Abstract
IN BRIEF This article reviews the evidence regarding the impact of postprandial glucose (PPG) on overall A1C and its relation to cardiovascular disease (CVD). To date, four randomized, controlled trials have evaluated the impact of PPG reduction on CVD; however, only one of these successfully demonstrated a positive effect. Despite this, epidemiological evidence does indicate a cardiovascular benefit of PPG reduction, and agents that can be used to manage PPG in people with type 2 diabetes are also discussed.
KEY POINTS
● Postprandial glucose (PPG) is a significant contributor to A1C that is often overlooked.
● Long-term goals cannot be achieved by targeting only fasting plasma glucose levels.
● PPG is an independent risk factor for cardiovascular disease.
● PPG should be measured after breakfast because post-breakfast excursions tend to be larger and more consistent, with lower day-to-day variation.
In people without diabetes, ingestion of food results in a transient increase in plasma glucose, which elicits a postprandial increase in the secretion of insulin from pancreatic β-cells and suppression of glucagon secretion from α-cells. In people with type 2 diabetes, however, this normal response to blood glucose spikes is dampened, primarily due to reduced insulin production resulting from β-cell dysfunction and loss combined with insulin resistance, leading to hyperglycemia (1). Hyperglycemia is associated with increased risk of microvascular complications such as retinopathy, neuropathy, and nephropathy, as well as macrovascular complications, including increased risk of myocardial infarction (MI), cardiovascular disease (CVD), and stroke (2).
The achievement of glycemic control is the key principle in diabetes management. A1C provides a good indication of overall glycemic control during the previous 2–3 months and remains the gold standard for assessing glycemic control in patients with diabetes (3,4). As a result, treatment guidelines for diabetes have historically focused on reducing A1C to specified targets. For example, the American Diabetes Association (ADA) recommends A1C targets ranging from <6.5% to <8.0% depending on factors such as patients’ health, comorbid conditions, and duration of diabetes (5). The International Diabetes Federation (IDF), the American Association of Clinical Endocrinologists (AACE), and the American College of Endocrinology recommend a target of <6.5% where possible, with individualization of goals depending on patients’ needs (6,7).
It is now well established that A1C levels are the result of a combination of both fasting plasma glucose (FPG) and postprandial glucose (PPG) levels (8,9), with the relative importance of each depending on factors such as the degree of glycemic control (9). Treatment may also influence this FPG-PPG relationship. For example, basal insulin primarily reduces FPG. Therefore, after its initiation in patients treated with oral antidiabetic drugs (OADs) with uncontrolled hyperglycemia, it is PPG that accounts for the majority (approximately two-thirds) of residual hyperglycemia (10). It has become increasingly apparent that long-term A1C target levels cannot be achieved by treating only FPG; rather, PPG must also be targeted by therapeutic strategies (11). Consequently, most treatment guidelines now include specific PPG targets alongside A1C and FPG targets. The ADA/European Association for the Study of Diabetes guidelines recommend targets of A1C <7.0%, FPG 80–130 mg/dL, and PPG <180 mg/dL (5); the IDF recommends targets of A1C <7.0%, FPG 115 mg/dL, and PPG <160 mg/dL (6); and AACE recommends targets of A1C <7.0%, FPG 110 mg/dL, and PPG ≤140 mg/dL (7).However, current strategies and therapies (i.e., metformin, sulfonylureas, thiazolidinediones, and basal insulins) are mainly effective in controlling FPG; the importance of PPG, particularly in maintaining long-term glycemic control, has been given less attention (12).
Studies have shown that, in addition to its contribution to overall A1C, PPG is an independent risk factor for CVD, with a demonstrated linear relationship of PPG and risk of cardiovascular (CV) death (13). Although a significant number of publications support PPG being an independent risk factor for CVD and death, data have varied (14–22). Furthermore, a prospective study was conducted in subjects with previously undiagnosed diabetes who had demonstrated no fasting hyperglycemia, in which subjects underwent oral glucose tolerance testing. This study concluded that, of those with isolated post-challenge hyperglycemia, women but not men showed a significantly increased risk of fatal CVD and heart disease compared to those without diabetes (23).
In this article, we review the evidence regarding the impact of PPG on overall A1C and its relationship to CVD in an attempt to help reach a consensus on the importance of controlling PPG in people with type 2 diabetes.
Impact of PPG on A1C
It is clear that A1C, as an index of overall glycemic control, is significantly affected by both FPG and PPG, although the data concerning the relative importance of each to A1C levels was initially varied. Monnier and Colette (9) investigated the relative contributions to A1C of FPG and PPG depending on the A1C level in an attempt to conciliate these different results. The group determined that the relative contributions changed, depending on whether patients’ diabetes was well controlled or not, with PPG excursions predominating at lower A1C levels, and FPG predominating at higher A1C levels. They calculated that the relative contribution of PPG is 70% in patients with A1C <7.3%, reducing to 30% in patients with A1C >10.2% (Figure 1) (9).
FIGURE 1.
