Abstract
Background:
Assessing the impact and effectiveness of HPV vaccines on anogenital warts in the United States can provide early indication of the success of vaccination programs as well as identify potential areas for improvement.
Methods:
Articles were identified from the PubMed, Medline, and Embase databases. Exclusion criteria were applied, and remaining studies were then classified as impact or effectiveness studies.
Results:
Eight eligible studies published through March 2018 were included. Population-based impact studies examining trends in diagnoses reported consistent declines in females ages 25 years and younger after 2006 when routine female vaccination began in the United States. Declines in males ages 25 years and younger were also seen; however, these declines were lower than those in females and more evident after routine male vaccination began in 2011. Among females and males older than 25 years, little to no change has been seen in the trends of anogenital warts since 2006. Studies that included the pre-vaccine era (before 2006) reported increasing trends during this period. After vaccine introduction, a reversal in these trends was observed. Effectiveness studies that included individual-level vaccination histories consistently demonstrated a lower risk of anogenital warts for those receiving at least one dose of the vaccine compared to those unvaccinated.
Conclusions:
These findings suggest that the degree of HPV vaccine impact has varied substantially by age and sex. Achieving the full prevention potential of HPV vaccines will likely require greater coverage among both females and males. Post-licensure estimates of effectiveness demonstrate the real-world benefit of the vaccine.
Human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States, with nearly 14 million new infections occurring every year.1 Greater than 90% of cases of anogenital warts are associated with two specific types of HPV, 6 and 11.2 Before introduction of the HPV vaccines in the United States, the prevalence of anogenital warts was estimated to be 5.6% among sexually active people 18 to 59 years old.3 Females and males 20 to 24 years of age experienced the greatest burden of anogenital warts, with incidence rates as high as 6.3 and 2.9 per 1000 person-years, respectively.4
Anogenital warts pose a significant challenge to both an individual’s health and the healthcare system. Diagnoses of anogenital warts are associated with negative psychosocial outcomes, including anxiety and depression.5 While clearance may occur spontaneously or with treatment, recurrences are common, occurring in approximately 30% of cases.6 Treatment can be lengthy, lasting months and requiring multiple healthcare visits. The national estimated direct cost associated with anogenital warts is over $220 million per year.7
HPV vaccination presents an opportunity to prevent nearly all cases of anogenital warts. Protection against HPV types 6 and 11 is provided by both the four-valent form of the HPV vaccine that has been available in the US since 2006 and the nine-valent vaccine that is in current use. Both vaccines were found to be highly efficacious against HPV types 6 and 11.8,9 Recommendations for routine administration to females at ages 11–12 years have been in place since 2006 in the United States, and similar recommendations for boys were introduced in 2011. Catch-up vaccination is recommended up to age 26 years for females and up to age 21 years for males; select high-risk males should be immunized to age 26 years.6 As a recommended preventive health service, cost of the vaccine is covered by all major private insurance companies in the US and the federal Vaccines for Children program for low-income families.10
The United States has achieved moderate levels of HPV vaccine coverage. As of 2016, 66% of adolescents between 13 and 17 years old had received at least 1 dose of the vaccine series and 49% completed the 2 or 3 dose series (depending on age at initiation).11 Because the United States does not have a national system in place for providing immunizations, practice-based immunization is the predominant mode of delivery. Missed opportunities for vaccination can occur in this setting for a number of reasons, including missed health care visits, lack of clinician recommendations, and low parental acceptance due to negative or ambivalent attitudes and lack of knowledge.12
While the efficacy of the vaccine has been established in numerous randomized trials, to determine its real-world benefit, it is important to assess both the population-level impact and post-licensure effectiveness of the vaccine outside of the tightly controlled settings of clinical trials. A recent systematic review and meta-analysis of HPV vaccine studies from around the world through 2016 reported high HPV vaccine impact on reducing anogenital warts. Significantly reduced risks of anogenital warts were observed for women 15 to 29 years old and men 15 to 24 years old when comparing the pre- and post-vaccination periods. The same review also reported that greater reductions in risk of anogenital warts were observed in countries with high coverage compared to countries with moderate coverage in both women and men.13 The purpose of this systematic literature review is to provide the first detailed review of the studies in the United States that assess the real-world impact and effectiveness of the HPV vaccine as it relates to anogenital warts including studies through 2018. In the US, where coverage of the vaccine is moderate, assessing the population-level trends and post-licensure individual-level effectiveness of the vaccine is crucial to identifying areas where the prevention potential of the vaccines is not being fully realized.
