Abstract
Various mechanisms in the mammalian body provide resilience against food deprivation and dietary stress. The ketone body β-hydroxybutyrate (BHB) is synthesized in the liver from fatty acids and represents an essential carrier of energy from the liver to peripheral tissues when the supply of glucose is too low for the body’s energetic needs, such as during periods of prolonged exercise, starvation, or absence of dietary carbohydrates. In addition to its activity as an energetic metabolite, BHB is increasingly understood to have cellular signaling functions. These signaling functions of BHB broadly link the outside environment to epigenetic gene regulation and cellular function, and their actions may be relevant to a variety of human diseases as well as human aging.
Keywords: metabolism, epigenetics, ketone bodies, fasting, aging
INTRODUCTION
Mammals have developed a variety of mechanisms for adapting to changes in the environment, particularly changes in food availability and nutrient stress. Many of these nutrient-responsive pathways have broader effects on health and are emerging as regulators of fundamental mechanisms of aging. Fasting and dietary restriction, for example, have long been the most consistently effective intervention to slow various effects of aging and prolong the life span of otherwise healthy mammals. It is increasingly understood that the effects of fasting involve the actions of specific molecular signaling pathways. Cellular energy metabolites act as key mediators of many of these pathways, linking the external environment to changes in cellular function (43). Nicotinamide adenine dinucleotide (NAD), for example, accepts high-energy electrons from reactions in the catabolism of glucose and fatty acids and transfers them to acceptor molecules to either produce ATP or perform energetically demanding metabolic reactions. However, in its oxidized form (NAD+), NAD is also a cofactor for sirtuin enzymes and poly-ADP-ribose polymerase (PARP), both of which consume NAD in the course of removing acyl groups from and adding poly-ADP to proteins, respectively. Sirtuins and PARP thereby regulate cellular functions ranging from gene expression and DNA damage repair to fatty acid metabolism (134). During times of fasting, or relative scarcity of cellular energy, more NAD is in the oxidized state, and sirtuins and PARP can be more active.
NAD+, a simple energy carrier, thereby acts as a fulcrum around which many cellular processes can be regulated in response to changes in the external environment. Such signaling metabolites include acetyl-CoA (coenzyme A), another carrier of high-energy bonds that is also substrate for a widely prevalent protein posttranslational modification (lysine acetylation), and S-adenosyl-methionine, which similarly is substrate for a common posttranslational modification of histones and other proteins (methylation) (43). Independent manipulation of these signaling molecules can recapitulate, or abrogate, some of the broader biological effects of environmental changes such as fasting or dietary restriction. For example, long-term dietary restriction can prevent the onset of common age-related hearing loss in C57BL/6 mice. However, dietary restriction in mice that carry a genetic knockout of the NAD-dependent sirtuin gene SIRT3 has no such beneficial effect (122). Inhibition of the TOR (target of rapamycin) signaling complex by rapamycin (46), activation of AMPK by metformin (81), or provision of NAD+ precursors (161) recapitulates some of the beneficial effects of dietary restriction on diseases of aging and longevity. Understanding the specific molecular actions of these signaling pathways and signaling metabolites that link changes in the environment to broad regulation of cellular functions will permit researchers to more precisely capture the therapeutic potential of metabolic or dietary changes to treat disease. It might also help explain the heterogeneous responses of individuals to such environmental changes, depending on their genetic or epigenetic capacity to generate and respond to these signaling metabolites.
Here, we review the signaling activities of the endogenous metabolite β-hydroxybutyrate (BHB). BHB is the most abundant ketone body in mammals. Ketone bodies are small molecules synthesized primarily in the liver from fats that circulate through the bloodstream during fasting, prolonged exercise, and when carbohydrates are restricted. They are taken up by tissues in need of energy, converted first to acetyl-CoA and then to ATP. Emerging evidence, however, shows that BHB not only is a passive carrier of energy but also has a variety of signaling functions both at the cell surface and intracellularly that can affect, for example, gene expression, lipid metabolism, neuronal function, and metabolic rate. Some of these effects are direct actions of BHB itself. Some are indirect effects governed by downstream metabolites into which BHB is converted, such as acetyl-CoA. We focus this review on BHB itself, referring to ketogenic diets only when necessary for translational context. Although ketogenic diets have been widely used both for research into the effects of ketone bodies and as therapeutics for conditions ranging from epilepsy to obesity, a ketogenic diet is a complex physiological state with many possible active components of which BHB is only one. Still, the signaling effects of BHB we summarize are likely relevant to the molecular mechanisms of interventions such as fasting, dietary restriction, and ketogenic diets. Altogether, these observations present a picture of a powerful molecule that offers both opportunities and cautions in its therapeutic application to common human diseases.
BHB: STRUCTURE AND METABOLISM
Ketone bodies are small, lipid-derived molecules that provide energy to tissues when glucose is scarce, such as during fasting or prolonged exercise. Over 80% of the human body’s stored energy resides in the fatty acids contained in adipose tissue (7). During fasting, after muscle and liver stores of glycogen are depleted, fatty acids are mobilized from adipocytes and transported to the liver for conversion to ketone bodies. Ketone bodies are then distributed via blood circulation to metabolically active tissues, such as muscle or brain, where they are metabolized into acetyl-CoA and eventually ATP (7). In humans, serum levels of BHB are usually in the low micromolar range but begin to rise to a few hundred micromolar after 12−-16 h of fasting, reaching 1−-2 mM after 2 days of fasting (13, 109) and 6−-8 mM with prolonged starvation (12). Similarly, serum levels of BHB can reach 1−-2 mM after 90 min of intense exercise (64). Consistent levels above 2 mM are also reached with a ketogenic diet that is almost devoid of carbohydrates (60). The term ketone bodies usually includes three molecules that are generated during ketogenesis: BHB, acetoacetate, and acetone. Most of the dynamic range in ketone body levels is in the form of BHB. When ketogenesis is activated, such as during fasting, blood levels of BHB rise much faster than either acetoacetate or acetone (74).
Regulation of Ketone Body Metabolism
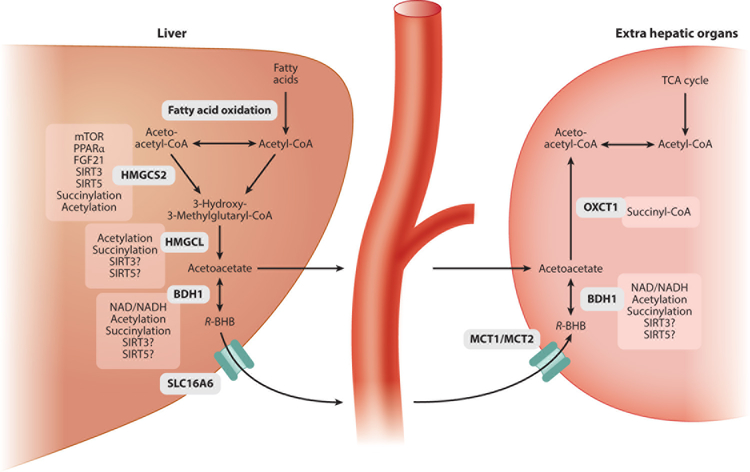
The biochemistry of ketone body production and utilization is well understood and has been recently summarized both in the literature and in textbooks (e.g., 7, 66, 92) (Figure 1). Two points are particularly relevant to understanding the signaling activities of BHB. First, the same enzyme, β-hydroxybutyrate dehydrogenase (BDH1; EC 1.1.1.30), interconverts BHB and acetoacetate in both the final step of ketogenesis and the first step of BHB utilization. BDH1 imparts chirality to BHB, as described below. Second, regulation of BHB synthesis is controlled via two principal mechanisms: substrate availability in the form of fatty acids and expression and activity of the enzyme HMG-CoA synthase (HMGCS2; EC 2.3.3.10). Ketogenesis occurs mostly in the liver (7), although expression of HMGCS2 may be sufficient to produce ketogenesis in other tissues (128, 160). Insulin and glucagon regulate ketogenesis primarily by modulating the availability of fatty acid substrates at the levels of mobilization from adipose tissue and importation into hepatic mitochondria (71). HMGCS2 gene expression is regulated by insulin/glucagon via acetylation and deacetylation of the FOXA2 transcription factor (144, 145, 136). FOXA2 deacetylation is controlled in part by the NAD-responsive enzyme SIRT1 (136). HMGCS2 gene expression is also regulated indirectly by the target of rapamycin complex mTORC1; mTORC1 inhibition is required for the activation of peroxisome proliferator-activated receptor alpha (PPARα) and fibroblast growth factor 21 (FGF21), both of which are required to induce ketogenesis (3, 4, 49, 117). The activity of HMGCS2 is regulated posttranslationally by succinylation and acetylation, regulated by the mitochondrial desuccinylase SIRT5 (104) and deacetylase SIRT3 (118), respectively. Altogether, this network of regulation centered on HMGCS2, involving substrate availability, transcriptional control, and posttranslational modification, lends tight temporal and spatial precision to BHB synthesis.
Figure 1.
Pathways of BHB metabolism and the regulation of key enzymes. Some of the molecules into which BHB is metabolized, such as acetyl-CoA, have signaling activities of their own, as do many of the cofactors involved in BHB metabolism, such as NAD. Abbreviations: BHB, β-hydroxybutyrate; BDH, β-hydroxybutyrate dehydrogenase; CoA, coenzyme A; FGF, fibroblast growth factor; HMGCS, HMG-CoA synthase; MCT, monocarboxylic acid transporter; mTOR, mechanistic target of rapamycin; NAD, nicotinamide adenine dinucleotide; PPARα, proliferator-activated receptor alpha; SIRT, sirtuin; TCA, tricarboxylic acid; VGLUT, vesicular glutamate transporter.
BHB Transport
BHB transport is relatively less well understood than its synthesis and utilization. As a small, polar molecule, BHB is readily soluble in water and blood (7). Several monocarboxylic acid transporters, including MCT1 and MCT2, carry BHB across the blood-brain barrier (99), and their expression can regulate brain BHB uptake (5). The monocarboxylate transporter SLC16A6 may be the key transporter for exporting BHB from the liver (52), but the putative transporters that facilitate the uptake of BHB into target tissues or its intracellular movement remain to be identified.
BHB Chirality
BHB is a chiral molecule at the 3′ hydroxyl group, an important feature in consideration of its signaling activities and possible therapeutic applications. There are two enantiomers, R/d and S/l. R-BHB is the normal product of human and mouse metabolism. This chiral specificity is introduced by BDH1, which catalyzes the final step in BHB synthesis by reducing the nonchiral 3′ carbonyl group of acetoacetate to the chiral 3′ hydroxyl group of BHB. BDH1 is also required for the utilization of BHB, by catalyzing the same reaction in reverse. As a result of the chiral specificity of BDH, only R-BHB is produced by normal metabolism and only R-BHB can be readily catabolized into acetyl-CoA and ATP. Fasting, exercise, caloric restriction, ketogenic diet, and any other state that results in endogenous production of BHB will produce only R-BHB.