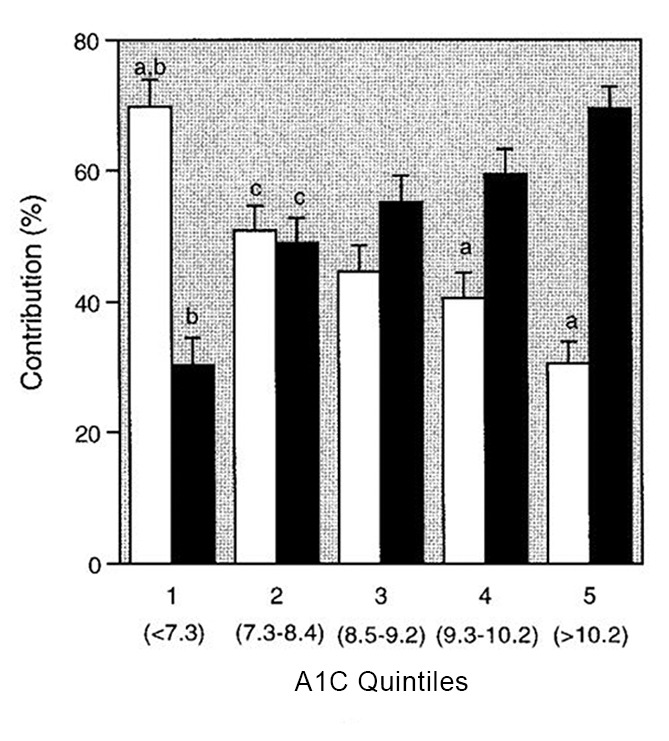
Relative contributions of postprandial (□) and fasting (■) hyperglycemia (%) to the overall diurnal hyperglycemia over quintiles of A1C. aSignificant difference between FPG and PPG (paired t test). bSignificantly different from all other quintiles (analysis of variance [ANOVA]). cSignificantly different from quintile 5 (ANOVA). Reprinted with permission from ref. 8.
A later analysis of data from six studies of treatment intensification with insulin or additional OADs supported these findings, determining that, where A1C is >7.0% despite OAD therapy, FPG dominates glucose exposure, contributing an average of 76–80% to hyperglycemia (10). The study also suggested that the type of antihyperglycemic treatment used may be more significant than the A1C level alone. Despite similar A1C levels, basal insulin reduced the FPG contribution to 32–41%, whereas alternative intensification regimens (i.e., insulin lispro, premixed insulin, or additional OADs) reduced the FPG contribution to 64–71% (10).
In a recent meta-analysis of 14 studies in patients with type 1 or type 2 diabetes, stronger correlations were found between PPG and A1C than between FPG and A1C (24). Furthermore, decreases in PPG resulted in greater A1C reductions than FPG reductions (24).
It has also been reported that, in type 2 diabetes, elevated PPG is one of the earliest abnormalities of glucose homeostasis, often arising before elevated FPG. This is distinctly exaggerated in patients with elevated FPG (25,26). Regardless of the exact contributions of each, the evidence clearly suggests that PPG and FPG are both significant contributors to A1C; therefore, both should be considered during treatment.
Impact of PPG on CVD
In addition to an increase in risk of microvascular complications, diabetes is associated with an overall two- to fourfold increased risk of developing CVD (1). Indeed, CVD is by far the single largest cause of mortality in patients with type 2 diabetes, accounting for up to 75–80% of deaths (2,27). Traditional risk factors for diabetes such as hypertension, obesity, and atherogenic dyslipidemia do not fully account for the increased risk of CVD associated with diabetes (2). Increased A1C levels are well known to be associated with increased CVD risk, implying the contribution of PPG to A1C is a significant factor in this increased risk (2). In addition, most epidemiological studies agree that PPG is a significant independent risk factor for CVD and MI, regardless of whether a person has diabetes (28,29).
Studies have also shown that, in addition to CV events, PPG is a predictor of CV-related and all-cause mortality, whereas it appears that FPG is not (30). In the Honolulu Heart Program conducted in Japanese-American men aged 45–68 years, there was an increased risk of coronary heart disease (CHD) in patients with abnormal oral glucose tolerance test results (14). Similar results were seen in the Baltimore Longitudinal Study of Aging, which concluded that, regardless of FPG and A1C, a higher 2-hour PPG level was associated with increased risks of CVD, CVD mortality, and all-cause mortality (16). The Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) study analyzed data from 10 prospective European cohort studies that included 15,388 men and 7,126 women aged 30–89 years. The authors concluded that 2-hour PPG values were a better predictor than FPG of death from all causes and CVD, with the largest number of excess deaths being observed in patients showing impaired glucose tolerance (IGT) after a 2-hour oral glucose test but normal FPG levels (17). Additional analysis showed that mortality associated with FPG concentration was largely dependent on 2-hour PPG levels. In this study, ∼33% of men and ∼44% of women who had diabetes according to the 2-hour PPG values were not identified as having diabetes according to their FPG levels, highlighting the diagnostic value of PPG measurement (17).
It is important to stress that the Honolulu Heart Program, the Baltimore Longitudinal Study of Aging, and DECODE were noninterventional studies that looked at subjects who did not have diagnosed diabetes. It is therefore unclear whether they can inform us on how to better treat those with diagnosed diabetes.
A post hoc analysis determined that a prandial strategy targeting PPG with three premeal doses of insulin lispro daily may be associated with lower risk of subsequent CV events than a basal strategy of twice-daily NPH or once-daily insulin glargine 100 units/mL in older patients (19). A caveat of this study, which should be considered, is that the magnitude of the differences in PPG levels between the two treatment regimens was smaller than expected, and the trial was eventually stopped due to lack of efficacy (18).
A number of other studies support the findings from these studies that PPG levels are linked to CVD. In a 14-year follow-up of patients with type 2 diabetes managed in routine clinical practice, PPG and A1C, but not FPG, were found to have similar predictive power for CV events and all-cause mortality (20), whereas a review of a large number of epidemiological studies concluded that PPG is, in fact, a more powerful risk factor than either A1C or FPG (31). In the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM), patients with IGT were randomized to receive either placebo or acarbose, an α-glucosidase inhibitor (AGI) that lowers PPG. In addition to a 25% relative risk reduction in the development of type 2 diabetes and a 34% risk reduction for hypertension, patients treated with acarbose had a 49% risk reduction of developing CV events (32). Additionally, in a substudy of STOP-NIDDM, patients treated with acarbose showed a reduced incidence of silent MIs compared to those receiving placebo (33). The Acarbose Cardiovascular Evaluation trial, conducted in Chinese patients with IGT and CHD, showed no significant difference between acarbose and placebo for incidence of primary five-point composite outcome (CV death, nonfatal MI, nonfatal stoke, hospital admission for unstable angina, and hospital admission for heart failure) or any secondary CV outcomes (34). However, patients treated with acarbose did have a reduced incidence of diabetes.