MATERIALS AND METHODS
The PubMed, Medline, and Embase databases were searched for relevant articles published during January 1, 2006 to March 12, 2018, using prespecified search terms. Search terms included the following subheadings: “papillomavirus vaccination,” “condylomata acuminata,” “incidence,” “prevalence,” “program evaluation,” “population surveillance,” and “epidemiological monitoring” (See Supplemental Content for complete search terms, http://links.lww.com/OLQ/A323). Duplicate references were then removed. The following exclusion criteria were then applied to the titles and abstracts of identified articles: studies not written in English, studies focused solely on the bivalent vaccine, review or editorial articles, modeling studies, cost-effectiveness analyses, clinical trials, studies set outside the United States, studies that did not measure the desired outcome of anogenital warts after introduction of vaccine, and conference abstracts with no associated papers. The full texts of the remaining articles were then assessed for eligibility using the same exclusion criteria. A Web of Science cited reference search was then performed using the eligible full-text articles to determine if any eligible articles were missed.
All eligible articles were then classified as either impact or effectiveness studies. Impact studies are those that are ecological in nature and describe population-based trends in the diagnoses of anogenital warts before and after introduction of HPV vaccines. Effectiveness studies are those that include individual-level vaccination histories and compared reductions in risks of warts between vaccinated and unvaccinated individuals in the post-licensure setting of use in real world conditions. Using quality assessment tables, all eligible articles were deemed of sufficient scientific rigor to include (see Supplemental Content for details, http://links.lww.com/OLQ/A324).
A standardized table was then used to extract the following information from each eligible full-text article: study location, data source, study population, sample size, data collection dates, and study design. Estimates of trends in anogenital warts or vaccine effectiveness against anogenital warts were then described for each included study, including results by available covariates, such as age and sex. This systematic review was registered with PROSPERO (registration number: 42018091189), an international database of prospectively registered systematic reviews with health-related outcomes. This review was found to be exempt from review by the Yale University Institutional Review Board.
RESULTS
Search Results
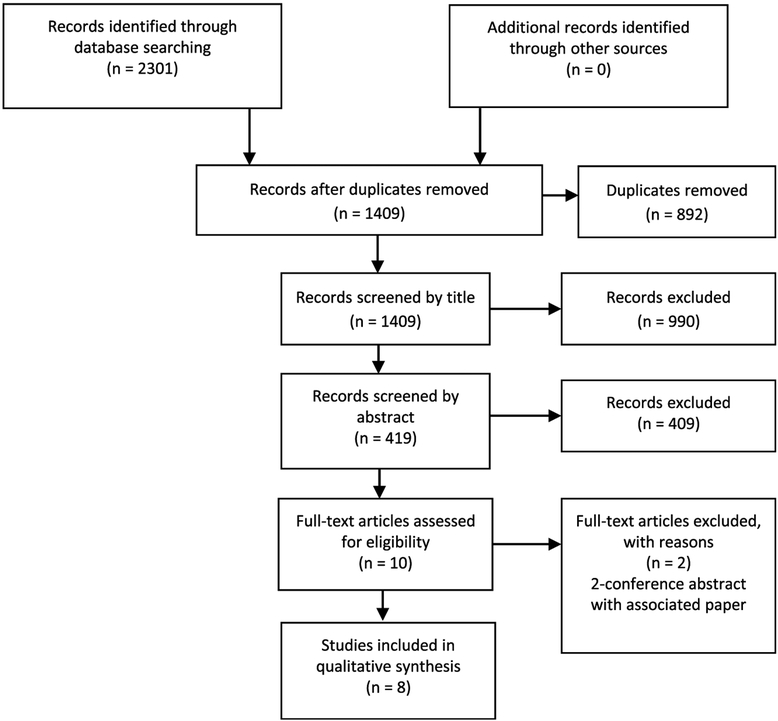
A total of 2,301 articles were initially identified. Of these, 892 were duplicates, leaving 1,409 unique references. After applying exclusion criteria to all titles, abstracts, and full text articles, 8 studies were determined to be eligible based on our inclusion criteria (Fig. 1). No other studies were identified in the Web of Science cited reference search. Of these eight studies, five were then classified as impact studies and three as effectiveness studies, and they are summarized below.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram detailing literature search and determination of articles for inclusion.
