Summary
In many settings, tumor-associated inflammation, supported mainly by innate immune cells, contributes to tumor growth. Initial innate activation triggers secretion of inflammatory, regenerative and anti-inflammatory cytokines, which in turn shape the adaptive immune response to the tumor. Here we review the current understanding of the intricate dialogue between cancer-associated inflammation and anti-tumor immunity. We discuss the changing nature of these interactions during tumor progression and the impact of the tissue environment on the anti-tumor immune response. In this context, we outline important gaps in understanding considering basic research and findings in the clinic. The future of cancer immunotherapy and its utility depend on improved understanding of these interactions and the ability to manipulate them in a predictable and beneficial manner.
Introduction: ENTRÉE
Tumor-associated inflammation was initially described by Virchow in the 19th century. It is now appreciated to be an important driver of malignant progression, and as such has become an extensively studied aspect of cancer biology and a focal point for drug development efforts. (Galdiero et al., 2018; Grivennikov et al., 2010; Ruffell and Coussens, 2015). As has been amply discussed (Grivennikov et al., 2010), cancer-associated inflammation can predate the appearance of visible tumors, being caused by a non-cancerous inflammatory conditions, such as colitis or hepatitis. It can also prepare the scene for malignant and metastatic lesions that are being supported through a variety of inflammatory cytokines, growth factors and angiogenesis-promoting proteins. Inflammation, in rare cases, may also contribute to accumulation of oncogenic mutations (Shaked et al., 2012). Conversely, oncogenic mutations and other signaling alterations inherent to cancer cells can promote tumor-elicited inflammation via production of chemokines and cytokines, alterations in tissue structure and oxygen pressure, and of particular importance in gastrointestinal and lung cancers, loss of barrier integrity that facilitates translocation of microbial products.
The majority of cell types involved in tumor-associated inflammation belong to the innate arm of the immune system, acting, by-and-large, in a tumor-supportive manner that should be contrasted with anti-tumor immunity, the working of the adaptive arm of the immune system. Realizing the incredible capacity of adaptive immune cells, whether they are expressing rearranged B and T cell receptors or secreting antibodies that recognize an infinite array of peptide and polysaccharide antigens, Burnet and Thomas came up with the immunosurveillance hypothesis (Ribatti, 2016). Lacking hard support and contradicted by the finding of little difference in cancer rates between immune-intact and immune-deficient experimental animals, immunosurveillance was replaced with the immunoediting hypothesis according to which early tumors avoid immune rejection by editing their tumor antigens (Ward et al., 2016). Alternatively, early tumors establish an immunosuppressive microenvironment (Zou, 2005). Importantly, tumor-associated inflammation and anti-cancer immunity are not necessarily antagonistic and as discussed below, the relationships between them are intricate, being time and context dependent.
The impact of tumor-associated inflammation on the cancerous cell itself and its role in malignant progression are extensively discussed elsewhere in this issue (Grivennikov & Greten). This review is focused on the delicate and ever-changing dialogue between tumor-associated inflammation and anti-cancer immunity. Understanding of this dialogue is essential for identifying strategies for implementation of more effective immunotherapies. As almost everything in biology is governed by the law of action and reaction, it is naïve to expect that the mere dismantling of tumor-promoting inflammation would activate anti-tumor immunity. Accordingly, here we focus on the interactions between tumor-associated inflammation and antitumor immunity, using gastrointestinal and liver cancers as the primary examples. We will use the Pas de Deux (French: step of two) ballet dance concept as an analogy to the intricate and fluid interactions between cancer-related inflammation and immunity. A “grand pas de deux” is typically structured in five parts: entrée (introduction), adagio (description and initiation), two variations (a solo for each dancer), and coda (conclusion and the final work). Our goal is to assemble the current understanding of these interactions into a framework that can lead to further investigation and the development of more effective cancer-directed immunotherapies. As coda, we highlight the many gaps in basic knowledge that need to be filled to move the field forward.
ADAGIO: Initiation and General Description
Our common perception of inflammation is that it is troublesome, annoying, and even damaging, a pathological response that needs to be prevented through the use of anti-inflammatory drugs. However, inflammation deserves much respect and appreciation, as it is one of the most primordial protective responses elicited by injury and infection, being essential for both tissue regeneration (Karin and Clevers, 2016) and innate immunity (Kotas and Medzhitov, 2015). Likewise, adaptive immunity is commonly viewed as highly beneficial and protective, but anyone who has suffered from allergy, dermatitis, or an auto-immune disease is fully aware of the nasty nature of uncontrolled immunity, coined by Ehrlich as “horror autotoxicus” (Fruton, 1957). A typical inflammatory response evoked by either injury or infection can be divided into three phases: initiation, amplification, and resolution. At each phase, acute inflammation has different effects on either cellular or humoral immunity (Iwasaki and Medzhitov, 2015). Triggered either by damage associated molecular patterns (DAMP) released by dying cells or pathogen associated molecular patterns (PAMP) released or presented by mislocalized commensals or invading pathogens, the initial inflammatory response entails degranulation of neutrophils and platelets and subsequent activation of tissue macrophages. The chemotactic peptides, lipid mediators (leukotrienes and prostaglandins), chemokines, and cytokines that are released during the initial inflammatory response lead to recruitment, differentiation, and activation of circulating monocytes and lymphocytes that congregate at the site of injury or infection or on the nascent tumor, which may be perceived as an irregularity in tissue structure. In addition to conversion of monocytes to macrophages, this response also involves activation of dendritic cells (DC), thereby augmenting their ability to take up antigens and present them to naïve T cells. Activated DC and macrophages also express a variety of co-stimulatory molecules that potentiate T cell activation as well as immunoregulatory cytokines, such as IL-10, IL-12, and IL-23, that dictate the nature of the ensuing immune response (Merad et al., 2013). Thus, during the initiation and amplification phases, inflammation plays an important immunostimulatory function and its complete suppression may compromise anti-tumor immunity. The resolution phase, however, is characterized by production of anti-inflammatory lipid mediators and cytokines such as resolvins, IL-10 and TGFβ, as well as growth factors (e.g. EGF family members), all of which are needed for termination of damaging inflammation, clearance of cell and microbial debris, and initiation of tissue repair and regeneration. To prevent over-stimulation of potentially damaging adaptive immunity, these molecules lead to further modulation of adaptive immunity, enhancing the generation of regulatory T and B cells that suppress ongoing immune activation (Figures 1). Interference with these reparative and anti-inflammatory factors may augment anti-tumor immunity but carries the risk of enhanced injury, bleeding, etc.
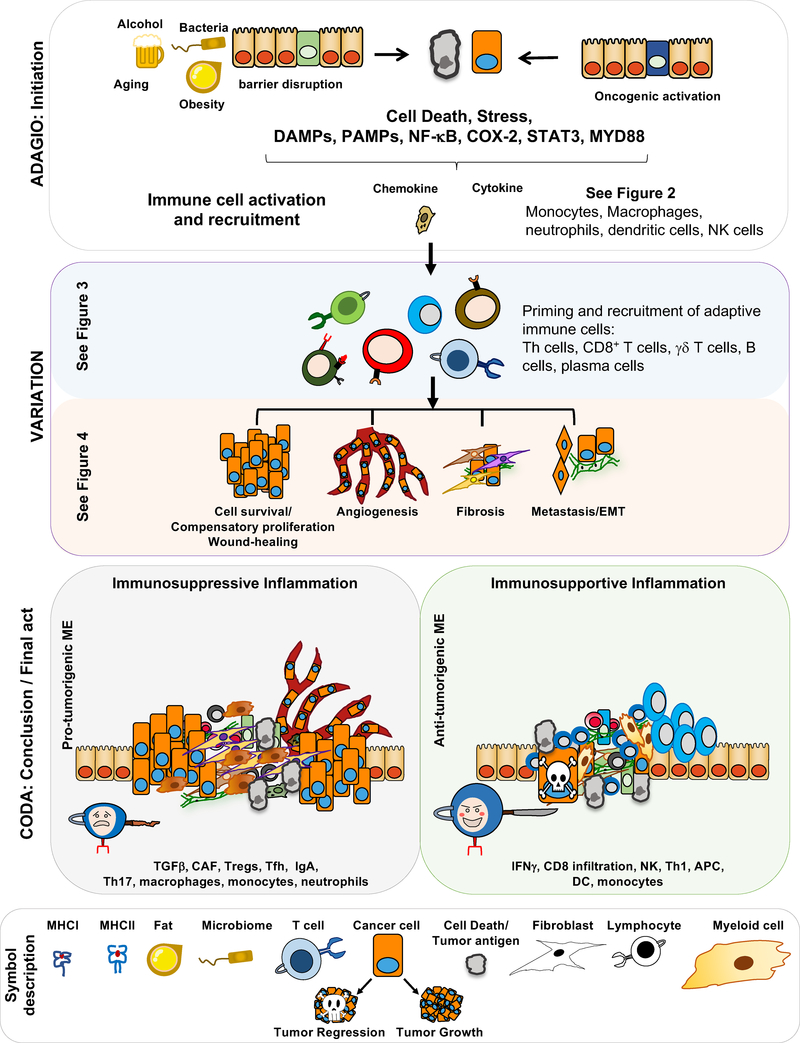
Figure 1: GRAND PAS DE DEUX: Inflammation, Immunity, and Cancer.
