in our cellular assays, Aβ40 and Aβ42 fibrils were able to induce aggregation of YFP-Aβ42 fusion proteins in a dose-dependent manner, and this aggregation was most efficient when YFP was fused to the N-terminus (Fig. 1D). We determined the fraction of cells that displayed punctate fluorescence due to the accumulation of aggregated YFP fusion proteins is a quantitative measure of prion-like activity. We designate the fraction of cells showing puncta as f(Aβ) (fraction of cells with puncta, in which the particular protein fused to YFP is expressed and shown in parentheses). This cell line was designated YFP-Aβ42.
Figure 1. Development of YFP-Aβ fusion proteins in HEK-293T cell lines for measuring Aβ aggregates in brains.
The indicated cell lines were developed to measure prion-like activity of preparations consisting of synthetic Aβ peptides and mouse brain-derived extracts based on their abilities to induce fluorescent aggregates which we refer to as puncta. (A) Diagram illustrating the Aβ constructs used in this study (left). Stably transfected HEK293T cells expressing an Aβ-YFP fusion construct underwent lipofectamine-based transduction with synthetic Aβ fibrils (right). (B) Representative confocal images of HEK293T cells expressing Aβ42 fused to YFP at the N-terminus (clone #1), which were treated with PBS (left; control) or exposed to synthetic Aβ40 fibrils (initial monomeric concentration, 1 μM) (right, exposed). The aggregates of Aβ-YFP appear as fluorescent yellow puncta. To measure prion-like activity, we counted the number of puncta-positive cells and expressed this as a percent of the total number of cells in the field of view (% positive cells). Lower panels are higher magnification images of white boxed areas in upper panels. Scale bars: 20 μm (upper panels) or 5 μm (lower panels). (C) HEK293T cells transfected with YFP-Aβ42 were treated with two different types of Aβ ranging from 0.03–100 nM (initial monomeric concentration): synthetic Aβ40 (left) or Aβ purified from TgAPP23 mouse brains (right). Puncta-inducing activity in the HEK293T cells was quantified 2 days after the initial exposure to various Aβ preparations. Data shown are mean ± SEM as determined from four images per well across four wells, and are representative of 3 independent experiments. (D) Cell lines stably expressing four different wildtype (WT) Aβ constructs (shown in panel A) were developed and puncta formation was compared with synthetic Aβ40 and Aβ42 isoforms. Quantification of Aβ in 16 monoclonal cell lines (four randomly chosen clones from each construct; see fig. S2A) was performed 2 days after exposure to increasing concentrations of synthetic Aβ40 or Aβ42 isoforms (1–100 nM) (see fig. S1). Data shown are mean ± SEM as determined from four images per well across four wells, , and are representative of 2 to 3 independent experiments. (E) Cell lines stably expressing four different Aβ40 constructs fused to YFP at the N-terminus (shown in panel A) were developed, and puncta formation was compared after exposure to synthetic Aβ40 and Aβ42 isoforms. Quantification of Aβ in 16 monoclonal cell lines (four randomly chosen clones from each construct; see fig. S2B) was performed two days after exposure to increasing concentrations of synthetic Aβ40 or Aβ42 isoforms (1–100 nM) (see fig. S1). Data shown are mean ± SEM as determined from four images per well across four wells, and are representative of 2 independent experiments.
We also examined the formation of cytoplasmic puncta in an analogous cell line designated YFP-Aβ40, which expressed the Aβ40 rather than the Aβ42 isoform. Aβ40 fibrils—but not Aβ42 fibrils—were able to induce bright puncta in this YFP-Aβ40 cell line. Thus, in contrast to the YFP-Aβ42 cell line, the YFP-Aβ40 cell line was Aβ isoform-specific (Fig. 1D and fig. S2A). We next introduced the familial mutations E22G, E22Q, and E22Δ into the YFP-Aβ40 cell lines (Fig. 1A). These cell lines showed high sensitivity for the formation of puncta when treated with wildtype (WT) Aβ40 fibrils, but not WT Aβ42 fibrils (Fig. 1E and fig. S2B). In contrast, synthetic WT Aβ40 fibrils were unable to induce f(Aβ) puncta in YFP-Aβ40 cells expressing the E22Δ mutation, a variant that is very prone to aggregation in vitro (45, 46) (Fig. 1E and Fig. S2B). These findings are similar to earlier biophysical studies measuring templating activities between WT Aβ and E22Δ Aβ (47). Thus, the YFP-Aβ40 cell line was useful for probing questions of isotype-specific induction of Aβ aggregation, whereas the YFP-Aβ42 cell line was more generally useful for measuring prion-like Aβ activity induced by exogenous addition of either Aβ40 or Aβ42 fibrils. Using the YFP-Aβ42 cell line, we determined quantitively the prion-like Aβ titer from serial dilutions of various preparations ranging from synthetic peptide fibrils to human AD brain homogenates.
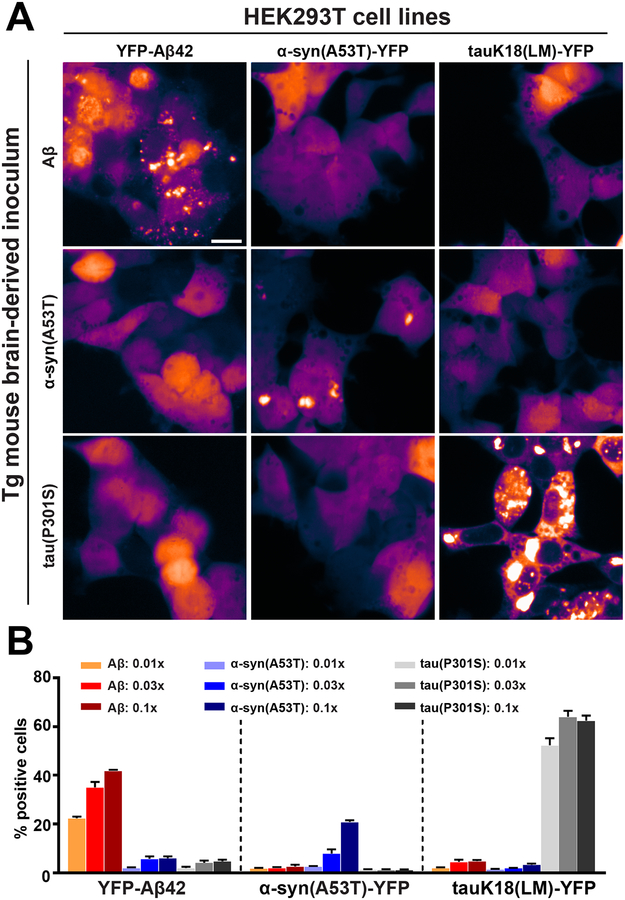
Next, we examined mouse brain-derived Aβ extracted using a sodium phosphotungstic acid (PTA) precipitation procedure (48). Aβ was extracted from the brains of transgenic mice (TgAPP23) expressing human mutant APP (49) that showed spontaneous Aβ deposition. The concentration of total Aβ determined by enzyme-linked immunosorbent assay (ELISA) that was required to stimulate intracellular f(Aβ) puncta formation in the YFP-Aβ42 cell line was approximately 10-fold lower than that for synthetic Aβ fibrils (Fig. 1C). We further defined the specificity of our bioassays using brain homogenates from transgenic mice carrying tau (50) or α-synuclein (51) mutations in the human MAPT or SNCA genes, respectively. These mice are commonly used to model the spontaneous accumulation of tau or α-synuclein aggregates in vivo. PTA-precipitated brain extracts from either mouse model did not stimulate f(Aβ) puncta formation in the YFP-Aβ42 cell line (Fig. 2). However, in cell lines expressing YFP fusions of the corresponding α-synuclein or tau mutant proteins, we observed intracellular prion-like activity of α-synuclein [f(syn)] or tau [f(tau)], respectively (Fig. 2).