Impact Studies
A total of five impact studies were identified (Table 1). The first report of trends in anogenital warts in the post-vaccine era by Bauer et al. was conducted in California among low-income individuals receiving family planning services and estimated vaccine impact by subtracting the rate of anogenital warts in the last year of observation to the rate in the first year (2010 and 2007, respectively). In this study, the largest declines in anogenital warts (34.8%) were observed among women younger than 21 years. Significant decreases were also observed among women and men ages 25 years and younger though no declines were seen in either men or women older than 25 years.14 In the first study using a national sample, Flagg et al. assessed the impact of HPV vaccines by estimating the annual trends of anogenital wart prevalence. Unlike the previous study from Bauer et al., this study also included data from the pre-vaccine periods (2003 to 2010), which allowed it to account for pre-existing trends. In this study, decreases in the prevalence of anogenital warts were seen in women aged 15 to 29 years and men aged 20 to 24 years. Declines in males did not begin until 2009, three years after introduction of female vaccination in 2006. Similar to Bauer et al., no declines were seen in either females 30 to 39 years old or males 25 to 39 years old.15
TABLE 1.
Included impact studies, study characteristics, and main results
| Reference | Setting | Population | Design | Results |
|---|---|---|---|---|
| Bauer 2012 AJPH | California, USA 2007–2010 | All males and females (n = 8,053,738) | Ecological Clinical encounter claims data from California Family PACT insurance program |
Difference in incidence rate between first and last study year (2007 vs. 2010): |
| Females | ||||
| Age < 21 years: 34.8% decrease (P < 0.001) | ||||
| Age 21–25 years: 10.0% decrease (P < 0.001) | ||||
| Age 26–30 years: 10.1% increase (P = 0.004) | ||||
| Age > 30 years: 9.4% increase (P = 0.005) | ||||
| Males | ||||
| Age < 21 years: 18.6% decrease (P < 0.001) | ||||
| Age 21–25 years: 11.2% decrease (P < 0.001) | ||||
| Age 26–30 years: 9.0% increase (P = 0.05) | ||||
| Age > 30 years: no change (P = 0.97) | ||||
| Flagg 2013 AJPH | USA January 2003–December 2010 | All males and females aged 10 to 39 (n = 64,130,456) | Ecological Commercial insurance plan claims found through Truven Health Marketscan Database |
Change in annual incidence rate trends during 2003–2010: |
| Females | ||||
| Age 15–19 years: upward trend between 2003 and 2006, downward trend between 2006 and 2010 (P < 0.001) | ||||
| Age 20–24 years: upward trend between 2003 and 2006 (P < 0.001), no change from 2007 to 2009, downward trend between 2009 and 2010 (P < 0.001) | ||||
| Age 25–29 years: upward trend between 2003 and 2009 (P < 0.001), downward trend between 2009 and 2010 (P < 0.001) | ||||
| Age 30–39 years: upward trend between 2003 and 2009 (P < 0.001), no change from 2009 to 2010 | ||||
| Males | ||||
| Age 20–24 years: upward trend between 2003 to 2009 (P < 0.001), downward trend between 2009 and 2010 (P = 0.001) | ||||
| Age 25–29 years: upward trend between 2003 to 2010 (P < 0.001) | ||||
| Age 30–39 years: upward trend between 2003 to 2009 (P < 0.001), no change from 2009 to 2010 | ||||
| Nsouli-Maktabi 2013 MSMR | USA January 1, 2000 - December 31, 2012 | Males and females all ages serving in active component of armed forces (n ranged from 1,544,029 in 2000 to 1,440,362 in 2012) | Ecological Defensive Medical Surveillance System |
Change in annual incidence rate trends between 2000–2012: |
| Females | ||||
| Age < 25 years: downward trend from 2007 to 2010 (no P-values given) | ||||
| Age ≥ 25 years: unchanged between 2000 and 2010, increase between 2010 and 2012 (no P-values given) | ||||
| Males | ||||
| Age < 25 years: unchanged between 2000 and 2010, increase between 2010 and 2012 (no P-values given) | ||||
| Age ≥ 25 years: unchanged between 2000 and 2010, increase between 2010 and 2012 (no P-values given) | ||||
