Abstract
Introduction:
Preoperative or definitive chemoradiation is an accepted treatment for locally advanced esophageal squamous cell carcinoma (ESCC). The MUNICON study showed that positron-emission tomography (PET) response following induction chemotherapy was predictive of outcomes in patients with gastroesophageal junction adenocarcinoma. We evaluated the predictive value of PET following induction chemotherapy in ESCC patients and assessed the impact of changing chemotherapy during radiation in PET nonresponders.
Methods:
We retrospectively reviewed all patients with locally advanced ESCC who received induction chemotherapy and chemoradiation; all patients had a PET before and after induction chemotherapy. Survival was calculated from date of repeat PET using Kaplan-Meier analysis and compared between groups using the log-rank test.
Results:
Of 111 patients, 70 (63%) were PET responders (defined as a 35% or more decrease in maximum standard uptake value) to induction chemotherapy. PET responders received the same chemotherapy during radiation. Of 41 PET nonresponders, 16 continued with the same chemotherapy and 25 were changed to alternative chemotherapy with radiation. Median progression-free survival (70.1 months versus 7.1 months, p < 0.01) and overall survival (84.8 months versus 17.2 months, p < 0.01) were improved for PET responders versus nonresponders. Median progression-free survival and overall survival for PET nonresponders who changed chemotherapy versus those who did not were 6.4 months versus 8.3 months (p = 0.556) and 14.1 versus 17.2 months (p = 0.81), respectively.
Conclusions:
PET after induction chemotherapy highly predicts for outcomes in ESCC patients who receive chemoradiation. However, our results suggest that PET nonresponders do not benefit from changing chemotherapy during radiation. Future trials should use PET nonresponse after induction chemotherapy to identify poor prognosis patients for novel therapies.
Keywords: Esophageal squamous cell carcinoma, Positron-emission tomography, Induction chemotherapy, Positron-emission tomography nonresponders, Chemoradiation
Introduction
Esophageal cancer is a highly lethal malignancy. Adenocarcinoma and squamous cell carcinoma (SCC) are the two main subtypes, and SCC histology accounts for approximately 25% of tumors in the United States but is the leading cause of esophageal cancer globally.1
Several phase III studies have shown a survival benefit for preoperative chemoradiation over surgery alone in patients with locally advanced esophageal SCC (ESCC).2,3 However, two phase III European studies showed no clear improvement in overall survival (OS) for subsequent surgery in ESCC patients who responded to upfront chemoradiation.4,5 Therefore, definitive chemoradiation is an accepted strategy for such patients, especially if they achieve a clinical complete response (cCR).
The predictive value of [18F]2-fluoro-deoxy-D-glucose (FDG)-positron-emission tomography (PET) in assessing outcome from preoperative chemotherapy in esophagogastric adenocarcinoma was evaluated in the MUNI-CON phase II study.6 Patients with gastroesophageal junction (GEJ) adenocarcinoma received 1 cycle of fluo-rouracil (5-FU)/cisplatin. Response was assessed by a PET 14 days later. PET responders (defined as a 35% or greater decrease in maximum standard uptake value [mSUV] of the primary tumor by retrospective analysis and prospective validation) continued with 12 weeks of chemotherapy before surgery, whereas PET nonresponders underwent immediate surgery. Compared to results of a prior prospective study in which all patients received preoperative chemotherapy irrespective of PET response, the results suggested no decrement in outcomes with early termination of ineffective chemotherapy and support early discontinuation of inactive preoperative chemotherapy in patients who are PET nonresponders.7
In addition, two studies have suggested that PET after induction chemotherapy may be useful in informing the appropriate preoperative therapy in patients with esophageal carcinoma by allowing identification of patients with early treatment failure. Outcomes may be improved in patients who are PET nonresponders through switching to an alternative chemotherapy during chemoradiation.8-10
In this study, we evaluated the predictive value of PET following induction chemotherapy before chemoradiation in a large cohort of ESCC patients. We also assessed the impact of changing chemotherapy during radiation in patients with suboptimal PET response following induction chemotherapy.