Figure 2. Specificity of the Aβ cellular assays.
Various Tg mouse brain inocula were assayed for their Aβ, tau and α-synuclein prion-like activities. (A) Representative confocal images of HEK293T cells stably expressing YFP-Aβ42 (top row), α-synuclein containing the A53T mutation fused to YFP (α-synA53T-YFP, middle row), or tauK18(LM)-YFP (bottom row) (see materials and methods for construct details). The cells were treated with APP-derived peptides from the brains of TgAPP23 mice (0.1 × PTA sample, left column), or Tgα-Syn*A53T mice (0.1 × PTA sample, middle column), or homozygous Tg0N4Rtau*P301S mice (0.1 × PTA sample, right column). Scale bar represents 20 μm. (B) Quantification of the responses of the YFP-Aβ42, α-synA53T-YFP, and tauK18(LM)-YFP cell lines two days after exposure to increasing concentrations of transgenic mouse brain-derived Aβ (0.01x–0.1x PTA sample; orange to dark red), α-synuclein carrying the A53T mutation (0.01x–0.1x PTA sample; light blue to dark blue), and tau carrying the P301S mutation (0.01×–0.1× PTA sample; light green to dark green). Data shown are mean ± SEM as determined from four images per well across four wells, and are representative of 4 independent experiments.
Increased prion-like Aβ titer, amyloid plaques, and gliosis in two transgenic mouse models of AD with age.
We found previously (32) that formation of Aβ peptides increased with age and correlated with measures of neuroinflammation and pathology in two well-studied AD mouse models expressing human mutant APP transgenes: TgAPP23 and TgCRND8 (49, 52). These two transgenic mouse strains exhibited progressive Aβ deposition with age (fig. S3A and D) as well as glial inflammation, neuronal dysfunction, and cognitive deficits (49, 52). To determine the progression of prion-like Aβ, we collected mouse brain samples over a ~2-year span in the slowly progressing TgAPP23 mouse model of AD. TgAPP23 mice display the first neuropathological brain changes between 6 and 9 months of age (49), with overt Aβ deposition appearing after ~1 year (390 days) (fig. S3A). Using our cellular assays, we found that the prion-like Aβ activity became detectable between 200–300 days of age, in parallel with the first appearance of amyloid plaques (fig. S3A) and of astrocytic gliosis, which was measured using a bioluminescent reporter gene driven by the glial fibrillary acidic protein (GFAP) promoter (fig. S3B) (32). The number of f(Aβ) puncta measured using our HEK293T YFP-Aβ42 cell line continued to increase as the animals approached ~2 years of age (732 days) (fig. S3A). We observed similar results in the more rapidly progressing TgCRND8 mouse model of AD (fig. S3D–F).
Human prion-like Aβ and tau accumulation as a function of age, gender, APOE ε4 genotype, and disease phenotype.
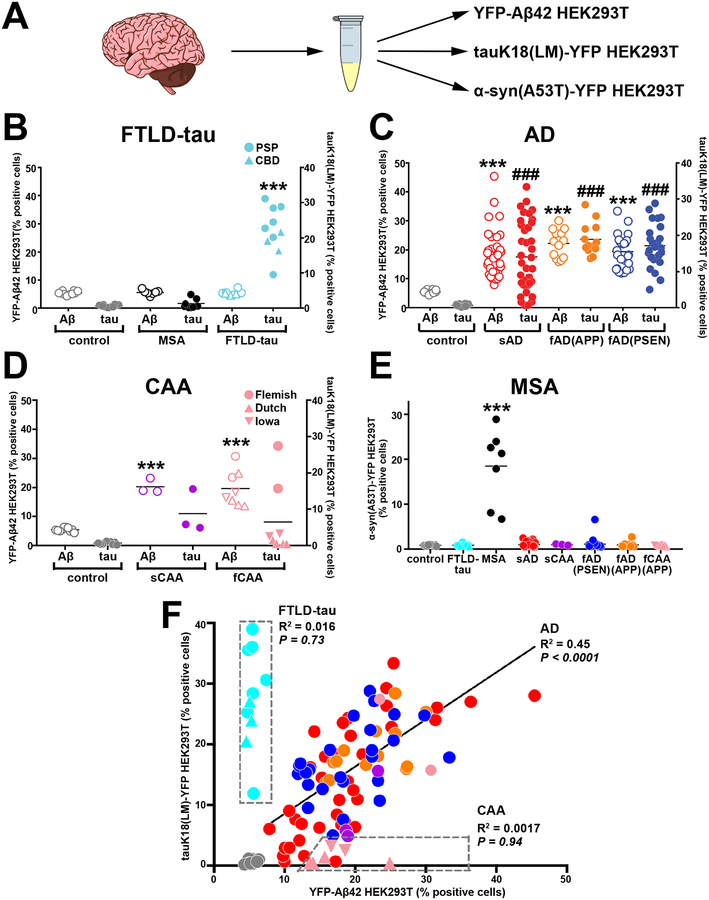
Our Aβ cellular assays in combination with analogous tau cellular assays(36, 38) enabled comparison of Aβ and tau prion-like activities in a collection of human postmortem brain tissue samples from patients with AD or other neurodegenerative diseases. We obtained postmortem human brain specimens from tissue banks (table S1) where the reported cause of death included a spectrum of neurodegenerative diseases ranging from “tau-only” dementias (e.g. frontotemporal lobar degeneration with tau-immunoreactive inclusions, FTLD-tau), to Aβ-centric familial CAA. More specifically, in addition to 10 aged control brains from people who died of non-neurological diseases, we examined two types of FTLD-tau brain tissue samples from 7 cases of progressive supranuclear palsy (PSP) and 3 cases of corticobasal degeneration (CBD). We also examined postmortem brain tissue from 37 patients with sporadic AD, 3 patients with sporadic CAA and 47 patients with familial AD or familial CAA bearing disease-causing mutations in APP, PSEN1, or PSEN2. For each brain sample, we used sodium phosphotungstic acid to selectively precipitate prion-like proteins from brain homogenates.
We next performed cellular assays for Aβ, tau, and α-synuclein using HEK293T cells expressing YFP-Aβ42, tau K18(LM)-YFP, or α-synuclein(A53T)-YFP, respectively (Fig. 3A). Elevated prion-like Aβ activities were detected in all of the AD and CAA brain samples (Fig. 3C and D). In each brain tissue sample, the value of f(Aβ) was at least 20 standard errors above the mean for the control brain samples, indicating that the prion-like Aβ puncta-inducing activity remained robust at the time of death in both AD and CAA and could be preserved by freezing. Additionally, all AD and CAA brain samples tested were devoid of puncta-inducing activity in the α-synuclein cellular assay, unlike postmortem brain samples from eight patients with multiple system atrophy (MSA) (Fig. 3E). Although tau puncta-inducing activity was detectable in all FTLD-tau brains, no measurable prion-like Aβ activity was found in these specimens (Fig. 3B), consistent with the classification of PSP and CBD as primary tauopathies.
Figure 3. Quantitation of postmortem human brain Aβ and tau in parallel cellular assays.