| Perkins 2015 STD | Boston area, USA 2004–2013 | All males and females aged 16 to 26 (n = 45,787) | Ecological De-identified medical record information from urban and community health centers |
Change in annual incidence rate trends between 2004 and 2013: |
| Females | ||||
| Age 16–26 years: rate of increase of 9.1% per year between 2004 and 2007 (P = 0.034), rate of decrease of 9.8% per year between 2007 and 2010 (P < 0.001), rate of decrease of 22.1% per year between 2011 and 2013 (P < 0.001) | ||||
| Males | ||||
| Age 16–26 years: rate of increase of 7.7% per year between 2004 and 2007 (P = 0.184), rate of decrease of 0.4% per year between 2007 and 2010 (P = 0.854), rate of decrease of 13.5% per year between 2011 and 2013 (P < 0.001) | ||||
| Flagg 2018 AJPH | USA January 2006–December 2014 | Males and females aged 15 to 39 N = 35,000,000 | Ecological Commercial insurance plan claims found through Truven Health Marketscan Database |
Change in annual incidence rate trends between 2006 and 2014: |
| Females | ||||
| Age 15–19 years: rate of decrease of 6.2% per year between 2006 and 2008 (P = 0.20), rate of decrease of 14.1% per year between 2009 and 2014 (P < 0.001) | ||||
| Age 20–24 years: unchanged between 2006 to 2009 (P = 0.56), rate of decrease of 12.9% per year between 2009 and 2014 (P < 0.001) | ||||
| Age 25–29 years: rate of increase of 12.9% per year between 2006 and 2009 (P = 0.009), rate of decrease of 6.0% per year between 2009 and 2014 (P = 0.001) | ||||
| Age 30–34 years: rate of increase of 17.6% per year between 2006 and 2009 (P = 0.009); unchanged between 2009 and 2014 (P = 0.25) | ||||
| Age 35–39 years: rate of increase of 11.8% per year between 2006 and 2009 (P = 0.002), rate of increase of 1.4% per between 2009 and 2014 (P = 0.030) | ||||
| Males | ||||
| Age 15–19 years: rate of increase of 16.9% per year between 2006 and 2009 (P = 0.08); rate of decrease of 5.4% per year between 2009 and 2014 (P = 0.048) | ||||
| Age 20–24 years: rate of increase of 20.6% per year between 2006 and 2009 (P = 0.020); rate of decrease of 6.5% per year between 2009 and 2014 (P = 0.005) | ||||
| Age 25–29 years: rate of increase of 22.5% per year between 2006 and 2009 (P = 0.007), rate of decrease of 1.7% per year between 2009 and 2014 (P = 0.17) | ||||
| Age 30–34 years: rate of increase of 22.6% per year between 2006 and 2009 (P = 0.010); rate of increase of 2.6% per year between 2009 and 2014 (P = 0.08) | ||||
| Age 35–39 years: rate of increase of 16.5% per year between 2006 and 2009 (P = 0.004), 2.4% per year increase between 2009 and 2014 (P = 0.025) |
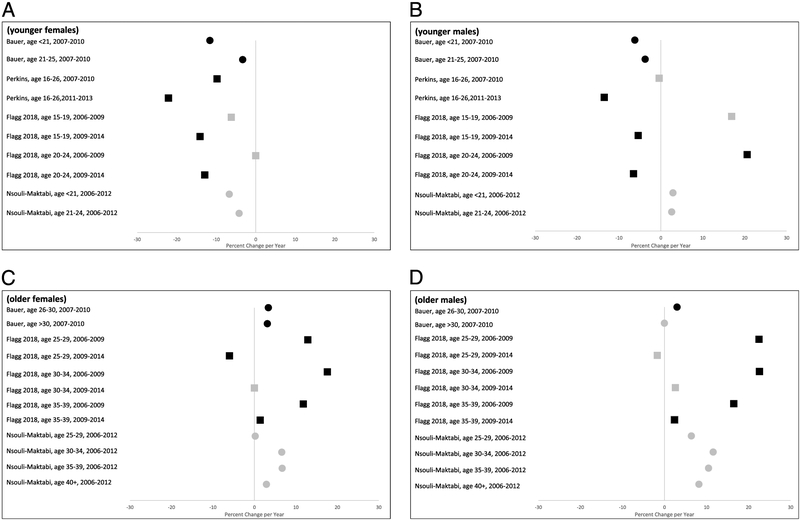
Three subsequent studies included later time periods (beyond 2010) that also encompassed the routine recommendations for male vaccination in 2011. In these studies, declines in rates were consistently demonstrated in females and males ages 25 years and younger. Perkins et al. looked at trends in diagnoses of anogenital warts among adolescents aged 16 to 26 years at community health centers between 2004 and 2013. In this study, an upward trend in the incidence of anogenital warts was observed among both males and females in the pre-vaccination era between 2004 and 2007. After vaccine introduction, a reversal in the direction of the trend (declining trend) was seen first in females (post-2007) and next in males (post-2011).16 A follow-up study was done by Flagg et al. that extended their previous national sample to include data up through 2014. In contrast to the first study where little or no impact was seen among men, this follow-up study demonstrated a clear and statistically significant trend reversal of a decline post-2011 among males aged 15 to 24 years.17 Nsouli-Maktabi et al. examined diagnoses in active duty armed services members and only observed declines in the annual rates of anogenital warts in females less than 25 years of age and no significant rate changes were seen among males of all ages and females aged 25 years and older.18 Visual display of results is presented in Figure 2.