A number of studies have given indications as to the underlying mechanisms for the association between PPG levels and CV risk. For example, a study of patients without diabetes showed that higher 1-hour PPG levels were significantly associated with increased arterial stiffness as determined by cardio-ankle vascular index values, a measure of the stiffness of the aorta, femoral artery, and tibial artery (35). Furthermore, in the Risk Factors in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study, there was a closer correlation between PPG and carotid intima-media thickness than FPG in patients with IGT (36).
Hypertriglyceridemia is also a risk factor for CVD and is amplified in the postprandial state, rising concomitantly with postprandial hyperglycemia. Despite this, evidence suggests a direct atherogenic role for postprandial hyperglycemia independent of that of lipids (29). Postprandial hypertriglyceridemia has been shown to be associated with increased carotid intima-media thickness in patients with diabetes, meaning that it may be an independent risk factor for early atherosclerosis in these patients (37). Furthermore, the progression of atherosclerosis has also been shown to be slowed and even reversed by therapies that reduce PPG (38).
PPG has been shown to stimulate oxidative stress, which has been implicated as the underlying cause of both macrovascular and microvascular complications in type 2 diabetes (39–42). Indeed, epidemiological and other studies have demonstrated a strong association between PPG and CV risk through oxidative stress, carotid intima-media thickness, and endothelial dysfunction (16,17). Glucose fluctuations have been shown to have a linear correlation with increased production of free radicals, and PPG induces overproduction of superoxide, which reacts with nitrous oxide to create derivatives that lead to endothelial damage (43). In the Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research study, treatment of patients with IGT with the angiotensin II receptor antagonist valsartan (which reduces blood pressure) did not affect CV outcomes. It should be noted, however, that the patients in this trial were not hypertensive, and valsartan was not used for blood pressure control (21). Thus, the observed effects of PPG on CV outcomes may not be explained by increased blood pressure associated with hyperglycemia.
In summary, in addition to its significant contribution to A1C, the literature strongly indicates that PPG is an independent risk factor for CVD. It remains unclear, however, whether the most important aspect of PPG is how frequently it is above the optimal range or whether it is its maximal values. Regardless of this, the impact of PPG on CVD implies that reducing PPG in patients with diabetes may be of significant benefit to their long-term prognosis and quality of life, even though demonstration of this benefit in randomized controlled trials has been elusive.
Monitoring PPG
Although widely used and recommended for monitoring glycemic control, the cost of A1C testing is high, which means its availability is very limited in resource-poor settings (24). Given that studies indicate that PPG and A1C have similar predictive power for CV outcomes, regular monitoring of PPG with plasma glucose testing, which is considerably less costly and easier to perform, may represent a viable and practical alternative that enables the improvement of overall glycemic control and reduced the risk of CV complications.
Data from an observational study of people with type 2 diabetes suggest that PPG readings preferably should be obtained after breakfast rather than after lunch or dinner because post-breakfast excursions tend to be larger and more consistent, with lower day-to-day variation (44). Furthermore, the median time to peak concentration in this study was ∼90 minutes, indicating that this is the time after the start of the meal that the reading should be taken (44). Postprandial self-monitoring of blood glucose (pp-SMBG) has been shown to be associated with improvements in glycemia, lipids, and weight, as well as exercise and dietary habits in subjects who have already reached their A1C goals; this provides a rationale for implementation of pp-SMBG when possible (45).
Diabetes organizations are increasingly recognizing that continuous glucose monitoring (CGM) may be an appropriate and useful diabetes management tool, especially in patients on insulin therapy. CGM technology is advancing rapidly; for example, the FreeStyle Libre and FreeStyle Libre Pro “flash” CGM systems do not require fingerstick calibration. While the Freestyle Libre system is intended to be used by patients for diabetes self-management, the FreeStyle Libre Pro is the first flash CGM system available for professional use in clinical practice. In a significant improvement over previous systems, the sensor is factory calibrated and can be continuously worn for up to 14 days, requiring no calibration via SMBG during that time period. Another system, the Dexcom G5, still requires calibration using SMBG; however, it has been granted a nonadjunctive indication by the U.S. Food and Drug Administration (FDA), meaning its readings alone can be used to modify therapy (46). More recently, the FDA approved the Dexcom G6 system, which does not require fingerstick calibration. Similarly, the FreeStyle Libre device does not require fingerstick SMBG calibration.
Reducing PPG
The most important and effective first step in diabetes management is to encourage patients to make lifestyle modifications, including increasing exercise and improving diet. However, diabetes is a progressive disease, and all patients will eventually require pharmacological treatment to maintain glycemic control. In general, treatment strategies to reduce A1C have focused on controlling FPG; however, as discussed, PPG is an important contributor to A1C. Controlling PPG is therefore a major unmet need, particularly in patients with longer durations of type 2 diabetes (12).
A number of treatment options are available to target PPG, including AGIs, amylin analogs, glinides, dopamine agonists, glucagon-like peptide 1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium–glucose cotransporter 2 (SGLT2) inhibitors, and rapid-acting insulins (Table 1) (3,47–62). Treatment guidelines provide algorithms for the intensification of therapy, including many of these agents, and recommend that specific strategies and choices should be based on patient- and disease-specific factors (5–7).
TABLE 1.