(A) The prion-like activities of Aβ and tau in postmortem human brain samples from patients with FTLD-tau, AD, CAA and MSA were added to three different HEK293T cell lines expressing: YFP-Aβ42, tauK18(LM)-YFP, or α-synA53T-YFP. (B–D) The prion-like activities of Aβ and tau in human brain tissue samples were quantified using the YFP-Aβ42 and tauK18(LM)-YFP cell lines. The cell lines were exposed to a 0.03x dilution of PTA-precipitated brain homogenates derived from 86 patients with sporadic or familial AD or CAA, 10 aged healthy controls, and 10 patients with FTLD-tau (7 PSP and 3 CBD). Data shown are mean ± SEM as determined from four images per well across four wells per sample. Statistical significance is indicated as *** (P<0.0001) for Aβ puncta-inducing prion-like activity compared to control, or### (P<0.0001) for tau puncta-inducing prion-like activity compared to control. (E) α-Synuclein abundance in brain homogenates was quantified as a percent of α-synA53T-YFP expressing cells positive for α-synuclein puncta. The α-synA53T-YFP cell line was exposed to a 0.03x dilution of PTA-precipitated brain homogenates from all postmortem brain samples. Data shown are mean ± SEM as determined from four images per well across four wells per sample. (F) Correlation analyses between Aβ load (x-axis) and tau load (y-axis). Shown is a summary plot for brain tissue from 75 AD cases (37 sporadic AD, red; 25 familial AD with the PSEN mutation, blue; 13 familial AD with the APP mutation, orange) compared to brain tissue from aged healthy controls (gray) and from patients with CAA (sporadic CAA, purple; familial CAA, pink) or FTLD-tau (PSP/CBD, cyan). Brain tissue from one sporadic AD patient lacked a Braak stage score and had comorbid Lewy body dementia; brain tissue samples from two sporadic AD patients were Braak stage III/IV; all three data points fell well within the range of other close-lying points and thus were not removed. Data representative of 3 independent experiments.
In familial CAA postmortem brain samples, Aβ puncta-inducing activity was uniformly elevated whereas tau activity was either low or absent. Postmortem brain tissue from AD patients carrying the Dutch (E22Q in Aβ or E693Q in APP) or Iowa (D22N) mutations showed substantial Aβ but insignificant tau puncta-inducing activity (Fig. 3D). Our findings are consistent with the absence of NFTs in the brains of these individuals (17, 18). Interestingly, elevated prion-like Aβ and tau activities were detected in postmortem brain tissue from two patients with AD carrying the Flemish (A21G) mutation (Fig. 3D). This finding confirms earlier reports of widespread NFTs and hyperphosphorylated tau in dystrophic neurites in such patient cohorts (53, 54). The two patients carrying the Flemish mutation (Fig. 3D) were slightly older at death (63 and 60 years old) than the patients carrying the Iowa and Dutch mutations (55.7 ± 2.9).
In a plot of f(Aβ) versus f(tau) in postmortem brain tissue from patients with different neurodegenerative diseases, the FTLD-tau cases were observed to lie near the y-axis in a distinct region comprising very low Aβ (Fig. 3F); for these cases, there was no correlation between the prion-like Aβ and tau titers. Brain tissue from familial CAA patients carrying the Dutch and Iowa mutations segregated along the x-axis (Fig. 3F). The remaining brain samples were broadly distributed along the diagonal with a modest but significant (P<0.0001) linear correlation between f(Aβ) and f(tau).
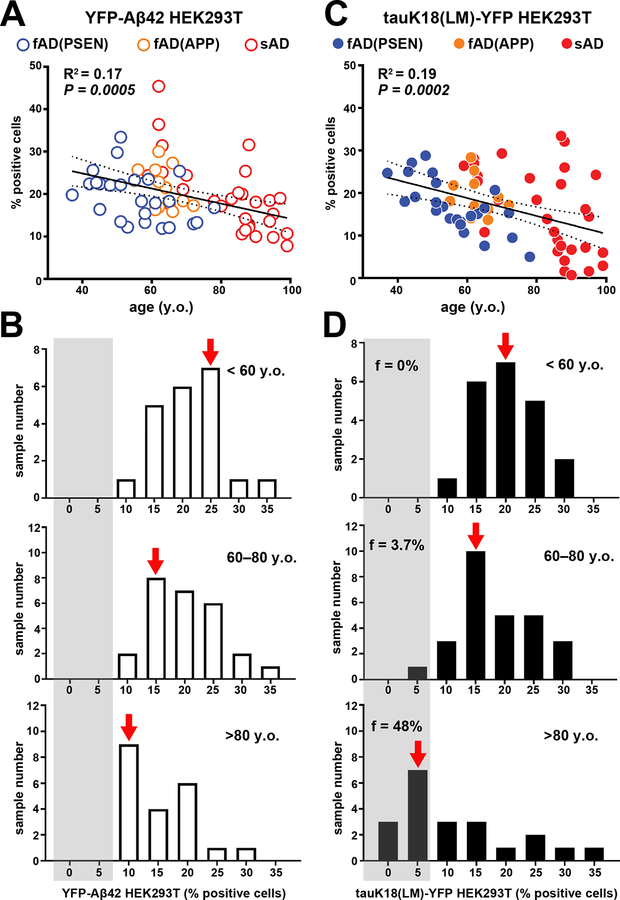
We next asked whether prion-like Aβ and tau activity in postmortem brain tissue at the time of death correlated with variables associated with the genetic background of the donor or the method of sample collection. No significant correlation was observed with respect to variables such as the type of brain bank and preservation method (table S1). However, correlations were observed between the longevity of the donor, the gender, and genetic background of AD patients despite the fact that all postmortem brain tissue had essentially the same confirmed neuropathology (CERAD neuritic plaque, C3; Braak stage V to VI; table S1). The values of f(Aβ) decreased modestly (R2 = 0.17) with respect to longevity (Fig. 4A, Fig. 4A, B), and f(tau) exhibited a similar linear decrease (R2 = 0.19) with age (Fig. 4C). Approximately half of the brain samples from patients who were 80 or older exhibited a low number of f(tau) puncta despite having reached Braak stage V or VI and CERAD score 3 (Fig. 4D). A preponderance of these elderly cases were female (fig. S5A, B), although we cannot rule out collection bias as the majority of the elderly patient donors were female. Overall, these linear trends were observed in brain tissue samples from a broad range of sporadic and familial AD patients.
Figure 4. Inverse correlation between longevity and self-propagating Aβ and tau activities.
The Aβ and tau prion activity in brain tissue from sporadic and familial AD samples (Braak stage V to VI) was plotted as a function of patient age at death (A): familial AD with the PSEN mutation, blue; familial AD with the APP mutation, orange; sporadic AD, red. Statistical values for correlation, linear regression, and 95% confidence intervals are shown. (B) Histogram of Aβ load for the same data set binned into three age groups: <60 years old (y.o., top); 60–80 years old (y.o., middle); >80 years old (y.o., bottom). (C) Tau load in brain tissue from sporadic and familial AD patients plotted as a function of patient age at death: familial AD with the PSEN mutation, blue; familial AD with the APP mutation, orange; sporadic AD, red. Statistical values for correlation, linear regression, and 95% confidence intervals are shown. (D) Histogram of tau abundance for the same data set binned into three age groups:<60 years old (y.o, top); 60–80 years old (y.o., middle); >80 years old (y.o., bottom). Fraction of total sample number in each age bin with 0%–5% tau-positive cells. The standard deviations of individual data points are similar to those in Figure 1 and are much smaller than the deviation from the regression line, indicating that measurement error did not contribute significantly to the deviations from the trend line. Data representative of 3 independent experiments.
Given that the ε4 allele of the gene encoding apolipoprotein E is the major risk factor for AD (55, 56), we compared the effect of the APOE ε4 allele on the values of f(Aβ) and f(tau) (fig. S4C, D). Correlation analysis showed that brain tissue from patients who were APOE ε4 non-carriers had lower quantities of tau aggregates than did brain tissue from APOE ε4 carriers, and this effect was particularly pronounced in patients over the age of 80 (fig. S4D). A similar trend was observed for Aβ although this fell short of 95% statistical confidence (P=0.057) for the given sample size (fig. S4C). Finally, a weak correlation between f(tau) and brain region was observed for samples taken from the frontal and temporal lobes (fig. S4F).
Correlations between longevity and APP, Aβ, tau, and tau phosphorylation.
