Abstract
Cytomegalovirus (CMV) infection is a significant public health problem. Congenital CMV infection is a leading infectious cause of long-term neurodevelopmental sequelae, including mental retardation and sensorineural hearing loss. Immune protection against mouse cytomegalovirus (MCMV) is primarily mediated by NK cells and CD8+ T cells, while CD4+ T cells are not needed for control of MCMV in majority of organs in immunocompetent adult mice. Here we set out to determine the role of CD4+ T cells upon MCMV infection of newborn mice. We provide evidence that CD4+ T cells are essential for clearance of MCMV infection in brain of neonatal mice and for prevention of recurrence of latent MCMV. In addition, we provide evidence that CD4+ T cells are required for induction and maintenance of tissue resident memory CD8+ T cells in the brain of mice perinatally infected with MCMV.
Keywords: mouse cytomegalovirus, tissue-resident memory T cells, brain pathology, CD4 T cells, congenital CMV infection
INTRODUCTION
Cytomegalovirus (CMV) infection is a significant public health problem. In immunocompetent individuals, infection with human CMV (HCMV) is usually asymptomatic. However, it is a major health concern in immunocompromised and immunologically immature individuals. Since cytomegaloviruses are species-specific, mouse CMV (MCMV) infection of mice is the most commonly used model to study the pathogenesis of CMV infection [1]. Immune protection against CMV is primarily mediated by NK cells and CD8+ T cells [2,3]. CD4+ T cells are not needed for control of MCMV in the majority of organs in immunocompetent adult mice, but they mediate IFN-γ dependent clearance of MCMV from salivary glands where viral immunoevasion prevents control by CD8+ T cells [4–7].
Congenital CMV infection is a leading infectious cause of long-term neurodevelopmental sequelae, including mental retardation and sensorineural hearing loss [8]. In contrast to HCMV, MCMV does not cross the placenta. However, the central nervous system in newborn mice is developmentally equivalent to the human fetus at 15 weeks of gestation, a period when HCMV infection in humans is most frequently acquired during pregnancy [9]. Therefore, we have been using MCMV-infected newborn mice to model congenital CMV infection [10]. Using this model, we have previously shown that inflammation in the brain is a major determinant of neurodevelopmental disorders [11,12]. However, the immune response is required in brain for viral clearance and therefore a delicate balance of immune response is necessary to provide virus control and reduce pathology [12]. We have previously shown that in perinatally infected mice, CD8+ T cells are essential for control of MCMV [13]. Furthermore, upon resolution of acute MCMV infection, CD8+ T cells persist in the brain as tissue-resident memory (CD8+ TRM) cells and prevent reactivation of latent virus [14]. In addition to CD8+ T cells, CD4+ T cells infiltrate the brain of MCMV infected newborn mice. However, whether they provide protection against acute or latent MCMV upon infection of neonatal mice remained unknown.
Here we set out to determine the role of CD4+ T cells upon MCMV infection of newborn mice. We provide evidence that CD4+ T cells contribute to the control of acute MCMV infection in newborn mice in several organs. CD4+ T cells were required for clearance of MCMV in salivary glands and surprisingly also in the brain. Furthermore, upon depletion of CD4+ T cells during MCMV latency, the reactivated virus was readily detected in the brain indicating their contribution in the prevention of virus reactivation. In addition, we provide evidence that CD4+ T cells are required for induction and maintenance of CD8+ TRM cells in the brain of mice perinatally infected with MCMV. Further studies are needed to elucidate the mechanisms of CD4+ T cell control of MCMV in newborn mice and their involvement in virus pathogenesis in the central nervous system.
MATERIALS AND METHODS
Mice and viruses
C57BL/6 and C57BL/6JHT/JHT mice were housed and bred under specific pathogen-free conditions at the Central Animal Facility, Faculty of Medicine, University of Rijeka in accordance with the guidelines contained in the International Guiding Principles for Biomedical Research Involving Animals. The Animal Welfare Committee at the University of Rijeka, Faculty of Medicine and National ethics committee approved all animal experiments (525-10/0255-17-4). Newborn mice were infected intraperitoneally (i.p.) with 200 PFU of MCMV. Adult, 8 week-old mice were infected i.p. with 200 000 PFU of MCMV. Tissue culture-derived MCMV reconstituted from BAC pSM3fr-MCK-2fl [15] was used in all experiments. Virus stocks for infection of newborn and adult mice were aliquoted and frozen at −80°C before use. Virus stocks and organs homogenates were titrated on murine embryonic fibroblasts (MEF) using standard procedures [16].