Figure 2.
Forest plots depicting the percent change per year in anogenital warts as reported by the included impact studies for: A younger females, B younger males, C older females, and D older males. Black symbols represent statistically significant values (P < 0.05) while grey symbols represent non-significant values or values where no P-value was reported. Squares represent percent changes presented in the original study, and circles represent percent changes calculated from raw data presented in the original study. Estimates presented are restricted to the post-vaccine era (2006 and later). Four of five studies are included in the Figure: data from the Flagg 2018 publication are updates from the Flagg 2013 publication; thus only data from the 2018 paper are included.
Effectiveness Studies
A total of three effectiveness studies were identified (Table 2). Two studies focused on effectiveness of the vaccine to prevent anogenital warts in females and estimated how the number and the timing of doses affected the risk of anogenital warts. Overall, they found that having 3 doses (the full series recommended at that time) provided the greatest protection. Both studies also reported effectiveness with fewer doses, though to a lesser degree than three. Hariri et al. studied female enrollees in an integrated healthcare delivery system in three areas in the United States. The study utilized a 6-month buffer period from last vaccine dose to account for possible undiagnosed anogenital warts before vaccination. They found that risk of anogenital warts decreased by 68% for 2 doses separated by greater than 6 months and 77% for 3 doses compared to those unvaccinated.19 Perkins et al. evaluated girls aged 9 to 18 years enrolled in commercial provider-sponsored insurance plans also found that the incidence rate of anogenital warts was 30.8% lower among those who received 3 doses (1.5 cases/1000 person-years) compared to those who received zero doses (2.17 cases/1000 person-years).20
TABLE 2.
Included effectiveness studies, study characteristics, and main results
| Reference | Setting | Population | Design | Results |
|---|---|---|---|---|
| Swedish 2014 PLOS ONE | New York City, USA 2007–2010 | HIV-negative men who have sex with men aged 26 or older (n = 313) | Post hoc analysis of nonconcurrent cohort; Medical records from an anorectal surgery practice | Relative risk and 95% confidence intervals for vaccinated with 3 doses compared to not vaccinated: 3 doses: HRR = 0.45 (0.22, 0.92) |
| Perkins 2017 STD | USA January 1, 2007 - December 31, 2013 | Females aged 9 to 18 (n = 387,906) | Retrospective cohort; Commercial insurance plan claims found through Truven Health Marketscan Database |
Relative risk and 95% confidence intervals for vaccinated with less than 3 doses, by number of doses, compared to vaccinated with 3 doses: |
| 0 doses: IRR = 1.90 (1.66, 2.18) | ||||
| 1 dose: IRR = 1.22 (1.05, 1.41) | ||||
| 2 doses: IRR = 1.12 (0.97, 1.29) | ||||
| Hariri 2018 AJE | Colorado, Georgia, and Pacific Northwest, USA August 1, 2006 - September 30, 2012 |
Females aged 15 to 22 with evidence of sexual activity (n = 64,517) | Retrospective cohort; Administrative and electronic health record data from nationwide integrated healthcare delivery system |
Relative risks and 95% confidence intervals for vaccinated, by number of doses, compared to not vaccinated: |
| With 6-month buffer from last dose | ||||
| 1 dose: HRR = 0.81 (0.60, 1.08) | ||||
| 2 doses <6 months apart: HRR = 0.91 (0.59, 1.41) | ||||
| 2 doses ≥6-month apart: HRR = 0.32 (0.17, 0.59) | ||||
| 3 doses: HRR = 0.23 (0.17, 0.31) | ||||
| With 12-month buffer from first dose | ||||
| 1 dose: HRR = 0.32 (0.20, 0.52) | ||||
| 2 doses <6 months apart: HRR = 0.72 (0.41, 1.25) | ||||
| 2 doses ≥6-month apart: HRR = 0.24 (0.13, 0.44) | ||||
| 3 doses: HRR = 0.20 (0.15, 0.27) |
The third study focused on the effectiveness of the vaccine in men who have sex with men aged 26 years and older through a post-hoc analysis of a cohort study. It found that risk for anogenital warts decreased by 55% with 3 doses of vaccine. This estimate was adjusted for potential confounders, including history of anogenital warts.21
DISCUSSION
More than 12 years have passed since HPV vaccination was implemented in the US. At this point in time, reviewing the evidence for impact and post-licensure effectiveness of HPV vaccines is crucial to determine if progress is being made at preventing HPV-associated diseases in real-world settings. Given that the majority of anogenital warts appear between 2–3 months from incident infection, this outcome may serve as one of the first clinical indicators of the real-world effect of the vaccine.22 Once determined, the impact and effectiveness may highlight potential areas of needed improvement in vaccination efforts to achieve the desired reductions in HPV infections and associated sequelae.