A schematic description of how cancer-associated inflammation develops, interacts with and modulates anti-tumor immunity. Importantly, inflammation can be either immunosuppressive or immunosupportive. For instance, the extent of myofibroblast activation and TGFβ production can tilt the inflammatory response in a more immunosuppressive direction.
This well-orchestrated and choreographed response assumes a considerably different face during chronic inflammation, the kind of inflammation associated with tissue destructive autoimmunity as well as tumor growth and progression. It is not always clear exactly how tumor-associated inflammation is initiated. It has been speculated that the disorderly structure formed by premalignant lesions is perceived by innate immune cells as tissue injury. The perception of tissue injury is further propagated by DAMP release from dying cancer cells and formation of hypoxic tumor cores (Tafani et al., 2016). In other cases, for instance cancers of the gastrointestinal system or the airways, adenoma formation results in loss of barrier integrity, leading to a chronic influx of PAMPs and even live commensals that maintain long-lasting, smoldering inflammation that is characterized by ongoing production of cytokines, such as IL-6, IL-23, IL-10, and IL-17 (Grivennikov et al., 2010, 2012). Chronic tumor-associated inflammation is also accompanied by ongoing production of TGFβ that, in addition to its ability to activate cancer-associated fibroblasts (CAF) and stimulate tumor angiogenesis, supports differentiation of immunosuppressive cell types, including regulatory T cells (Treg) and IgA-expressing plasmocytes (Massagué, 2008; Sanjabi et al., 2009; Shalapour et al., 2017). Another highly important and multifaceted inflammatory and regulatory cytokine is VEGF. While being the major mediator of vascular permeability during the initiation phase, VEGF stimulates formation of new blood vessels during resolution and healing (Ferrara et al., 2003). Healing, which is often perturbed during chronic autoimmunity, favors immunosuppression over immune activation, thereby boosting tumor growth, progression, and eventual immune evasion.
Here, we will mainly focus on how inflammation directly shapes the development and response of CD8+ T cells, CD4+ T helper (Th) cells and B cells, the main adaptive immune cell types, mostly through production of immunoregulatory cytokines and chemokines, and how these influence adaptive immune cells’ response to cancer cells. We will not deeply discuss innate LC (iLC), γδ T cells or NK T cells (Chou and Li, 2018; Fleming et al., 2017; Pahl and Cerwenka, 2017; Zitti and Bryceson, 2018). We will also focus on how inflammation regulates non-hematopoietic cells and thereby indirectly shapes adaptive cancer immunity.
Main Inflammation Inducers: Stress and Damage Signals
Tumor growth results in disturbance of tissue architecture and stress. Some of this stress, as exemplified by the hypoxic response, is due to the much higher glycolytic rate of rapidly dividing cancer cells compared to the surrounding normal cells and the slow growth of newly-formed tumor blood vessels. Tumor hypoxia results in HIF-1α induction, CAF activation and secretion of TGFβ (Ammirante et al., 2014) and a large number of chemokines that trigger the influx of diverse myeloid and lymphoid cell types, including pro-angiogenic monocytes and macrophages (Cruz et al., 2017; Dolan et al., 2006). Thus, in addition to inflammation, tumor hypoxia supports formation of an immunosuppressive microenvironment (Barsoum et al., 2014). Another important consequence of tumor growth within a barrier epithelium, as represented by formation of colonic adenomas, is barrier disruption, which is followed by an influx of microbial products, such as endotoxin and nucleic acids, that activate resident tissue macrophages and DC through engagement of Toll-like receptors (TLRs), including TLR2, 4, and 9 and activation of transcription factor MyD88 (Grivennikov et al., 2012). Many types of cancers also exhibit endoplasmic reticulum (ER) stress, an important contributor to tumorigenesis (Wang and Kaufman, 2014). ER stress induced by saturated fatty acids and cholesterol (Nakagawa et al., 2014; Wang et al., 2006) or different metabolic disorders further enhances inflammation by activating JNK–AP-1 and IKK-NF-κB signaling cascades that stimulate the production of pro-inflammatory cytokines (e.g., TNF, IL-6, IL1β) that support tumorigenesis, while modulating immune cell function, as outlined above (Garg et al., 2012). ER stress within DC can result in immunosuppression (Cubillos-Ruiz et al., 2015). Within cancer cells, most stress responses have also been linked to immunogenic cell death-associated release of DAMPs and upregulation of “eat-me signals” that were proposed to support cross-presentation and priming of cytotoxic CD8+ T cells (CTL) (Kepp et al., 2013, 2015).
Most cancers exhibit oxidative stress and many chemotherapeutic drugs further enhance production of reactive oxygen species (ROS) within cancer cells (Conklin, 2004). Oxidative stress can result in damage to both nuclear and mitochondrial DNA, and non-oxidized DNA fragments that are released to the cytoplasm lead to activation of cGAS-STING signaling, thereby culminating in type I IFN induction (Chen et al., 2016). Type I IFN potentiates T cell activation but can also enhance pro-tumorigenic inflammation (Liu et al., 2018). Oxidized DNA fragments, however, especially oxidized and fragmented mitochondrial DNA, lead to activation of the NLRP3 inflammasome, thereby inducing pro-tumorigenic IL-1β and IL-18 (Zhong et al., 2018). Stressed cells, cancer cells included, express stress antigens, such as CD1d and NKG2D, that engage NK receptors and lead to NK cell activation, which can have direct antitumorigenic effects (Bauer, 1999) but can also support tumor-promoting inflammation (Ogura et al., 2018). Extreme hypoxia and cell stress can result in cell death. Whereas apoptotic cell death is usually tolerogenic, necroptotic cell death can trigger inflammation or immune stimulation (Green et al., 2009). Certain chemotherapeutic drugs, such as anthracyclines, oxaliplatin, and methotrexate, were reported to cause a more poorly defined form of cell death referred to as immunogenic cell death, by virtue of its ability to enhance adaptive immunity to model antigens injected into mice (Galluzzi et al., 2017). While the exact mechanism of immunogenic cell death is nebulous, it is well accepted that DAMPs engage TLRs and thereby lead to DC maturation and enhanced antigen uptake and cross-presentation (Zelenay and Sousa, 2013). Altogether, this enhances T cell priming although it also leads to production of pro-tumorigenic inflammatory cytokines. Immunogenic cell death, however, is not essential for T cell priming, as DC can also sample antigens that are expressed by live cells and cross-present them to naïve T cells, leading to formation and expansion of effector T cells (Cruz et al., 2017). Moreover, by releasing DAMPs (e.g. IL-1α, HMGB1) and inducing molecules like TIM-1, CD154 and CD47 through oxidative stress, therapy-induced cancer cell death and hypoxia can be either immunosuppressive or support development of immunosuppressive cells (Griffith and Ferguson, 2011; Liu et al., 2015; Seifert et al., 2016; Shalapour et al., 2015; Steinman, 2012; Xiao et al., 2015; Ye et al., 2018).
VARIATIONS: Hematopoietic Cells, Particularly Myeloid Cells, in the Inflamed TME
The initiation of a successful anti-tumor immune response requires T cell priming which is mediated by antigen presenting cells (APC), the most common of which are DC that pick up tumor antigens and present them to naïve T cells (Guermonprez et al., 2002; Steinman, 2012). Different inflammatory signaling pathways modulate antigen delivery to APC. ER stress suppresses DC development and supports tumor progression (Cubillos-Ruiz et al., 2015), whereas mitophagy in epithelial cells supports anti-tumor immunity by increasing the cross-dressing of DC with MHCI molecules (Ziegler et al., 2018). Cross-dressing, or trogocytosis, is the mechanism in which entire peptide–MHCI complexes from donor cells are transferred to the DC, which presents them on its surface (Cruz et al., 2017; Dolan et al., 2006). Inflammation and inflammatory signals induce DC maturation and enhance their expression of various co-stimulatory molecules (Blanco et al., 2008) (Figure 2).