Materials and Methods
Study Design and Patients
This retrospective study included patients treated at Memorial Sloan Kettering Cancer Center (MSK) between July 2002 and February 2017. All patients with locally advanced ESCC without distant metastases who had undergone induction chemotherapy and concurrent chemoradiation were evaluated. Surgery was performed on an individualized basis. All patients underwent a PET scan at baseline and following induction chemotherapy. Patients also underwent baseline staging with computed tomography (CT) and endoscopic ultrasound. All pathology was reviewed and confirmed at MSK. A waiver of authorization to review these data was approved by the institutional review board.
Statistical Analysis
Disease and treatment characteristics were summarized using frequency and percentage for categorical variables and the median and range for continuous variables. Fisher’s exact test and the Wilcoxon rank-sum test were used for comparisons.
Based on previous findings, we used a cutoff of a 35% decrease in mSUV in the primary tumor after induction chemotherapy to divide patients into two groups: PET responders (35% or greater) and PET nonresponders (less than 35%).6,7 Progression-free survival (PFS) was calculated from date of repeated PET to date of progression or death, whichever occurred first. Overall survival (OS) was calculated from date of repeated PET to date of death. Patients who were alive and did not experience an event were censored at the date of last follow-up. OS and PFS were estimated using Kaplan-Meier methods and compared between groups using the log-rank test.
Results
Patients
One-hundred eleven patients were included in our analysis; patient characteristics are summarized in Table 1. The median age was 64 years (range, 21–87 years), 62% of patients were male, and the median Karnofsky performance status was 80%. Seventy percent had node-positive tumors on endoscopic ultrasound. The only statistically significant difference in baseline characteristics between PET responders and nonresponders was mSUV uptake on PET (15.1 in PET responders vs. 11.6 in PET nonresponders, p= 0.009). Forty-two percent (n = 48) of patients underwent baseline PET imaging at an outside center and imaging was reviewed at MSK in 63% (n = 30) of these patients. Adherence to PET scan protocol, in which scans were performed at 60 ± 10 minutes after injection of at least 7 mCi of FDG could be verified from radiology reports in 74 baseline PET scans (67%) and in 80 (69%) repeat PET scans.
Table 1.
Patient Characteristics
| All Patients | PET Responders | PET Nonresponders | ||
|---|---|---|---|---|
| (n = 111) | (n = 70) | (n = 41) | p Value | |
| Age, years (range) | 64 (21-87) | 64 (36-87) | 65 (21-87) | 0.439 |
| Sex | 0.685 | |||
| Male | 69(62.2) | 42 (60) | 27 (65.9) | |
| Female | 42 (37.8) | 28 (40) | 14 (34.1) | |
| KPS (range) | 80 (70-90) | 90 (70-90) | 80 (70-90) | 0.157 |
| Tumor location | 0.886 | |||
| Upper | 27 (24.8) | 16 (22.9) | 11 (26.8) | |
| Mid | 43 (38.7) | 29 (41.4) | 14 (34.1) | |
| Lower | 32 (28.8) | 19 (27.1) | 13 (31.7) | |
| GE junction | 9 (8.1) | 6 (8.6) | 3 (7.3) | |
| Baseline staging | 0.254 | |||
| uT1-3N0 | 17 (15.3) | 13 (18.6) | 4 (9.8) | |
| uT2-3Nx | 6 (5.4) | 3 (4.3) | 3 (7.3) | |
| uT2-3N+ | 74 (66.7) | 50 (71.4) | 24 (58.5) | |
| uT4N0 | 2 (1.8) | 1 (1.4) | 1 (2.4) | |
| uT4N+ | 4 (3.6) | 1 (1.4) | 3 (7.3) | |
| Unknown | 8 (7.2) | 2 (2.9) | 6 (14.6) | |
| Baseline mSUV (range) | 13.5 (3.1-43.2) | 15.1 (3.9-35.5) | 11.6 (3.1-43.2) | 0.009 |
| Induction chemotherapy | 0.223 | |||
| Platinum/paclitaxel | 61 (55) | 41 (58.6) | 20 (48.8) | |
| Platinum/irinotecan | 43 (38.7) | 25 (35.7) | 19 (46.3) | |
| Irinotecan/docetaxel ± cisplatin | 6 (5.4) | 4 (5.7) | 2 (4.9) | |
| Capecitabine/oxaliplatin | 1 (0.9) | 1 (1.4) | 0 (0) |
Values are shown as n (%) unless otherwise noted. Bold values indicate significance.