Post-vaccine introduction, the most consistent finding among all studies was a reduced rate of anogenital warts among females 25 years of age and younger. Two of the studies (Perkins et al., Flagg et al.) reported that the incidence rate of anogenital warts was actually rising during the pre-vaccine era.15,16 Evaluating vaccine impact with data gathered only during the post-vaccine period may not account for the previous secular trends and thus could over- or under-estimate impact attributed to the vaccine. Assuming there were no systemic or extraneous influences on anogenital warts diagnoses during those study periods, a reversal from an upward to a downward trend post-vaccine introduction provides compelling evidence that HPV vaccines have reversed this trend and are contributing to the reduction of anogenital warts among younger females.
In contrast to the strong evidence of vaccine impact in females ages 25 years or younger, data to support real-world impact among men and women older than 25 years are limited. The little or no population-level impact seen in these groups are likely due to either their lower likelihood of vaccination or to vaccination after natural exposure given their age when the vaccine was introduced. Because the vaccine is prophylactic and not therapeutic, limited declines might be expected in these age groups during this review period. For males ages 25 and younger, declines in diagnoses of anogenital warts did occur, but the timing of these declines varied. Observed declines prior to routine male vaccination in 2011 could reflect some degree of herd immunity provided by vaccination of females. Studies that showed declines after male vaccination was routinely recommended likely reflect the direct effect of male vaccination. With a shorter period of time available to asses the direct impact on males, future monitoring of these trends is important.
Direct assessment in studies that compared vaccinated to unvaccinated populations is critical in the post-licensure period to determine real-world vaccine effectiveness as it is being used in clinical practice. These studies also demonstrate the benefit of the vaccine on reducing risk of anogenital warts at the individual level. These studies reported a reduced risk of anogenital warts for individuals who received at least one dose of the vaccine compared to those who were unvaccinated. This reduction in risk was seen in both females and men who have sex with men and demonstrated a dose–response effect (increased effectiveness with increasing number of doses of the vaccine received). More research is needed to assess if the effectiveness of the vaccine will vary significantly with the most recent 2-dose schedule.
Countries with moderate HPV vaccination coverage similar to the US have achieved comparable declines in anogenital warts. For example, in Germany, one year after vaccine introduction, there was a 23% reduction in incidence of anogenital warts among females aged 15 to 19 years.23 Similar to data from the US, evidence of vaccine impact among males in other countries have been mixed. For example, a study in Ontario, Canada did not observe decreases in males of any age after beginning female vaccination while another study set in Quebec during the same time period found decreases of 21% in males aged 15 to 19 years.24,25 These findings suggest that in countries with moderate vaccine uptake like the US, herd immunity may only play a low to modest role in preventing disease. Estimates of vaccine effectiveness in the US were also comparable to that observed in other counties; a provincial cohort study in Canada found a 56% vaccine effectiveness against anogenital warts among those who received three doses before the age of 18.26 Similarly, an effectiveness study set in Sweden found a 76% vaccine effectiveness for those who received three doses before the age of 20.27
In countries with national immunization delivery systems and high HPV vaccine uptake, reductions in anogenital warts have been greater than those observed in the United States. In an analysis of the national patient registry for Denmark, a statistically significant decline of 45.3% was observed in females aged 16–17 years old after introduction of the four-valent vaccine.28 In Australia, the diagnoses of anogenital warts were nearly eliminated in women younger than 21 years old, decreasing by 92.6% from 2007 to 2011.29 In addition to the overall greater reductions in anogenital warts in women residing in high vaccine coverage countries, these countries may also experience a greater herd immunity effect. A study in Australia found an 81.8% decrease in diagnoses of anogenital warts in males younger than 21 years old.29 These findings demonstrate the substantial prevention potential of HPV vaccines for the US if higher coverage could be achieved.