Flow cytometry
Flow cytometry was performed according to the guidelines for the use of flow cytometry and cell sorting in immunological studies [17]. Lymphocytes from the brain were isolated using standard protocols. Briefly, mice were perfused with cold PBS and each brain was collected in RPMI 1640 with 3% FCS and mechanically dissociated. A 30% Percoll/brain homogenate suspension was underlaid with 70% Percoll in PBS and then centrifuged at 1050 g for 25 min. Cells in the interphase were collected for further analysis. Splenic leukocytes were prepared using standard protocols. Before staining of lymphocytes, Fc receptors were blocked using 2.4G2 antibody. The following antibodies, purchased from ThermoFisher were used: anti-mouse CD8α (clone 53-6.7), anti-mouse CD45.2 (clone 104), anti-mouse CD4 (clone RM4-5), anti-mouse CD69 (clone H1.2F3), anti-mouse CD103 (clone 2E7), anti-mouse IFN-γ (clone XMG1.2), anti-mouse CD11a (clone M17/4), anti-mouse/human T-bet (clone 4B10) and anti-mouse CXCR3 (clone CXCR3-173). Fixable Viability Dye (ThermoFisher) was used to exclude dead cells. IFNγ producing MCMV-specific CD4+ T cells were determined according to the previously described protocol [18]. In short, mice were treated intraperitoneally with 1 ml of 3% Thioglycolate 4 days before harvesting peritoneal macrophages (PECs). PECs were pulsed overnight with 100 ug/ml of uninfected or MCMV-infected MEF lysates. The following day, lymphocytes were isolated from brains and spleens of perfused mice and co-incubated with pulsed PECs in 4:1 ratio overnight in the presence of Brefeldin A and Monensin (ThermoFisher). Production of IFN-γ was determined by intracellular staining. All samples were acquired using FACSAriallu and data were analyzed using FlowJo v10 (Tree Star) software.
Depletion of T cells
Depletion of CD4+ and/or CD8+ T cells in newborn mice was performed by i.p. injection of 50 μg of CD4+ T cell-depleting antibody (clone YTS 191.1) and/or CD8+ T cell-depleting antibody (clone YTS 169.4) diluted in PBS every 3 days starting from postnatal day (PND) 2 until the termination of experiment. Depletion of CD4+ T cells in adult mice was performed by i.p. injection of 150 μg of CD4+ T cell-depleting antibody (YTS 191.1) once a week for four weeks.
Immunohistochemical analysis
MCMV IE1 staining was performed on formalin–fixed and paraffin-embedded tissue sections (3μm). After de-paraffinization and rehydration, antigen retrieval was performed in sodium citrate buffer (pH 6.0). Endogenous peroxidases were blocked with Peroxidase blocking solution (Dako). Samples were stained with MCMV IE1 antibody (clone IE1.01), biotin goat anti mouse Ig (BD) and streptavidin-POD (Roche). DAB (Dako) was used as the substrate. Samples were counterstained with hematoxylin. The analysis was performed using the Olympus BX40 microscope and Olympus digital camera (DP71).
Statistical analysis
Statistical analysis was performed using the Prism 5 software (GraphPad Software Inc.). Mann-Whitney U test was used to determine differences between groups. P<0.05 was considered to be statistically significant (*, P < 0.05; **, P < 0.01). In all figures, only statistically significant differences are indicated.
RESULTS
CD4+ T cells are necessary for control of MCMV in brain of newborn mice
We have previously shown that CD8+ T cells are essential for control of MCMV in newborn mice [13]. However the role of CD4+ T cells remained elusive. To determine the requirement for CD4+ T cells in control of MCMV in newborn mice we have depleted CD4+ T cells in MCMV infected newborn mice (Fig. 1). MCMV titers were significantly higher in all the observed organs in the CD4+ T cell-depleted group compared to undepleted controls. As expected, based on previous studies in adult mice, MCMV persisted at higher levels in salivary glands of CD4+ T cell-depleted mice as compared to the control group. Surprisingly, the similar was observed in the brain of newborn infected mice. These data indicate the important role of CD4+ T cells in control of MCMV infection in the brain of infected newborn mice.
Figure 1. CD4+ T cells control MCMV in newborn mice.
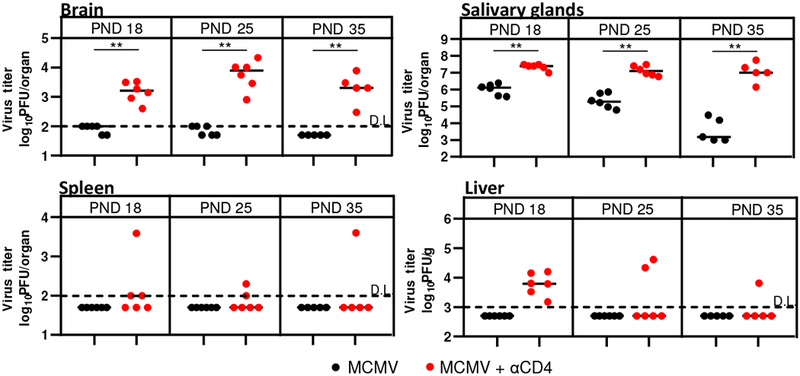
Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1. Organs were collected at the indicated postnatal days and viral titers were determined by plaque assay. Titers in the organs of individual mice are shown (circles, n=5-6). Horizontal bars represent medians; data were analyzed using Mann–Whitney U test. Asterisks denote significant values: *P < 0.05; **P < 0.01; DL, detection limit.