S-BHB itself is not a normal product of human metabolism. However, S-BHB-CoA is a transient intermediate in the final round of β-oxidation of fatty acids. Under normal circumstances, it should not persist long enough to leave the mitochondrion or circulate in the blood. Experiments involving infusions of labeled R-BHB, S-BHB, or mixtures thereof into rats or pigs found that S-BHB is converted mostly to R-BHB (74); the molecular pathway for this is not known, but it may occur through conversion of S-BHB to acetyl-CoA and then production of R-BHB from acetyl-CoA. At least some of the S-BHB is eventually converted to CO2, presumably also after being metabolized to acetyl-CoA. S-BHB is metabolized much more slowly than R-BHB is (139), so that infusion of the same amount of S-BHB may result in higher and more sustained blood levels of S-BHB compared with a similar infusion of R-BHB (19). We discuss the chiral specificity of BHB signaling activities below.
DIRECT SIGNALING ACTIONS OF BHB
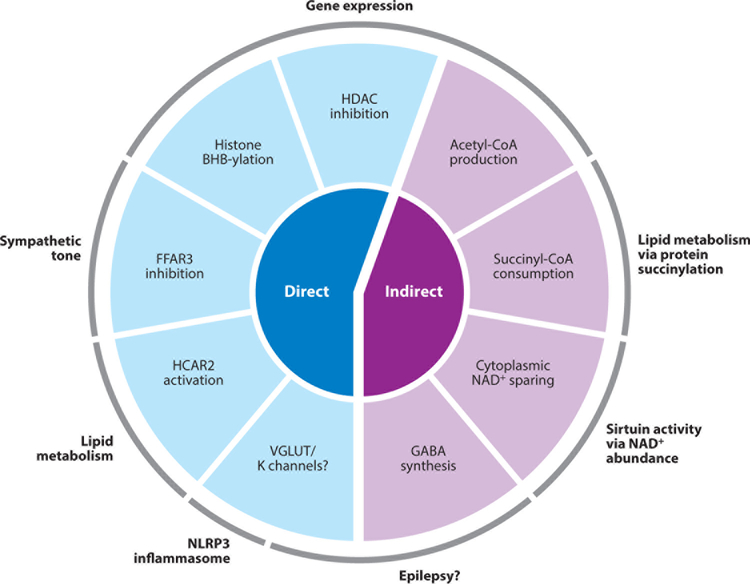
Several direct signaling actions of BHB have been described, including binding to cell-surface receptors, competitive inhibition of enzymes, as a substrate for protein posttranslational modification, and modulation of ion channel activity (Figure 2).
Figure 2.
Schematic of direct and indirect signaling functions of the ketone body BHB. Indirect signaling functions require catabolism to other molecules, whereas direct signaling functions are actions of BHB itself. The major downstream effects of signaling functions are noted. Abbreviations: BHB, β-hydroxybutyrate; CoA, coenzyme A; FFAR3, free fatty acid receptor 3; GABA, γ-amino-butyric acid; HDAC, histone deacetylase; HCAR2, hydroxycarboxylic acid receptor 2; NAD, nicotinamide adenine dinucleotide; VGLUT, vesicular glutamate transporter.
BHB Inhibits Class I Histone Deacetylases
BHB inhibits class I histone deacetylases (HDACs) (41), a family of proteins that have important roles in regulating gene expression by deacetylating lysine residues on histone and nonhistone proteins (reviewed in References 85, 90, and 151). Class I HDACs (e.g., HDAC1, HDAC2, HDAC3, and HDAC8) are small, mostly nuclear proteins that consist primarily of a deacetylase domain, and are usually found in large regulatory multiprotein complexes. Histone hyperacetylation is generally associated with activation of gene expression; so as a broad generality, class I HDAC activity suppresses gene expression. Many nonhistone proteins, including NF-κB, TP53, MYC, and MYOD1, among others (34), are also subject to HDAC-mediated deacetylation and regulation.
BHB inhibits HDAC1, HDAC3, and HDAC4 (classes I and IIa) in vitro with an IC50 of 2−-5 mM. BHB treatment of certain cultured cells induces dose-dependent histone hyperacetylation, particularly at histone H3 lysines 9 and 14 (119). Fasting, which increases plasma BHB levels, is associated with increases in histone acetylation in a number of mouse tissues by Western blot analysis (119), as well as in the liver by quantitative mass spectrometry (148). BHB can also increase histone H3 acetylation in vitro in macrophages (154) and neurons (121). Treating mice with BHB via an osmotic pump increases histone hyperacetylation, particularly in the kidney, and causes specific changes in gene expression, including induction of forkhead box O3 (Foxo3a), the mammalian ortholog of the stress-responsive transcriptional factor DAF16 that regulates life span in C. elegans (59). Induction of Foxo3a appears to be a direct effect of HDAC inhibition, as HDAC1 and HDAC2 are found on its promoter, knockdown of both relieves HDAC-mediated Foxo3a repression, and BHB causes hyperacetylation of histones at the Foxo3a promoter that results in increased Foxo3a expression (119). BHB regulates Bdnf (brain derived neurotrophic factor) expression in the mouse brain by a similar mechanism after exercise. Exercise increases BHB levels and Bdnf expression in the hippocampus, and Bdnf expression is increased by treating hippocampal slices with BHB or by infusing BHB intraventricularly. Treating primary neurons with BHB increases histone H3 acetylation, reduces Bdnf promoter occupancy by HDAC2 and HDAC3, and increases Bdnf expression (121).
Inhibition of class I HDACs appears to be a direct effect of BHB. Enzymatic inhibition in vitro is observed using immunopurified FLAG-tagged HDAC proteins with a synthetic peptide substrate, which should provide no opportunity for BHB metabolism or secondary effects (119). Competitive inhibition of the catalytic site is the likely mechanism. Crystal structures of the human class I HDAC8 bound to several hydroxamic acid inhibitors (123, 131), as well as modeling of other inhibitors, show that a carbonic or hydroxamic acid group of an inhibitor is commonly bound to the catalytic zinc at the bottom of the hydrophobic active site channel of the HDAC (138). BHB is structurally similar to the canonical HDAC inhibitor butyrate. Butyrate demonstrates the kinetics of a competitive inhibitor (116), suggesting that its carboxylic acid group might chelate the catalytic zinc in a manner similar to that of other acidic moieties on HDAC inhibitors. The structures of butyrate, BHB, and acetoacetate differ only in the oxidation state of the 3′ carbon. Increasing oxidation may be a barrier to binding within the hydrophobic channel of the HDAC active site, and as expected, the IC50 of the three compounds for HDAC1 increases with the oxidation state (116, 119).
β-Hydroxybutyrylation of Proteins
In addition to inhibiting enzymes involved in the regulation of protein posttranslational modifications such as HDACs, BHB can itself modify proteins at the posttranslational level. Lysine β-hydroxybutyrylation [K(BHB)] was detected via mass spectrometry as a histone modification in yeast, fly, mouse, and human cells (148). Western blot analysis detected that histone K(BHB) increases in human cells in proportion to treatment with exogenous BHB, and increases in mouse liver in proportion to increases in plasma BHB levels with either fasting of normal mice or induction of diabetic ketoacidosis with streptozotocin. At least 40 BHB-modified histone lysine sites were detected in human cells or mouse liver by mass spectrometry, including sites critical for transcriptional regulation such as H3K9. Fasting increased the relative abundance of K(BHB) at these sites by up to 40-fold (148).
Chromatin immunoprecipitation (ChIP) with specific antibodies also revealed that K(BHB) of H3K9 is preferentially associated with the promoters of actively transcribed genes (148). A comparison of ChIP data and RNAseq expression data from fasted mouse liver found a strong correlation between genes with the greatest increase in expression and genes with the greatest increase in K(BHB) at the promoter. H3K9 K(BHB) is correlated with other activation markers, such as acetylated H3K9 and trimethylated H3K4, but has an association with gene activation independent of these other two markers, suggesting an independent function (148). What that function is remains to be determined. The scope of β-hydroxybutyrylation as a posttranslational modification is not clear: It is not known whether nonhistone proteins are modified, or whether K(BHB) exists in organs other than the liver. It is also not known whether any enzymes catalyze the addition or removal of K(BHB), providing opportunities for specific targeting and regulation. The wide diversity of lysine acylations removed by the various mammalian sirtuins (1) hints that hydroxybutyrylation might be removed by a specific sirtuin. In all, histone K(BHB) may be the nexus of an important new network of gene regulatory activity associated with fasting and other conditions linked to increase BHB production.
Cell Surface Receptors: HCAR2 and FFAR3
BHB binds to at least two cell surface G-protein-coupled receptors (GPCRs), HCAR2 (hydroxycarboxylic acid receptor 2) and FFAR3 (free fatty acid receptor 3). These are among several GPCRs with fatty acid ligands that have important roles in metabolism and metabolic disease (9, 70). HCAR2 (also known as HCA2, PUMA-G, and Gpr109) is a Gi/o-coupled GPCR that was first identified as a nicotinic acid receptor (130). It was later shown to bind and be activated by BHB, with an EC50 of 0.7 mM (125). HCAR2 activation reduces lipolysis in adipocytes (95, 125), which might perhaps represent a feedback mechanism to regulate the availability of the fatty acid precursors of ketone body metabolism. However, elevated levels of free fatty acids in plasma from dysregulated adipocytes are also thought to contribute to insulin resistance through a variety of mechanisms, including proinflammatory cytokines, oxidative stress, and endoplasmic reticulum stress (10). Pharmacological agonists of HCAR2 reduce both plasma free fatty acids and plasma glucose levels in humans with type 2 diabetes (21). Infusion of BHB has long been observed to reduce the concentrations of nonesterified fatty acids in plasma, even when insulin levels (which also regulate fatty acid release from adipocytes) are held constant (109). In retrospect, this reduction of free fatty acids in plasma may be due to HCAR2 activation.
HCAR2 is also expressed in a variety of other cell types, including immune cells, microglia, and colonic epithelial cells, in which its activation induces anti-inflammatory effects (38). In the central nervous system, this is associated with neuroprotection mediated in part by the activation of IP3-dependent intracellular calcium release, alterations in prostaglandin production, and downstream inhibition of NF-κB activation (reviewed in Reference 96). More specifically, HCAR activates a neuroprotective subset of macrophages via the production of PGD2 by COX1 (103). The neuroprotective effect of ketogenic diet in a mouse stroke model is abrogated in Hcar2 knockout mice, and it appears that activation of HCAR2 on brain-infiltrating macrophages/monocytes is crucial to this neuroprotection (103). Finally, HCAR2 activation in neurons can potentiate glutaminergic signaling, which in one particular region of the brain helps regulate blood pressure and heart rate (106).