Figure 2: Inflammation in the early stages of cancer.
Stress, cell death, obesity, bacterial infection and translocation of microbial components across disrupted barriers induce innate immune cell activation and increased expression of chemokines and cytokines that promote infiltration of adaptive immune cells to the site of tissue stress or injury. Moreover, myeloid cells, especially DC, take up antigens and present them to T cells to induce CTL activation. Despite increasing antigen release, cell death can also be immunosuppressive and tolerogenic, thereby suppressing CTL activation. In a similar manner, monocytes and macrophages can suppress CTL-mediated tumor rejection through expression of IL-10, ARG1, IDO, and TGFβ.
Although tumor-associated DCs have been described (Böttcher and Reis e Sousa, 2018; Merad et al., 2013), the most common myeloid cells in the tumor microenvironment (TME) are tumor-associated macrophages (TAM). The phenotype and function of these highly plastic cells are easily altered and adapted in response to various environmental and metabolic cues (Mantovani et al., 2017; Ruffell and Coussens, 2015). Obviously, initial inflammatory activation results in production of cytokines, such as IL-1β, TNF, and IL-6, that promote tumor growth as well as VEGF that supports neo-angiogenesis. However, even during late phases of inflammatory activation, TAMs support tumor progression through production of immunosuppressive cytokines (Figure 2), PD-L1 (Kuang et al., 2009), B7-H4 (Kryczek et al., 2006) and indoleamine 2,3-dioxygenase (IDO), a tryptophan hydrolyzing enzyme that attenuates effector T cell functions (DeNardo and Ruffell, 2019; Ruffell and Coussens, 2015). Immunosuppressive TAMs also produce TGFβ and IL-10 (Figure 2). Similar tumor-supporting functions can be expressed by inflammatory monocytes and granulocytes, which produce VEGF, ARG1, PGE2, and IL-10 (Veglia et al., 2018).
Pro- and Anti- Inflammatory Cytokines as Modulators of T Cell Responses
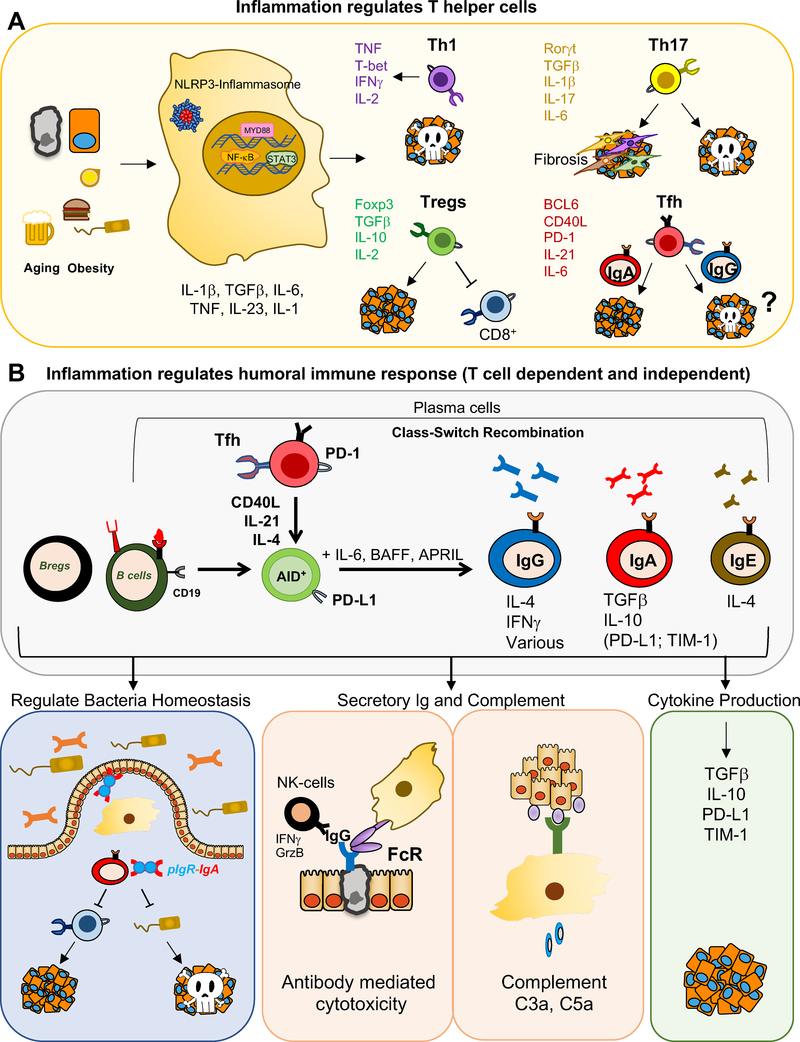
Tumor resident myeloid cells secrete numerous cytokines that exert either pro-inflammatory or anti-inflammatory effects. The impact that these cytokines have on malignant cells and components of the tumor microenvironment has been reviewed (Galdiero et al., 2018). Here we restrict our discussion to their impact on anti-tumor immunity. A major pro-inflammatory cytokine is IL-6. In addition to its well-studied proliferation, and survival-supporting effects on normal and malignant epithelial cells, mediated through STAT3 and YAP transcription factors (Taniguchi and Karin, 2018), IL-6 is an immunomodulatory cytokine that promotes Th cell differentiation and effector functions (Dienz and Rincon, 2009), and also affects a subset of naïve CD8+ T cells (Yang et al., 2016). IL-6 can support Th2 differentiation by up-regulating IL-4 production, which is not as anti-tumorigenic as Th1 differentiation and was even shown to support tumor growth (De Monte et al., 2011; Diehl et al., 2002). IL-6 can also act immunosuppressively by inhibiting IFN-γ production and Th1 differentiation via SOCS1 induction (Diehl et al., 2000). Inhibition of Th1 differentiation precludes the mounting of a robust anti-tumor CTL response by making CD8+ T cells helpless (Bevan, 2004). IL-6 together with TGFβ contributes to Th17 cell differentiation (Bettelli et al., 2006; Ivanov et al., 2006), thereby supporting early tumorigenesis (Grivennikov et al., 2012; Jin et al., 2019). IL-6 also induces IL-21 production by CD4+ T cells (Diehl et al., 2012; Dienz et al., 2009; Suto et al., 2008) and is required for generation of T follicular helper (Tfh) cells (Nurieva et al., 2008), in which it leads to BCL6 induction through STAT1 activation (Choi et al., 2013). By inducing Tfh differentiation and IL-21 production, IL-6 indirectly promotes class-switch recombination (CSR) which requires activation-induced cytidine deaminase (AID) induction, converting B cells to plasma cells, thereby enhancing antibody secretion (Diehl et al., 2012; Dienz et al., 2009; Suto et al., 2008) (Figure 3). The generation of IgA-producing plasma cells, as discussed below, suppresses tumor-directed CTL responses in prostate and liver cancers (Shalapour et al., 2015, 2017). Altogether, IL-6 shifts anti-tumor immunity to a tumor-supportive immunosuppressive response, an important aspect of its tumor-promoting function that so far has been overlooked (Figure 3).
Figure 3: Role of Hematopoietic Cells, Particularly Adaptive Immune Cells in Anti-Tumor Immunity in Inflamed TME.
A) Development of different Th cell subsets in the context of an inflammatory tumor environment. Inflammation supports inhibitors of CTL activation and impairs CTL activators. B) Humoral immunity- good or bad? By-and-large, humoral immunity can suppress CTL activation and thereby support tumor growth, but it can also facilitate NK cell activation, through antibody-mediated cytotoxicity and thereby enhancing anti-tumor immunity.
However, the role of regulatory T cells (Treg) and Th17 cells seem to be tumor type and context dependent. Th17 cells seem to support the initiation of malignant transformation in liver, colon and lung (Grivennikov et al., 2012; Jin et al., 2019; Knochelmann et al., 2018), however they may have an antitumorigenic function in late stages of colon cancer and melanoma (Muranski et al., 2008; Viaud et al., 2013). Tregs, on the other hand, suppress inflammation and development of colorectal adenomas but support development of liver tumors, breast cancer metastasis and advanced carcinomas, when they have the ability to suppress CD8+ T cells (Saito et al., 2016; Tanaka and Sakaguchi, 2017). In fact, part of the anti-tumor activity of CTLA4 blocking antibodies is probably due to conversion of Treg into effector T cells (Paterson et al., 2015; Wei et al., 2018). Treg can also support cancer through secretion of cytokines in breast cancer (Tan et al., 2011).