CD4+ T cells control MCMV independently of CD8+ T cells in newborn mice
To understand if the impaired control of MCMV in CD4+ T cell-depleted mice is due to lack of help for CD8+ T cells, we have simultaneously depleted both CD4+ and CD8+ T cells (Fig. 2). Individual depletion of CD8+ T cells or CD4+ T cells resulted in an increase of virus titers in both spleen and brain as compared to the undepleted control group. Simultaneous depletion of both CD8+ and CD4+ T cells resulted in a further increase in viral titers in spleen and brain as compared to individually CD8+ and CD4+ T cell-depleted groups (Fig. 2). Altogether, these data indicate that CD4+ T cells contribute to the control of MCMV infection at least partially independent of CD8+ T cells in newborn mice.
Figure 2. CD4+ and CD8+ T cell-dependent control of MCMV in newborn mice.

Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1 and depleted for the indicated T cell populations every 3 days starting from PND 2. Organs were collected on PND 14, and viral titers were determined by plaque assay. Titers in the organs of individual mice are shown (circles, n= 6). Horizontal bars represent medians; data were analyzed using Mann–Whitney U test. Asterisks denote significant values: *P < 0.05; **P < 0.01; DL, detection limit.
Virus-specific CD4+ T cells persist in the brain of mice perinatally infected with MCMV
We have previously shown that virus-specific CD8+ T lymphocytes infiltrate the brain of newborn mice infected with MCMV, and persist in this organ essentially for a lifetime as tissue resident cells [14,13]. CD4+ T cells also infiltrate the brain, and persist in this organ for up to 120 days [14]. CD4+ T cells persisting in the brain expressed signature markers of TRM cells, such as CD69. Here we extend this notion and show that CD69+CD4+ T cells can be found in the brain of mice infected perinatally even 1.5 years after infection, i.e. for the lifetime of mice (Fig. 3A). In addition to CD69, brain CD4+ T cells expressed markers of Th1 CD4+ T cells CD11a, T-bet and CXCR3 (Fig. 3B) [19]. In accordance with their Th1 phenotype, following stimulation of brain-derived lymphocytes with macrophages pulsed with lysates of MCMV infected MEF, approximately 30% of CD4+ T cells in the brain produced IFN-γ (Fig. 3C and 3D). At the same time, in spleen less than 5% of CD4+ T cells produced IFN-γ, indicating that virus specific CD4+ T cells are enriched in brain tissue. The observed frequency of IFN-γ producing CD4+ T cells was similar to the frequency of IFN-γ producing CD8+ T cells in the brain (Fig. 3C). Altogether, these data indicate that virus-specific CD4+ T cells persist in brain for the lifetime of mice perinatally infected with MCMV.
Figure 3. Virus-specific CD4+T cells are enriched in the brain of perinatally infected mice and persist as TRM cells.
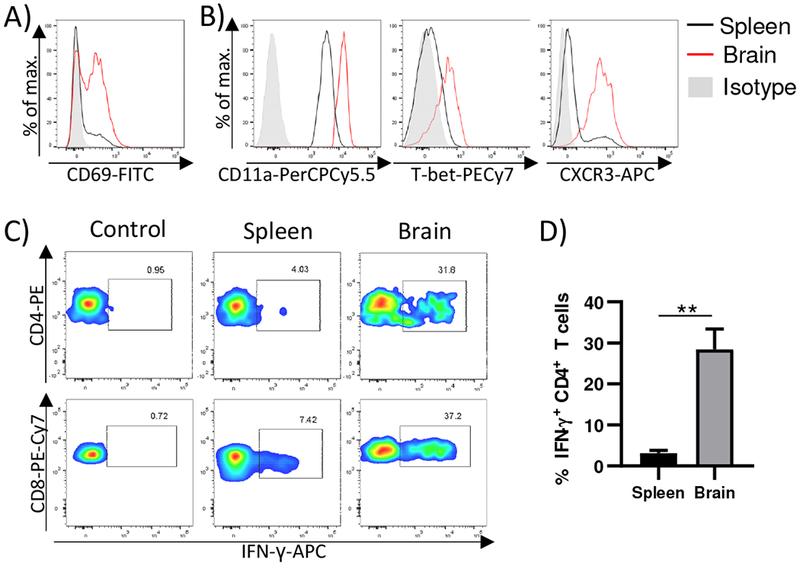
Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1. A) Representative expression of CD69 on CD4+ T cells isolated from brain and spleen 1.5 years after infection is shown. B) Representative expression of CD11a, T-bet and CXCR3 on CD69+ CD4+ T cells isolated from brain and spleen 1.5 years after infection is shown. (C-D). One year after infection, splenic and brain lymphocytes were isolated and stimulated with macrophages pulsed with lysates of MCMV infected MEFs. The frequency of IFN-γ producing CD4+ and CD8+ T cells was determined after 24 h stimulation. Representative flow cytometry plots (C) and quantification (D) are shown. 3-5 organs were pooled per sample (number of samples=5).