Activation of HCAR2 in the gut epithelium by short-chain fatty acids produced by bacterial fermentation of dietary fiber activates the NLRP3 inflammasome and maintains gut membrane integrity (79). The mechanism for inflammasome activation appears to be potassium efflux stimulated by intracellular calcium release. Inflammasome activation is beneficial in mouse models of colitis, and appears to explain the benefit of a high-fiber diet in these conditions, but stands in interesting contrast to the role of BHB in suppressing NLRP3 inflammasome activation described below.
BHB is also a ligand for FFAR3 (also known as GPR41). FFAR3 is another Gi/o-protein-coupled receptor that is highly expressed in sympathetic ganglions (62) throughout the body of mice (94). Ffar3 knockout mice have reduced basal oxygen consumption and body temperature but are then insensitive to the usual further sympathetic depression seen during prolonged fasting (62). Antagonism of FFAR3 by BHB suppresses sympathetic tone and heart rate and may be responsible for the sympathetic depression commonly seen during fasting (62). However, an electrophysiological study in rats later reported that BHB in fact acts as an agonist of FFAR3 (146), which regulates voltage-dependent calcium channels. In fact, other endogenous short-chain fatty acids, including acetate, butyrate, and propionate, have been reported as agonists of FFAR3 (11).
Further work is needed to confirm the activity of BHB on FFAR3, but a substantial literature is emerging on other biological functions of FFAR3 relevant to human health. One such function is glucose homeostasis. Deletion of Ffar3 (together with Ffar2) in mouse pancreatic β cells improves insulin secretion and glycemic control on a high-fat diet (126). Genetic gain- and loss-of-function models of Ffar3 alone similarly show that lower FFAR3 signaling increases insulin secretion from pancreatic β islet cells (133). An alternative route by which FFAR3 affects glucose metabolism is via activation by gut-derived propionate in the periportal afferent neural system. This feeds into a brain-gut signaling circuit that induces intestinal gluconeogenesis, with beneficial effects on glucose and energy homeostasis (17).
FFAR3 also regulates inflammation. Activation of FFAR3 by gut-derived propionate helps rescue allergic airway inflammation in mice by reducing the capacity of lung-resident dendritic cells to promote TH2-mediated inflammation (129). Similarly, activation of FFAR2 and FFAR3 by acetate suppresses the expression of inflammatory cytokines in human monocytes, which suggests FFAR2/3 agonists may help ameliorate inflammatory bowel diseases (2). As these examples illustrate, whether activation or inhibition of FFAR3 is beneficial to health may be highly dependent on the specific tissue and disease contexts.
Membrane Channel and Transporter Regulation
Two threads of indirect evidence imply a role for BHB in the modulation of potassium flux across the plasma membrane. Inhibition of potassium efflux is the suggested mechanism by which BHB inhibits activation of the NLRP3 inflammasome (154). An extensive series of careful in vitro experiments excluded other known direct and indirect signaling functions of BHB, including HDACs, HCAR2, and metabolism to acetyl-CoA. The event most upstream of inflammasome activation that is affected by BHB treatment is prevention of the decline in intracellular potassium in response to inflammasome-activating signals (154). This effect is the reverse of HCAR2 activation stimulating potassium efflux.
Application of acetoacetate or R-BHB to brain slices reduces the rate of firing from γ-amino-butyric acid (GABA)ergic neurons, and pharmacological inhibition of ATP-sensitive K (KATP) channels or knockout of the Kir6.2 subunit abrogates the effect (78). Although this might suggest that BHB could increase K channel opening, several lines of evidence indicate that the change in KATP activity is more likely an indirect effect of the absence of glucose on local intracellular ATP concentrations. The nonmetabolized enantiomer S-BHB did not have a similar effect on neuron firing (78); a later study showed that the change in KATP channel opening is due to reduced glycolysis in the presence of BHB (76) and that providing sufficient BHB and oxygen to maintain normal cellular ATP production can prevent the change in KATP activity (77).
BHB appears to have a direct regulatory effect on the neuronal vesicular glutamate transporter VGLUT2 (56). Cl− is an allosteric activator of glutamate uptake in an in vitro system consisting of proteoliposomes containing purified VGLUT2. Acetoacetate, BHB, and pyruvate inhibit this Cl−-dependent glutamate uptake, with kinetics indicating competition for the Cl− binding site. All these effects require the anions to be exposed to the extravesicular side of the membrane transporter. Acetoacetate is >10-fold more potent than BHB at inhibiting VGLUT2, but the IC50 of 3.75 mM for BHB still suggests that biologically relevant effects on glutamate uptake might occur at physiological low-millimolar BHB concentrations. Cl−-dependent activation of other VGLUT transporters were similarly inhibited by acetoacetate, but the vesicular GABA transporter VGAT was not. Consistent with these biochemical findings, acetoacetate inhibits glutamate release from neurons in a manner suggesting a reduced vesicular quantity of glutamate (56). In other contexts, however, BHB enhances glutaminergic transmission by increasing neurotransmitter release (121). Nevertheless, VGLUT inhibition may be a mechanism by which BHB can reduce excitatory glutamate neurotransmission without affecting inhibitory GABA neurotransmission.
Enantiomeric Specificity of Direct Signaling Activities
As noted above, BHB is a chiral molecule, and R-BHB is the enantiomer generated and readily consumed in normal mammalian metabolism. Signaling functions or other effects that depend on the rapid catabolism of BHB therefore are relevant only to the R-enantiomer. Signaling functions that are direct actions of BHB, however, might be recapitulated in part or in full by S-BHB depending on the stereoselectivity of the proteins involved. Indeed, several of the direct signaling functions described here have been reported to be nonstereoselective in the literature.
S-BHB can bind the GPCR HCAR2, albeit with somewhat lower affinity than R-BHB. An in vitro assay with modified Chinese hamster ovary cells expressing human HCAR2, using calcium flux as the readout, found that both R-BHB and S-BHB showed robust, similar receptor activation at concentrations of 15 mM. A quantitative binding assay using radiolabeled GTP to measure nucleotide exchange found that the EC50 was 0.7 mM for R-BHB and 1.6 mM for S-BHB with human HCAR2, and 0.3 mM and 0.7 mM, respectively, with mouse HCAR2. Acetoacetate was inactive (125). The chirality of BHB used to test activation of FFAR was not reported (62).
S-BHB can also block inflammasome activation (154). The use of caspase-1 activation in lipopolysaccharide-treated bone marrow--derived macrophage cells as an in vitro assay of NLRP3 inflammasome activation showed that S-BHB effectively blocks inflammasome activation, albeit perhaps with lower efficacy than R-BHB.
The steroselectivity of HDAC inhibition by BHB remains to be explored, but if, as described above, the relevant biochemical action is chelation of a catalytic zinc at the base of a hydrophobic channel, then the chirality of the trailing 3′ hydroxyl group may not be a critical determinant of activity. There is at least one suggestion that both R-BHB and S-BHB can increase histone acetylation in vitro (154).
The chirality of histone K(BHB) may be important to its biological function. The method used to detect K(BHB) could not distinguish modification with R-BHB from modification with S-BHB (148), but it is likely that both K(S-BHB) and K(R-BHB) are present. First, K(BHB) was detected in yeast and flies, and although generation of S-BHB-CoA as an intermediate of lipid β-oxidation is a conserved pathway in these metazoans, mitochondrial ketogenesis of R-BHB is not. Nor is Saccharomyces cerevisiae known to use polymers of R-BHB as an energy store, as is common in prokaryotes (18). Second, although fasting increases synthesis of R-BHB in mouse liver, it also increases flux of S-BHB-CoA as an intermediary of fatty acid oxidation. A number of factors probably influence whether K(R-BHB) or K(S-BHB) predominates. By analogy with other protein acylations, K(BHB) is likely formed from CoA-activated BHB. β-Oxidation produces S-BHB-CoA from an acyl-CoA precursor. Free R-BHB (as well as free S-BHB) must undergo enzymatic activation by a CoA synthase, which has long been observed to occur (109), although the precise CoA synthase involved and cellular compartment in which the activation occurs are unknown. The balance of K(R-BHB) versus K(S-BHB) in the liver might therefore depend on the rate and site of activation of R-BHB and on the efficiency of extramitochondrial transport of both activated forms. The balance in extrahepatic tissue might also depend on the relative utilization of fatty acids versus BHB for energy. Neurons, for example, which do not utilize fatty acids (7), would likely favor K(R-BHB) under ketogenic conditions. Whether the chirality of K(BHB) matters depends on its biological function, which is not yet understood. If the function is simply to occupy the lysine site and prevent alternative modifications, the chirality may be irrelevant. If the function is to serve as a binding site for other proteins, the 3′ hydroxyl group of BHB should remain both chiral and accessible to influence the binding of any putative K(BHB) recognition proteins.
INDIRECT SIGNALING ACTIONS VIA BHB CATABOLISM
In addition to its direct signaling activities, BHB might exert additional signaling effects in the course of its catabolism to acetyl-CoA and ATP. These reflect the signaling activities of other intermediate metabolites, including acetyl-CoA, succinyl-CoA, and NAD. Finally, catabolism of BHB can alter the steady state of downstream metabolic pathways, such as those that regulate neurotransmitter synthesis. When considering the possible medical applications of BHB precursors, note that R-BHB should be much more potent at eliciting these indirect signaling actions than S-BHB is, as S-BHB is catabolized much more slowly and through a different route.
Production of Acetyl-CoA, Substrate for Protein Acetylation
Catabolism of BHB into acetyl-CoA should raise intracellular acetyl-CoA levels, favoring both enzymatic and nonenzymatic protein acetylation. This effect complements HDAC inhibition by BHB but may have broader effects in multiple cellular compartments. Protein acetylation in mitochondria appears to be particularly sensitive to acetyl-CoA flux, as a variety of states associated with increased lipid utilization---including dietary restriction, fasting, and high-fat diets---increase mitochondrial protein acetylation. This effect on mitochondrial acetylation occurs despite the fact that neither acetyltransferases nor the HDACs that are inhibited by BHB enter mitochondria (48). Acetylation---and deacetylation by SIRT3---is widespread in mitochondria (105) and regulates the function of several mitochondrial enzymes, including HMGCS2 (118) and the long-chain acyl-CoA dehydrogenase (51).