Other important immunomodulatory cytokines produced by activated macrophages and DC are IL-12 and IL-23, which are heterodimers that share a common p40 subunit (Teng et al., 2015). Whereas IL-12 has anti-tumorigenic activity in skin cancer, IL-23 is pro-tumorigenic in both skin and colon (Grivennikov et al., 2012; Langowski et al., 2006; Teng et al., 2010). However, IL-23 receptors are not expressed by epithelial cells, and pro-tumorigenic IL-23 activity in early colorectal cancer depends on its ability to expand IL-17 producing cells, including Th17 and γδT cells (Grivennikov et al., 2010). Unlike IL-23, IL-17A, the predominant pro-tumorigenic IL-17 family member, acts directly on tumor progenitors that express IL-17 receptor A (Wang et al., 2014) and its signal transducing subunit, Act1 (Wu et al., 2012; Zepp et al., 2017). Moreover, expression of IL-23 receptor (IL-23R) in tumor-associated Treg enables STAT3 activation on IL-23 binding, inducing upregulation of Foxp3 and increasing secretion of immunosuppressive IL-10, thus constituting an IL-23-mediated tumor-supportive inflammation (Kortylewski et al., 2009) (Figure 3A). At the same time, IL-23 inhibits IL-12 dependent anti-tumor immunity.
During the resolution phase and in chronic tumor-associated inflammation, macrophages produce IL-10 and TGFβ, both of which have strong immunosuppressive activity (Sanjabi et al., 2009). IL-10 can inhibit the function of a variety of immune cells, thus playing an important role in dampening T cell mediated inflammation and immunity, while supporting chronic inflammation (Corinti et al., 2001; Moore et al., 2001). In fact, IL-10 is a multifunctional cytokine that has diverse effects on most immune cells, with the ability to inhibit activation and effector function of T cells as well as monocytes and macrophages (Moore et al., 2001). IL-10 also regulates growth and/or differentiation of CTL, Th cells, B cells, NK cells, mast cells, granulocytes, DCs, keratinocytes, and endothelial cells. However, IL-10 can also exert a stimulatory effect on CD8+ T cells, aiding in tumor regression (Emmerich et al., 2012; Mumm et al., 2011). Conversely, it was recently shown that IL-10 can directly modulate CD8+ T cell activation and function through modification of cell surface protein glycosylation, increasing the antigenic threshold required for T cell activation (Smith et al., 2018). Although initially identified as a growth factor and later found to be a tumor suppressor, TGFβ plays a central role in supporting and regulating tumor development and metastasis as well as tumor-directed immune responses (Li and Flavell, 2008; Massagué, 2008). Particularly, TGFβ exerts a pleiotropic effect on adaptive immune cells, regulating both effector and regulatory CD4+ Th cells, CD8+ CTLs, and supporting generation of immunosuppressive IgA+ plasma cells (Shalapour et al., 2017; Travis and Sheppard, 2014) (Figure 3B). TGFβ receptors are expressed on both immune and non-immune cells and were shown to regulate immune cell functions (Travis and Sheppard, 2014) as well as have critical effects on innate immune cells and fibroblasts, some of which are discussed below.
A specialized group of cytokines, remotely related to IL-10 and produced by different cell types, especially plasmacytoid DC, are type I and II IFNs. Although the primary function of type I IFNs is innate antiviral immunity, they also exert potent immunostimulatory activity that augments effector T cell functions (Corrales et al., 2015; Woo et al., 2015). Type II IFN or IFNγ is produced by diverse cell types, including activated Th1 and CTLs. Although IFNγ has an important immunostimulatory function mediated by upregulation of MHC-I class I (HLA-A,B,C) molecules and their associated antigen processing and presentation machinery (Zhou, 2009), its continuous production induces PD ligand 1 (PD-L1) and leads to exhaustion of chronically stimulated effector CD8+ T cells (Benci et al., 2016). This regulatory response has evolved as a protective mechanism that prevents collateral damage during anti-viral immunity and is triggered by IFNγ in cooperation with IL-10 (Sanjabi et al., 2009). However, the same response is also mounted by cancer cells as a way to suppress immunosurveillance (Alspach et al., 2018; Sharma et al., 2017). The dual effects of IFNγ on anti-tumor immunity have been discussed (Mandai et al., 2016) and were even compared to the dual effects of IL-10 (Wilke et al., 2011). Therapeutic manipulations capable of dismantling IFNγ-induced immune dysfunction while retaining strong immunostimulatory effects, other than PD-L1 or PD-1 blocking antibodies, remain a major challenge.
Immunosuppressive B Cells and Plasmocytes
First identified in treatment refractory prostate cancer (PCa), ISP are derived from naïve B cells that are recruited into the TME by CAF-generated CXCL13 and CXCL12 (Ammirante et al., 2014; Shalapour et al., 2015). Upon encounter of B-cell receptor (BCR) specific-antigens, that could be bacterial-, food-, or tumor-derived and further exposure to TGFβ and other cytokines including IL-21 from circulatory Tfh cells, lymphotoxin β (LTβ), IL-33, and IL-10, naïve B cells undergo CSR to replace their surface-expressed IgM-BCR with surface-expressed IgA (Cerutti, 2008; Shalapour et al., 2015, 2017). IgA+ plasmocytes, which are generated in response to chronic inflammation (Figure 3B), have the ability to not only suppress CTL activation in the context of cancer development or during cancer therapy (Shalapour et al., 2015, 2017) but can also regulate and dampen neuroinflammation (Rojas et al., 2019). These anti-inflammatory and immunosuppressive effects are in line with the well-established homeostatic and regulatory role of IgA+ plasmocytes in mucosal immunity (Gutzeit et al., 2014; Macpherson et al., 2018; Mantis et al., 2011). However, the primary role of IgA is also to maintain bacterial homeostasis, therefore IgA+ plasmocytes may suppress the growth of tumors that depend on microbial signals.
B cells and humoral immunity have also been described to regulate anti-tumor immunity through other mechanisms, either by expressing cytokines such as IL-10 or IL-35, inducing antibody-mediated cytotoxicity through NK cells, or activating the complement system components C5a or C3a, which seem to either activate or suppress anti-tumor immunity in a context dependent manner (Gunderson et al., 2016; Markiewski et al., 2008; Mauri and Menon, 2015; Medler et al., 2018; Pylayeva-Gupta et al., 2016) (Figure 3B). However, the ways by which different B cell types manifest their immunosuppressive effects remain poorly understood.
Microbe-Induced Inflammation and Modulation of Tumor Immunity
The microbiota plays a fundamental role in regulating gut immune and tissue homeostasis, nutrient absorption, and energy metabolism. The microbiome also has a central role in induction, training and function of the entire host immune system. Gut microbes can activate and support antigen presentation through TLR signaling and modulate T cell homeostasis through induction of IFNγ, IL-1 and IL-7. Importantly, gut microbes support Treg and Th17 development (Arpaia et al., 2013; Dmitrieva-Posocco et al., 2019; Furusawa et al., 2013; Ivanov et al., 2009; Shalapour et al., 2010) and control humoral immunity, especially IgA production (Gutzeit et al., 2014; Nakajima et al., 2018). Therefore, the microbiota has a pivotal role in modulating anti-cancer immunity as well as gastrointestinal inflammation. Expansion of IL-17 producing cells supports tumor growth in colon, liver and lung and is often elicited by translocation of commensal microbes and their products (Gomes et al., 2016; Grivennikov et al., 2012; Jin et al., 2019). Microbial translocation can lead to chronic inflammation and accumulation of immunosuppressive IgA+ plasma cells (Rojas et al., 2019; Rosser et al., 2014; Shalapour et al., 2017). However, bacteria can also induce NKT cell activation and stimulate anti-tumor immunity (Ma et al., 2018). Moreover, the gut microbiota was shown to regulate the response to checkpoint inhibitors through APC differentiation and maturation, thereby enhancing T cell priming (Gopalakrishnan et al., 2018; Matson et al., 2018; Routy et al., 2018).
However, it is still not clear whether the interaction between commensal microbes and the immune system entails innate recognition or whether it also affects adaptive immune recognition, which may support or suppress anti-tumor immunity. In a context dependent manner, bacteria support tumor development, particularly in non-alcoholic steatohepatitis (NASH)-induced hepatocellular carcinoma, colon and lung cancer, by inducing IL-17 and inflammation. However, commensal microbes also support DC and NK cell activation to enhance anti-tumor immunity, particularly in melanomas, but their exact contribution needs to be further analyzed and tested on a case-by-case basis.