FDA-Approved Pharmacological Interventions That Target Postprandial Hyperglycemia*
| Agent | Mode of Action | A1C Reduction, % | PPG Reduction, mmol/L (mg/dL) | CV Benefit |
|---|---|---|---|---|
| AGIs | ||||
| Acarbose | Inhibits carbohydrate digestion, delaying absorption | 0.4–0.8 | 4.0 (72) | |
| Miglitol | Inhibits carbohydrate digestion, delaying absorption; enhances GLP-1 activity | 0.2–0.8 | 1.5–3.5 (27–63) | |
| Amylin analogs | ||||
| Pramlintide | Slows gastric emptying; suppresses glucagon activity; increases satiety | 0.6 | 2.0 (36) | |
| Glinides | ||||
| Repaglinide | Stimulates insulin release | 0.6–1.5 | 2.6 (47) | |
| Nateglinide | Stimulates insulin release | 0.5–0.8 | 2.6 (47) | |
| Insulin | ||||
| Rapid-acting | 1.5–2.5 | |||
| SGLT2 inhibitors | ||||
| Canagliflozin | Inhibition of glucose reuptake in the kidney; short-term inhibition of intestinal SLGT1 at higher doses | 0.8–1.0 | 2.4–3.3 (43–59) | ✓ |
| Dapagliflozin | Inhibition of glucose reuptake in the kidney | 0.6–1.0 | 3.6–3.8 (65–68) | |
| Empagliflozin | Inhibition of glucose reuptake in the kidney | 0.7–0.8 | 2.0–2.6 (36–47) | |
| Incretin-based agents | ||||
| GLP-1 receptor agonists | ||||
| Exenatide | Enhances insulin secretion; inhibits glucagon release after eating; delays gastric emptying; promotes satiety | 0.5–1.0† | 3.6 (65) | |
| Short-acting: predominant effect on PPG | ||||
| Long-acting: predominant effect on FPG | ||||
| Liraglutide | Enhances insulin secretion; inhibits glucagon release after eating | 1.0–1.5† | 1.7–2.7 (31–49) | ✓ |
| Predominant effect on FPG | ||||
| Lixisenatide | Enhances insulin secretion; inhibits glucagon release after eating; delays gastric emptying; promotes satiety | 0.5–0.9 | 3.1–5.9 (56–106) | |
| Predominant effect on PPG | ||||
| DPP-4 inhibitors | ||||
| Sitagliptin | Inhibits DPP-4, increasing levels of GLP-1 | 0.6–0.8 | 2.8 (50) | |
| Saxagliptin | Inhibits DPP-4, increasing levels of GLP-1 | 0.6–0.8 | 2.8 (50) | |
| Combination agents | ||||
| iDegLira | Complementary action of basal insulin on FPG and GLP-1 receptor agonist on PPG | 0.8–1.9 | Not reported | |
| iGlarLixi | Complementary action of basal insulin on FPG and GLP-1 receptor agonist on PPG | 1.1–1.6 | 4.7–5.7 (85–103) | |
Adapted from refs. 3, 96, and 97, with additional data from refs. 47–62.
Used as monotherapy or in combination with other antidiabetic agents.
Assuming starting value ≥8%.
Insulin Therapy
In patients treated with basal insulin who are not achieving glycemic targets, preprandial rapid-acting insulin analogs or premixed insulin formulations consisting of intermediate and rapid-acting insulin are often initiated (63). Rapid-acting insulins are a well-established and effective treatment for patients requiring prandial control. However, adverse effects associated with rapid-acting insulin analogs, including weight gain and increased risk of hypoglycemia, mean that patients and health care providers are often reluctant to initiate their use. In addition, data from population-based studies suggest that this approach may not be optimal, in terms of both long-term glycemic control and CV outcomes (12).
AGIs
AGIs significantly reduce PPG-dependent insulinotropic polypeptide (gastric inhibitory polypeptide) secretion and are effective at reducing PPG by altering the intestinal absorption of carbohydrates (64,65). In general, AGIs have modest A1C-lowering effects and a low risk of hypoglycemia and require frequent dosing (7,66), and the action of AGIs means that undigested carbohydrates reach the colon, resulting in flatulence and diarrhea (67). Although this effect may lessen over time, gastrointestinal side effects make AGIs difficult to tolerate for many patients, and this has limited their use (7).
Amylin Analogs
Amylin analogs target PPG by suppressing post-meal glucagon activity, slowing gastric emptying, and increasing satiety. This significantly reduces PPG and improves glycemic control when added to insulin and has a beneficial effect on weight (68). They are generally used as a supplement to basal insulin therapy in patients who are not meeting glycemic targets (69). The major disadvantages of amylin analogs are the need for multiple daily injections due to their short duration of action and increased risk of nausea. There is also in an increased risk of hypoglycemia, although this is lower than with rapid-acting insulins (12).
Glinides
Glinides are short-acting insulinotropic agents that rapidly increase insulin secretion and reduce PPG (70). Glinides are associated with an increased risk of hypoglycemia, although this is lower than that of sulfonylureas. They are also associated with weight gain, require frequent dosing, and have only modest A1C-lowering effects (7,66).
SGLT2 Inhibitors
SGLT2 inhibitors lower plasma glucose by inhibition of glucose reuptake in the kidney and so reduce both FPG and PPG (71). Canagliflozin 300 mg (maximum recommended dose) was shown to have provided greater reductions in PPG and insulin excursions, possibly related to a combination of renal SGLT2 inhibition and delayed absorption of ingested glucose due to intestinal SGLT1 inhibition (49). This insulin-independent mechanism of action means that they are not associated with weight gain, have a low risk of hypoglycemia, and can be used at any stage of type 2 diabetes. They have similar A1C-lowering efficacy to other OADs (7,66). Patients with type 2 diabetes and high risk for CV events who were treated with empagliflozin have been shown to have lower rates of a composite outcome of death from CV causes, nonfatal MI, or nonfatal stroke, as well as death from any cause, compared to placebo (71), and this was also seen in the canagliflozin CANVAS research program (72).
A disadvantage of SGLT2 inhibitors is that they result in elevated excretion of glucose in the urine, which is associated with urinary tract and genital infections in patients (particularly women) (73). Additionally, canagliflozin carries a black-box warning for lower-limb amputation, with an approximately twofold increased risk observed in patients with type 2 diabetes either with established CVD or at risk of CVD (74). However, it should be considered that the absolute risk remains low.