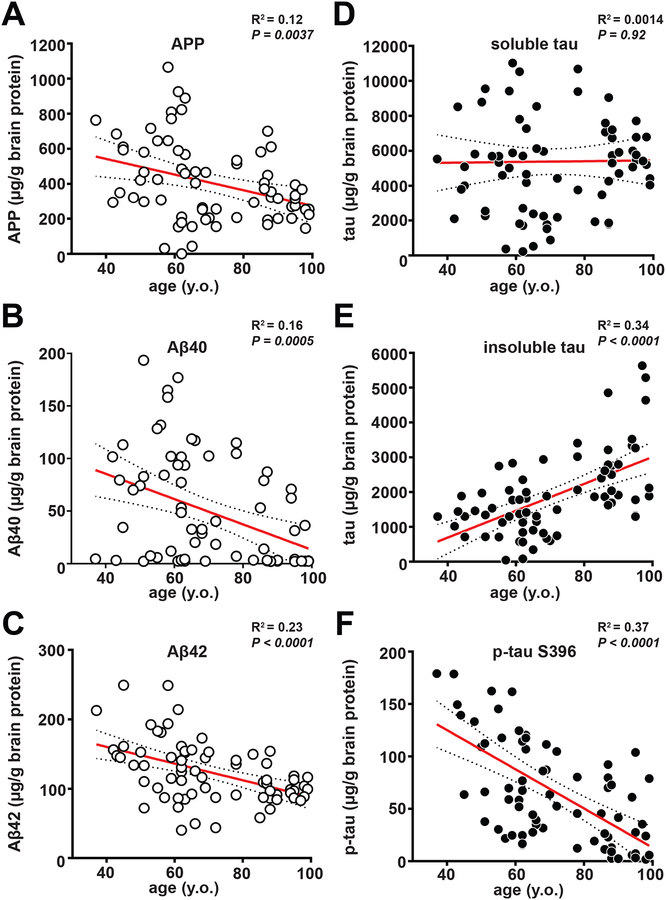
It is possible that the inter-subject variations in the quantities of f(Aβ) and f(tau) simply reflect differences in APP and MAPT expression or the concentrations of misfolded forms of these proteins. Alternatively, individuals with higher f(Aβ) and f(tau) might have biochemically or physically distinct forms exhibiting greater intrinsic stability or potency. To differentiate among these possibilities, we measured the total abundance of various forms of soluble and insoluble Aβ and tau in postmortem brain samples using ELISA. The abundance of APP, Aβ40, and Aβ42 showed a slight (R2 = 0.12–0.23) but significant trend (P<0.005 in all cases) toward lower values with respect to age.
Markedly different trends were observed when the abundance of various forms of the tau protein were quantified in postmortem brain samples. There was no significant relationship between age at death and concentrations of total soluble tau (Fig. 5D), as measured using an antibody that was specific for all splice forms of tau protein. Furthermore, the amount of total insoluble tau (Fig. 5E) increased with age in contrast to the decrease in prion-like tau activity seen in Fig. 4C. We therefore examined the abundance of insoluble phosphorylated tau (p-tau) in the postmortem brain samples because the amount of p-tau is known to be associated with clinical severity (57, 58). Antibodies for three different phosphorylated tau epitopes (S396, S199, T231) provided similar results: each antibody showed that unlike total insoluble tau, the extent of insoluble p-tau decreased linearly with age at death (Fig. 5F and fig. S5B, C).
Figure 5. Measurement of the expression level of APP, and the amounts of soluble and insoluble Aβ and tau species as a function of the age at death of AD patients.
ELISA was used to measure different proteins in brain samples (Braak stage V to VI) from patients with familial or sporadic AD. The following proteins were measured: (A) APP in the clarified brain homogenate (PBS soluble), (B, C) Aβ40 and Aβ42 (formic acid soluble), respectively, (D) total tau in the clarified brain homogenate (PBS soluble), (E) total tau (formic acid soluble), and (F) phosphorylated-tau (phosphorylated epitope Serine396, formic acid soluble). All data are plotted as a function of patient age at death (y.o., years old). Statistical values for correlation, linear regression, and 95% confidence interval are shown. The measurements were made in duplicate and are much smaller than the deviation from the regression line, indicating that measurement error did not contribute significantly to the deviations from the trend line. Data representative of 1 to 2 independent experiments.
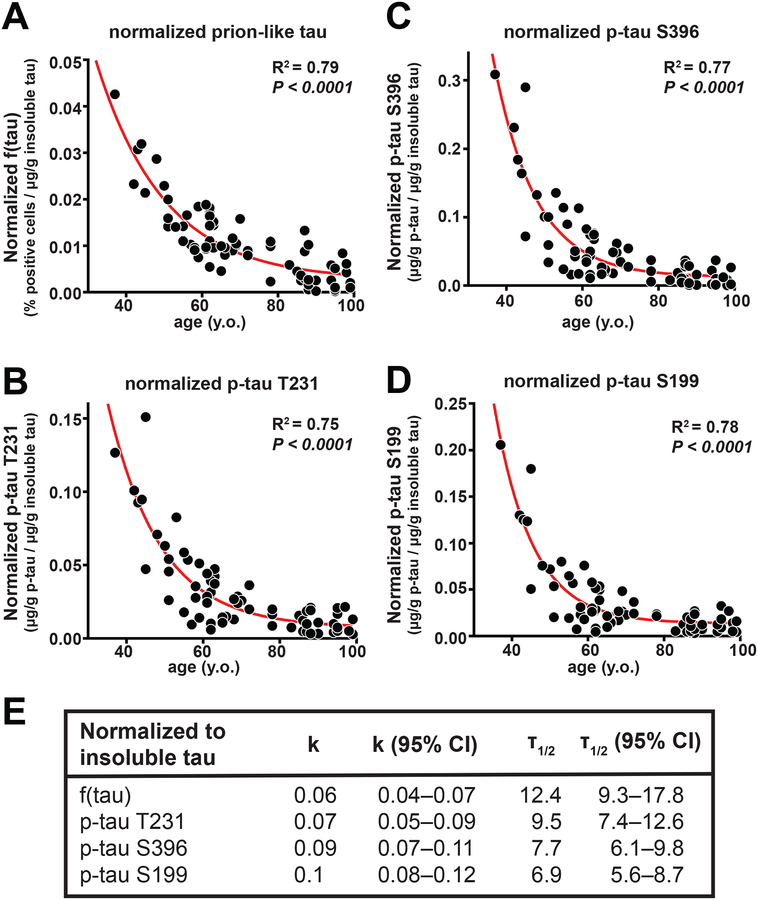
We next normalized prion-like tau abundance, relative to the total insoluble tau concentration, to provide a measure of the specific activity of the insoluble tau within a given brain sample. The data are described by an exponential decay (R2=0.79) over five half-lives of observations. This correlation is in part a result of the data spanning a wide range of ages at death from 37 to 99 years, resulting in a large range of the dependent variables relative to sampling and other errors. If a smaller age-range were considered, the overall range of specific activities would be smaller, while experimental and other errors would remain approximately the same leading to a lower correlation coefficient. Thus, the exponential nature of the process is most clearly revealed by including as wide a range of ages as available.
The extent of tau phosphorylation as a function of age was similarly evaluated by calculating the amount of a given p-tau epitope in the insoluble tau fraction relative to total insoluble tau. Again, a single exponential decay was observed over five half-lives, with R2 values ranging from 0.75 to 0.78 (P<0.0001), depending on the epitope used to quantify insoluble p-tau (Fig. 6B–D). The half-life of prion-like tau obtained from the normalized cellular assay data (Fig. 6A and E) was 12 years, whereas the corresponding half-lives for p-tau ranged from 7 to 10 years. The 95% confidence intervals for each of the half-lives overlapped (Fig. 6E), so it was not possible to distinguish whether the differences in half-lives were meaningful. At present, we can conclude that both prion-like tau activity and the extent of tau phosphorylation decreased about two-fold for each decade of longevity. Thus, for example, on average, an individual who died at age 40 would have about 25 or 32-fold higher prion-like tau than would an individual who lived five decades longer and died at age 90 (when normalized for total insoluble tau). Our data indicate that the biophysical and biochemical changes that accompany tau phosphorylation correlate with disease progression at a young age in AD patients, with mutations in APP or PSEN accounting for most of these cases.