Our review did have some limitations. Because of the small number of eligible studies, our review was descriptive and narrative, and no formal meta-analysis was conducted. However, the included studies did cover a wide range of populations, including national samples, men who have sex with men, and adolescents. Furthermore, as with any systematic literature review, there exists the possibility of publication bias. However, included studies did have both positive and negative results, reflecting a range of findings.
Even with suboptimal uptake of the vaccine, the United States has experienced significant declines in the rates of anogenital warts. However, the magnitude of declines and effects for some populations (e.g., men) have been modest compared to countries with higher uptake, suggesting that the full prevention potential of these vaccines has not yet been reached in the United States. Ongoing monitoring is needed to continue to assess progress, and greater efforts to increase immunization are needed to further reduce the incidence of anogenital warts and their associated individual and public health consequences in the US.
Supplementary Material
Conflict of Interest and Sources of Funding:
Dr Niccolai reports previous work as a Scientific Advisor for Merck. All other authors declare no conflicts of interest. This work was supported in part by the National Institutes of Health (grant no. R01AI123204; to L.M.N.), American Cancer Society (grant PF-16-016-01; to C.R.O.), and Robert E. Leet and Clara Guthrie Patterson Trust Mentored Research Award (to C.R.O.).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2008. Sex Transm Dis 2013; 40:187–193. [DOI] [PubMed] [Google Scholar]
- 2.Greer CE, Wheeler CM, Ladner MB, et al. Human papillomavirus (HPV) type distribution and serological response to HPV type 6 virus-like particles in patients with genital warts. J Clin Microbiol 1995; 33:2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinh T-H, Sternberg M, Dunne EF, et al. Genital Warts Among 18- to 59-Year-Olds in the United States, National Health and Nutrition Examination Survey, 1999–2004. Sex Transm Dis 2008; 35:357–360. [DOI] [PubMed] [Google Scholar]
- 4.Camenga DR, Dunne EF, Desai MM, et al. Incidence of Genital Warts in Adolescents and Young Adults in an Integrated Health Care Delivery System in the United States Before Human Papillomavirus Vaccine Recommendations. Sex Transm Dis 2013; 40:534–538. [DOI] [PubMed] [Google Scholar]
- 5.Woodhall SC, Jit M, Soldan K, et al. The Impact of Genital Warts: Loss of Quality of Life and Cost of Treatment in Eight Sexual Health Clinics in the UK. Sex Transm Infect 2011; 87:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz LE, Dunne EF, Saraiya M, et al. Human Papillomavirus Vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2014; 63:1–30. [PubMed] [Google Scholar]
- 7.Hoy T, Singhal PK, Willey VJ, et al. Assessing Incidence and Economic Burden of Genital Warts with Data from a US Commercially Insured Population. Curr Med Res Opin 2009; 25:2343–2351. [DOI] [PubMed] [Google Scholar]
- 8.Joura EA, Giuliano AR, Iversen O-E, et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N Engl J Med 2015; 372:711–723. [DOI] [PubMed] [Google Scholar]
- 9.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent Vaccine against Human Papillomavirus to Prevent Anogenital Diseases. N Engl J Med 2007; 356:1928–1943. [DOI] [PubMed] [Google Scholar]
- 10.Niccolai LM, Hansen CE, Credle M, et al. Parents’ Views on Human Papillomavirus Vaccination for Sexually Transmissible Infection Prevention: A Qualitative Study. Sex Health 2014; 11:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker TY, Elam-Evans LD, Yankey D, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2017. MMWR Morb Mortal Wkly Rep 2018; 67(33):909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niccolai LM, North AL, Footman A, et al. Lack of School Requirements and Clinician Recommendations for Human Papillomavirus Vaccination. J Public Health Res 2018; 7:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drolet M, Benard E, Perez N, et al. Population-Level Impact and Herd Effects Following Human Papillomavirus Vaccination Programs: Evidence from Post-Vaccination Studies in High-Income Countries Presented at: Eurogen 2017; 2017; Amsterdam. [Google Scholar]