DPP-4 Inhibitors
DPP-4 inhibitors (i.e., sitagliptin, vildagliptin, saxagliptin, and linagliptin) are incretin-based therapies that provide another way of targeting PPG. They are associated with weight loss and lower risk of hypoglycemia than rapid-acting insulin. DPP-4 inhibitors prevent DPP-4 from degrading native incretins such as GLP-1, which in turn activate the GLP-1 receptor. This results in DPP-4 inhibitors and GLP-1 receptor agonists having similar effects resulting from activation of the GLP-1 receptor; however, GLP-1 receptor agonists have been shown to provide superior glycemic control compared to DPP-4 inhibitors (3,75), effectively stimulating glucose-dependent insulin secretion and suppressing postprandial glucagon levels, and thereby reducing PPG (76).
Unlike rapid-acting insulin, DPP-4 inhibitors have a neutral effect on hypoglycemia and weight. Some may carry a risk of congestive heart failure; however, this is uncertain due to short follow-ups and low-quality evidence of studies (77). Recent analysis has suggested that only saxagliptin in the SAVOR-TIMI 53 trial resulted in increased hospitalization for heart failure (78,79).
GLP-1 Receptor Agonists
GLP-1 receptor agonists are also an incretin-based therapy, meaning they have similar effects to DPP-4 inhibitors, resulting from activation of the GLP-1 receptor (3). As with DPP-4 inhibitors, this activation results in stimulation of insulin secretion and suppression of glucagon secretion from the pancreas in a glucose-dependent manner (11,80,81). They are also associated with a lower risk of hypoglycemia than rapid-acting insulins.
Because their mechanism of action differs from that of DPP-4 inhibitors, GLP-1 receptor agonists are associated with weight loss, whereas DPP-4 inhibitors are weight neutral; however, both classes are superior to rapid-acting insulins, which are associated with weight gain. The mechanism by which GLP-1 receptor agonists promote weight loss is multifactorial and involves both the brain and the gastrointestinal tract. They slow gastric emptying and increase satiety to varying degrees, resulting in reduced food intake and associated weight loss (11,82,83).
Evidence suggests that GLP-1 receptor agonists result in better glycemic control and greater weight loss than DPP-4 inhibitors (75). A study has shown that GLP-1 receptors are required to be present in the central nervous system, while another study showed that a small peptide GLP-1 receptor agonist can penetrate the brain, subsequently activating certain neurons to stimulate weight loss (84,85).
Short-acting GLP-1 receptor agonists such as lixisenatide and exenatide have a predominant effect on PPG and are associated with a greater effect on gastric emptying. Longer-acting GLP-1 receptor agonists such as liraglutide, exenatide long-acting release, albiglutide, and dulaglutide have more of an effect on FPG and a lesser effect on gastric emptying. It seems that continuous stimulation of the GLP-1 receptor can attenuate this effect of gastric emptying via tachyphylaxis (5,86).
Overall, GLP-1 receptor agonists are associated with a lower incidence of hypoglycemia compared to insulin (87). Trials investigating the CV safety of GLP-1 receptor agonists show comparable (88,89) or reduced (90,91) CV outcomes compared to placebo in patients with diabetes. A meta-analysis of these four trials suggested a class effect of GLP-1 receptor agonists for improving CV outcomes. This analysis suggested that treatment with a GLP-1 receptor agonist results in a significant (10%) reduction in relative risk for three major adverse cardiac events (CV mortality, nonfatal MI, and nonfatal stroke). Furthermore, there were relative risk reductions of 13% for CV mortality and 12% for all-cause mortality. There was no identified impact of GLP-1 receptor agonist therapy on fatal and nonfatal MI, fatal and nonfatal stroke, hospital admission for unstable angina, or hospital admission for heart failure (92).
It should be noted that it is unlikely that these improved CV outcomes can be attributed solely to reduced PPG. The meta-analysis included studies of longer-acting GLP-1 receptor agonists; although these do have a modest impact on PPG, it is likely a result of reduced basal glycemia. They have little to no sustained impact on the rate of gastric emptying, which is the factor most important for reduced PPG excursions seen with short-acting GLP-1 receptor agonists (85,93). The authors of the meta-analysis speculated that the CV effects may be related to antiatherogenic mechanisms, which affect common CV risk factors such as blood pressure, anti-inflammatory pathways, cardiac output, ischemic conditioning, and endothelial function (92).
Another meta-analysis, which included a greater number of studies and compared GLP-1 receptor agonist treatment to placebo or any other non–GLP-1 receptor agonist drugs, showed similar results. Patients with type 2 diabetes treated with a GLP-1 receptor agonist had lower all-cause mortality, CV mortality, and MI rates, whereas no significant differences were seen for stroke or heart failure (94).
Given their complementary modes of action, the combination of a GLP-1 receptor agonist and basal insulin is potentially an attractive option to manage both PPG and FPG. Titratable fixed-ratio combinations of basal insulin glargine and the GLP-1 receptor agonist lixisenatide (iGlarLixi), and of insulin degludec and liraglutide (iDegLira), were approved by the FDA in 2016 and are now on the market. In clinical trials, once-daily injections of these formulations have been shown to result in greater A1C reduction than basal insulin or a GLP-1 receptor agonist alone. These trials have also shown weight gain associated with basal insulin therapy to be mitigated, hypoglycemia to be reduced, and gastrointestinal adverse effects to be reduced compared to GLP-1 receptor agonists alone (60–62,95).
Conclusion
Studies have consistently demonstrated that PPG is a significant contributor to A1C and is also an independent risk factor for CVD. Long-term epidemiological studies and meta-analyses show that PPG, far more than FPG, is a predictor of CV risk. PPG is especially important to patients with diabetes who have achieved their FPG goal but whose A1C remains high. In patients with an A1C of ≥10.2%, PPG only contributes up to ∼30% of 24-hour A1C; however, in patients closer to goal (A1C ≤7.3%), PPG contributes ∼70% of 24-hour A1C (8). Therefore, patients who have achieved FPG goals but still have elevated A1C should consider PPG-targeting therapeutics.