Figure 6. The abundance of self-propagating and phosphorylated tau decreased exponentially over six decades in patients with AD.
The specific activity of prion-like tau in AD brain homogenates decreased in parallel with a reduction in hyperphosphorylated tau. (A) Prion-like tau abundance measured in AD brain tissue samples (Braak stage V to VI) was normalized to the adjusted value of insoluble tau as measured by ELISA (see Fig. 5E). Shown are normalized data plotted as a function of AD patient age at death and fitted using an exponential decal model equation (one-phase decay). (B) Phosphorylated-tau (phosphorylated on Threonine 231; p-tauT231) was measured in AD brain samples normalized to the adjusted value of insoluble tau as measured by ELISA (see Fig. 5E). Normalized data were plotted as a function of AD patient age at death and fitted using an exponential decay model equation (one-phase decay). To normalize the data, the prion-like tau values from Fig. 4 or the p-tau concentration from Fig. 5 were divided by concentration of total insoluble tau obtained from the regression line for total tau versus age at death for AD patients shown in Fig. 5E. (C) Phosphorylated-tau (phosphorylated on Serine 396; p-tauS396) was measured in AD brain samples normalized to the adjusted value of insoluble tau as measured by ELISA. (D) Phosphorylated-tau (phosphorylated on Serine 199; p-tauS199) was measured in AD samples normalized to the adjusted value of insoluble tau as measured by ELISA. (E) Statistical values for correlation, decay constant (K), half-life (T), and their respective 95% confidence intervals (CI) are shown.
Discussion
A wealth of evidence argues that both Aβ and tau adopt pathological conformations leading to prion-like spreading throughout the brain during AD pathogenesis. The findings reported here establish the presence of both prion-like Aβ and tau proteins in the brains of patients who died of either sporadic or inherited AD. Moreover, studies by us and others in cellular and transgenic mouse models have previously demonstrated prion-like tau in the brains of patients who died of FTLD-tau (36, 38, 59–61). Our results extend those earlier studies by demonstrating that postmortem brain tissue from FTLD-tau patients was devoid of both prion-like Aβ and α-synuclein proteins.
The linear trends in f(Aβ) and f(tau) with longevity were observed over a range of sporadic and familial forms of AD. Thus, what had appeared to be a set of disparate disorders can now be seen as a continuous spectrum, with the defining feature being the spreading of prion-like Aβ and tau through the CNS. Our findings from earlier molecular genetic studies and those described here begin to create a more complete view of the chemical processes that feature in the pathogenesis of AD, because they allow a clear definition between inactive inert tau and Aβ versus their active prion-like forms.
Both human and animal studies argue that pathologically misfolded Aβ initiates formation of prion-like tau (18, 21, 62, 63) in AD. Presumably, the formation of prion-like Aβ begins in one or more brain regions and then spreads to others. The movement of PrPSc prions and other prion-like proteins such as α-synuclein, tau, and possibly Aβ42 from one CNS region to another argues for trans-synaptic spread (64–69). The apparent spread of prion-like proteins in the CNS is reflected by their regional distribution and has been well-documented in neuropathological studies (70, 71). The identification of distinct prion-like Aβ conformational strains in AD brains with different etiologies has been particularly informative (72–75). It remains to be determined which of these conformations are related to distinct disease phenotypes or which are associated with selective prion-like tau formation. Using the bioassays reported here, we can now correlate the presence of a given conformer with prion-like tau or and/or prion-like Aβ activities across a variety of phenotypic manifestations of AD.
Recent studies report that the minimal size of a biologically active tau aggregate may range from a monomer (76, 77) to linear aggregates of ~100 nm in length (78). Pentameric or smaller tau aggregates were unable to support prion-like tau activity (79). Additionally, tau phosphorylation may also contribute to the conformation of prion-like tau as shown in previous studies where immunodepletion of p-tau in brain extracts used as inocula abolished prion-like tau activity in recipient cells (80, 81) or in animal models (79). These findings are consistent with our data demonstrating a relationship between prion-like tau activity and the extent of tau phosphorylation in brain samples from AD patients obtained at different ages of death (Figures 5 and 6).
Development of a new cellular assay for prion-like Aβ peptides has permitted us to compare prion-like Aβ generated using synthetic Aβ fibrils versus transgene-encoded Aβ preparations. Our cellular assay is also useful for measuring Aβ prion-like activities generated by allele-specific Aβ subtypes. It also has enabled parallel quantification of prion-like Aβ and prion-like tau activities, providing a direct quantitative comparison of these actively propagating species, rather than comparisons of inert protein deposits. Our data also show that the AD patients with greatest longevity had lower concentrations of both prion-like Aβ and prion-like tau at the time of death compared to patients who died at younger ages from AD-related disease. Previous studies showed that the abundance of NFTs correlated well with the extent of brain atrophy and cognitive decline in AD (29, 82). Notably, these studies focused only on total insoluble tau. By examining the age of death as a variable, we found that low prion-like tau activity correlated with greater longevity.
Notably, both the extent of prion-like Aβ activity and the abundance of APP, Aβ40, and Aβ42 decreased with longevity in a roughly synchronous manner. This finding is consistent with the hypothesis that prion-like Aβ features early during the formation of pathological tau tangles. Moreover, measurable prion-like Aβ activity was found in the oldest patients, suggesting that it continues to participate throughout AD pathogenesis. However, the R2 values that we found ranged from 0.12 to 0.2 indicating that many factors ranging from the methods of sample collection to genetic factors appeared to have a sizable influence on the observed correlation. Clearly, genetic factors such as the APOE ε4 allele and TREM2 variants, which have been implicated in Aβ metabolism and clearance, can strongly increase the risk of AD (56, 83). Although we found interesting trends with respect to the APOE ε4 genotype and gender, we will need to perform larger studies that carefully sample all of the different APOE genotypes.
The strong associations among longevity, prion-like tau activity, and tau hyperphosphorylation are particularly intriguing. Such findings are consistent with the greater contribution of tau versus Aβ protein misfolding to the AD phenotype as measured by neurological dysfunction and neuropathological lesions. One particularly striking result is the accumulation of insoluble tau that increases with a greater age at death in contrast to phosphorylated insoluble tau, which decreases with increasing longevity (Figures 4 and 5). Although these relationships were clear from examining the extent of prion-like tau formation and phosphorylation per gram of total brain protein, these findings became more striking when the data were normalized according to the abundance of insoluble tau (Figure 6). Our findings argue that all insoluble tau is not equally neurotoxic and that biochemical events such as phosphorylation influence the formation of prion-like tau or modulate tau toxicity. It remains to be determined whether the low abundance of prion-like tau in long-lived AD patients is a result of a slowly replicating tau conformer or is due to host factors that more readily clear prion-like conformers or shift prion-like tau toward a more inert amyloid state (e.g., insoluble total tau). Thus, future work aimed at the development of diagnostic reagents and effective therapeutics will need to focus on prion-like tau activity and its associated posttranslational modifications rather than total insoluble tau.
Measuring both prion-like Aβ and tau abundance in postmortem brain samples is likely to have many applications. Antemortem detection of Aβ and tau activities in the CSF or blood of patients with AD, as shown for PrP prions (84–86), may provide more informative diagnostic tools to stage disease and measure efficacy of putative effective therapeutics. Also, our findings may help to illuminate both the successes and failures of pharmaceutical approaches that target Aβ and the Aβ-tau axis. The availability of paired cellular bioassays for measuring prion-like Aβ and prion-like tau should contribute to future drug discovery programs for AD.