Non-Hematopoietic Cells and Fibroblasts that Regulate Adaptive Immunity
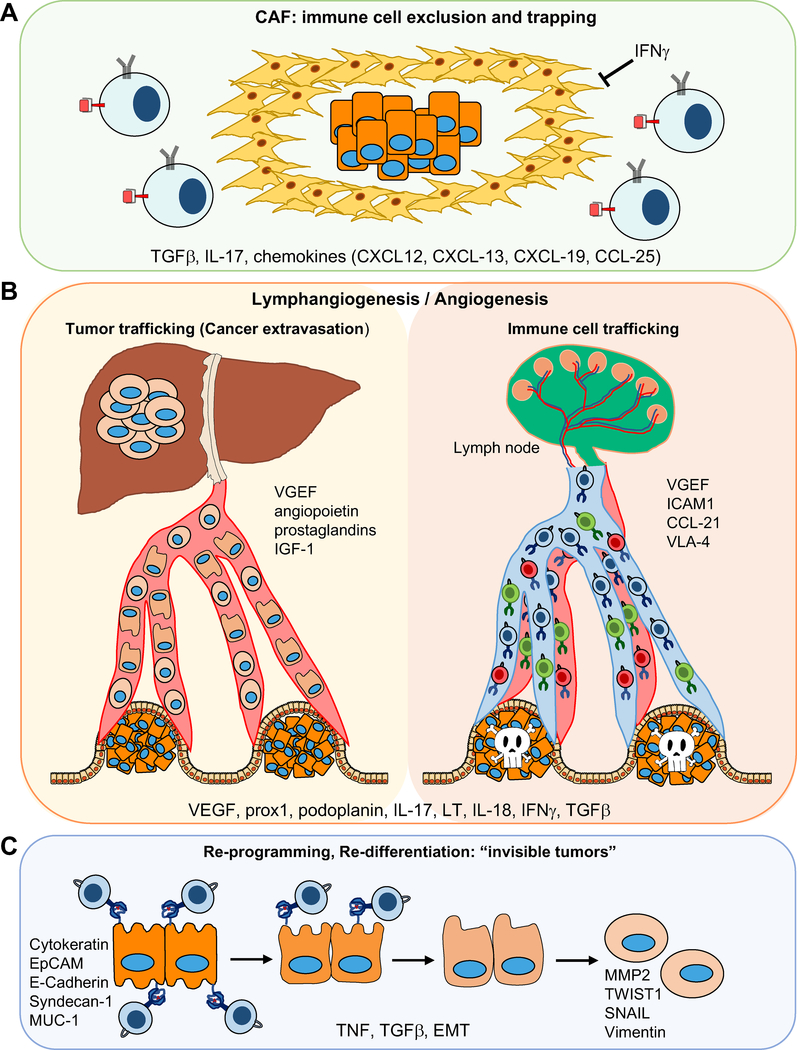
Tumor hypoxia caused by either rapid tumor growth or as a result of drug treatment leads to CAF activation in a manner dependent on autocrine TGFβ signaling. Although CAFs have been known to support tumor growth, their ability to recruit immune cells and modulate anti-tumor immunity was discovered only recently. This modulation of tumor immunity by CAFs is very much context dependent and can be either positive or negative depending on the tumor type (Kalluri, 2016; Kato et al., 2018; Lakins et al., 2018; Özdemir et al., 2014). In addition to immunosuppressive TGFβ and IL-6, activated CAF produce CXCL13 and many other chemokines and cytokines, including CXCL12, CXCL19, CCl25, and IL-7 (Ammirante et al., 2014; Shalapour et al., 2010, 2017), that recruit lymphocytes and affect their differentiation and homeostasis. By contrast to their ability to recruit immune cells through chemokine production, CAFs can physically block the entry of immune cells into the tumor parenchyma and trap them within the collagen-rich peritumoral stroma (Mariathasan et al., 2018)(Figure 4A). The latter effect can be disrupted by using TGFβ neutralizing antibodies.
Figure 4: Role of Non-hematopoietic Cells in Anti-Tumor Immunity in the Inflamed TME.
A) Immune cell exclusion by CAFs. B) Angiogenesis and lymphangiogenesis; building the road for tumor and immune cell trafficking. C) Cancer cell re-programming, EMT.
Roles of Inflammation-Induced Lymphatic Vessels and Tertiary Lymphoid Structures
Lymphatic vessels drain fluids and macromolecules from tissues and take up lipids (chylomicrons) in the intestine. They transport antigens and leukocytes between peripheral tissues, lymph nodes (LNs), and the blood and therefore are important for induction and regulation of immune responses. The lymphatic vasculature enables transport of DC and antigens into tumor-adjacent draining LN, thereby regulating anti-tumor immunity (Figure 4). Lymphatic vessels can also promote tumor tolerance (Lucas et al., 2018; Lund et al., 2016; Vranova, 2014) and even aid in malignant progression by contributing to the dissemination and metastatic spread of many carcinomas (Stacker et al., 2014). Mediators of inflammatory lymphangiogenesis like VEGF-A and VEGF-C/VEGF-D, which signal through VEGFR-2 and/or VEGFR-3, are produced by either leukocytes or stromal cells, and TAMs are important sources of VEGF-A and VEGF-C. Both TAMs and neutrophils can modulate lymphangiogenesis in the inflamed as well as tumor-draining LNs by producing mediators, such as IL-17 or IL-8, that induce lymphangiogenesis. Lymphotoxins (LTα and LTβ) have also been implicated in inflammatory lymphangiogenesis, particularly in the context of tertiary lymphoid organs (TLO), which were described to exert conflicting effects on tumor development (Dieu-Nosjean et al., 2014; Finkin et al., 2015). Importantly, some inflammatory cytokines can inhibit lymphangiogenesis and angiogenesis. IFNγ, in particular, coming from Th1 and NK cells, was shown to inhibit angiogenesis and thereby support tumor regression and probably inhibit metastasis (Kammertoens et al., 2017). IFNγ also inhibits CAF activation and fibrosis (Baroni et al., 1996; Luo et al., 2013). Furthermore, inhibition of TGFβ can induce lymphangiogenesis (Oka et al., 2008).
CODA
Although chronic inflammation generates an immunosuppressive microenvironment that supports tumor development and suppresses anti-tumor immunity, uninflamed tumors do not respond to checkpoint inhibitors, particularly anti-PD(L)1 drugs whose action depends on presence of exhausted CD8+ T cells. Although it is not entirely clear why certain types of cancers are not inflamed, it was suggested that the lack of inflammatory infiltrates may be due to the scarcity of damaged DNA, which is needed for activation of the cGAS-STING pathway and type I IFN production. Alternatively, the absence of chemokine and cytokine production by cancer cells results in poor lymphocyte recruitment and homing to the tumor tissue (Gajewski et al., 2017; Trujillo et al., 2018). It should be noted that only a slight correlation between the tumoral content of T cells (also known as T cell inflammation) and immunogenic peptides has been observed (Spranger et al., 2016). Although production of neo-antigens often correlates with checkpoint inhibitor responses in some cancers, there are additional factors that affect tumor immunogenicity. For instance, chronic inflammation contributes to resistance to cancer immunotherapy, and resistance to anti-CTLA4 therapy can be conferred by IFNγ-signaling, which induces PD-L1 expression. In this case, responsiveness to immune checkpoint inhibitors (ICI) is restored by combining anti-CTLA4 and anti-PD(L)1 drugs (Benci et al., 2016).
On the other hand, cell death and inflammation induce TGFβ, which promotes CAF activation and fibrosis, resulting in accumulation of a fibrotic tumor stroma that excludes T cells from the tumor and leads to ICI resistance (Mariathasan et al., 2018; Tauriello et al., 2018). Epithelial-to-mesenchymal transition (EMT), which can also be regulated by the TME, inflammatory cytokines, and factors like TGFβ, modulates the response to immunotherapy and contributes to immunosuppression, as mesenchymal tumors are more likely to recruit immunosuppressive cells, have higher PD-L1 expression and downregulate MHCI expression. Thus, mesenchymal tumors are more likely to escape CTL-mediated killing. However, the exact mechanisms by which EMT affects anti-tumor immunity remain unknown (Chae et al., 2018; Dongre and Weinberg, 2019; Dongre et al., 2017). Interestingly, it has been shown both in mouse and human studies that inflammation and TNF can induce reprogramming of melanoma cells (dedifferentiation), resulting in elevated expression of neural crest markers (e.g. NGFR). Dedifferentiated melanoma cells lack expression of melanocytic antigen (MART-1) and therefore acquired resistance to adoptive T cell immunotherapy (Landsberg et al., 2012; Mehta et al., 2018).