Despite this association, studies have not consistently shown improved CV outcomes in patients taking PPG-lowering therapy. Although it is possible that PPG is merely a marker or surrogate for CV risk, it may be that the designs of studies conducted to date have been insufficient to fully answer this question (93). Overall, the data suggest that reducing PPG excursions may be protective against CVD. A range of available treatments can be used to target PPG, including rapid-acting insulin analogs, GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors.
Control of PPG should be considered equally as important as FPG control in people with type 2 diabetes. However, despite the increasing apparent importance of PPG, it is commonly ignored by primary care providers (PCPs) in lieu of FPG control. Often, PCPs have been trained to address FPG first and therefore believe that management of PPG is of lesser importance. Many PCPs have also acquired a familiarity and comfort with the use of basal insulin. However, basal insulin is effective only up to a tipping point of ∼0.5 units/kg; thereafter, further titration will likely result in hypoglycemia and weight gain.
Many patients are able to achieve an FPG target while their A1C remains high. At this point, agents targeting PPG are essential. However, these often require an additional injection, the use of carbohydrate counting, titration, or other nuances for which PCPs often have not been trained and would therefore be likely to refer such patients to an endocrinologist. Newer agents such as GLP-1 receptor agonists or fixed-ratio combinations may be added to basal insulin and can mitigate some of the issues of weight gain while not increasing the risk of hypoglycemia. However, there may be additional side effects with these agents, including increased gastrointestinal adverse effects, that will need to be managed.
Acknowledgments
Acknowledgments
The authors received writing/editorial support in the preparation of this manuscript provided by Nick Patterson, PhD, and Rasilaben Vaghjiani, PhD, of Excerpta Medica.
Funding
This review, and the writing/editorial support for it, were funded by Sanofi US, Inc.
Duality of Interest
K.S.H. received honoraria for lectures from Amgen, AMH, Boehringer Ingelheim, Janssen, Mannkind, Novo Nordisk, and Primed and honoraria for consulting from Intarcia. B.R.H. is a consultant for Janssen, Merck, and Sanofi. O.O. is a consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Merck, Novo Nordisk, and Sanofi.
Author Contributions
All authors contributed equally to the idea and development of the manuscript. K.S.H. is the guarantor of this work and, as such, had full access to all the materials in the study and takes responsibility for the integrity of the materials and the accuracy of the dissemination of the materials.
References
- 1.Madsbad S. Impact of postprandial glucose control on diabetes-related complications: how is the evidence evolving? J Diabetes Complications 2016;30:374–385 [DOI] [PubMed] [Google Scholar]
- 2.Båvenholm PN, Efendic S. Postprandial hyperglycaemia and vascular damage: the benefits of acarbose. Diab Vasc Dis Res 2006;3:72–79 [DOI] [PubMed] [Google Scholar]
- 3.Blevins T. Control of postprandial glucose levels with insulin in type 2 diabetes. Postgrad Med 2011;123:135–147 [DOI] [PubMed] [Google Scholar]
- 4.Fysekidis M, Cosson E, Banu I, Duteil R, Cyrille C, Valensi P. Increased glycemic variability and decrease of the postprandial glucose contribution to HbA1c in obese subjects across the glycemic continuum from normal glycemia to first time diagnosed diabetes. Metabolism 2014;63:1553–1561 [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S61–S70 [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation Guideline Development Group Global guideline for type 2 diabetes. Diabetes Res Clin Pract 2014;104:1–52 [DOI] [PubMed] [Google Scholar]
- 7.Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract 2017;23:207–238 [DOI] [PubMed] [Google Scholar]
- 8.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 9.Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract 2006;12(Suppl. 1):42–46 [DOI] [PubMed] [Google Scholar]
- 10.Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011;34:2508–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallwitz B. Implications of postprandial glucose and weight control in people with type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S322–S325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riddle MC. Basal glucose can be controlled, but the prandial problem persists: it’s the next target! Diabetes Care 2017;40:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceriello A, Hanefeld M, Leiter L, et al. . Postprandial glucose regulation and diabetic complications. Arch Intern Med 2004;164:2090–2095 [DOI] [PubMed] [Google Scholar]
- 14.Donahue RP, Abbott RD, Reed DM, Yano K. Postchallenge glucose concentration and coronary heart disease in men of Japanese ancestry: Honolulu Heart Program. Diabetes 1987;36:689–692 [DOI] [PubMed] [Google Scholar]
- 15.Laws A, Marcus EB, Grove JS, Curb JD. Lipids and lipoproteins as risk factors for coronary heart disease in men with abnormal glucose tolerance: the Honolulu Heart Program. J Intern Med 1993;234:471–478 [DOI] [PubMed] [Google Scholar]
- 16.Sorkin JD, Muller DC, Fleg JL, Andres R. The relation of fasting and 2-h postchallenge plasma glucose concentrations to mortality: data from the Baltimore Longitudinal Study of Aging with a critical review of the literature. Diabetes Care 2005;28:2626–2632 [DOI] [PubMed] [Google Scholar]
- 17.DECODE Study Group, European Diabetes Epidemiology Group Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405 [DOI] [PubMed] [Google Scholar]
- 18.Raz I, Wilson PW, Strojek K, et al. . Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raz I, Ceriello A, Wilson PW, et al. . Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care 2011;34:1511–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavalot F, Pagliarino A, Valle M, et al. . Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011;34:2237–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NAVIGATOR Study Group; McMurray JJ, Holman RR, Haffner SM, et al. . Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med 2010;362:1477–1490 [DOI] [PubMed] [Google Scholar]