CD4+ T cells are required for generation and maintenance of CD8+ TRM cells
CD4+ T cells are important for formation of CD8+ TRM cells in some organs [20]. Upon MCMV infection of newborn mice, CD4+ T cells are needed for efficient generation of CD8+ TRM cells in the brain (Fig. 4A and [14]). To understand if CD4+ T cells are continuously required for maintenance of CD103 expression by CD8+ T cells in the brain, we injected CD4+ T cell-depleting antibody for one month into one-year-old mice perinatally infected with MCMV (Fig. 4C). To prevent the development of antibody response to the CD4+ T cell-depleting antibody (of rat origin), we have used B-cell deficient mice (C57BL76JHT/JHT). We did not detect CD4+ T cells in brains of mice which received CD4+T cell-depleting antibodies for one month (data not shown). Importantly, CD8+ T cells in the brain of CD4+ T cell-depleted mice did not express CD103, indicating that CD4+ T cells are required for maintenance of CD8+ TRM cells as well.
Figure 4. CD4+ T cells are required for generation and maintenance of CD8+ TRM cells in the MCMV-infected brain.
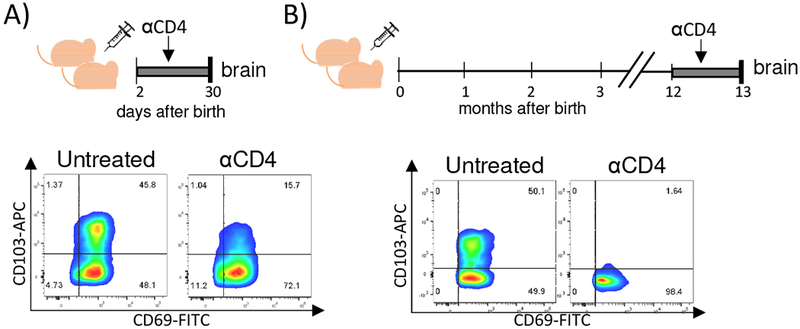
A) Newborn C57BL/6 mice were injected i.p. with 200 PFU of MCMV on PND 1. Starting with PND 2 mice were depleted of CD4+ T cells for one month, followed by lymphocyte isolation and analysis. B) Newborn C57BL/6JHT/JHT mice were injected i.p. with 200 PFU of MCMV on PND 1. One year after perinatal infection, C57BL/6JHT/JHT mice were CD4+ T cell-depleted for one month followed by lymphocyte isolation and analysis. Representative expression of TRM markers CD69 and CD103 by CD8+ T cells is shown.
Depletion of CD4+ T cells during latency in perinatally infected mice results in virus reactivation in the brain
To determine the role of CD4+ T cells in control of latent MCMV in the brain of perinatally infected mice, we applied the depletion regimen of CD4+ T cell as shown in Fig. 5A. We observed significantly more IE1+ cells in CD4+ T cell-depleted mice infected with MCMV perinatally as compared to control mice (Fig. 5B). Expectedly, we did not observe any IE1+ cells in brains of latently infected mice which were infected as adults. These data indicate that CD4+ T cells are not only required for control of primary MCMV infection in brain, but also for prevention of reactivation of latent virus in the brain of perinatally infected mice.
Figure 5. The absence of CD4+ T cells leads to an increase in virus reactivation in the brain.