PET, positron-emission tomography; GE, gastroesophageal; KPS, Karnofsky performance scale; mSUV, maximum standardized uptake value.
PET Response After Induction Chemotherapy
Patients received a variety of induction chemotherapy regimens as shown in Table 1. PET imaging was undertaken a median of 8 days (range, 1–32 days) from completion of induction chemotherapy. Seventy of the 111 patients (63%) had 35% or greater decrease in mSUV of the primary tumor. Of these, 41 (59%) received induction carboplatin/paclitaxel, 25 (36%) received platinum/irinotecan, 3 (4%) received docetaxel/irinotecan ± cisplatin (on a phase I study), and 1 (1%) received capecitabine/oxaliplatin. Twenty of the 41 PET nonresponders (49%) were treated with induction platinum/paclitaxel, whereas 19 (46%) received platinum/irinotecan and 2 (5%) received docetaxel/irinotecan. Regarding type of induction chemotherapy received, there was no difference between PET responders versus PET nonresponders (p= 0.223)
Chemotherapy Change With Radiation for PET Nonresponders
PET responders (70 of 111 patients) continued with the same chemotherapy during radiation. Most patients received 28 fractions of 1.8 Gy administered over approximately 5.5 weeks (total 50.4 Gy). In general, patients who recieived cisplatin/irinotecan and docetaxel/irinotecan ± cisplatin continued on the same dose/schedule with radiation. Patients who continued with weekly carboplatin/paclitaxel during radiation typically continued the same dose of carboplatin but paclitaxel was reduced from 70–80 mg/m2 to 40–50 mg/m2.
Of the 41 PET nonresponders, 16 (39%) continued the same chemotherapy during chemoradiation. Twenty-five (61%) of the PET nonresponders were changed to alternative chemotherapy during radiation. Twenty-four patients who received platinum- or taxane-based induction chemotherapy crossed over to fluoropyrimidine-based therapy. One patient who received induction platinum-based therapy received salvage taxane-based therapy.
Outcomes
Treatment outcomes are shown in Table 2. Most patients (n = 98; 88%) underwent comprehensive re-staging following chemoradiation (repeat CT and/or PET/CT scan and repeat endoscopy with biopsy). Six patients (5%) did not undergo any re-staging at the end of treatment. Three patients died during or after chemoradiation due to aspiration pneumonia, pneumonitis, and cardiopulmonary failure of unknown etiology, respectively. Two patients had a decline in performance status after treatment and went to rehabilitation, one due to bilateral aspiration pneumonia in the setting of a tracheo-esophageal fistula, and one due to seizures secondary to post-stroke epilepsy. One patient underwent esophagectomy without re-staging.
Table 2.
Treatment Outcomes
| PET Nonresponders (n = 41) |
||||
|---|---|---|---|---|
| All Patients (n = 111), % |
PET Responders (n = 70), % |
No chemotherapy Change (n = 16), % |
Chemotherapy Change (n = 25), % |
|
| Clinical CR | ||||
| Yes | 82 (74) | 59 (84) | 8 (50) | 15 (60) |
| No | 24 (23) | 8 (11) | 6 (37.5) | 10 (40) |
| N/A | 5 (4.5) | 3 (4) | 2 (12.5) | - |
| Surgery | ||||
| Yes | 31 (27) | 22 (31) | 5 (31) | 4 (16) |
| No | 80 (72) | 48 (68.6) | 11 (69) | 21 (84) |
| R0 resection (n = 31) | ||||
| Yes | 30 (97) | 21 (100) | 5 (100) | 4 (80) |
| No | 1 (3) | 0 | 0 | 1 (20) |
| Pathologic CR (n = 31) | ||||
| Yes | 8 (26) | 7 (32) | 0 | 1 (25) |
| No | 23 (74) | 15 (68) | 5 (100) | 3 (75) |
Values are shown as n (%) unless otherwise noted.