- 22.Takao T, Suka M, Yanagisawa H, Iwamoto Y. Impact of postprandial hyperglycemia at clinic visits on the incidence of cardiovascular events and all‐cause mortality in patients with type 2 diabetes. J Diabetes Investig 2017;8:600–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men: the Rancho Bernardo Study. Diabetes Care 1998;21:1236–1239 [DOI] [PubMed] [Google Scholar]
- 24.Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health 2015;73:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association Postprandial blood glucose. Diabetes Care 2001;24:775–778 [DOI] [PubMed] [Google Scholar]
- 26.Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med 2004;164:486–491 [DOI] [PubMed] [Google Scholar]
- 27.Peter R, Okoseime OE, Rees A, Owens DR. Postprandial glucose: a potential therapeutic target to reduce cardiovascular mortality. Curr Vasc Pharmacol 2009;7:68–74 [DOI] [PubMed] [Google Scholar]
- 28.Qiao Q, Dekker JM, de Vegt F, et al. . Two prospective studies found that elevated 2-hr glucose predicted male mortality independent of fasting glucose and HbA1c. J Clin Epidemiol 2004;57:590–596 [DOI] [PubMed] [Google Scholar]
- 29.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005;54:1–7 [DOI] [PubMed] [Google Scholar]
- 30.Cavalot F, Petrelli A, Traversa M, et al. . Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006;91:813–819 [DOI] [PubMed] [Google Scholar]
- 31.Beisswenger P, Heine RJ, Leiter LA, Moses A, Tuomilehto J. Prandial glucose regulation in the glucose triad: emerging evidence and insights. Endocrine 2004;25:195–202 [DOI] [PubMed] [Google Scholar]
- 32.Chiasson JL. Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) trial. Endocr Pract 2006;12(Suppl. 1):25–30 [DOI] [PubMed] [Google Scholar]
- 33.Zeymer U, Schwarzmaier-D’assie A, Petzinna D, Chiasson JL; STOP-NIDDM Trial Research Group . Effect of acarbose treatment on the risk of silent myocardial infarctions in patients with impaired glucose tolerance: results of the randomised STOP-NIDDM trial electrocardiography substudy. Eur J Cardiovasc Prev Rehabil 2004;11:412–415 [DOI] [PubMed] [Google Scholar]
- 34.Holman RR, Coleman RL, Chan JCN, et al. ; ACE Study Group : Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:877–886 [DOI] [PubMed] [Google Scholar]
- 35.Tsuboi A, Ito C, Fujikawa R, Yamamoto H, Kihara Y. Association between the postprandial glucose levels and arterial stiffness measured according to the cardio-ankle vascular index in non-diabetic subjects. Intern Med 2015;54:1961–1969 [DOI] [PubMed] [Google Scholar]
- 36.Hanefeld M, Koehler C, Henkel E, Fuecker K, Schaper F, Temelkova‐Kurktschiev T. Post‐challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima‐media thickness: the RIAD Study: risk factors in impaired glucose tolerance for atherosclerosis and diabetes. Diabet Med 2000;17:835–840 [DOI] [PubMed] [Google Scholar]
- 37.Teno S, Uto Y, Nagashima H, et al. . Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care 2000;23:1401–1406 [DOI] [PubMed] [Google Scholar]
- 38.Leiter LA, Ceriello A, Davidson JA, et al. ; International Prandial Glucose Regulation Study Group. Postprandial glucose regulation: new data and new implications. Clin Ther 2005;27(Suppl. B):S42–S56 [DOI] [PubMed] [Google Scholar]
- 39.Monnier L, Mas E, Ginet C, et al. . Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006;295:1681–1687 [DOI] [PubMed] [Google Scholar]
- 40.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 41.Ceriello A, Falleti E, Motz E, et al. . Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress. Horm Metab Res 1998;30:146–149 [DOI] [PubMed] [Google Scholar]
- 42.Cominacini L, Pasini AF, Garbin U, et al. . E-selectin plasma concentration is influenced by glycaemic control in NIDDM patients: possible role of oxidative stress. Diabetologia 1997;40:584–589 [DOI] [PubMed] [Google Scholar]
- 43.Giugliano D, Ceriello A, Esposito K. Glucose metabolism and hyperglycemia. Am J Clin Nutr 2008;87:217S–222S [DOI] [PubMed] [Google Scholar]
- 44.Cichosz SL, Fleischer J, Hoeyem P, et al. . Assessment of postprandial glucose excursions throughout the day in newly diagnosed type 2 diabetes. Diabetes Technol Ther 2013;15:78–83 [DOI] [PubMed] [Google Scholar]
- 45.Zhang DA, Katznelson L, Li M. Postprandial glucose monitoring further improved glycemia, lipids, and weight in persons with type 2 diabetes mellitus who had already reached hemoglobin A1c goal. J Diabetes Sci Technol 2012;6:289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther 2017;19(Suppl. 2):S4–S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stenlöf K, Cefalu WT, Kim KA, et al. . Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavalle-González FJ, Januszewicz A, Davidson J, et al. . Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013;56:2582–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polidori D, Sha S, Mudaliar S, et al. . Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care 2013;36:2154–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care 2012;35:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010;375:2223–2233 [DOI] [PubMed] [Google Scholar]
- 52.Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 2011;13:928–938 [DOI] [PubMed] [Google Scholar]
- 53.Häring HU, Merker L, Seewaldt-Becker E, et al. ; EMPA-REG METSU Trial Investigators . Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2013;36:3396–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Häring HU, Merker L, Seewaldt-Becker E, et al. ; EMPA-REG MET Trial Investigators. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014;37:1650–1659 [DOI] [PubMed] [Google Scholar]
- 55.Kovacs CS, Seshiah V, Swallow R, et al. ; EMPA-REG PIO Trial Investigators. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 2014;16:147–158 [DOI] [PubMed] [Google Scholar]
- 56.Riddle MC, Aronson R, Home P, et al. . Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riddle MC, Forst T, Aronson R, et al. . Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 2013;36:2497–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 2013;36:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodbard HW, Bode BW, Harris SB, et al. ; Dual Action of Liraglutide and Insulin Degludec (DUAL) IV Trial Investigators. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with type 2 diabetes: the DUAL IV trial. Diabet Med 2017;34:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gough SCL, Bode B, Woo V, et al. ; NN9068-3697 (DUAL-I) Trial Investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naïve patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885–893 [DOI] [PubMed] [Google Scholar]