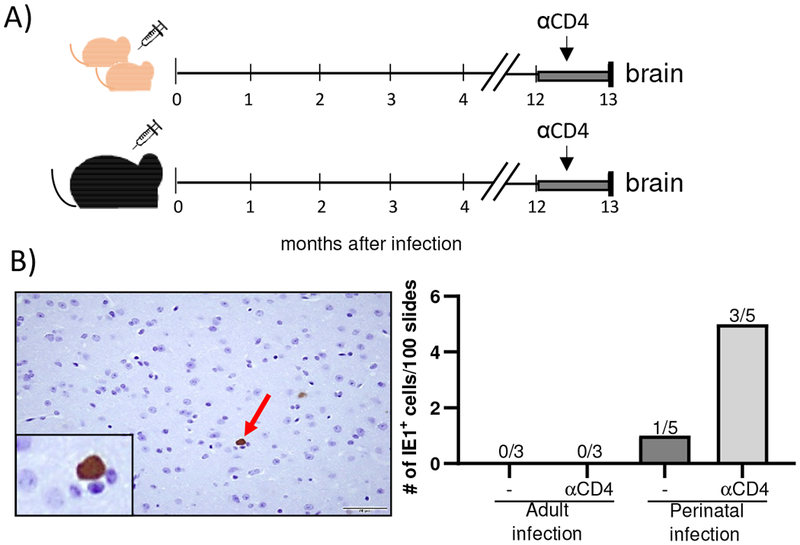
(A) Newborn C57BL/6JHT/JHT mice were injected i.p. with 200 PFU of MCMV on PND 1. Adult C57BL/6JHT/JHT mice were injected with 200 000 PFU. One year after infection mice were depleted of CD4+ T cells for one month before their brains were harvested. B) Brains were analyzed for MCMV IE1 expression using immunohistological analysis. A representative analysis of IE1+ cell in the brain is shown (x40; left). Quantification of IE1+ cells is shown on the right. The number of brains in which IE1+ cells were identified is shown above bars.
DISCUSSION
In immunocompetent individuals, control of CMV is readily established, and the infection is usually asymptomatic, but in immunocompromised and immunologically immature hosts, virus control is inefficient, and can result in morbidity and death. Therefore, understanding the immune response to the infection under such conditions is of great importance. Immune response to CMV in immunocompetent hosts has been studied extensively [2,1]. Protection against CMV is provided by different immune cell subsets, most importantly CD8+ T cells and NK cells. CD4+ T cells are not needed for control of MCMV in adult immunocompetent mice, with the exception of salivary glands [4]. However, under different immunodeficient conditions, CD4+ T cells can provide a certain level of protection against MCMV infection [21–23]. The role of CD4+ T cells in protection against MCMV in immunologically immature newborn mice was so far unknown. Here, we have determined the role of CD4+ T cells in control of MCMV in newborn mice, with the emphasis on the brain, a prime organ of long-term sequelae following congenital CMV infection.
In our study, the absence of CD4+ T cells resulted in impaired control of MCMV in newborn mice, which is not the case for adult mice. Moreover, after perinatal MCMV infection, CD4+ T cells stayed in the brains of infected animals over the lifetime. MCMV does not infect the brain of immunocompetent adult mice, however, following intracerebral MCMV infection CD4+ T cells were not important for virus control, with CD8+ T cells providing protection in a perforin-dependent manner [36]. The explanation why CD4+ T cells are needed for efficient MCMV control in newborn mice, but not in adult mice, remains unknown. Neonatal CD4+ T cell response is skewed towards Th2 response rather than to Th1 [24,25]. though it was shown that Th1-promoting inflammatory treatments, certain live viruses or DNA vaccines can overcome this bias and induce Th1 responses [26]. Little is known about the CD4+ T cell-mediated control of HCMV during congenital infection. HCMV-specific CD4+ T cells were identified in congenitally infected infants, and they are impaired in frequency and function as compared to CD4+ T cells in CMV infected adults [27–29]. However, their role in the protection against infection is still unclear.
One of the possible explanations for impaired MCMV control in CD4+ T cell-depleted mice could be lack of help resulting in impaired CD8+ T cell responses. Here we show that this is probably not the case at least in early days after infection. Namely, simultaneous depletion of CD4+ and CD8+ T cells further increased viral titers in brain and spleen as compared to a single depleted group lacking either CD4+ or CD8+T cells. Since CD8+ T cells are essential for the survival of newborn mice after MCMV infection [13]. the effect of CD8+ T cell deficiency on the viral burden in later days after infection could not be determined. However, this leaves the possibility of multiple roles of CD4+ T cells in different phases of infection. In line with this, we showed that at later times after infection absence of CD4+ T cells results in impaired generation and maintenance of CD8+ TRM cells in the brain of MCMV infected newborn animals. Tissue-resident memory T cells are the first line of defense against reinfections and reactivations, and they have been identified in mice and men [20]. In MCMV infected newborn mice CD8+ TRM cells are generated in the brain after PND 11 [14]. It was shown previously that CD4+ T cell help is required for CD8+ TRM development in lungs. However, they were not important once CD8+ TRM cells were established [30]. Similarly, CD4+ regulatory T cells (Treg) were required for generation of CD8+ TRM cells in the brain upon intracranial injection of MCMV in adult mice [31], and upon West Nile virus infection where Treg dependent TGF-β production was required for the expression of CD103 by CD8+ T cells [32]. Recently, it was shown that in the brain of mice persistently infected with polyomavirus, CD8+ TRM cells require CD4+ T cell help for their induction and maintenance [33]. Similarly, in our model, depletion of CD4+ T cells immediately after infection of newborn mice resulted in the diminished formation of CD8+ TRM cells in the brain. In addition, we have also observed that CD4+ T cells are important for the maintenance of CD8+ TRM cells in the brain. Namely, depletion of CD4+ T cells in latently infected mice for one month resulted in loss of CD103+ CD8+ T cells in brain. This was associated with increased numbers of MCMV IE1+ cells in brain tissue. Whether impaired generation of CD8+ TRM cells in brain upon depletion of CD4+ T cells is the reason for impaired MCMV control in brain following PND 18, remains to be verified.