Increased acetyl-CoA pools also affect nuclear protein acetylation. Mitochondrial acetylcarnitine is a source of acetyl-CoA for histone acetylation (80). Export of acetyl-CoA from the mitochondria is accomplished via a citrate shuttle mediated by citrate synthase inside mitochondria and ATP citrate lyase inside the cytoplasm (141). ATP citrate lyase is a key enzyme in fatty acid biosynthesis, but its role in facilitating acetyl-CoA export from mitochondria is also required for the increase in histone acetylation that occurs with growth factor stimulation (141). An alternative pathway for acetyl-CoA export from mitochondria is via the enzymes carnitine acetyltransferase and carnitine/acylcarnitine translocase (89). Indeed, a muscle-specific knockout of carnitine acetyltransferase in mice compromises glucose tolerance and decreases metabolic flexibility (89), demonstrating the importance of intracellular acetyl-CoA transport to overall metabolic health.
Consumption of Succinyl-CoA, Substrate for Protein Succinylation
Utilization of BHB in peripheral tissues uses succinyl-CoA to donate CoA to acetoacetate. This consumption of succinyl CoA may affect the balance of lysine succinylation, which, like acetylation, is widespread in mitochondria (104) and present across diverse organisms (140). A substantial fraction of these succinylation sites are regulated by the mitochondrial desuccinylase, the sirtuin SIRT5 (104). HMGCS2 has long been known to be succinylated, a modification that reduces its activity (102); HMGCS2 enzymatic activity in the liver is suppressed by succinylation and restored by desuccinylation mediated by SIRT5 (104). The mechanism of lysine succinylation is not clearly understood; succinyltransferase is not known to exist in mammalian cells, and because both liver succinyl-CoA abundance and succinylation of HMGCS2 are reduced in rats after treatment with glucagon (101, 102), it is possible that succinylation is primarily a nonenzymatic process dependent on local concentrations of succinyl-CoA. Although HMGCS2 itself is not expressed in the peripheral tissues that utilize BHB, enzymes in many other key mitochondrial pathways, including fatty acid oxidation, branched-chain amino acid catabolism, and the tricarboxylic acid (TCA) cycle, are heavily succinylated and regulated by SIRT5 (104). By analogy with acetylation (and the effect of succinylation on HMGCS2), these pathways may be activated by a reduction in succinylation. Consumption of succinyl-CoA during BHB utilization and consequent reduction in mitochondrial protein succinylation may therefore regulate many of these crucial mitochondrial pathways in peripheral tissues, perhaps favoring the switch to lipid-dependent energy usage.
Cytoplasmic and Mitochondrial NAD:NADH Equilibrium
Cellular NAD balance is emerging as a crucial factor in metabolic disease and aging (124). NAD utilization during BHB metabolism differs from that during glucose metabolism in two important respects. Fewer NAD+ molecules are consumed per acetyl-CoA produced when BHB is used than when glucose is used, and the cellular compartment in which NAD+ is consumed is different. Metabolism of one molecule of glucose to two molecules of acetyl-CoA involves conversion of four molecules of NAD+ into NADH. Two of these molecules are converted in the cytosol during glycolysis; the other two are converted in the mitochondrion by pyruvate decarboxylase. The cytosolic NADH are shuttled into mitochondria, potentially depleting the cytoplasmic NAD+ pool with high glucose utilization. By contrast, metabolism of one BHB molecule to the same two molecules of acetyl-CoA involves conversion of only two molecules of NAD+ into NADH, both in the mitochondrion by BDH1, thereby preserving the cytoplasmic NAD+ pool (7). The cytoplasmic and mitochondrial NAD pools are relatively distinct, so the preservation of cytoplasmic NAD+ by BHB may have important cellular effects. NAD+ is a cofactor for sirtuin deacylases (such as nuclear/cytoplasmic SIRT1) as well as poly-ADP-ribose polymerase (PARP) (134). Consumption of NAD+ by PARP or overproduction of NADH may promote age-related diseases by decreasing the activity of sirtuins (53). Conversely, repletion of NAD+ by exogenous feeding with nicotinamide mononucleotide improves glucose tolerance in both high-fat-diet-fed and aged mice (153). The relative sparing of NAD+ by utilizing BHB vis à vis glucose may therefore have important consequences for metabolic diseases and diabetes.
In the mitochondrion, BHB utilization is both determined by and changes the NAD:NADH equilibrium, with implications for signaling. The mitochondrial BHB:AcAc equilibrium is so closely linked to the NAD:NADH equilibrium that early metabolic studies of the liver used the former as a proxy for the latter (65). This tight relationship suggests that any influence that pushes the mitochondrial NAD:NADH equilibrium toward NADH (such as consumption of NAD by sirtuins or reduced activity of NADH dehydrogenase/complex I) will reduce BHB consumption. This reduction could increase the local concentration of BHB available for direct signaling functions within and around the mitochondrion, but also potentially reduce the flux of BHB transiting the cytoplasm and nucleus toward mitochondria.
However, BHB utilization also affects the mitochondrial redox state. Increased utilization of BHB is associated with pushing the NAD:NADH equilibrium toward NADH, as well as increasing the oxidation state of coenzyme Q (115). Owing to the increased heat of combustion of BHB compared with that of pyruvate, BHB also increases the efficiency of ATP production from the mitochondrial proton gradient and reduces the production of free radicals (132). Changes in free radical production might alter the activity of signaling networks that sense and respond to mitochondrial free radicals, such as p66Shc (33).
Neurotransmitter Synthesis
The potential mechanisms of action of ketogenic diets in treating epilepsy remain complex and controversial and have been the subject of several thorough reviews (47, 83, 156). One mechanism consistently proposed, however, involves how the downstream effects of BHB catabolism on the abundance or flux of other intermediate metabolites might alter the biosynthesis of the inhibitory neurotransmitter GABA.
The biosynthesis of GABA in inhibitory GABAergic neurons begins with the synthesis of glutamine in astrocytes. Glutamine is exported from astrocytes to neurons, where it undergoes conversion to glutamate and then decarboxylation to GABA. An alternative fate for glutamate in neurons is donation of its amino moiety to oxaloacetate, producing aspartate and α-ketoglutarate. Studies of isotopically labeled BHB show that it is used as a substrate for the synthesis of glutamine and other amino acids (158). Data from clinical studies of children on ketogenic diet for epilepsy show that cerebrospinal fluid GABA levels are higher on ketogenic diet, and the highest levels correlate with best seizure control (16).
BHB may affect GABA production via increased synthesis and/or pushing the fate of glutamate toward GABA and away from aspartate. Studies in synaptosomes show that BHB increases the content of glutamate and decreases that of aspartate (25). Studies of ketogenic diet in rodents similarly show that less glutamate is converted to aspartate (158). In cultured astrocytes, the presence of acetoacetate reduces the conversion of labeled glutamate to aspartate (157). Infusion of labeled BHB into rats fed a normal diet rapidly increases the levels of all components of this pathway (glutamine, glutamate, GABA, and aspartate) (113). Even when ketotic states are not associated with an increase in overall GABA levels, the proportion of glutamate shunted to GABA production is increased (84). The reason for this shunt may be the effect of BHB catabolism on TCA cycle intermediates. The relatively greater efficiency of BHB at generating acetyl-CoA compared with that of glucose, described above, increases the flux of acetyl-CoA through the TCA cycle. This increases the proportion of oxaloacetate required to condense with acetyl-CoA to permit its entry into the TCA cycle, reducing the availability of free oxaloacetate to participate in glutamate deamination to aspartate. By indirectly tying up oxaloacetate, BHB pushes the fate of glutamate toward GABA (156). Altogether, one effect of BHB catabolism, alone or as part of a ketogenic diet, appears to increase the capacity of GABAergic neurons to rapidly generate GABA from glutamate (156).
Although BHB is structurally reminiscent of GABA itself, evidence of a direct effect of BHB on activating GABA receptors is lacking. Neither BHB nor acetoacetate alters GABA currents in cultured rodent cortical neurons (22) or in rat hippocampal neurons (127). BHB did enhance the function of GABAA receptors expressed in Xenopus oocytes, but only modestly and at concentrations of 10 mM or higher (150), leaving the physiological significance of this effect unclear. However, GABOB (γ-amino-β-hydroxybutyric acid) is an endogenous agonist of GABA receptors that differs structurally from BHB only in the presence of the γ-amino moiety. It is biochemically plausible that BHB might be a direct substrate for GABOB synthesis, but no such aminotransferase is known to exist. Nor has any pathway for conversion of GABA to GABOB yet been identified in mammals. Of interest in consideration of BHB precursors as therapeutics, the enantiomer of GABOB that would be derived from S-BHB has the more potent antiepileptic affect and is a stronger GABAB receptor agonist (149).
BHB SIGNALING IN REGULATION OF METABOLISM
The integration of the various direct and indirect signaling functions of BHB appears to broadly help the organism adapt to a fasting state. The transition to a fasting state is already under way when ketogenesis is activated in the liver. BHB production might further promote that transition in extrahepatic tissues while also fine-tuning the control of lipid and glucose metabolism.
The combinatorial effects of BHB on gene expression, described below, might be expected to generally facilitate the activation of new transcriptional programs. The enrichment of histone K(BHB) at genes activated by fasting suggests that this might be particularly important for activating fasting-related gene networks, although this could also reflect a nonspecific association with activated transcription.
Inhibition of class I HDACs by BHB could play a major role in metabolic reprogramming, according to studies of HDAC knockout mice and of HDAC inhibitors. HDAC3 regulates expression of gluconeogenic genes (86), and HDAC3 knockout mice have reduced fasting glucose and insulin levels (8, 26, 63). In fact, chronic treatment with the HDAC inhibitor butyrate essentially keeps mice metabolically normal on a high-fat diet, with lower glucose and insulin levels, better glucose tolerance, reduced weight gain, and improved respiratory efficiency (32). Butyrate also provides some of these benefits even to mice already obese from being fed a high-fat diet (32). Similarly, inhibition of class I HDACs, but not class II HDACs, increases mitochondrial biogenesis, improves insulin sensitivity, and increases metabolic rate and oxidative metabolism in a mouse diabetes model (31). The mechanism for these metabolic benefits of class I HDAC inhibition may be upregulation of PGC1α (Ppargc1a) in in a variety of tissues by relief of HDAC3-mediated transcriptional repression (31, 32). Transcription of Fgf21 is similarly upregulated via inhibition of HDAC3 by butyrate, activating ketogenesis in obese mice (72). Several single nucleotide polymorphisms in HDAC3 have been associated with an elevated risk of type 2 diabetes in a Chinese population (159).
Activation of HCAR2 reduces lipolysis in adipocytes; because the availability of fatty acids in the liver is a critical determinant of ketogenesis (and is strongly regulated by insulin), this may provide a self-feedback mechanism to limit the production of BHB. Why this mechanism is insufficient to prevent dysregulated BHB production in insulin-deficient states such as diabetic ketoacidosis, and whether it could be potentiated to treat such states, such as through HCAR2 agonists, is unclear.