- 61.Rosenstock J, Aronson R, Grunberger G, et al. ; Investigators LixiLan-O Trial. Benefits of lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled with oral agents: the LixiLan-O randomized trial. Diabetes Care 2016;39:2026–2035 [DOI] [PubMed] [Google Scholar]
- 62.Aroda VR, Rosenstock J, Wysham C, et al. ; LixiLan-L Trial Investigators. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 2016;39:1972–1980 [DOI] [PubMed] [Google Scholar]
- 63.Rhinehart AS. Adding GLP-1 receptor agonist therapy to basal insulin for postprandial glucose control. Clin Diabetes 2015;33:73–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sumi K, Ohkura T, Yamamoto N, et al. . Long-term miglitol administration suppresses postprandial glucose-dependent insulinotropic polypeptide secretion. Diabetol Int 2013;4:190–196 [Google Scholar]
- 65.DiNicolantonio JJ, Bhutani J, O’Keefe JH. Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015;2:e000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 67.Hanefeld M. Cardiovascular benefits and safety profile of acarbose therapy in prediabetes and established type 2 diabetes. Cardiovasc Diabetol 2007;6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pullman J, Darsow T, Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc Health Risk Manag 2006;2:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edelman S, Garg S, Frias J, et al. . A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care 2006;29:2189–2195 [DOI] [PubMed] [Google Scholar]
- 70.Owens DR, Luzio SD, Ismail I, Bayer T. Increased prandial insulin secretion after administration of a single preprandial oral dose of repaglinide in patients with type 2 diabetes. Diabetes Care 2000;23:518–523 [DOI] [PubMed] [Google Scholar]
- 71.Zinman B, Wanner C, Lachin JM, et al. . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 72.Mahaffey KW, Neal B, Perkovic V, et al. , on behalf of the CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (canagliflozin cardiovascular assessment study). Circulation 2018;137:323–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Investig 2014;5:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janssen. Invokana prescribing information. Available from www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVOKANA-pi.pdf. Accessed 4 April 2018
- 75.Brunton S. GLP‐1 receptor agonists vs. DPP‐4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract 2014;68:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanimoto M, Kanazawa A, Hirose T, et al. . Comparison of sitagliptin with nateglinide on postprandial glucose and related hormones in drug‐naïve Japanese patients with type 2 diabetes mellitus: a pilot study. J Diabetes Invest 2015;6:560–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li L, Li S, Deng K, et al. . Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ 2016;352:i610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scirica BM, Braunwald E, Raz I, et al. ; SAVOR-TIMI 53 Steering Committee and Investigators. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2015;132:e198. [DOI] [PubMed] [Google Scholar]
- 79.Scheen AJ. Cardiovascular outcome studies with incretin-based therapies: comparison between DPP-4 inhibitors and GLP-1 receptor agonists. Diabetes Res Clin Pract 2017;127:224–237 [DOI] [PubMed] [Google Scholar]
- 80.Ahrén B, Galstyan G, Gautier JF, et al. . Postprandial glucagon reductions correlate to reductions in postprandial glucose and glycated hemoglobin with lixisenatide treatment in type 2 diabetes mellitus: a post hoc analysis. Diabetes Ther 2016;7:583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fineman MS, Cirincione BB, Maggs D, Diamant M. GLP-1 based therapies: differential effects on fasting and postprandial glucose. Diabetes Obes Metab 2012;14:675–688 [DOI] [PubMed] [Google Scholar]
- 82.Larsen PJ. Mechanisms behind GLP-1 induced weight loss. Br J Diabetes Vasc Dis 2008;8(Suppl. 2):S34–S41 [Google Scholar]
- 83.Ottney A. Glucagon-like peptide-1 receptor agonists for weight loss in adult patients without diabetes. Am J Health Syst Pharm 2013;70:2097–2103 [DOI] [PubMed] [Google Scholar]
- 84.Secher A, Jelsing J, Baquero AF, et al. . The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 2014;124:4473–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest 2014;124:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werner U. Effects of the GLP-1 receptor agonist lixisenatide on postprandial glucose and gastric emptying—preclinical evidence. J Diabetes Complications 2014;28:110–114 [DOI] [PubMed] [Google Scholar]
- 87.Li Z, Zhang Y, Quan X, et al. . Efficacy and acceptability of glycemic control of glucagon-like peptide-1 receptor agonists among type 2 diabetes: a systematic review and network meta-analysis. PLoS One 2016;11:e0154206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pfeffer MA, Claggett B, Diaz R, et al. ; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257 [DOI] [PubMed] [Google Scholar]
- 89.Holman RR, Bethel MA, Mentz RJ, et al. ; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 91.Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bethel MA, Patel RA, Merrill P, et al. ; EXSCEL Study Group . Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113 [DOI] [PubMed] [Google Scholar]
- 93.Owens DR, Monnier L, Hanefeld M. A review of glucagon‐like peptide‐1 receptor agonists and their effects on lowering postprandial plasma glucose and cardiovascular outcomes in the treatment of type 2 diabetes mellitus. Diabetes Obes Metab 2017;19:1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monami M, Zannoni S, Pala L, et al. . Effects of glucagon-like peptide-1 receptor agonists on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Int J Cardiol 2017;240:414–421 [DOI] [PubMed] [Google Scholar]
- 95.Lingvay I, Pérez Manghi F, García-Hernández P, et al. ; DUAL V. Investigators. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated haemoglobin in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA 2016;315:898–907 [DOI] [PubMed] [Google Scholar]
- 96.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 2010;33:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh AK, Singh R. Recent cardiovascular outcome trials of antidiabetic drugs: a comparative analysis. Indian J Endocrinol Metab 2017;21:4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]