CR, complete response; PET, positron-emission tomography; N/A, not available
Of 70 PET responders, 59 (84%) achieved a cCR, defined as the absence of metastatic disease on imaging, decreased extent and/or standard uptake value (SUV) of the primary tumor compared to baseline, and negative endoscopic biopsy. Eight of 16 PET nonresponders (50%) who received the same chemotherapy with radiation and 15 of 25 PET nonresponders (60%) who changed chemotherapy during radiation achieved a cCR. Although there was a higher cCR rate in the patients who were PET responders versus those who were not (p <0.01), the cCR rate was not different in the PET nonresponder patients who changed chemotherapy compared to the PET nonresponders who did not (p = 0.86).
Thirty-one (27%) patients underwent elective surgery, and 30 (97%) had R0 resections. Eight patients (7%) were treated on the phase II study which mandated surgery, 11 (10%) underwent elective surgery having achieved a cCR, 8 (7%) underwent surgery for locally persistent disease, and 4 (4%) underwent salvage surgery for locally recurrent disease. The pathologic complete response (pCR) rate was 32% (n = 7) in the PET responders. None of the five PET nonresponders who continued with the same chemotherapy during radiation achieved a pCR, whereas one of three PET nonresponders who changed chemotherapy with radiation obtained a pCR.
Of the 41 PET nonresponder patients, 30 relapsed (73%). Of these, 13 (43%) were distant, 8 (27%) were both local and distant, and 9 (30%) were local relapses. In PET responders (n = 70), 24 relapsed (34%). Of these, 8 were distant (33%), 7 (29%) were local and distant, and 9 patients (38%) relapsed locally.
Survival
At a median follow-up of 41.8 months, the median PFS and OS in all patients were 21 months (95% confidence interval [CI]: 13–62 months) and 45 months (95% CI: 27–85 months), respectively. When stratified by PET response status, median PFS was 70 months for PET responders and 7 months for nonresponders (p < 0.01). Median OS was 85 months in the PET responder group and 17 months in the nonresponder group (p < 0.01).
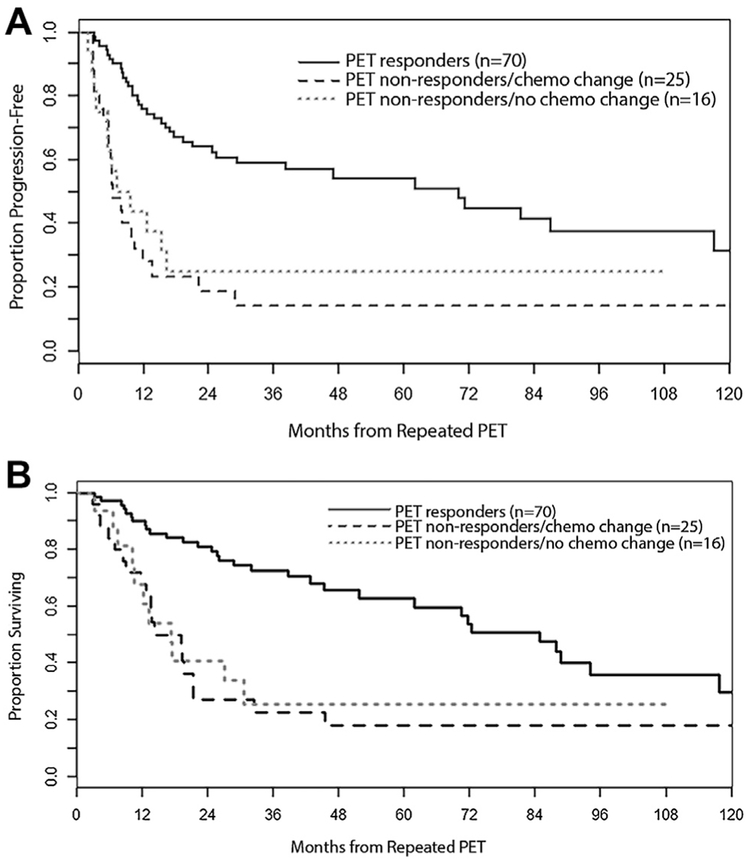
We further stratified the PET nonresponder group based on whether they received the same or alternative chemotherapy during radiation (Fig. 1). The median PFS of PET nonresponders who switched chemotherapy during radiation was not significantly different from those who continued with the same regimen and radiation (6 months versus 8 months, p = 0.556). The median OS of PET nonresponders who changed chemotherapy was 14 months versus 17 months for those who continued with the same chemotherapy (p = 0.81).