MATERIALS AND METHODS
Study design.
The aim of our study was to develop a rapid, quantitative cell-based assay to measure biologically active Aβ derived from postmortem human brain tissue. We first engineered HEK293T cell lines that were sensitive to Aβ40 and Aβ42, and validated the assay using synthetic Aβ fibrils that had previously been shown to be transmissible when injected into transgenic mouse brains (38). We tested our cell lines with these Aβ fibril preparations in three independent experiments. The sample size and time-points (including end-points) were chosen for cell and animal experiments based on our previous work (34, 37, 38, 75). We used the minimum number of animals required to obtain a significant difference based on the expected variability, and all mice were randomly assigned and gender-balanced. De-identified human postmortem brain tissue was collected from several brain bank repositories located in the United States, Europe, and Australia (table S1). We replaced the identity of the samples with an internal code, and the investigator performing the experiments was blinded to the sample identity during testing and analysis. Sampling and experimental replicates for each experiment are listed in the figure legends.
Cell line development.
Constructs encoding human WT and mutant Aβ42 or Aβ40 fused with yellow fluorescent protein (YFP) at the N- or C-terminus were introduced into the pIRESpuro3 vector (Clontech), were transfected to HEK293T cells (ATCC), and monoclonal cell lines were generated and maintained as described (37). Cell lines expressing human α-synuclein with the A53T mutation or human tau containing the repeat domain of 4R tau with the mutations P301L and V337M fused with YFP were generated as described (37, 38).
Development of the cellular assay.
The cellular bioassay for Aβ was developed as described (37, 38). Briefly, cells were plated in a 384-well plate at a density of 3,000 cells/well (70 μL/well) with 0.1 μg/mL Hoechst 33342 (Thermo Fisher). A mixture of Lipofectamine 2000 (1.5% final volume; Thermo Fisher), OptiMEM (78.5% final volume; Thermo Fisher) and sample (20% final volume) were incubated at room temperature for 2 hours and plated in four replicate wells (10 μL/well). Plates were then incubated and imaged every 24 hours on the IN Cell 6000 (GE Healthcare) for 3–4 days. Images of both the DAPI and FITC channels were collected from four different regions in each well. The images were analyzed using the IN Cell Developer software with an algorithm developed to identify intracellular aggregates only in live cells.
Preparation of synthetic Aβ fibrils.
Preparation of synthetic Aβ fibrils was performed as described (34). Briefly, the WT Aβ40 and WT Aβ42 peptides were purchased from Bachem. Lyophilized peptides were dissolved to 5 mg/mL hexafluoroisopropanol and separated in 200 μg aliquots. Hexafluoroisopropanol was evaporated in a speedvac and stored at –20 °C. For conversion, the dried peptide film was solubilized in 20 μL dimethyl sulfoxide (DMSO) and diluted with 980 μL of aqueous buffer solutions containing 10 mM sodium phosphate. Samples were incubated at 37°C for 72 hours in 1.5 mL centrifugation tubes under constant agitation at 900 rpm. The resulting samples were spun down for 1 hour at 100,000 × g, and the pellet was resuspended in 100 μL phosphate buffered saline (PBS) at 2 mg/mL. Samples were further analyzed or diluted, snap-frozen in liquid nitrogen, and stored at −80 °C.
Phosphotungstic acid precipitation of Aβ peptides and tau protein in postmortem brain samples.
Phosphotungstic acid (PTA) precipitation of human postmortem brain samples was performed as described (50, 87). Briefly, 10% brain homogenate was incubated in 2% sarkosyl and 0.5% benzonase (Sigma) at 37°C with constant agitation (1,200 rpm) in an orbital shaker for 2 hours. Sodium PTA was dissolved in double-distilled H2O, and the pH was adjusted to 7.0. PTA was added to the solution to a final concentration of 2%, which was then incubated overnight under the same conditions. The sample was centrifuged at 16,100 × g for 30 minutes at room temperature, and the supernatant was removed. The resulting pellet was resuspended in 2% sarkosyl in PBS and 2% PTA in ddH2O, pH 7.0. The sample was again incubated for at least 1 hour prior to a second centrifugation. The supernatant was again removed, and the pellet was resuspended in PBS using 10% of the initial starting volume and stored at −80°C.
To establish bioassays for measuring prion-like Aβ and tau loads, we prepared a dilution series (e.g. 0.01, 0.03, and 0.1x) of all PTA-precipitated brain samples to perform the initial experiments. Once we established the dilution factor that best suited the majority of all samples, we performed subsequent experiments with only one or two dilution factors in order to conserve sample stocks. Using this approach, we ensured that our aggregation-inducing activity measurements were well within the dynamic range of the bioassay.
Formic acid extraction of insoluble proteins in postmortem brain tissue for ELISA.
Fifty microliters of formic acid was added to 25 μL 10% brain homogenate and placed in an ultracentrifuge tube. The samples were vortexed, sonicated for 20 minutes at 37°C in a water-bath sonicator, and then centrifuged at 100,000 × g for 1 hour. We removed 50 μL of the supernatant and neutralized it with 950 μL of neutralization buffer in a low binding tube. The neutralization buffer consisted of 1M Tris Base and 500 mM dibasic sodium phosphate with no pH adjustment. (If a very small pellet or layer of lipids formed at the top of the supernatant, we aspirated the sample from the middle of the supernatant to maximize the protein in the extract.) Samples were aliquoted into low binding tubes and flash frozen in liquid nitrogen. The following ELISA kits from Thermo-Fisher Scientific were used according to the manufacturer’s protocol: APP (KHB0051), Aβ40 (KHB3481), Aβ42 (KHB3441), total tau (KHB0041), p-tau S396 (KHB7031), p-tau S199 (KHB7041), and p-tau T231 (KHB8051). The Aβ43 ELISA kit was from IBL-America (27710). Each sample was analyzed in duplicate. We adjusted the raw ELISA values to total brain protein (grams) in the clarified 10% brain homogenate as determined by bicinchoninic acid assay (Pierce/Thermo-Fischer Scientific).
Transgenic mice.
TgAPP23 mice, which express human APP (751-amino acid isoform) containing the Swedish mutation under the control of the Thy-1.2 promoter, were maintained on a C57BL/6 background. TgCRND8 mice, which express human APP (695-amino acid isoform) with the Swedish and Indiana mutations under the control of the hamster Prnp promoter, were maintained on a mixed B6/C3 background. TgGfap-luc mice, which express firefly luciferase under the control of the murine Gfap promoter, were a gift from Caliper Life Sciences and were maintained on an FVB/N background. To create bigenic mice, TgAPP23 and TgCRND8 mice were crossed with TgGfap-luc animals and were screened for the presence of both transgenes. Aged homozygous Tg0N4Rtau*P301S and Tgα-Syn*A53T mice were used to provide control brain samples (80, 88). Animals were maintained in an AAALAC-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Bioluminescence imaging.
Bioluminescence imaging of the brains of bigenic TgAPP23:Gfap-luc mice and TgCRND8:Gfap-luc mice was performed as previously described (32). Isoflurane-anesthetized, head-shaved mice were imaged (60 second-exposure) after receiving an intraperitoneal (i.p.) injection of 50 μL 30 mg/mL D-luciferin potassium salt solution (Gold Biotechnology) that was prepared in PBS, pH 7.4 (a dose of ~60 mg/kg). Brain bioluminescence values were calculated from images displaying surface radiance using circular regions of interest and then were converted to total photon flux (photons per second) using Living Image software version 4.4 (PerkinElmer).
Statistical analysis.
Statistical analyses were performed with GraphPad Prism version 7. Data are shown as mean ± SEM. Comparisons between multiple groups was performed using one-way ANOVA with post-hoc Dunnett’s test. We used linear regression analysis for correlation plots. For two-group (frequency distribution) comparisons, we used a nonparametric Mann-Whitney U test. A value of P<0.05 was considered significant. The exponential decay equation model (one phase decay) used the least squares (ordinary) fitting method with no constraints applied.