HCAR2 was originally identified as a niacin receptor, spurring efforts to develop more specific agonists to capture the therapeutic benefits of niacin on cardiovascular risk or glycemic control that were thought to be due to HCAR2’s effects on the levels of free fatty acid in blood. An HCAR2 agonist, GSK256073, transiently reduces levels of free fatty acids in blood but has only a modest effect on glycemic control in type 2 diabetes mellitus (20). Two other HCAR2 agonists similarly lowered free fatty acids but without otherwise altering the lipid profile in humans, while niacin was found to produce beneficial changes to the lipid profile in Hcar2 knockout mice (69). Thus, the model that niacin (and by extension BHB) exerts beneficial effects on cardiovascular risk through activation of HCAR2 in adipose tissue is probably too simplistic. It may even be the case that other cell types such as macrophages might mediate the therapeutic effects of HCAR2 agonists (30).
Interpreting the metabolic effects of BHB mediated by FFAR3 depends on whether BHB acts as an agonist or antagonist, and in which contexts. Strong evidence suggests that BHB antagonizes FFAR3 to reduce sympathetic activity, resulting in reduced heart rate, body temperature, and metabolic rate. If BHB also antagonizes FFAR3 in other contexts, it could improve insulin secretion from pancreatic β islet cells and impair intestinal gluconeogenesis. Altogether, these findings suggest that BHB would improve glycemic control, though a decrease in metabolic rate could be obesogenic.
The important role of the NLRP3 inflammasome in regulating obesity-associated inflammation and metabolic dysfunction has been extensively reviewed (15, 45). Briefly, the NLRP3 inflammasome appears to mediate an inflammatory response to nutrient excess and mitochondrial dysfunction. Mice deficient in NLRP3 are grossly normal when fed chow but are protected from obesity and insulin resistance when fed a high-fat diet. One proposed mechanism is through a reduction in inflammasome-induced IL-1β, which otherwise inhibits insulin signaling in adipocytes and hepatocytes while inducing pancreatic β-cell dysfunction. While NLRP3 may have an important homeostatic role in the response to day-to-day nutrient fluctuations, its chronic activation by nutrient excess may contribute to the development of metabolic disease---and inhibition of the NLRP3 inflammasome by BHB might ameliorate these maladaptations.
BHB SIGNALING IN REGULATION OF GENE EXPRESSION
Regulation of gene expression is the most common theme that emerges from the direct and indirect signaling functions of BHB. Histones are at the nexus of this theme, with their posttranslational modifications, including acetylation, succinylation, and β-hydroxybutyrylation. Histone acetylation is a well-understood mechanism for both broad and specific regulation of gene expression, and BHB can alter histone acetylation by directly inhibiting HDACs and by indirectly promoting acetyltransferase activity via acetyl-CoA flux. The biology of histone β-hydroxybutyrylation largely remains to be elucidated but is an area that should receive strong interest for its potential relevance to gene expression reprogramming in response to metabolic stimuli. Lysine succinylation is another histone posttranslational modification (147) and is regulated by the sirtuin SIRT7 in the context of the DNA damage response (73). Finally, alterations in cytoplasmic and nuclear NAD+ levels can affect the activity of sirtuins, which deacylate a number of histone tail residues (54).
The net integration of all these effects might be expected to facilitate gene transactivation, particularly of quiescent genes that are activated in response to stimuli. Two well-characterized examples of how HDAC inhibition (and acetyltransferase activation) can activate gene expression include reactivation of HIV latency and activation of lineage-specific genes during muscle differentiation. The HIV provirus sits in a transcriptionally inactive state in resting T cells, providing a reservoir of potential virus production that is impossible for current antiretroviral therapy to eradicate (44). The class I HDACs HDAC1, HDAC2, and HDAC3 help maintain this transcriptionally silenced state, as components of several transcriptional repressor complexes, by deacetylating histone tails. Ordinarily, a key step in the reactivation of HIV transcription is the recruitment of complexes containing the acetyltransferase p300 to acetylate histone tails. Inhibition of HDACs with vorinostat or panobinostat also promotes histone acetylation, resulting in the loss of latency and reactivation of HIV transcription (44). In vivo, such HDAC inhibitors broadly reactivate transcription from a diverse pool of latent proviruses (6).
The differentiation of a satellite cell (i.e., muscle stem cell) into the muscle lineage involves the transcriptional activation of a sequence of differentiation-promoting transcription factors (see Reference 120 for a review). One of these factors, MYOD1, further activates a wide range of muscle-specific genes. HDACs, including HDAC1 and HDAC2, reside at the promoters of many of these MYOD1 targets, maintaining histones in a deacetylated state. Activation of the genes requires dissociation of the HDAC from the promoter, and often recruitment of a p300 complex to promote histone acetylation. HDAC inhibitors potentiate this process, much as in HIV latency, and both increase acetylation at MYOD1 target promoters and increase myogenic differentiation. One contrast with HIV latency is that many gene promoters in satellites cells are maintained in a poised or bivalent state, possessing both activating and repressing histone modifications (120). Thus, whereas HIV latency is an example of HDAC inhibition waking a gene from deep silencing, muscle differentiation is an example of potentiating or lowering the threshold of activation for a gene already poised to do so. A similar example of activating poised genes may be relevant to cognition and dementia (see below).
How histone K(BHB) participates in epigenetic regulation largely remains to be determined. K(BHB) was particularly enriched after fasting at genes that were upregulated by fasting (148). Other stimuli or contexts expected to change gene expression were not examined, nor were cells or animals treated with BHB in the context of such stimuli. Thus, we do not yet know whether K(BHB) is specifically associated with a gene network that is responsive to fasting, or whether it is generally associated with newly transactivated promoters. We also have yet to understand the biological meaning of K(BHB)---whether it is an active signal that stimulates transcription through regulatory or binding interactions with the protein machinery of chromatin remodeling and transactivation, or whether it is a bystander modification emplaced at fasting-activated promoters owing to the confluence of increased BHB-CoA levels and newly accessible histone tails.
An additional layer of regulation can occur on nonhistone proteins involved in gene expression, as these posttranslational modifications are not restricted to histones. Acetylation and succinylation occur throughout the proteome and can affect protein function. It is possible that β-hydroxybutyrylation will be found to as well. Acetylation of nonhistone proteins is a critical step in both HIV latency and muscle differentiation, where acetylation activates the transcription factors Tat (44) and MYOD1 (120), respectively. The multilayered effects of BHB on gene expression can be illustrated with HMGCS2 itself, the rate-limiting enzyme in BHB synthesis. As described above, the transcription factor FOXA2 helps control Hmgcs2 transcription. FOXA2 is itself acetylated by EP300 in a reaction using acetyl-CoA as the acetyl group donor (increased by BHB). FOXA2 is deacetylated by a class I HDAC (inhibited by BHB) and/or by the sirtuin SIRT1, which requires NAD+ as a cofactor (increased by BHB). Initiation of Hmgcs2 transcription by activated FOXA2 might involve displacing or deactivating HDACs (inhibited by BHB) to permit emplacement of activating histone marks such as H3K9 acetylation by acetyltransferases (via acetyl-CoA increased by BHB). As this example illustrates, the overall impact of BHB might generally favor initiation or upregulation of transcription, but the effects on any specific loci could vary depending on the posttranslational regulation of the proteins involved at that site.
BHB IN THE BRAIN: EPILEPSY, DEMENTIA, AND COGNITION
The ketogenic diet has been clinically used for decades to treat epilepsy and currently has a wide range of therapeutic applications, mostly in childhood epilepsies (143). Despite this extensive clinical history, the mechanism of action of the ketogenic diet remains controversial (107). In fact, whether BHB itself is necessary or even active in the therapeutic effect of ketogenic diets is controversial, and the evidence varies between animal models (107). The various possible mechanisms of the antiepileptic effect of ketogenic diets have been reviewed (83, 107). Several of the signaling activities described above may be relevant, particularly modulation of potassium channels, FFAR3 activation, and promotion of GABA synthesis. Epigenetic modifications may also contribute to neuronal hyperexcitability and the long-term effects of epilepsy on the brain through persistent changes in gene expression (112). REST (RE1-silencing transcription factor) is a transcriptional repressor that recruits HDACs, among other chromatin-modifying enzymes, to help silence target genes. REST expression is increased in neurons after seizures and promotes aberrant neurogenesis. However, whether REST activity is helpful or harmful for seizure control differs in different seizure models (112). The contribution of epigenetic modifiers, including those that are modulated by BHB, to epilepsies and their long-term effects requires much further study.
Ironically, given the decades of study on the role of ketogenic diets in epilepsy, more molecular detail is known about the potential mechanisms of BHB in ameliorating dementia. Two major threads link BHB signaling with dementia: epigenetic modifications and neuronal hyperexcitability. There is a growing literature on the importance of epigenetic regulation in learning and memory, specifically in mouse models of dementia. Age-related impairments in learning and memory in wild-type mice are associated with alterations in histone acetylation (98), and treatment with HDAC inhibitors improves memory performance in both young and aged mice (42, 98). HDAC inhibitors also improve cognition in the CK-p25 dementia mouse model (28). HDAC2 appears to be the crucial mediator of these effects, as overexpression of HDAC2, but not HDAC1, impairs learning and memory in wild-type mice (42). Conversely, Hdac2 knockout mice show improved memory formation, which is not further improved by HDAC inhibitors (42). HDAC2 expression is increased in the brains of two mouse dementia models as well as the brains of humans with Alzheimer’s disease (39). One model of how HDAC inhibitors regulate cognition is via epigenetic priming, reminiscent of the poised transcriptional state of genes involved in muscle differentiation (40). The broader role of epigenetics in cognition and neurodegenerative disease has been reviewed (67, 68, 100).
The worlds of epilepsy and dementia have been linked through the finding that mouse models of Alzheimer’s disease show neuronal hyperexcitability and epileptiform spikes from dysfunctional inhibitory interneurons (97, 135). Epilepsy, an extreme manifestation of this hyperexcitability, is associated with more rapid cognitive decline in patients with Alzheimer’s disease (137). Promising treatments that reduce epileptiform spikes, including at least one commonly used antiepileptic drug, improve cognition in these models (114, 135). The various signaling activities by which BHB acts in epilepsy may thus be relevant to ameliorating cognitive decline in Alzheimer’s disease. In small studies, provision of BHB precursor molecules improves cognition in an Alzheimer’s mouse model (58) and in a patient with Alzheimer’s disease (93). Further exploration of the links between BHB signaling, epilepsy, and dementia may prove fruitful in generating new translational therapies.