Figure 1.
(A) Progression-free and (B) overall survival stratified by positron-emission tomography (PET) response status and change in chemotherapy during radiation.
Given that carboplatin/paclitaxel with radiation is a current standard of care, we evaluated patients who received induction carboplatin/paclitaxel (n = 61; 55%).3 Median PFS and OS were 29 months (95% CI: 15–71 months) and 46 months (95% CI: 26–62 months), respectively. There were 41 PET responders, and 20 PET nonresponders, 8 of whom who continued the same chemotherapy during radiation and 12 who changed chemotherapy during radiation. Median PFS (47 months versus 7 months, p= 0.0026) and OS (52 months versus 17 months, p = 0.0095) were improved amongst PET responders versus nonresponders. However, there was no difference in PFS among patients who were PET nonresponders and changed chemotherapy compared to those who did not (7 months versus 11 months, p = 0.456). The median OS in PET nonresponders who changed chemotherapy was 14 months versus 17 months in PET nonresponders who did not change chemotherapy (p = 0.597).
Discussion
In this study, we reviewed the benefit of PET scan following induction chemotherapy before chemoradiation in a large cohort of ESCC patients. This data supports the use of PET after induction chemotherapy to predict outcomes in ESCC patients who receive chemoradiation. Our results also suggest that patients who are PET nonresponders after induction may not benefit from changing chemotherapy during radiation. However, a definitive conclusion cannot be drawn given the small patient numbers.
In addition to the MUNICON study discussed above, two Japanese studies retrospectively evaluated PET response in ESCC patients.11,12 They identified mSUV changes of 70% and 55% respectively, as optimal cutoffs for predicting outcomes. In a subsequent review of therapeutic PET response in patients with ESCC who received preoperative chemotherapy, patients with a complete metabolic response following chemotherapy had improved disease-free survival and OS.13
Our findings in conjunction with those from previous studies provide compelling evidence that PET assessment of chemotherapy is highly predictive of outcomes following subsequent surgery or chemoradiation (with or without surgery). In our series, the median PFS and OS rates of PET responders versus nonresponders were not only highly significant but clinically relevant. The median OS of PET responders was approximately 4.5 years longer than that of PET nonresponders.
PET responders had higher baseline SUV uptake than PET nonresponders (15.1 versus 11.6, p = 0.009). Whether a more FDG-avid tumor is associated with worse outcomes is unclear. An earlier study from our group of esophageal adenocarcinoma patients treated with surgery alone noted that a higher SUV in the primary tumor was associated with worse outcomes.14 Similarly, a series from MD Anderson suggested that higher baseline SUV was associated with lack of cCR and poorer OS in patients with esophageal cancer who received definitive chemoradiation.15 However, data from our group (in the era before PET-directed treatment) found that initial SUV did not predict survival in patients with esophageal adenocarcinoma treated with preoperative chemoradiation; although the low SUV group did have a higher likelihood of residual nodal disease and a lower likelihood of pCR.16 In SCC, a study from the same group of investigators who led the MUNICON study noted a numerically but not statistically significant difference in baseline SUV in 38 patients treated with preoperative chemoradiation who were pathologic responders versus those without a pathologic response (9.6 versus 3.5, p = 0.23).17
Response in nodal disease was not assessed in this study as response assessment in nodal disease has never been prospectively validated and was not included in metabolic assessment in the MUNICON or CALGB 80803 studies. Furthermore, some patients did not have FDG-avid nodal disease and in some cases FDG uptake in the primary tumor obscured lymph node activity making mSUV measurements difficult to determine.