Supplementary Material
Figure S1. Development of YFP-Aβ fusion HEK-293T cell lines: synthetic Aβ fibril inocula and puncta-inducing kinetics..
Figure S2. Development of YFP-Aβ fusion HEK-293T cell lines: Individual clones
Figure S3. Amyloid plaque pathology, astrogliosis, and prion-like Aβ abundance during disease progression in TgAPP23 and TgCRND8 mice.
Figure S4. APOE ε4 status, gender, and brain region influence the extent of prion-like Aβ and tau abundance in AD postmortem brain tissue
Figure S5. Correlation of different Aβ and tau species with age at death of AD patients.
Table S1. Source of postmortem human brain tissue samples.
Date File S1
Acknowledgments:
We thank S. Kelley (Abraham Lincoln High School, San Francisco, CA) for assistance with statistical analysis. Funding: This work was supported by grants from the National Institutes of Health (NIH) (# AG002132 and AG031220), as well as by the Brockman Foundation, the Dana Foundation, the Glenn Foundation, the Oak Meadow Foundation, the Rainwater Charitable Foundation, and the Sherman Fairchild Foundation. C.C. and W.F.D. are also supported by National Institute on Aging grant (# AG061874). W.F.D. is additionally supported by a National Institute of General Medical Sciences grant (# GM122603). J.S. was supported by the Alzheimer’s Association (grant # 2015-NIRG-339935). Human brain tissue was received from the UCSF Neurodegenerative Disease Brain Bank, which is supported by the NIH (# AG023501 and AG19724 to W.W.S.), the Tau Consortium, and the Consortium for Frontotemporal Dementia Research. Human brain tissue was provided by the Brain Bank at Karolinska Institutet (KI), Stockholm, Sweden, which received financial support from StratNeuro at KI, Swedish Brain Power, and Stockholm County Council. Human brain tissue samples also were provided by the Massachusetts Alzheimer’s Disease Research Center (director, M. P. Frosch), which received financial support from the NIH (# P50 AG005134). Autopsy brain tissue was obtained from the University of Washington Neuropathology Core, which is supported by the Alzheimer’s Disease Research Center (# AG05136), the Adult Changes in Thought Study (# AG006781), and the Morris K. Udall Center of Excellence for Parkinson’s Disease Research (# NS062684). C.D.K. is supported by the Nancy and Buster Alvord Endowment. Autopsy brain tissue was also supplied by King’s College, University of London (Department of Clinical Neuroscience), the University of Edinburgh (Department of Neuropathology), and the Manchester Brain Bank (University of Manchester), which is part of the Brains for Dementia Research Initiative, jointly funded by the Alzheimer’s Society and Alzheimer’s Research UK. We thank the Queen Square Brain Bank for Neurological Disorders [supported by the Reta Lila Weston Trust for Medical Research, the Progressive Supranuclear Palsy (Europe) Association, and the Medical Research Council] at the UCL Institute of Neurology, University College London, for provision of the UK human brain tissue samples. The Sydney Brain Bank is supported by Neuroscience Research Australia and the University of New South Wales. G.H. is a National Health and Medical Research Council of Australia Senior Principal Research Fellow (#1079679). Human brain tissue also was provided by the Rush Alzheimer’s Disease Center, Rush University Medical Center (director, D. A. Bennett) (88), which has received financial support from the NIH (# P30AG10161 and R01AG15819). Author contributions: A.A., C.C., J.S., and S.B.P. designed the research. A.A., C.C., J.S., W.Y., B.M.R., and J.C.L. performed the experiments. A.L.W. codeveloped the tauK18(LM)-YFP and alpha-syn(A53T)-YFP cell lines. G.H., S.v.D., M.I., L.L., C.G., T.D.B., C.D.K., and W.W.S. contributed postmortem human brain samples, generated neuropathology scores, and acquired APOE genotypes. A.A., C.C., J.S., W.F.D., and S.B.P. analyzed the data. A.A., C.C., W.F.D., and S.B.P. wrote the paper. C.C. and S.B.P. supervised the study. Competing interests: The Institute for Neurodegenerative Diseases (UCSF) has a research collaboration with Daiichi Sankyo (Tokyo, Japan). S.B.P. is the chair of the scientific advisory board of Alzheon Inc. and a member of the scientific advisory board of ViewPoint Therapeutics, neither of which contributed support for this study. W.F.D. is a member of the scientific advisory boards of Alzheon Inc., Pliant, Longevity, Cytegen, Amai, and ADRx Inc., none of which contributed support for this study. W.W.S. received consulting fees from Bristol Myers-Squibb, Merck Inc., and Biogen Idec. A.L.W., A.A., and S.B.P. are coinventors on patent # WO/2017/172764 entitled “Modified cell line and method of determining tauopathies.” Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials. All postmortem human brain samples came from tissue banks listed in table S1 and were obtained through material transfer agreements with the indicated institutions associated with the samples.
Footnotes
Data availability: All data associated with this study are included in the paper or supplementary materials. All postmortem human brain samples came from tissue banks listed in table S1 and were obtained through materials transfer agreements with the indicated institutions associated with the samples.
References:
- 1.Alzheimer A, Über eigenartige Krankheitsfälle des späteren Alters. Zentralbl. Gesamte Neurol. Psychiatr 4, 356–385 (1911). [Google Scholar]
- 2.Glenner GG, Wong CW, Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun 120, 885–890 (1984). [DOI] [PubMed] [Google Scholar]
- 3.Brion JP, Couck AM, Passareiro E, Flament-Durand J, Neurofibrillary tangles of Alzheimer’s disease: an immunohistochemical study. J. Submicrosc. Cytol 17, 89–96 (1985). [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I et al. , Abnormal phosphorylation of the microtubule-associated protein (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosik KS, Joachim CL, Selkoe DJ, Microtubule-associated protein tau is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A 83, 4044–4048 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock NJ, Mirra SS, Binder LI, Hansen LA, Wood JG, Filamentous aggregates in Pick’s disease, progressive supranuclear palsy, and Alzheimer’s disease share antigenic determinants with microtubule-associated protein, tau. Lancet 2, 1211 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Ayers JI, Giasson BI, Borchelt DR, Prion-like spreading in tauopathies. Biol. Psychiatry 83, 337–346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condello C, Stöhr J, Aβ propagation and strains: Implications for the phenotypic diversity in Alzheimer’s disease. Neurobiol. Dis 109, 191–200 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Prusiner SB, A unifying role for prions in neurodegenerative diseases. Science 336, 1511–1513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prusiner SB, in Prion Biology, Prusiner SB, Ed. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2017), pp. 1–15. [Google Scholar]
- 11.Hsiao K et al. , Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature 338, 342–345 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Goate A et al. , Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Goate A, Hardy J, Twenty years of Alzheimer’s disease-causing mutations. J. Neurochem 120 (Suppl. 1), 3–8 (2012). [DOI] [PubMed] [Google Scholar]
- 14.TCW J, Goate AM, in Prion Diseases, Prusiner SB, Ed. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2017), pp. 203–213. [Google Scholar]
- 15.Hutton M et al. , Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Spillantini MG, Bird TD, Ghetti B, Frontotemporal dementia and parkinsonism linked to chromosome 17: A new group of tauopathies. Brain Pathol. 8, 387–402 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Duinen SG et al. , Hereditary cerebral haemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 84, 5991–5994 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maat-Schieman MLC et al. , Glial reactions and the clearance of amyloid β protein in the brains of patients with hereditary cerebral hemorrhage with amyloidosis-Dutch type. Acta Neuropathol. 107, 389–398 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Viola KL, Klein WL, Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 129, 183–206 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karran E, De Strooper B, The amyloid cascade hypothesis: Are we poised for success or failure? J. Neurochem 139 (Suppl. 2), 237–252 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Gotz J, Chen F, van Dorpe J, Nitsch RM, Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293, 1491–1495 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Lewis J et al. , Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Bennett RE et al. , Enhanced tau aggregation in the presence of amyloid β. Am. J. Pathol 187, 1601–1612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z et al. , Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesne SE, Toxic oligomer species of amyloid-β in Alzheimer’s disease, a timing issue. Swiss Med. Wkly 144, w14021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musiek ES, Holtzman DM, Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat. Neurosci 18, 800–806 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thal DR, Rüb U, Orantes M, Braak H, Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Murray ME et al. , Clinicopathologic and11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138, 1370–1381 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR Jr. et al. , Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Luehmann M et al. , Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Eisele YS et al. , Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330, 980–982 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watts JC et al. , Bioluminescence imaging of Abeta deposition in bigenic mouse models of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 108, 2528–2533 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stöhr