Another paradoxical effect of inflammation on anti-tumor immunity is related to obesity-induced metabolic inflammation. Many epidemiological, clinical and animal model studies have confirmed and documented the effects of obesity on cancer incidence and mortality, largely mediated via obesity-induced barrier disruption and chronic inflammation (Nakagawa et al., 2014; Park et al., 2010; Shalapour et al., 2017; Sun and Karin, 2012). However, peripheral adipose depots also have a profound influence on adaptive immunity (DiSpirito and Mathis, 2015; Maurizi et al., 2018), increasing immune ageing and T cell exhaustion and dysfunction (Shalapour et al., 2017; Wang et al., 2019). Moreover, linoleic acid, a fatty acid that accumulates during hepatic steatosis, was suggested to induce oxidative damage and mediate selective loss of intrahepatic CD4+ T cells to support HCC development (Ma et al., 2016). More recent studies, however, showed that obesity is associated with increased efficacy of PD-(L)1 blockade in tumor-bearing mice and cancer patients (Wang et al., 2019). This surprising result may be attributed to obesity-induced immunosuppression which increases PD-L1 expression either in tumors or through effects on PD-L1+ immunosuppressive IgA+ plasmocytes (Shalapour et al., 2017), macrophages or even adipocytes (Shirakawa et al., 2016; Wu et al., 2018). While suppressing immunosurveillance, such changes may render the obese patient more susceptible to the beneficial effects of anti-PD-(L)1 therapy, but this remains to be validated in the clinic.
In conclusion, the intricate and ever-changing dialogue between inflammation and immunity in the context of cancer development, progression and treatment provides a fertile ground and many challenges for future research. How to boost anti-tumor immunity without losing innate immune defenses or conversely, triggering a cytokine storm, remains to be determined, as well as identifying the best way for dismantling the immunosuppressive aspects of chronic inflammation while leaving its immunostimulatory side intact. Another important but poorly understood concept in tumor immunology is T cell exhaustion. Why do tumor directed CD8+ T cells become exhausted after chronic antigenic stimulation, but self-directed T cells in autoimmunity remain activated for the duration of the disease? Notwithstanding these mysteries, it has become clear that inflammation-mediated immunosuppression is a major player in cancer biology and its improved understanding is critical for future progress at the translational front.
Chronic inflammation supports tumor growth and is often immunosuppressive, but under certain conditions, it can enhance anti-tumor immunity. Shalapour and Karin review the intricate and dynamic dialogue between tumor-associated inflammation and anti-cancer immunity, focusing on the impact of inflammatory signals on the adaptive immune response and the implications for cancer immunotherapies.
ACKNOWLEDGMENTS
We thank R. K. Ngu for illustration and figure preparation and C. Lichtenstern for manuscript assistance. S.S. was supported by PCF-Young Investigator Award and SCRC for ALPD & Cirrhosis funded by the NIAAA (P50 AA011999). Work in M.K. laboratory was supported by grants from the NIH (R01 AI043477, P01 CA128814, R01 CA211794) and Tower Cancer Research Foundation. Additional support came from U01AA027681 to S.S. and M.K., P01 CA128814 to M.K./Ze’ev Ronai and Padres Pedal the Cause C3 award #PTC2018.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Alspach E, Lussier DM, and Schreiber RD (2018). Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol 11, a028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Shalapour S, Kang Y, Jamieson CAM, and Karin M (2014). Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc. Natl. Acad. Sci. U. S. A 111, 14776–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, and Benedetti A (1996). Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology 23, 1189–1199. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, Koti M, Siemens DR, and Graham CH (2014). Mechanisms of Hypoxia-Mediated Immune Escape in Cancer. Cancer Res. 74, 7185–7190. [DOI] [PubMed] [Google Scholar]
- Bauer S (1999). Activation of NK Cells and T Cells by NKG2D, a Receptor for Stress-Inducible MICA. Science 285, 727–729. [DOI] [PubMed] [Google Scholar]
- Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et al. (2016). Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 167, 1540–1554.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, and Kuchroo VK (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238. [DOI] [PubMed] [Google Scholar]
- Bevan MJ (2004). Helping the CD8+ T-cell response. Nat. Rev. Immunol 4, 595–602. [DOI] [PubMed] [Google Scholar]
- Blanco P, Palucka AK, Pascual V, and Banchereau J (2008). Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 19, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher JP, and Reis e Sousa C (2018). The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 4, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Ha S-J, Elsaesser H, Sharpe AH, Freeman GJ, and Oldstone MBA (2008). IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sci 105, 20428–20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A (2008). The regulation of IgA class switching. Nat. Rev. Immunol 8, 421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YK, Chang S, Ko T, Anker J, Agte S, Iams W, Choi WM, Lee K, and Cruz M (2018). Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Sun L, and Chen ZJ (2016). Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol 17, 1142–1149. [DOI] [PubMed] [Google Scholar]
- Choi YS, Eto D, Yang JA, Lao C, and Crotty S (2013). Cutting Edge: STAT1 Is Required for IL-6-Mediated Bcl6 Induction for Early Follicular Helper Cell Differentiation. J. Immunol. 190, 3049–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C, and Li MO (2018). Re(de)fining Innate Lymphocyte Lineages in the Face of Cancer. Cancer Immunol. Res 6, 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin KA (2004). Chemotherapy-Associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther 3, 294–300. [DOI] [PubMed] [Google Scholar]
- Corinti S, Albanesi C, la Sala A, Pastore S, and Girolomoni G (2001). Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol 166, 4312–4318. [DOI] [PubMed] [Google Scholar]
- Corliss BA, Azimi MS, Munson J, Peirce SM, and Murfee WL (2016). Macrophages: An Inflammatory Link between Angiogenesis and Lymphangiogenesis. Microcirculation 23, 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo SR, Lemmens E, Banda T, Leong JJ, et al. (2015). Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 11, 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FM, Colbert JD, Merino E, Kriegsman BA, and Rock KL (2017). The Biology and Underlying Mechanisms of Cross-Presentation of Exogenous Antigens on MHC-I Molecules. Annu. Rev. Immunol 35, 149–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. (2015). ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 161, 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, and Protti MP (2011). Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med 208, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, and Ruffell B (2019). Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol 19, 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, and Rincón M (2000). Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 13, 805–815. [DOI] [PubMed] [Google Scholar]
- Diehl S, Chow C-W, Weiss L, Palmetshofer A, Twardzik T, Rounds L, Serfling E, Davis RJ, Anguita J, and Rincón M (2002). Induction of NFATc2 Expression by Interleukin 6 Promotes T Helper Type 2 Differentiation. J. Exp. Med 196, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl SA, Schmidlin H, Nagasawa M, Blom B, and Spits H (2012). IL-6 Triggers IL-21 production by human CD4+ T cells to drive STAT3-dependent plasma cell differentiation in B cells. Immunol. Cell Biol 90, 802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, and Rincon M (2009). The effects of IL-6 on CD4 T cell responses. Clin. Immunol 130, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, et al. (2009). The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J. Exp. Med 206, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean M-C, Goc J, Giraldo NA, Sautès-Fridman C, and Fridman WH (2014). Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 35, 571–580. [DOI] [PubMed] [Google Scholar]
- DiSpirito JR, and Mathis D (2015). Immunological contributions to adipose tissue homeostasis. Semin. Immunol 27, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva-Posocco O, Dzutsev A, Posocco DF, Hou V, Yuan W, Thovarai V, Mufazalov IA, Gunzer M, Shilovskiy IP, Khaitov MR, et al. (2019). Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity 50, 166–180.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan BP, Gibbs KD, and Ostrand-Rosenberg S (2006). Dendritic Cells Cross-Dressed with Peptide MHC Class I Complexes Prime CD8+ T Cells. J. Immunol 177, 6018–6024. [DOI] [PubMed] [Google Scholar]
- Dongre A, and Weinberg RA (2019). New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol 20, 69. [DOI] [PubMed] [Google Scholar]
- Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, and Weinberg RA (2017). Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res. 77, 3982–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich J, Mumm JB, Chan IH, LaFace D, Truong H, McClanahan T, Gorman DM, and Oft M (2012). IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 72, 3570–3581. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber H-P, and LeCouter J (2003). The biology of VEGF and its receptors. Nat. Med 9, 669–676. [DOI] [PubMed] [Google Scholar]
- Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, Browning JL, Goossens N, Nakagawa S, Gunasekaran G, et al. (2015). Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat. Immunol 16, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming C, Morrissey S, Cai Y, and Yan J (2017). γδ T Cells: Unexpected Regulators of Cancer Development and Progression. Trends Cancer 3, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruton JS (1957). The Collected Papers of Paul Ehrlich. Yale J. Biol. Med 29, 628. [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, and Spranger S (2017). Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment In Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy, Kalinski P, ed. (Springer International Publishing; ), pp. 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdiero MR, Marone G, and Mantovani A (2018). Cancer Inflammation and Cytokines. Cold Spring Harb. Perspect. Biol 10, a028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, and Kroemer G (2017). Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol 17, 97–111. [DOI] [PubMed] [Google Scholar]
- Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, and Agostinis P (2012). ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol. Med 18, 589–598. [DOI] [PubMed] [Google Scholar]
- Gomes AL, Teijeiro A, Burén S, Tummala KS, Yilmaz M, Waisman A, Theurillat J-P, Perna C, and Djouder N (2016). Metabolic Inflammation-Associated IL-17A Causes Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell 30, 161–175. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Ferguson T, Zitvogel L, and Kroemer G (2009). IMMUNOGENIC AND TOLEROGENIC CELL DEATH. Nat. Rev. Immunol 9, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TS, and Ferguson TA (2011). Cell Death in the Maintenance and Abrogation of Tolerance: The Five Ws of Dying Cells. Immunity 35, 456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, and Karin M (2010). Immunity, inflammation, and cancer. Cell 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu G-Y, Österreicher CH, Hung KE, et al. (2012). Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P, Valladeau J, Zitvogel L, Théry C, and Amigorena S (2002). Antigen Presentation and T Cell Stimulation by Dendritic Cells. Annu. Rev. Immunol 20, 621–667. [DOI] [PubMed] [Google Scholar]
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, et al. (2016). Bruton Tyrosine Kinase–Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 6, 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit C, Magri G, and Cerutti A (2014). Intestinal IgA production and its role in hostmicrobe interaction. Immunol. Rev 260, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Im SJ, Araki K, and Ahmed R (2017). Cytokine-Mediated Regulation of CD8 T-Cell Responses During Acute and Chronic Viral Infection. Cold Spring Harb. Perspect. Biol a028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, and Littman DR (2006). The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. (2009). Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 139, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, and Medzhitov R (2015). Control of adaptive immunity by the innate immune system. Nat. Immunol 16, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, et al. (2019). Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 176, 998–1013.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R (2016). The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598. [DOI] [PubMed] [Google Scholar]
- Kammertoens T, Friese C, Arina A, Idel C, Briesemeister D, Rothe M, Ivanov A, Szymborska A, Patone G, Kunz S, et al. (2017). Tumour ischaemia by interferon-γ resembles physiological blood vessel regression. Nature 545, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, and Clevers H (2016). Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, Komoto S, Katsube R, Ninomiya T, Tazawa H, et al. (2018). Cancer-Associated Fibroblasts Affect Intratumoral CD8+ and FoxP3+ T Cells Via IL6 in the Tumor Microenvironment. Clin. Cancer Res 24, 4820–4833. [DOI] [PubMed] [Google Scholar]
- Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L, et al. (2013). Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 24, 311–318. [DOI] [PubMed] [Google Scholar]
- Kepp O, Semeraro M, Bravo-San Pedro JM, Bloy N, Buqué A, Huang X, Zhou H, Senovilla L, Kroemer G, and Galluzzi L (2015). eIF2α phosphorylation as a biomarker of immunogenic cell death. Semin. Cancer Biol 33, 86–92. [DOI] [PubMed] [Google Scholar]
- Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, and Paulos CM (2018). When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol 15, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, and Yu H (2009). Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 15, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, and Medzhitov R (2015). Homeostasis, Inflammation, and Disease Susceptibility. Cell 160, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, Chen L, and Zou W (2006). Cutting Edge: Induction of B7-H4 on APCs through IL-10: Novel Suppressive Mode for Regulatory T Cells. J. Immunol 177, 40–44. [DOI] [PubMed] [Google Scholar]
- Kuang D-M, Zhao Q, Peng C, Xu J, Zhang J-P, Wu C, and Zheng L (2009). Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med 206, 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakins MA, Ghorani E, Munir H, Martins CP, and Shields JD (2018). Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat. Commun 9, 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, Fatho M, Lennerz V, Wölfel T, Hölzel M, et al. (2012). Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 490, 412–416. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, and Oft M (2006). IL-23 promotes tumour incidence and growth. Nature 442, 461–465. [DOI] [PubMed] [Google Scholar]
- Li MO, and Flavell RA (2008). TGF-β: A Master of All T Cell Trades. Cell 134, 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L, Jiang Y, Fei Y, Zhu C, Tan R, et al. (2018). Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563, 131–136. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yan W, Tohme S, Chen M, Fu Y, Tian D, Lotze M, Tang D, and Tsung A (2015). Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J. Hepatol 63, 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas ED, Finlon JM, Burchill MA, McCarthy MK, Morrison TE, Colpitts TM, and Tamburini BAJ (2018). Type 1 IFN and PD-L1 Coordinate Lymphatic Endothelial Cell Expansion and Contraction during an Inflammatory Immune Response. J. Immunol 201, 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, et al. (2016). Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Invest 126, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X-Y, Takahara T, Kawai K, Fujino M, Sugiyama T, Tsuneyama K, Tsukada K, Nakae S, Zhong L, and Li X-K (2013). IFN-γ deficiency attenuates hepatic inflammation and fibrosis in a steatohepatitis model induced by a methionine- and choline-deficient high-fat diet. Am. J. Physiol.-Gastrointest. Liver Physiol 305, G891–G899. [DOI] [PubMed] [Google Scholar]
- Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, Stroncek DF, Terabe M, Kapoor V, ElGindi M, et al. (2016). NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature 531, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. (2018). Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Yilmaz B, Limenitakis JP, and Ganal-Vonarburg SC (2018). IgA Function in Relation to the Intestinal Microbiota. Annu. Rev. Immunol 36, 359–381. [DOI] [PubMed] [Google Scholar]
- Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, and Konishi I (2016). Dual Faces of IFNγ in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin. Cancer Res 22, 2329–2334. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, and Corthésy B (2011). Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Marchesi F, Malesci A, Laghi L, and Allavena P (2017). Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol 14, 399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, et al. (2018). TGFβ attenuates tumour response to PDL1 blockade by contributing to exclusion of T cells. Nature 554, 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, and Lambris JD (2008). Modulation of the antitumor immune response by complement. Nat. Immunol 9, 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J (2008). TGFβ in Cancer. Cell 134, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, and Gajewski TF (2018). The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, and Menon M (2015). The expanding family of regulatory B cells. Int. Immunol 27, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi G, Guardia LD, Maurizi A, and Poloni A (2018). Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol 233, 88–97. [DOI] [PubMed] [Google Scholar]
- Medler TR, Murugan D, Horton W, Kumar S, Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin M, Margolin AA, et al. (2018). Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy. Cancer Cell 34, 561–578.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Kim YJ, Robert L, Tsoi J, Comin-Anduix B, Berent-Maoz B, Cochran AJ, Economou J, Tumeh PC, Puig-Saus C, et al. (2018). Immunotherapy resistance by inflammation-induced dedifferentiation. Cancer Discov. 8, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M, Sathe P, Helft J, Miller J, and Mortha A (2013). The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol 31, 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, and O’Garra A (2001). Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol 19, 683–765. [DOI] [PubMed] [Google Scholar]
- Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al. (2011). IL-10 Elicits IFNγ-Dependent Tumor Immune Surveillance. Cancer Cell 20, 781–796. [DOI] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. (2008). Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112, 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J, et al. (2014). ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26, 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Vogelzang A, Maruya M, Miyajima M, Murata M, Son A, Kuwahara T, Tsuruyama T, Yamada S, Matsuura M, et al. (2018). IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med 215, 2019–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CT, and Oldstone MBA (2014). IL-10: Achieving Balance During Persistent Viral Infection. Curr. Top. Microbiol. Immunol 380, 129–144. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang Y, Watowich SS, Jetten AM, Tian Q, et al. (2008). Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 29, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Sato-Matsushita M, Yamamoto S, Hori T, Sasahara M, Iwakura Y, Saiki I, Tahara H, and Hayakawa Y (2018). NK Cells Control Tumor-Promoting Function of Neutrophils in Mice. Cancer Immunol. Res 6, 348–357. [DOI] [PubMed] [Google Scholar]
- Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, Komuro A, Kano MR, and Miyazono K (2008). Inhibition of endogenous TGF-β signaling enhances lymphangiogenesis. Blood 111, 4571–4579. [DOI] [PubMed] [Google Scholar]
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. (2014). Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 25, 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl J, and Cerwenka A (2017). Tricking the balance: NK cells in anti-cancer immunity. Immunobiology 222, 11–20. [DOI] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu G-Y, He G, Ali SR, Holzer RG, Österreicher CH, Takahashi H, and Karin M (2010). Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 140, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AM, Lovitch SB, Sage PT, Juneja VR, Lee Y, Trombley JD, ArancibiaCárcamo CV, Sobel RA, Rudensky AY, Kuchroo VK, et al. (2015). Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med 212, 1603–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, and Bar-Sagi D (2016). IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 6, 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D (2016). The concept of immune surveillance against tumors: The first theories. Oncotarget 8, 7175–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas OL, Pröbstel A-K, Porfilio EA, Wang AA, Charabati M, Sun T, Lee DSW, Galicia G, Ramaglia V, Ward LA, et al. (2019). Recirculating Intestinal IgA-Producing Cells Regulate Neuroinflammation via IL-10. Cell 176, 610–624.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, and Mauri C (2014). Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production. Nat. Med 20, 1334–1339. [DOI] [PubMed] [Google Scholar]
- Routy B, Chatelier EL, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. (2018). Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97. [DOI] [PubMed] [Google Scholar]
- Ruffell B, and Coussens LM (2015). Macrophages and Therapeutic Resistance in Cancer. Cancer Cell 27, 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et al. (2016). Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med 22, 679–684. [DOI] [PubMed] [Google Scholar]
- Sanjabi S, Zenewicz LA, Kamanaka M, and Flavell RA (2009). Anti-inflammatory and proinflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol 9, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. (2016). The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked H, Hofseth LJ, Chumanevich A, Chumanevich AA, Wang J, Wang Y, Taniguchi K, Guma M, Shenouda S, Clevers H, et al. (2012). Chronic epithelial NF- B activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc. Natl. Acad. Sci 109, 14007–14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Deiser K, Sercan Ö, Tuckermann J, Minnich K, Willimsky G, Blankenstein T, Hämmerling GJ, Arnold B, and Schüler T (2010). Commensal microflora and interferon-γ promote steady-state interleukin-7 production in vivo. Eur. J. Immunol 40, 2391–2400. [DOI] [PubMed] [Google Scholar]
- Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, Willimsky G, Ammirante M, Strasner A, Hansel DE, et al. (2015). Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S, Lin X-J, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, et al. (2017). Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551, 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Hu-Lieskovan S, Wargo JA, and Ribas A (2017). Primary, Adaptive and Acquired Resistance to Cancer Immunotherapy. Cell 168, 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa K, Yan X, Shinmura K, Endo J, Kataoka M, Katsumata Y, Yamamoto T, Anzai A, Isobe S, Yoshida N, et al. (2016). Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Invest 126, 4626–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, Boukhaled GM, Condotta SA, Mazouz S, Guthmiller JJ, Vijay R, Butler NS, Bruneau J, Shoukry NH, Krawczyk CM, et al. (2018). Interleukin-10 Directly Inhibits CD8+ T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity 48, 299–312.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, Gajewski AP, Andrade J, and Gajewski TF (2016). Density of immunogenic antigens does not explain the presence or absence of the T-cell–inflamed tumor microenvironment in melanoma. Proc. Natl. Acad. Sci 113, E7759–E7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, and Achen MG (2014). Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 14, 159–172. [DOI] [PubMed] [Google Scholar]
- Steinman RM (2012). Decisions About Dendritic Cells: Past, Present, and Future. Annu. Rev. Immunol 30, 1–22. [DOI] [PubMed] [Google Scholar]
- Sun B, and Karin M (2012). Obesity, inflammation, and liver cancer. J. Hepatol 56, 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, et al. (2008). Development and characterization of IL21–producing CD4+ T cells. J. Exp. Med 205, 1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, Fini M, and Russo MA (2016). The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell. Longev 2016, 3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, and Karin M (2011). Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL–RANK signalling. Nature 470, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, and Sakaguchi S (2017). Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K, and Karin M (2018). NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol 18, 309–324. [DOI] [PubMed] [Google Scholar]
- Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, et al. (2018). TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554, 538–543. [DOI] [PubMed] [Google Scholar]
- Teng MWL, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Möller A, Hill GR, Iwakura Y, Oft M, and Smyth MJ (2010). IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc. Natl. Acad. Sci. U. S. A 107, 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng MWL, Bowman EP, McElwee JJ, Smyth MJ, Casanova J-L, Cooper AM, and Cua DJ (2015). IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med 21, 719–729. [DOI] [PubMed] [Google Scholar]
- Travis MA, and Sheppard D (2014). TGF-β Activation and Function in Immunity. Annu. Rev. Immunol 32, 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JA, Sweis RF, Bao R, and Luke JJ (2018). T Cell–Inflamed versus Non-T Cell–Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res 6, 990–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Perego M, and Gabrilovich D (2018). Myeloid-derived suppressor cells coming of age. Nat. Immunol 19, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. (2013). The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 342, 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranova M (2014). Lymphatic Vessels in Inflammation. J. Clin. Cell. Immunol 05. [Google Scholar]
- Wang M, and Kaufman RJ (2014). The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 14, 581–597. [DOI] [PubMed] [Google Scholar]
- Wang D, Wei Y, and Pagliassotti MJ (2006). Saturated Fatty Acids Promote Endoplasmic Reticulum Stress and Liver Injury in Rats with Hepatic Steatosis. Endocrinology 147, 943–951. [DOI] [PubMed] [Google Scholar]
- Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu L-W, et al. (2014). Interleukin-17 Receptor A Signaling in Transformed Enterocytes Promotes Early Colorectal Tumorigenesis. Immunity 41, 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, Mirsoian A, Minnar CM, Stoffel KM, Sturgill IR, et al. (2019). Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med 25, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP, Gubin MM, and Schreiber RD (2016). The Role of Neoantigens in Naturally Occurring and Therapeutically Induced Immune Responses to Cancer. Adv. Immunol 130, 25–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Duffy CR, and Allison JP (2018). Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 8, 1069–1086. [DOI] [PubMed] [Google Scholar]
- Wilke CM, Wei S, Wang L, Kryczek I, Kao J, and Zou W (2011). Dual biological effects of the cytokines interleukin-10 and interferon-γ. Cancer Immunol. Immunother 60, 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-R, Corrales L, and Gajewski TF (2015). Innate Immune Recognition of Cancer. Annu. Rev. Immunol 33, 445–474. [DOI] [PubMed] [Google Scholar]
- Wu B, Sun X, Gupta HB, Yuan B, Li J, Ge F, Chiang H-C, Zhang X, Zhang C, Zhang D, et al. (2018). Adipose PD-L1 Modulates PD-1/PD-L1 Checkpoint Blockade Immunotherapy Efficacy in Breast Cancer. OncoImmunology 7, e1500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zepp J, and Li X (2012). Function of Act1 in IL-17 family signaling and autoimmunity. Adv. Exp. Med. Biol 946, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Brooks CR, Sobel RA, and Kuchroo VK (2015). Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J. Immunol. Baltim. Md 1950 194, 1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Masters AR, Fortner KA, Champagne DP, Yanguas-Casás N, Silberger DJ, Weaver CT, Haynes L, and Rincon M (2016). IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21–producing B helper CD8+ T cells. J. Exp. Med 213, 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, Zhang T, Cao Y, Pan H, Zhang L, et al. (2018). Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J. Immunother. Cancer 6, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenay S, and Sousa CR e (2013). Adaptive immunity after cell death. Trends Immunol. 34, 329–335. [DOI] [PubMed] [Google Scholar]
- Zepp JA, Zhao J, Liu C, Bulek K, Wu L, Chen X, Hao Y, Wang Z, Wang X, Ouyang W, et al. (2017). IL-17A–Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis. J. Immunol 199, 3849–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin X, Wong J, Ding S, Seki E, Schnabl B, et al. (2018). New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F (2009). Molecular Mechanisms of IFN-γ to Up-Regulate MHC Class I Antigen Processing and Presentation. Int. Rev. Immunol 28, 239–260. [DOI] [PubMed] [Google Scholar]
- Ziegler PK, Bollrath J, Pallangyo CK, Matsutani T, Canli Ö, De Oliveira T, Diamanti MA, Müller N, Gamrekelashvili J, Putoczki T, et al. (2018). Mitophagy in Intestinal Epithelial Cells Triggers Adaptive Immunity during Tumorigenesis. Cell 174, 88–101.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitti B, and Bryceson YT (2018). Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. 42, 37–46. [DOI] [PubMed] [Google Scholar]
- Zou W (2005). Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 5, 263–274. [DOI] [PubMed] [Google Scholar]