Whether CD4+ T cells residing in the brain are important for the observed findings, or peripheral CD4+ T cells are also involved remains beyond the scope of this study. There are still technical challenges to directly discriminate the role of brain resident T cells. This seems to be even more demanding in the case of CD4+ T cells as at least in the skin they have been shown to be in equilibrium with the circulation in the steady state, and that they can modulate the expression of CD69 [34]. Whether this is the case for brain CD4+ T cells induced by MCMV infection remains to be determined.
In conclusion, in this study, we provided evidence that CD4+ T cells play an important role in the control of acute MCMV infection in brain of newborn mice. Similarly, in the absence of CD4+ T cells during latency, MCMV reactivates in the brain of latently infected mice. Altogether, we show that CD4+ T cells are an important antiviral mediator in MCMV-infected newborn mice.
Acknowledgments
We thank Edvard Ražić, Dijana Rumora, Miro Samsa, Edvard Marinović, Ante Miše and Tina Rudančić for the excellent technical support. The study was supported by NIH (1RO1AI089956-01A1) (to W.J.B. and S.J.), by the European Regional Development Fund (KK.01.1.1.01.0006) (to S.J.) and University of Rijeka (uniri-biomed-18-297 to I.B.).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
This article is part of the Special Issue on Immunological Imprinting during Chronic Viral Infection
Conflict of interest:
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Reddehase MJ, Lemmermann NAW (2018) Mouse Model of Cytomegalovirus Disease and Immunotherapy in the Immunocompromised Host: Predictions for Medical Translation that Survived the “Test of Time”. Viruses 10 (12). doi: 10.3390/vl0120693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S (2003) Pathogenesis of murine cytomegalovirus infection. Microbes Infect 5 (13):1263–1277 [DOI] [PubMed] [Google Scholar]
- 3.Reddehase MJ, Mutter W, Münch K, Bühring HJ, Koszinowski UH (1987) CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol 61 (10):3102–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonjić S, Mutter W, Weiland F, Reddehase MJ, Koszinowski UH (1989) Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J Exp Med 169 (4):1199–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton SM, Mandaric S, Torti N, Zimmermann A, Hengel H, Oxenius A (2011) Absence of cross-presenting cells in the salivary gland and viral immune evasion confine cytomegalovirus immune control to effector CD4T cells. PLoS Pathog 7 (8):e1002214. doi: 10.1371/journal.ppat.1002214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucin P, Pavic I, Polic B, Jonjic S, Koszinowski UH (1992) Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J Virol 66 (4):1977–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddehase MJ, Jonjic S, Weiland F, Mutter W, Koszinowski UH (1988) Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol 62 (3):1061–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boppana SB, Ross SA, Fowler KB (2013) Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 57 Suppl 4:S178–181. doi: 10.1093/cid/cit629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slavuljica I, Kveštak D, Huszthy PC, Kosmac K, Britt WJ, Jonjić S (2015) Immunobiology of congenital cytomegalovirus infection of the central nervous system—the murine cytomegalovirus model. Cell Mol Immunol 12 (2):180–191. doi: 10.1038/cmi.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cekinovic D, Lisnic VJ, Jonjic S (2014) Rodent models of congenital cytomegalovirus infection. Methods Mol Biol 1119:289–310. doi: 10.1007/978-l-62703-788-4_16 [DOI] [PubMed] [Google Scholar]