Inhibition of the NLRP3 inflammasome could also prevent cognitive decline and dementia. β-amyloid protein, which aggregates into the amyloid plaques characteristic of Alzheimer’s disease, activates the NLRP3 inflammasome in microglia, the resident macrophage population in the brain, releasing inflammatory cytokines including IL-1β (reviewed in References 29 and 35). This activation is evident in the brain of humans with both mild cognitive impairment and Alzheimer’s disease, and Alzheimer’s mouse models that carry deficiencies in NLRP3 inflammasome components are protected from β-amyloid deposition and cognitive decline (50). Microglia, as critical mediators of brain inflammation, may be the site of integration of various BHB-related signals, including HCAR2 activation.
BHB INTERACTIONS WITH AGING PATHWAYS
The hypothesis that BHB may play a broad role in regulating longevity and the effects of aging comes in part from the observation that many of the interventions that most consistently extend longevity across a wide range of organisms, such as dietary restriction and fasting, intrinsically involve ketogenesis and the production of BHB in mammals (92). The effects of such regimens on invertebrate, rodent, and human health have been reviewed and can include extended longevity, cognitive protections, reductions in cancer, and immune rejuvenation (75, 82). More specific interventions that promote ketogenesis, such as transgenic overexpression of FGF21, also extend life span in rodents (163). BHB itself extends longevity in C. elegans (24), and whether it would do so in rodents remains to be investigated.
Several of the signaling functions of BHB described above broadly regulate longevity and diseases of aging pathways, most prominently HDAC inhibition and inflammasome inhibition. The data from invertebrate organisms showing that reduction in class I HDAC activity extends life span, and generally acts through similar pathways as dietary restriction, have been reviewed (92). Briefly, deletion of Rpd3, the yeast and fly homolog of mammalian class I HDACs, extends replicative life span by 40−-50% in S. cerevisiae (61). Rpd3 deletion enhances ribosomal DNA silencing (61), the same mechanism by which overexpression of the sirtuin Sir2 enhances replicative longevity in S. cerevisiae (57). Drosophilids heterozygous for a null or hypomorphic Rpd3 allele show a 30−-40% extension of life span, with no further increase with caloric restriction (111). Both caloric restriction and reduced Rpd3 activity increase expression of Sir2 (111). Conversely, mutations in Sir2 block life span extension by either caloric restriction or Rpd3 mutations (110). In both organisms, then, modest reductions in HDAC activity (stronger reductions are lethal) extend life span via the same mechanisms as in dietary restriction and Sir2 expression.
Other possible longevity mechanisms downstream of Rpd3 in invertebrates include autophagy, which is regulated by histone acetylation of specific genes (152), and enhanced proteostasis through increased chaperone expression (164).
No life span data yet exist for reduced HDAC function in rodents. However, Hdac2 knockout mice display impaired IGF-1 signaling and are 25% smaller than normal (165), a potential longevity phenotype (87). Hdac2 knockout is also protective in models of tumorigenesis (165). Conditional knockouts in mouse embryonic fibroblasts and embryonic stem cells demonstrated roles for HDAC1 and HDAC2 in hematopoiesis (142) and stem cell differentiation (23). By analogy to the modest reductions in class I HDACs that enhance longevity in invertebrates, it may be of interest to determine whether heterozygous HDAC1/2 knockout mice, or mice treated with low-dose pharmacological HDAC inhibitors, have enhanced longevity.
An inducible compound heterozygote knockout of HDAC1 and HDAC2 does suppress one translatable age-related phenotype, cardiac hypertrophy, as do HDAC inhibitors (88). HDAC inhibitors ameliorate cardiac dysfunction in mouse diabetes models (14) and prevent maladaptive cardiac remodeling (162). The mechanism for the effect on cardiac hypertrophy appears to be inhibition of HDACs that suppress the activity of a mechanistic mTOR complex (88). This is one of several examples of intersections between BHB, its signaling effects, and mTOR/rapamycin, a canonical longevity-regulating pathway (55). As described above, mTOR is also a checkpoint in the activation of ketogenesis; inhibition of mTORC1 is required to activate the transcription factors and hormones that control ketogenesis (4, 117).
Inhibition of NLRP3 inflammasome activation might also have broad effects on aging and longevity, as reviewed in References 27 and 37. The NLRP3 inflammasome in particular has a wide range of activating stimuli, many of which accumulate with age such as urate, amyloid, cholesterol crystals, and excess glucose. The age-related phenotypes that may be ameliorated by its inhibition are similarly diverse: insulin resistance, bone loss, cognitive decline, and frailty. In two such examples, BHB inhibited NLRP3 inflammasome activation in urate crystal--activated macrophages, and ketogenic diet ameliorated flares of gout arthritis in rats (36). Whether genetic or pharmacological inhibition of the NLRP3 inflammasome would extend mammalian life span remains unknown, but the potential certainly exists for translational application to human diseases of aging.
APPLICATION AND FUTURE DIRECTIONS
The ketone body BHB expresses a variety of molecular signaling functions, in addition to its role as a glucose-sparing energy carrier, that may influence a broad range of human diseases. There is sufficient evidence for several significant human diseases, including type 2 diabetes mellitus and Alzheimer’s dementia, in model organisms to justify human studies of BHB or a BHB-mimetic intervention. The diversity of age-associated diseases and pathways affected by BHB signaling suggests that therapies derived from BHB may hold promise for broadly enhancing health span and resilience in humans (91).
The translation of these effects into therapies that improve human health span requires the pursuit of two converging strategies: deeper mechanistic understanding of the downstream effects of BHB signals and improved systems for the targeted delivery of BHB for both experimental and therapeutic goals. Deeper mechanistic understanding would solidify some of the transitive connections described above. For example, BHB inhibits HDACs, and HDAC inhibition protects against cognitive decline in rodents; but does BHB protect against cognitive decline? Via HDAC inhibition? Which gene promoters are targeted? Establishing such links would permit rational design of human studies to test specific effects of BHB, with plausible biomarkers and intermediate outcomes. Improved delivery systems would facilitate both animal and human studies.
BHB-mimetic drugs, or ketomimetics, would recapitulate the desired activity of BHB. The key obstacles to exogenous delivery of BHB are its nature as an organic acid and the rapid catabolism of R-BHB. The quantity of exogenous BHB required to sustain blood levels over a long period would likely be harmful because of either excessive salt load or acidosis. Alternatively, approaches to ketomimetics include (a) the use of agents that activate endogenous ketogenesis in an otherwise normal dietary context, (b) the delivery of BHB prodrugs or precursors that avoid the acid/salt problem, and (c) the use of agents that phenocopy specific downstream signaling events. The last approach, such as using HCAR2 agonists or HDAC inhibitors, is tempting, but a perhaps crucial advantage of adapting BHB itself is utilizing the existing endogenous transporters and metabolite gradients to bring BHB to its sites of action. Esters of BHB are a promising approach to delivering BHB as a prodrug, but the expense of synthesis is challenging. Confirming whether such synthetic compounds need be enantiomerically pure, or indeed whether S-BHB has better pharmacokinetics for the desired signaling function, might help reduce cost.
The ketone body BHB, a fasting fuel and fasting signal, is emerging as a poster child of the endogenous metabolite that transmits signals from the environment to affect cellular function and human health. Researchers have made important strides in understanding the signaling functions of BHB, many of which have crucial implications for the management of human diseases. A deeper knowledge of the endogenous actions of BHB, and improved tools for delivering BHB or replicating its effects, offers promise for the improvement of human health span and longevity.
ACKNOWLEDGMENTS
We thank Sarah Gardner for assistance with figures. This work is supported by Gladstone intramural funds (E.V.) and NIH K08AG048354 (J.C.N.).
ACRONYMS AND DEFINITIONS
- γ-amino-butyric acid (GABA)
an inhibitory neurotransmitter
- BHB
β-hydroxybutyrate
- Class I histone deacetylases (HDACs)
small, mostly nuclear HDACs with important roles in gene transcription
- GABOB
γ-amino-β-hydroxybutyric acid
- K(BHB)
lysine β-hydroxybutyrylation
- NAD
nicotinamide adenine dinucleotide
- R-BHB
R-enantiomer of BHB (normal ketone body)
- Rpd3
invertebrate homolog of class I HDACs
- S-BHB
S-enantiomer of BHB
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Anderson KA, Green MF, Huynh FK, Wagner GR, Hirschey MD. 2014. SnapShot: mammalian sirtuins. Cell 159(4):956–956.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang ZW, Er JZ, Tan NS, Lu JH, Liou YC, et al. 2016. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci. Rep 6:34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. 2009. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150:4931–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. 2007. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–37 [DOI] [PubMed] [Google Scholar]
- 5.Balietti M, Casoli T, Di Stefano G, Giorgetti B, Aicardi G, Fattoretti P. 2010. Ketogenic diets: an historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res. Rev 9:273–79 [DOI] [PubMed] [Google Scholar]
- 6.Barton KM, Palmer SE. 2016. How to define the latent reservoir: tools of the trade. Curr. HIV/AIDS Rep 13:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg JM, Tymoczko JL, Stryer L. 2012. Biochemistry New York: Freeman [Google Scholar]
- 8.Bhaskara S, Knutson SK, Jiang G, Chandrasekharan MB, Wilson AJ, et al. 2010. Hdac3 is essential for the maintenance of chromatin structure and genome stability. Cancer Cell 18:436–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blad CC, Tang C, Offermanns S. 2012. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat. Rev. Drug Discov 11:603–19 [DOI] [PubMed] [Google Scholar]
- 10.Boden G 2011. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes 18:139–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, et al. 2003. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem 278:11312–19 [DOI] [PubMed] [Google Scholar]
- 12.Cahill GF Jr. 2006. Fuel metabolism in starvation. Annu. Rev. Nutr 26:1–22 [DOI] [PubMed] [Google Scholar]
- 13.Cahill GF Jr, Herrera MG, Morgan AP, Soeldner JS, Steinke J, et al. 1966. Hormone-fuel interrelationships during fasting. J. Clin. Investig 45:1751–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YF, Du JF, Zhao YT, Zhang L, Lv GR, et al. 2015. Histone deacetylase (HDAC) inhibition improves myocardial function and prevents cardiac remodeling in diabetic mice. Cardiovasc. Diabetol 14:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coll RC, O’Neill LAJ, Schroder K. 2016. Questions and controversies in innate immune research: What is the physiological role of NLRP3? Cell Death Discov 2:16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlin M, Elfving A, Ungerstedt U, Amark P. 2005. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res 64:115–25 [DOI] [PubMed] [Google Scholar]
- 17.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, et al. 2014. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96 [DOI] [PubMed] [Google Scholar]
- 18.Dedkova EN, Blatter LA. 2014. Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front. Physiol 5:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desrochers S, Dubreuil P, Brunet J, Jette M, David F, et al. 1995. Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am. J. Physiol 268:E660–67 [DOI] [PubMed] [Google Scholar]
- 20.Dobbins R, Byerly R, Gaddy R, Gao F, Mahar K, et al. 2015. GSK256073 acutely regulates NEFA levels via HCA2 agonism but does not achieve durable glycaemic control in type 2 diabetes. A randomised trial. Eur. J. Pharmacol 755:95–101 [DOI] [PubMed] [Google Scholar]
- 21.Dobbins RL, Shearn SP, Byerly RL, Gao FF, Mahar KM, et al. 2013. GSK256073, a selective agonist of G-protein coupled receptor 109A (GPR109A) reduces serum glucose in subjects with type 2 diabetes mellitus. Diabetes Obes. Metab 15:1013–21 [DOI] [PubMed] [Google Scholar]
- 22.Donevan SD, White HS, Anderson GD, Rho JM. 2003. Voltage-dependent block of N-methyl-d-aspartate receptors by the novel anticonvulsant dibenzylamine, a bioactive constituent of l-(+)-β-hydroxybutyrate. Epilepsia 44:1274–79 [DOI] [PubMed] [Google Scholar]
- 23.Dovey OM, Foster CT, Cowley SM. 2010. Histone deacetylase 1 (HDAC1), but not HDAC2, controls embryonic stem cell differentiation. PNAS 107:8242–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC. 2014. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging 6:621–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erecinska M, Nelson D, Daikhin Y, Yudkoff M. 1996. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J. Neurochem 67:2325–34 [DOI] [PubMed] [Google Scholar]