- 14.Bauer HM, Wright G, Chow J. Evidence of Human Papillomavirus Vaccine Effectiveness in Reducing Genital Warts: An Analysis of California Public Family Planning Administrative Claims Data, 2007–2010. Am J Public Health 2012; 102:833–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flagg EW, Schwartz R, Weinstock H. Prevalence of Anogenital Warts Among Participants in Private Health Plans in the United States, 2003–2010: Potential Impact of Human Papillomavirus Vaccination. Am J Public Health 2013; 103:1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins RB, Legler A, Hanchate A. Trends in Male and Female Genital Warts Among Adolescents in a Safety-Net Health Care System 2004–2013: Correlation with Introduction of Female and Male Human Papillomavirus Vaccination. Sex Transm Dis 2015; 42:665–668. [DOI] [PubMed] [Google Scholar]
- 17.Flagg EW, Torrone EA. Declines in Anogenital Warts Among Age Groups Most Likely to Be Impacted by Human Papillomavirus Vaccination, United States, 2006–2014. Am J Public Health 2018; 108:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nsouli-Maktabi H Incidence of Genital Warts Among U.S. Service Members Before and After the Introduction of the Quadrivalent Human Papillomavirus Vaccine. MSMR 2013; 20(2):17. [PubMed] [Google Scholar]
- 19.Hariri S, Schuler MS, Naleway AL, et al. Human Papillomavirus Vaccine Effectiveness Against Incident Genital Warts Among Female Health-Plan Enrollees, United States. Am J Epidemiol 2018; 187:298–305. [DOI] [PubMed] [Google Scholar]
- 20.Perkins RB, Lin M, Wallington SF, et al. Impact of Number of Human Papillomavirus Vaccine Doses on Genital Warts Diagnoses Among a National Cohort of U.S. Adolescents. Sex Transm Dis 2017; 44(6):365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swedish KA, Goldstone SE. Prevention of Anal Condyloma with Quadrivalent Human Papillomavirus Vaccination of Older Men Who Have Sex with Men. PLoS One 2014; 9:e93393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winer RL, Kiviat NB, Hughes JP, et al. Development and Duration of Human Papillomavirus Lesions, after Initial Infection. J Infect Dis 2005; 191:731–738. [DOI] [PubMed] [Google Scholar]
- 23.Mikolajczyk RT, Kraut AA, Horn J, et al. Changes in Incidence of Anogenital Warts Diagnoses After the Introduction of Human Papillomavirus Vaccination in Germany—An Ecologic Study. Sex Transm Dis 2013; 40:28–31. [DOI] [PubMed] [Google Scholar]
- 24.Guerra FM, Rosella LC, Dunn S, et al. Early Impact of Ontario’s Human Papillomavirus (HPV) Vaccination Program on Anogenital Warts (AGWs): A Population-Based Assessment. Vaccine 2016; 34:4678–4683. [DOI] [PubMed] [Google Scholar]
- 25.Steben M, Ouhoummane N, Rodier C, et al. The Early Impact of Human Papillomavirus Vaccination on Anogenital Warts in Québec Canada. J Med Virol 2018; 90:592–598. [DOI] [PubMed] [Google Scholar]
- 26.Willows K, Bozat-Emre S, Righolt CH, et al. Early Evidence of the Effectiveness of the Human Papillomavirus Vaccination Program Against Anogenital Warts in Manitoba, Canada: A Registry Cohort Study. Sex Transm Dis 2017; Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 27.Leval A, Herweijer E, Ploner A, et al. Quadrivalent Human Papilloma-virus Vaccine Effectiveness: A Swedish National Cohort Study. J Natl Cancer Inst 2013; 105:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baandrup L, Blomberg M, Dehlendorff C, et al. Significant Decrease in the Incidence of Genital Warts in Young Danish Women After Implementation of a National Human Papillomavirus Vaccination Program. Sex Transm Dis 2013; 40:130–135. [DOI] [PubMed] [Google Scholar]
- 29.Ali H, Donovan B, Wand H, et al. Genital Warts in Young Australians Five Years into National Human Papillomavirus Vaccination Programme: National Surveillance Data. BMJ 2013; 346:f2032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.