J et al. , Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. U.S.A. 109, 11025–11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stöhr J et al. , Distinct synthetic Aβ prion strains producing different amyloid deposits in bigenic mice. Proc. Natl. Acad. Sci. U.S.A. 111, 10329–10334 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI, Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem 287, 19440–19451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders DW et al. , Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woerman AL et al. , Propagation of prions causing synucleinopathies in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 112, E4949–E4958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woerman AL et al. , Tau prions from Alzheimer’s disease and chronic traumatic encephalopathy patients propagate in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 113, E8187–E8196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wurth C, Guimard NK, Hecht MH, Mutations that reduce aggregation of the Alzheimer’s Aβ42 peptide: An unbiased search for the sequence determinants of Aβ amyloidogenesis. J. Mol. Biol 319, 1279–1290 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Kim W et al. , A high-throughput screen for compounds that inhibit aggregation of the Alzheimer’s peptide. ACS Chem. Biol 1, 461–469 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Ochiishi T et al. , Development of new fusion proteins for visualizing amyloid-β oligomers in vivo. Sci. Rep 6, 22712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu JX et al. , Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154, 1257–1268 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt M et al. , Peptide dimer structure in an Aβ(1–42) fibril visualized with cryo-EM. Proc. Natl. Acad. Sci. USA 112, 11858–11863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gremer L et al. , Fibril structure of amyloid-β(1–42) by cryo-electron microscopy. Science 358, 116–119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nilsberth C et al. , The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat. Neurosci 4, 887–893 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Elkins MR et al. , Structural polymorphism of Alzheimer’s β-amyloid fibrils as controlled by an E22 switch: A solid-state NMR study. J. Am. Chem. Soc 138, 9840–9852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cloe AL, Orgel JPRO, Sachleben JR, Tycko R, Meredith SC, The Japanese mutant Aβ (ΔE22-Aβ(1–39)) forms fibrils instantaneously, with low-thioflavin T fluorescence: seeding of wild-type Aβ(1–40) into atypical fibrils by ΔE22-Aβ(1–39). Biochemistry 50, 2026–2039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine DJ et al. , Mechanism of scrapie prion precipitation with phosphotungstate anions. ACS Chem. Biol 10, 1269–1277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sturchler-Pierrat C et al. , Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. USA 94, 13287–13292 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen B et al. , Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci 22, 9340–9351 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giasson BI et al. , Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Chishti MA et al. , Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem 276, 21562–21570 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Cras P et al. , Presenile Alzheimer dementia characterized by amyloid angiopathy and large amyloid core type senile plaques in the APP 692Ala-->Gly mutation. Acta Neuropathol. 96, 253–260 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Kumar-Singh S et al. , Dense-core senile plaques in the Flemish variant of Alzheimer’s disease are vasocentric. Am. J. Pathol 161, 507–520 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verghese PB, Castellano JM, Holtzman DM, Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 10, 241–252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao N, Liu C-C, Qiao W, Bu G, Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol. Psychiatry 83, 347–357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT, Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 103, 26–35 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K, Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clavaguera F et al. , Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol 11, 909–913 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frost B, Jacks RL, Diamond MI, Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem 284, 12845–12852 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woerman AL et al. , Kinetics of human mutant tau prion formation in the brains of two transgenic mouse lines. JAMA Neurol. 74, 1464–1472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolmont T et al. , Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP x Tau transgenic mice. Am. J. Pathol 171, 2012–2020 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasconcelos B et al. , Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. 131, 549–569 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW, Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med 14, 504–506 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Li JY et al. , Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med 14, 501–503 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Desplats P et al. , Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA 106, 13010–13015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iba M et al. , Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathol. 130, 349–362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye L et al. , Progression of seed-induced Aβ deposition within the limbic connectome. Brain Pathol. 25, 743–752 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu JW et al. , Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci 19, 1085–1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Braak H et al. , Pattern of brain destruction in Parkinson’s and Alzheimer’s diseases. J. Neural Transm. 103, 455–490 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Braak H, Del Tredici K, in Prion Biology, Prusiner SB, Ed. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2017), pp. 377–399. [Google Scholar]
- 72.Watts JC et al. , Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc. Natl. Acad. Sci. U.S.A. 111, 10323–10328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qiang W, Yau WM, Lu JX, Collinge J, Tycko R, Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 541, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rasmussen J et al. , Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 114, 13018–13023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Condello C et al. , Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 115, E782–E791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirbaha H et al. , Inert and seed-competent tau monomers suggest structural origins of aggregation. elife 7, e36584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharma AM, Thomas TL, Woodard DR, Kashmer OM, Diamond MI, Tau monomer encodes strains. eLife 7, e37813 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falcon B et al. , Conformation determines the seeding potencies of native and recombinant tau aggregates. J. Biol. Chem 290, 1049–1065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson SJ et al. , Short fibrils constitute the major species of seed-competent tau in the brains of mice transgenic for human P301S tau. J. Neurosci 36, 762–772 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Furman JL et al. , Widespread tau seeding activity at early Braak stages. Acta Neuropathol. 133, 91–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson NR et al. , Evidence for sortilin modulating regional accumulation of human tau prions in transgenic mice. Proc. Natl. Acad. Sci. USA 114, E11029–E11036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xia C et al. , Association of in vivo [18F]AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 74, 427–436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Condello C, Yuan P, Grutzendler J, Microglia-mediated neuroprotection, TREM2, and Alzheimer’s disease: Evidence from optical imaging. Biol. Psychiatry 83, 377–387 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edgeworth JA et al. , Detection of prion infection in variant Creutzfeldt-Jakob disease: a blood-based assay. Lancet 377, 487–493 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Concha-Marambio L et al. , Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med 8, 370ra183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bongianni M et al. , Diagnosis of human prion disease using real-time quaking-induced conversion testing of olfactory mucosa and cerebrospinal fluid samples. JAMA Neurol. 74, 155–162 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Lee IS, Long JR, Prusiner SB, Safar JG, Selective precipitation of prions by polyoxometalate complexes. J. Am. Chem. Soc 127, 13802–13803 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Holmes BB et al. , Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl. Acad. Sci. U.S.A. 111, E4376–E4385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bennett DA et al. , Religious Orders Study and Rush Memory and Aging Project. J. Alzheimers Dis 64, S161–S189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Development of YFP-Aβ fusion HEK-293T cell lines: synthetic Aβ fibril inocula and puncta-inducing kinetics..
Figure S2. Development of YFP-Aβ fusion HEK-293T cell lines: Individual clones
Figure S3. Amyloid plaque pathology, astrogliosis, and prion-like Aβ abundance during disease progression in TgAPP23 and TgCRND8 mice.
Figure S4. APOE ε4 status, gender, and brain region influence the extent of prion-like Aβ and tau abundance in AD postmortem brain tissue
Figure S5. Correlation of different Aβ and tau species with age at death of AD patients.
Table S1. Source of postmortem human brain tissue samples.
Date File S1