- 26.Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, et al. 2002. The retinoblastoma-histone deacetylase 3 complex inhibits PPARγ and adipocyte differentiation. Dev. Cell 3:903–10 [DOI] [PubMed] [Google Scholar]
- 27.Feldman N, Rotter-Maskowitz A, Okun E. 2015. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev 24:29–39 [DOI] [PubMed] [Google Scholar]
- 28.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. 2007. Recovery of learning and memory is associated with chromatin remodelling. Nature 447:178–82 [DOI] [PubMed] [Google Scholar]
- 29.Freeman LC, Ting JPY. 2016. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem 136:29–38 [DOI] [PubMed] [Google Scholar]
- 30.Gaidarov I, Chen XH, Anthony T, Maciejewski-Lenoir D, Liaw C, Unett DJ. 2013. Differential tissue and ligand-dependent signaling of GPR109A receptor: implications for anti-atherosclerotic therapeutic potential. Cell. Signal 25:2003–16 [DOI] [PubMed] [Google Scholar]
- 31.Galmozzi A, Mitro N, Ferrari A, Gers E, Gilardi F, et al. 2013. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 62:732–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, et al. 2009. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gertz M, Steegborn C. 2010. The lifespan-regulator p66Shc in mitochondria: redox enzyme or redox sensor? Antioxid. Redox Signal 13:1417–28 [DOI] [PubMed] [Google Scholar]
- 34.Glozak MA, Sengupta N, Zhang X, Seto E. 2005. Acetylation and deacetylation of non-histone proteins. Gene 363:15–23 [DOI] [PubMed] [Google Scholar]
- 35.Gold M, El Khoury J. 2015. β-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin. Immunopathol 37:607–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, et al. 2017. β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep 18:2077–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberg EL, Dixit VD. 2015. Drivers of age-related inflammation and strategies for healthspan extension. Immunol. Rev 265:63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graff EC, Fang H, Wanders D, Judd RL. 2016. Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2. Metab. Clin. Exp 65:102–13 [DOI] [PubMed] [Google Scholar]
- 39.Graff J, Rei D, Guan JS, Wang WY, Seo J, et al. 2012. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature 483:222–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graff J, Tsai LH. 2013. Histone acetylation: molecular mnemonics on the chromatin. Nat. Rev. Neurosci 14:97–111 [DOI] [PubMed] [Google Scholar]
- 41.Gregoretti IV, Lee YM, Goodson HV. 2004. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J. Mol. Biol 338:17–31 [DOI] [PubMed] [Google Scholar]
- 42.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, et al. 2009. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gut P, Verdin E. 2013. The nexus of chromatin regulation and intermediary metabolism. Nature 502:489–98 [DOI] [PubMed] [Google Scholar]
- 44.Hakre S, Chavez L, Shirakawa K, Verdin E. 2012. HIV latency: experimental systems and molecular models. FEMS Microbiol. Rev 36:706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haneklaus M, O’Neill LAJ. 2015. NLRP3 at the interface of metabolism and inflammation. Immunol. Rev 265:53–62 [DOI] [PubMed] [Google Scholar]
- 46.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, et al. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartman AL, Gasior M, Vining EPG, Rogawski MA. 2007. The neuropharmacology of the ketogenic diet. Pediatr. Neurol 36:281–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He W, Newman JC, Wang MZ, Ho L, Verdin E. 2012. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol. Metab 23:467–76 [DOI] [PubMed] [Google Scholar]
- 49.Hegardt FG. 1999. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem. J 338 (Pt. 3):569–82 [PMC free article] [PubMed] [Google Scholar]
- 50.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, et al. 2013. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, et al. 2010. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464:121–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DY, Schlegel A. 2012. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev 26:282–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imai SI, Guarente L. 2014. NAD+ and sirtuins in aging and disease. Trends Cell Biol 24:464–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jing H, Lin HN. 2015. Sirtuins in epigenetic regulation. Chem. Rev 115:2350–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson SC, Rabinovitch PS, Kaeberlein M. 2013. mTOR is a key modulator of ageing and age-related disease. Nature 493:338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, et al. 2010. Metabolic control of vesicular glutamate transport and release. Neuron 68:99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaeberlein M 2010. Lessons on longevity from budding yeast. Nature 464:513–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, et al. 2013. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 34:1530–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kenyon CJ. 2010. The genetics of ageing. Nature 464:504–12 [DOI] [PubMed] [Google Scholar]
- 60.Kim DY, Rho JM. 2008. The ketogenic diet and epilepsy. Curr. Opin. Clin. Nutr. Metab. Care 11:113–20 [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Benguria A, Lai CY, Jazwinski SM. 1999. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol. Biol. Cell 10:3125–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, et al. 2011. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). PNAS 108:8030–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. 2008. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J 27:1017–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koeslag JH, Noakes TD, Sloan AW. 1980. Post-exercise ketosis. J. Physiol 301:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krebs HA, Gascoyne T. 1968. The redox state of the nicotinamide-adenine dinucleotides in rat liver homogenates. Biochem. J 108:513–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laeger T, Metges CC, Kuhla B. 2010. Role of β-hydroxybutyric acid in the central regulation of energy balance. Appetite 54:450–55 [DOI] [PubMed] [Google Scholar]
- 67.Landgrave-Gómez J, Mercado-Gómez O, Guevara-Guzmán R. 2015. Epigenetic mechanisms in neurological and neurodegenerative diseases. Front. Cell. Neurosci 9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HWM, et al. 2015. The epigenetics of aging and neurodegeneration. Prog. Neurobiol 131:21–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lauring B, Taggart AKP, Tata JR, Dunbar R, Caro L, et al. 2012. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci. Transl. Med 4:148ra115. [DOI] [PubMed] [Google Scholar]
- 70.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. 2013. Short chain fatty acids and their receptors: new metabolic targets. Transl. Res.: J. Lab. Clin. Med 161:131–40 [DOI] [PubMed] [Google Scholar]
- 71.LeRoith D, Taylor SI, Olefsky JM. 2004. Diabetes Mellitus: A Fundamental and Clinical Text Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- 72.Li H, Gao Z, Zhang J, Ye X, Xu A, et al. 2012. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes 61:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Shi L, Yang SD, Yan RR, Zhang D, et al. 2016. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat. Commun 7:12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lincoln BC, Des Rosiers C, Brunengraber H. 1987. Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch. Biochem. Biophys 259:149–56 [DOI] [PubMed] [Google Scholar]
- 75.Longo VD, Panda S. 2016. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 23:1048–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lund TM, Ploug KB, Iversen A, Jensen AA, Jansen-Olesen I. 2015. The metabolic impact of β-hydroxybutyrate on neurotransmission: Reduced glycolysis mediates changes in calcium responses and KATP channel receptor sensitivity. J. Neurochem 132:520–31 [DOI] [PubMed] [Google Scholar]
- 77.Lutas A, Birnbaumer L, Yellen G. 2014. Metabolism regulates the spontaneous firing of substantia nigra pars reticulata neurons via KATP and nonselective cation channels. J. Neurosci 34:16336–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma WY, Berg J, Yellen G. 2007. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J. Neurosci 27:3618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macia L, Tan J, Vieira AT, Leach K, Stanley D, et al. 2015. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun 6:6734. [DOI] [PubMed] [Google Scholar]
- 80.Madiraju P, Pande SV, Prentki M, Madiraju SR. 2009. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenet.: Off. J. DNA Methylation Soc 4:399–403 [DOI] [PubMed] [Google Scholar]
- 81.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, et al. 2013. Metformin improves healthspan and lifespan in mice. Nat. Commun 4:2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mattson MP, Longo V, Harvie M. 2016. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev pii:S1568–1637(16)30251–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McNally MA, Hartman AL. 2012. Ketone bodies in epilepsy. J. Neurochem 121:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melo TM, Nehlig A, Sonnewald U. 2006. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem. Int 48:498–507 [DOI] [PubMed] [Google Scholar]
- 85.Mihaylova MM, Shaw RJ. 2013. Metabolic reprogramming by class I and II histone deacetylases. Trends Endocrinol. Metab 24:48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mihaylova MM, Vasquez DS, Ravnskjaer K, Denechaud PD, Yu RT, et al. 2011. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell 145:607–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Milman S, Atzmon G, Huffman DM, Wan JX, Crandall JP, et al. 2014. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 13:769–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morales CR, Li DL, Pedrozo Z, May HI, Jiang N, et al. 2016. Inhibition of class I histone deacetylases blunts cardiac hypertrophy through TSC2-dependent mTOR repression. Sci. Signal 9:ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, et al. 2012. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 15:764–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.New M, Olzscha H, La Thangue NB. 2012. HDAC inhibitor-based therapies: Can we interpret the code? Mol. Oncol 6:637–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newman JC, Milman S, Hashmi SK, Austad SN, Kirkland JL, et al. 2016. Strategies and challenges in clinical trials targeting human aging. J. Gerontol. A Biol. Sci. Med. Sci 71:1424–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newman JC, Verdin E. 2014. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab 25:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. 2015. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s Dement 11:99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, et al. 2015. Expression of the short chain fatty acid receptor Gpr41/Ffar3 in autonomic and somatic sensory ganglia. Neuroscience 290:126–37 [DOI] [PubMed] [Google Scholar]
- 95.Offermanns S, Colletti SL, Lovenberg TW, Semple G, Wise A, IJzerman AP. 2011. International union of basic and clinical pharmacology. LXXXII: nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B). Pharmacol. Rev 63:269–90 [DOI] [PubMed] [Google Scholar]
- 96.Offermanns S, Schwaninger M. 2015. Nutritional or pharmacological activation of HCA2 ameliorates neuroinflammation. Trends Mol. Med 21:245–55 [DOI] [PubMed] [Google Scholar]
- 97.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, et al. 2007. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci 27:5903–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, et al. 2010. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328:753–56 [DOI] [PubMed] [Google Scholar]
- 99.Pellerin L, Bergersen LH, Halestrap AP, Pierre K. 2005. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J. Neurosci. Res 79:55–64 [DOI] [PubMed] [Google Scholar]
- 100.Penney J, Tsai LH. 2014. Histone deacetylases in memory and cognition. Sci. Signal 7:re12. [DOI] [PubMed] [Google Scholar]
- 101.Quant PA, Tubbs PK, Brand MD. 1989. Treatment of rats with glucagon or mannoheptulose increases mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase activity and decreases succinyl-CoA content in liver. Biochem. J 262:159–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quant PA, Tubbs PK, Brand MD. 1990. Glucagon activates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in vivo by decreasing the extent of succinylation of the enzyme. Eur. J. Biochem 187:169–74 [DOI] [PubMed] [Google Scholar]
- 103.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, et al. 2014. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun 5:3944. [DOI] [PubMed] [Google Scholar]
- 104.Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, et al. 2013. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18:920–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, et al. 2013. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. PNAS 110:6601–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rezq S, Abdel-Rahman AA. 2016. Central GPR109A activation mediates glutamate-dependent pressor response in conscious rats. J. Pharmacol. Exp. Ther 356:456–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rho JM. 2015. How does the ketogenic diet induce anti-seizure effects? Neurosci. Lett 637:4–10 [DOI] [PubMed] [Google Scholar]
- 108.Deleted in proof.