Although several studies have shown a worse outcome for PET nonresponders, a critical question is whether lack of response on PET scan can be overcome in some of these patients with a change and/or augmentation of treatment. The use of PET to guide subsequent chemotherapy during radiation has been explored in esophageal adenocarcinoma patients. In MUNICON 2, PET response after 5-FU/cisplatin in GEJ adenocarcinoma patients was used to determine subsequent treatment strategy.18 PET nonresponders received chemoradiation with cisplatin before surgery, whereas PET responders were treated with chemotherapy and surgery. The PET nonresponders still had inferior outcomes. However, cisplatin was administered as a single agent with a low dose of radiation (32 Gy), and was associated with sub-optimal PET response when administered as induction therapy in combination with 5-FU in these patients.
An alternative strategy is to change chemotherapy in PET nonresponders. In the CALGB 80803 study, patients with esophageal/GEJ adenocarcinoma received induction with either carboplatin/paclitaxel or FOLFOX (bolus and infusional 5-FU/leucovorin/oxaliplatin).9 PET responders continued with the same regimen during radiation before surgery, whereas PET nonresponders received the alternative regimen with radiation followed by surgery. Preliminary results showed a pCR rate of 17% and 19% in PET nonresponders who changed from carboplatin to FOLFOX and from FOLFOX to carboplatin/paclitaxel respectively, compared to a historic rate of 3% in a retrospective analysis performed at MSK.19 Furthermore, median OS was 47.3 months in PET responders versus 28.9 months in PET nonresponders (p= 0.09).10 Based on comparison with historic controls, changing chemotherapy in PET nonresponders appears to improve survival in this patient population.
In contrast, our analysis in ESCC patients indicates that PET nonresponders who receive alternative chemotherapy during radiation do not derive benefit from this strategy and continue to have poor outcomes. The short survival in PET nonresponders suggests these patients may have underlying aggressive biology that cannot be countered by changing chemotherapy during radiation.
Preliminary results of a study evaluating the optimal radiation dose during definitive chemoradiation for inoperable ESCC showed no difference in locoregional PFS, PFS, or OS in patients treated with 60-Gy radiation versus standard-dose radiation in combination with cisplatin/docetaxel.20
The SCOPE2 trial () is also prospectively evaluating radiotherapy dose escalation in a randomized phase II/III trial in patients with esophageal adenocarcinoma and SCC receiving definitive chemoradiation. Patients will be randomized to standard or high-dose radiation and patients who are PET nonresponders to initial chemotherapy with cisplatin/capecitabine will also be eligible to be randomized to alternative chemotherapy with carboplatin/paclitaxel along with either standard or high-dose radiation.
However, the majority of patients in the PET nonresponder group in our study experienced distant failure (70%), suggesting that intensification of locoregional therapy with radiation dose escalation is unlikely to be a promising strategy and more effective alternative systemic therapies are urgently needed in this group.
Our study has several limitations including its retrospective nature, nonstandardized induction chemotherapy, and that surgery was not mandated for all patients. Although 65 patients (58%) had baseline PET imaging performed at MSK, for the remaining 48 patients (42%) the SUV uptake was based on the outside scan. However, we reviewed all PET imaging for 30 of these patients and re-measured SUV values. No significant discrepancies were noted between these measurements and the original values.
In summary, our observations confirm that PET has strong prognostic utility in ESCC. However, the strategy of changing chemotherapy in PET nonresponders does not appear to be beneficial in this population. Future clinical trials should use PET after induction chemotherapy to identify the subgroup of patients likely to experience poor outcomes and to consider for experimental strategies, such as the addition of immune checkpoint inhibitors. Future molecular analysis of tumors from PET responders and nonresponders may uncover markers of response and mechanisms of chemoresistance.