- 11.Kosmac K, Bantug GR, Pugel EP, Cekinovic D, Jonjic S, Britt WJ (2013) Glucocorticoid treatment of MCMV infected newborn mice attenuates CNS inflammation and limits deficits in cerebellar development. PLoS Pathog 9 (3):el003200. doi: 10.1371/journal.ppat.1003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brizić I, Hiršl L, Britt WJ, Krmpotić A, Jonjić S (2017) Immune responses to congenital cytomegalovirus infection. Microbes Infect. doi: 10.1016/j.micinf.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bantug GR, Cekinovic D, Bradford R, Koontz T, Jonjic S, Britt WJ (2008) CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol 181 (3):2111–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brizić I, Šušak B, Arapović M, Huszthy PC, Hiršl L, Kveštak D, Juranić Lisnić V, Golemac M, Pernjak Pugel E, Tomac J, Oxenius A, Britt WJ, Arapović J, Krmpotić A, Jonjić S (2018) Brain-resident memory CD8+ T cells induced by congenital CMV infection prevent brain pathology and virus reactivation. Eur J Immunol. doi: 10.1002/eji.201847526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan S, Krause J, Prager A, Mitrovic M, Jonjic S, Koszinowski UH, Adler B (2011) Virus progeny of murine cytomegalovirus bacterial artificial chromosome pSM3fr show reduced growth in salivary Glands due to a fixed mutation of MCK-2. J Virol 85 (19):10346–10353. doi: 10.1128/JVI.00545-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brizić I, Lisnić B, Brune W, Hengel H, Jonjić S (2018) Cytomegalovirus Infection: Mouse Model. Curr Protoc lmmunol:e51. doi: 10.1002/cpim.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cossarizza A, Chang HD, Radbruch A, Akdis M, Andrä I, Annunziato F, Bacher P, Barnaba V, Battistini L, Bauer WM, Baumgart S, Becher B, Beisker W, Berek C, Blanco A, Borsellino G, Boulais PE, Brinkman RR, Büscher M, Busch DH, Bushnell TP, Cao X, Cavani A, Chattopadhyay PK, Cheng Q, Chow S, Clerici M, Cooke A, Cosma A, Cosmi L, Cumano A, Dang VD, Davies D, De Biasi S, Del Zotto G, Della Bella S, Dellabona P, Deniz G, Dessing M, Diefenbach A, Di Santo J, Dieli F, Dolf A, Donnenberg VS, Dörner T, Ehrhardt GRA, Endl E, Engel P, Engelhardt B, Esser C, Everts B, Dreher A, Falk CS, Fehniger TA, Filby A, Fillatreau S, Follo M, Förster I, Foster J, Foulds GA, Frenette PS, Galbraith D, Garbi N, García-Godoy MD, Geginat J, Ghoreschi K, Gibellini L, Goettlinger C, Goodyear CS, Gori A, Grogan J, Gross M, Grützkau A, Grummitt D, Hahn J, Hammer Q, Hauser AE, Haviland DL, Hedley D, Herrera G, Herrmann M, Hiepe F, Holland T, Hombrink P, Houston JP, Hoyer BF, Huang B, Hunter CA, Iannone A, Jäck HM, Jávega B, Jonjic S, Juelke K, Jung S, Kaiser T, Kalina T, Keller B, Khan S, Kienhöfer D, Kroneis T, Kunkel D, Kurts C, Kvistborg P, Lannigan J, Lantz O, Larbi A, LeibundGut-Landmann S, Leipold MD, Levings MK, Litwin V, Liu Y, Lohoff M, Lombardi G, Lopez L, Lovett-Racke A, Lubberts E, Ludewig B, Lugli E, Maecker HT, Martrus G, Matarese G, Maueröder C, McGrath M, Mclnnes I, Mei HE, Melchers F, Melzer S, Mielenz D, Mills K, Mirrer D, Mjösberg J, Moore J, Moran B, Moretta A, Moretta L, Mosmann TR, Müller S, Müller W, Münz C, Multhoff G, Munoz LE, Murphy KM, Nakayama T, Nasi M, Neudörfl C, Nolan J, Nourshargh S, O’Connor JE, Ouyang W, Oxenius A, Palankar R, Panse I, Peterson P, Peth C, Petriz J, Philips D, Pickl W, Piconese S, Pinti M, Pockley AG, Podolska MJ, Pucillo C, Quataert SA, Radstake TRDJ, Rajwa B, Rebhahn JA, Recktenwald D, Remmerswaal EBM, Rezvani K, Rico LG, Robinson JP, Romagnani C, Rubartelli A, Ruckert B, Ruland J, Sakaguchi S, Sala-de-Oyanguren F, Samstag Y, Sanderson S, Sawitzki B, Scheffold A, Schiemann M, Schildberg F, Schimisky E, Schmid SA, Schmitt S, Schober K, Schüler T, Schulz AR, Schumacher T, Scotta C, Shankey TV, Shemer A, Simon AK, Spidlen J, Stall AM, Stark R, Stehle C, Stein M, Steinmetz T, Stockinger H, Takahama Y, Tarnok A, Tian Z, Toldi G, Tornack J, Traggiai E, Trotter J, Ulrich H, van der Braber M, van Lier RAW, Veldhoen M, Vento-Asturias S, Vieira P, Voehringer D, Volk HD, von Volkmann K, Waisman A, Walker R, Ward MD, Warnatz K, Warth S, Watson JV, Watzl C, Wegener L, Wiedemann A, Wienands J, Willimsky G, Wing J, Wurst P, Yu L, Yue A, Zhang Q, Zhao Y, Ziegler S, Zimmermann J (2017) Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 47 (10):1584–1797. doi: 10.1002/eji.201646632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walton SM, Wyrsch P, Munks MW, Zimmermann A, Hengel H, Hill AB, Oxenius A (2008) The dynamics of mouse cytomegalovirus-specific CD4 T cell responses during acute and latent infection. J Immunol 181 (2):1128–1134 [DOI] [PubMed] [Google Scholar]