- 109.Robinson AM, Williamson DH. 1980. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev 60:143–87 [DOI] [PubMed] [Google Scholar]
- 110.Rogina B, Helfand SL. 2004. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. PNAS 101:15998–6003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rogina B, Helfand SL, Frankel S. 2002. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science 298:1745. [DOI] [PubMed] [Google Scholar]
- 112.Roopra A, Dingledine R, Hsieh J. 2012. Epigenetics and epilepsy. Epilepsia 53:2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roy M, Beauvieux MC, Naulin J, El Hamrani D, Gallis JL, et al. 2015. Rapid adaptation of rat brain and liver metabolism to a ketogenic diet: an integrated study using 1H- and 13C-NMR spectroscopy. J. Cereb. Blood Flow Metab 35:1154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez PE, Zhu L, Verret L, Vossel KA, Orr AG, et al. 2012. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. PNAS 109:E2895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, et al. 1995. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9:651–58 [DOI] [PubMed] [Google Scholar]
- 116.Sekhavat A, Sun JM, Davie JR. 2007. Competitive inhibition of histone deacetylase activity by trichostatin A and butyrate. Biochem. Cell Biol 85:751–58 [DOI] [PubMed] [Google Scholar]
- 117.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. 2010. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468:1100–4 [DOI] [PubMed] [Google Scholar]
- 118.Shimazu T, Hirschey MD, Hua L, Dittenhafer-Reed KE, Schwer B, et al. 2010. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab 12:654–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, et al. 2013. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339:211–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sincennes MC, Brun CE, Rudnicki MA. 2016. Concise review: epigenetic regulation of myogenesis in health and disease. Stem Cells Transl. Med 5:282–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, et al. 2016. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife pii:e15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Someya S, Yu W, Hallows WC, Xu JZ, Vann JM, et al. 2010. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, et al. 2004. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12:1325–34 [DOI] [PubMed] [Google Scholar]
- 124.Stein LR, Imai S. 2012. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab 23:420–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, et al. 2005. (d)-β-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem 280:26649–52 [DOI] [PubMed] [Google Scholar]
- 126.Tang C, Ahmed K, Gille A, Lu S, Grone HJ, et al. 2015. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med 21:85–89 [DOI] [PubMed] [Google Scholar]
- 127.Thio LL, Wong M, Yamada KA. 2000. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology 54:325–31 [DOI] [PubMed] [Google Scholar]
- 128.Thumelin S, Forestier M, Girard J, Pegorier JP. 1993. Developmental changes in mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene expression in rat liver, intestine and kidney. Biochem. J 292(Pt. 2):493–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, et al. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med 20:159–66 [DOI] [PubMed] [Google Scholar]
- 130.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, et al. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med 9:352–55 [DOI] [PubMed] [Google Scholar]
- 131.Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, et al. 2004. Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. PNAS 101:15064–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Veech RL. 2004. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 70:309–19 [DOI] [PubMed] [Google Scholar]
- 133.Veprik A, Laufer D, Weiss S, Rubins N, Walker MD. 2016. GPR41 modulates insulin secretion and gene expression in pancreatic β-cells and modifies metabolic homeostasis in fed and fasting states. FASEB J 30:3860–69 [DOI] [PubMed] [Google Scholar]
- 134.Verdin E 2015. NAD+ in aging, metabolism, and neurodegeneration. Science 350:1208–13 [DOI] [PubMed] [Google Scholar]
- 135.Verret L, Mann EO, Hang GB, Barth AM, Cobos I, et al. 2012. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149:708–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.von Meyenn F, Porstmann T, Gasser E, Selevsek N, Schmidt A, et al. 2013. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Cell Metab 17:436–47 [DOI] [PubMed] [Google Scholar]
- 137.Vossel KA, Beagle AJ, Rabinovici GD, Shu HD, Lee SE, et al. 2013. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol 70:1158–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang DF, Helquist P, Wiech NL, Wiest O. 2005. Toward selective histone deacetylase inhibitor design: homology modeling, docking studies, and molecular dynamics simulations of human class I histone deacetylases. J. Med. Chem 48:6936–47 [DOI] [PubMed] [Google Scholar]
- 139.Webber RJ, Edmond J. 1977. Utilization of l(+)-3-hydroxybutyrate, d(−)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J. Biol. Chem 252:5222–26 [PubMed] [Google Scholar]
- 140.Weinert BT, Schölz C, Wagner SA, Iesmantavicius V, Su D, et al. 2013. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep 4:842–51 [DOI] [PubMed] [Google Scholar]
- 141.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. 2009. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324:1076–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wilting RH, Yanover E, Heideman MR, Jacobs H, Horner J, et al. 2010. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J 29:2586–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winesett SP, Bessone SK, Kossoff EHW. 2015. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev. Neurother 15:621–28 [DOI] [PubMed] [Google Scholar]
- 144.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. 2004. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature 432:1027–32 [DOI] [PubMed] [Google Scholar]
- 145.Wolfrum C, Besser D, Luca E, Stoffel M. 2003. Insulin regulates the activity of forkhead transcription factor Hnf-3β/Foxa-2 by Akt-mediated phosphorylation and nuclear/cytosolic localization. PNAS 100:11624–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Won YJ, Lu VB, Puhl HL 3rd, Ikeda SR. 2013. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci 33:19314–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xie ZY, Dai JBA, Dai LZ, Tan MJ, Cheng ZY, et al. 2012. Lysine succinylation and lysine malonylation in histones. Mol. Cell. Proteom 11:100–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie ZY, Zhang D, Chung DJ, Tang ZY, Huang H, et al. 2016. Metabolic regulation of gene expression by histone lysine β-hydroxybutyrylation. Mol. Cell 62:194–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamamoto I, Absalom N, Carland JE, Doddareddy MR, Gavande N, et al. 2012. Differentiating enantioselective actions of GABOB: a possible role for threonine 244 in the binding site of GABAC ρ1 receptors. ACS Chem. Neurosci 3:665–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang L, Zhao J, Milutinovic PS, Brosnan RJ, Eger EI, Sonner JM. 2007. Anesthetic properties of the ketone bodies ss-hydroxybutyric acid and acetone. Anesthesia Analgesia 105:673–79 [DOI] [PubMed] [Google Scholar]
- 151.Yang XJ, Seto E. 2008. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol 9:206–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yi C, Ma M, Ran L, Zheng J, Tong J, et al. 2012. Function and molecular mechanism of acetylation in autophagy regulation. Science 336:474–77 [DOI] [PubMed] [Google Scholar]
- 153.Yoshino J, Mills KF, Yoon MJ, Imai S. 2011. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14:528–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, et al. 2015. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med 21:263–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deleted in proof.
- 156.Yudkoff M, Daikhin Y, Melo TM, Nissim I, Sonnewald U, Nissim I. 2007. The ketogenic diet and brain metabolism of amino acids: relationship to the anticonvulsant effect. Annu. Rev. Nutr 27:415–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yudkoff M, Daikhin Y, Nissim I, Grunstein R, Nissim I. 1997. Effects of ketone bodies on astrocyte amino acid metabolism. J. Neurochem 69:682–92 [DOI] [PubMed] [Google Scholar]
- 158.Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. 2001. Brain amino acid metabolism and ketosis. J. Neurosci. Res 66:272–81 [DOI] [PubMed] [Google Scholar]
- 159.Zeng Z, Liao R, Yao Z, Zhou W, Ye P, et al. 2014. Three single nucleotide variants of the HDAC gene are associated with type 2 diabetes mellitus in a Chinese population: a community-based case-control study. Gene 533:427–33 [DOI] [PubMed] [Google Scholar]
- 160.Zhang D, Yang H, Kong X, Wang K, Mao X, et al. 2011. Proteomics analysis reveals diabetic kidney as a ketogenic organ in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab 300:E287–95 [DOI] [PubMed] [Google Scholar]
- 161.Zhang HB, Ryu D, Wu YB, Gariani K, Wang X, et al. 2016. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352:1436–43 [DOI] [PubMed] [Google Scholar]
- 162.Zhang L, Qin X, Zhao Y, Fast L, Zhuang SG, et al. 2012. Inhibition of histone deacetylases preserves myocardial performance and prevents cardiac remodeling through stimulation of endogenous angiomyogenesis. J. Pharmacol. Exp. Ther 341:285–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, et al. 2012. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1:e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao Y, Sun H, Lu J, Li X, Chen X, et al. 2005. Lifespan extension and elevated hsp gene expression in Drosophila caused by histone deacetylase inhibitors. J. Exp. Biol 208:697–705 [DOI] [PubMed] [Google Scholar]
- 165.Zimmermann S, Kiefer F, Prudenziati M, Spiller C, Hansen J, et al. 2007. Reduced body size and decreased intestinal tumor rates in HDAC2-mutant mice. Cancer Res 67:9047–54 [DOI] [PubMed] [Google Scholar]