Acknowledgments
Supported by the National Cancer Institutes (NCI) Cancer Center Support Grant P30 CA 008748. The authors thank Erin Patterson, Department of Surgery, Memorial Sloan Kettering Cancer Center, for editorial assistance.
Footnotes
Disclosure: Dr. Molena has received personal fees from Novadaq. Dr. Rusch has received supportfrom Genelux, Inc., Genentech, and Bristol-Myers Squibb. Dr. Goodman is an advisory board member for Reno-voRx. Dr. Janjigian has received grants from Boehringer Ingelheim, Bayer, Genentech/Roche, Bristol-Myers Squibb, Eli Lilly, and Merck; and has received personal fees from Bayer, Bristol-Myers Squibb, Eli Lilly, Merck, Merck Serono, Pfizer, and Imugene. The remaining authors declare no conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 4.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–2317. [DOI] [PubMed] [Google Scholar]
- 5.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. [DOI] [PubMed] [Google Scholar]
- 6.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8: 797–805. [DOI] [PubMed] [Google Scholar]
- 7.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692–4698. [DOI] [PubMed] [Google Scholar]
- 8.Ilson DH, Minsky BD, Ku GY, et al. Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer. 2012;118:2820–2827. [DOI] [PubMed] [Google Scholar]
- 9.Goodman K, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): a randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol. 2017;35:1 [abstr]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman KA, Hall N, Bekaii-Saab TS, et al. Survival outcomes from CALGB 80803 (Alliance): a randomized phase II trial of PETscan-directed combined modality therapy for esophageal cancer. J Clin Oncol. 2018;36;4012–4012. [Google Scholar]
- 11.Makino T, Miyata H, Yamasaki M, et al. Utility of response evaluation to neo-adjuvant chemotherapy by (18)F-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148:908–918. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara R, Yamamoto S, Iishi H, et al. Predicting the effects of chemoradiotherapy for squamous cell carcinoma of the esophagus by induction chemotherapy response assessed by positron emission tomography: toward PET-response-guided selection of chemoradiotherapy or esophagectomy. Int J Clin Oncol. 2012;17:225–232. [DOI] [PubMed] [Google Scholar]
- 13.Yanagawa M, Tatsumi M, Miyata H, et al. Evaluation of response to neoadjuvant chemotherapy for esophageal cancer: PET response criteria in solid tumors versus response evaluation criteria in solid tumors. J Nucl Med. 2012;53:872–880. [DOI] [PubMed] [Google Scholar]
- 14.Rizk N, Downey RJ, Akhurst T, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg. 2006;81:1076–1081. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki A, Xiao L, Hayashi Y, et al. Prognostic significance of baseline positron emission tomography and importance of clinical complete response in patients with esophageal or gastroesophageal junction cancer treated with definitive chemoradiotherapy. Cancer. 2011;117: 4823–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizk NP, Tang L, Adusumilli PS, et al. Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J Thorac Oncol. 2009;4:875–879. [DOI] [PubMed] [Google Scholar]
- 17.Wieder HA, Brucher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22: 900–908. [DOI] [PubMed] [Google Scholar]
- 18.zum Buschenfelde CM, Herrmann K, Schuster T, et al. (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med. 2011;52:1189–1196. [DOI] [PubMed] [Google Scholar]
- 19.Ku GY, Kriplani A, Janjigian YY, et al. Change in chemotherapy during concurrent radiation followed by surgery after a suboptimal positron emission tomography response to induction chemotherapy improves outcomes for locally advanced esophageal adenocarcinoma. Cancer. 2016;122:2083–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Zhu W, Zheng X, et al. A multi-center, randomized, prospective study evaluating the optimal radiation dose of definitive concurrent chemoradiation for inoperable esophageal squamous cell carcinoma. J Clin Oncol. 2018;36:4013–4013. [Google Scholar]