- 19.Schreiner D, King CG (2018) CD4+ Memory T Cells at Home in the Tissue: Mechanisms for Health and Disease. Front Immunol 9:2394. doi: 10.3389/fimmu.2018.02394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller SN, Mackay LK (2016) Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16 (2):79–89. doi: 10.1038/nri.2015.3 [DOI] [PubMed] [Google Scholar]
- 21.Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH (1990) Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol 64 (11):5457–5464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeitziner SM, Walton SM, Torti N, Oxenius A (2013) Adoptive transfer of cytomegalovirus-specific effector CD4+ T cells provides antiviral protection from murine CMV infection. Eur J Immunol 43 (11):2886–2895. doi: 10.1002/eji.201343690 [DOI] [PubMed] [Google Scholar]
- 23.Reuter JD, Wilson JH, Idoko KE, van den Pol AN (2005) CD4+ T-cell reconstitution reduces cytomegalovirus in the immunocompromised brain. J Virol 79 (15):9527–9539. doi: 10.1128/JVI.79.15.9527-9539.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H (2004) IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 20 (4):429–440 [DOI] [PubMed] [Google Scholar]
- 25.Simon AK, Hollander GA, McMichael A (2015) Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282 (1821):20143085. doi: 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debock I, Flamand V (2014) Unbalanced Neonatal CD4(+) T-Cell Immunity. Front Immunol 5:393. doi: 10.3389/fimmu.2014.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huygens A, Lecomte S, Tackoen M, Olislagers V, Delmarcelle Y, Burny W, Van Rysselberge M, Liesnard C, Larsen M, Appay V, Donner C, Marchant A (2015) Functional exhaustion limits CD4+ and CD8+ T-cell responses to congenital cytomegalovirus infection. J Infect Dis 212 (3):484–494. doi: 10.1093/infdis/jiv071 [DOI] [PubMed] [Google Scholar]
- 28.Lidehäll AK, Engman ML, Sund F, Malm G, Lewensohn-Fuchs I, Ewald U, Tötterman TH, Karltorp E, Korsgren O, Eriksson BM (2013) Cytomegalovirus-specific CD4 and CD8 T cell responses in infants and children. Scand J Immunol 77 (2):135–143. doi: 10.1111/sji.12013 [DOI] [PubMed] [Google Scholar]
- 29.Hayashi N, Kimura H, Morishima T, Tanaka N, Tsurumi T, Kuzushima K (2003) Flow cytometric analysis of cytomegalovirus-specific cell-mediated immunity in the congenital infection. J Med Virol 71 (2):251–258. doi: 10.1002/jmv.10477 [DOI] [PubMed] [Google Scholar]
- 30.Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM (2014) CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41 (4):633–645. doi: 10.1016/j.immuni.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad S, Hu S, Sheng WS, Singh A, Lokensgard JR (2015) Tregs modulate lymphocyte proliferation, activation, and resident-memory T-cell accumulation within the brain during MCMV infection. PLoSOne 10 (12):e0145457. doi: 10.1371/journal.pone.0145457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham JB, Da Costa A, Lund JM (2014) Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J Immunol 192 (2):683–690. doi: 10.4049/jimmunol.1202153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mockus TE, Shwetank, Lauver MD, Ren HM, Netherby CS, Salameh T, Kawasawa Yl, Yue F, Broach JR, Lukacher AE (2018) CD4 T cells control development and maintenance of brain-resident CD8 T cells during polyomavirus infection. PLoS Pathog 14 (10):el007365. doi: 10.1371/journal.ppat.1007365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins N, Jiang X, Zaid A, Macleod BL, Li J, Park CO, Haque A, Bedoui S, Heath WR, Mueller SN, Kupper TS, Gebhardt T, Carbone FR (2016) Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Commun 7:11514. doi: 10.1038/ncommsll514 [DOI] [PMC free article] [PubMed] [Google Scholar]


