Abstract
IN BRIEF Diabetic ketoacidosis (DKA) is a life-threatening complication that frequently occurs at diagnosis of type 1 diabetes, occurs more commonly when a patient is misdiagnosed, is the leading cause of death in children with type 1 diabetes, and is associated with worse long-term outcomes. Our retrospective online survey found that 25% of all participants were misdiagnosed and that misdiagnosis was associated with an 18% increased risk for DKA compared to those correctly diagnosed. Adult providers should consider type 1 diabetes when diagnosing type 2 diabetes, and pediatric providers should rule out type 1 diabetes when a patient reports nonspecific viral symptoms.
Type 1 diabetes is an autoimmune disease with an estimated incidence of 21.7/100,000 youths in the United States; a reliable estimate for the incidence of type 1 diabetes in adults is not available. Multiple studies indicate that the incidence of type 1 diabetes continues to rise globally (1–4).
Diabetic ketoacidosis (DKA) is a life-threatening complication of type 1 diabetes that occurs in a significant number of patients at diagnosis (5–9). The large, multisite SEARCH for Diabetes in Youth study has reported that DKA is present in 30% of youth with new-onset type 1 diabetes. Known risk factors for DKA at diagnosis are young age, ethnic minority status, limited or lack of health insurance, lower parent education, lower family income, existing mental disorders, lower BMI, puberty, and preceding infection (8–15). Other risk factors for DKA at diagnosis are the misdiagnosis and delayed treatment of hyperglycemia-related symptoms (16,17).
Misdiagnosis is correlated with increased rates of DKA (13,16). Patients may be misdiagnosed or overlooked at disease onset because the initial symptoms of type 1 diabetes may be nonspecific (2). Many children who are newly diagnosed with type 1 diabetes have seen health care providers (HCPs) within the previous 4 weeks, suggesting that hyperglycemia is initially missed in some patients (2,11,18). Among adults, a misdiagnosis of type 2 diabetes is more common. This presumably occurs secondary to a low index of suspicion, difficulty distinguishing diabetes subtypes based on clinical phenotype, and slower progression to insulin deficiency compared to children (19–24).
DKA at diagnosis is associated with higher initial A1C levels, a lower frequency of partial remission, and less residual β-cell function (5,8,25). Moreover, DKA is the leading cause of death in children and young adults with type 1 diabetes (5,6,10,26). Other consequences of DKA are prolonged recovery, morphologic and functional brain changes associated with adverse neurocognitive outcomes, lengthier hospital stays, and high health care expenses (15,17,27,28).
In the United States, we have been unable to leverage the modifiable risk factors to reduce the incidence of DKA at type 1 diabetes onset (5,12,27). In fact, the rate appears to be increasing (3,29). There are very limited data describing the experience of type 1 diabetes diagnosis from the adult patient and pediatric caregiver perspectives. Furthermore, very little information exists regarding differences in type 1 diabetes diagnosis associated with symptom onset and age. The aim of this study is to report the prevalence of missed diagnosis of type 1 diabetes and DKA as reported by patients and caregivers in the United States.
Design and Methods
Participants
Adults with type 1 diabetes and parents of children with type 1 diabetes were invited to complete an online questionnaire about their diagnosis experiences. Participants were contacted through the T1D Exchange clinic registry and the T1D Exchange online patient community (myGlu.org). T1D Exchange connects researchers, clinicians, funders, and innovators and offers them real-world and clinical data to inform type 1 diabetes product development, clinical care, and innovation. An e-mail invitation was sent to individuals who had previously agreed to be contacted for research purposes. Participants were also recruited through T1D Exchange-Glu social media accounts.
Eligible respondents were >18 years of age and either had type 1 diabetes or were the parent or guardian of a child with type 1 diabetes. Respondents who did not report age or year of diagnosis or who were not diagnosed in the United States were excluded from analysis.
Measures
The survey used in this study was developed by a multidisciplinary research team, including clinicians, researchers, patient advocates, and individuals with type 1 diabetes. Items targeted the type 1 diabetes diagnosis experience and were presented using a multiple-choice format. Regarding diagnosis, respondents were asked, “Were you/w
Was your child misdiagnosed with another condition before being diagnosed with type 1 diabetes?” All study materials were approved by the institutional review board at the Jaeb Center for Health Research in Tampa, Fla. Each participant provided informed consent before completing the survey. Participants completed the survey from their own electronic devices between 20 June 2016 and 9 February 2017.
Analyses
Statistical analyses were completed using R, version 3.4.1 Single Candle software (R Foundation for Statistical Computing, Vienna, Austria) (30). Comparison testing included t tests, analysis of variance (ANOVA), χ2 tests, and risk ratios. Analyses focused on comparing experiences between patients who were diagnosed at <18 years of age and those who were diagnosed as adults.
Results
Demographics
Eighty-three percent of the 3,030 participants who completed the survey met the inclusion criteria. Twenty-two percent identified as a parent or guardian of someone with type 1 diabetes.
Additional demographics can be found in Table 1.
TABLE 1.
Participant Demographics
| Overall (n = 2,526) | Diagnosis During Adulthood (n = 856; 34%) | Diagnosis During Childhood (n = 1,670; 66%) | |
|---|---|---|---|
| Age, years | 35.34 (19.1, 1–90) | 48.51 (15.1, 19–90) | 28.56 (17.2, 1–84) |
| Age at diagnosis, years | 16.3 (13.3, <1–74) | 31.52 (11.5, 18–74) | 8.56 (4.5, <1–17) |
| Female sex | 1,669 (66.4) | 559 (65.8) | 1,110 (66.7) |
| White race | 2,366 (93.7) | 812 (94.9) | 1,554 (93.1) |
| Private health insurance at diagnosis | 1,929 (76.4) | 690 (80.6) | 1,239 (74.2) |
| Current insulin delivery | |||
| CSII | 1,815 (71.9) | 579 (67.6) | 1,236 (74.0) |
| MDI regimen (pen or syringe) | 872 (34.5) | 319 (37.3) | 553 (33.1) |
| Inhalable | 12 (0.5) | 8 (0.9) | 4 (0.2) |
| No insulin | 9 (0.4) | 4 (0.5) | 5 (0.3) |
| Current CGM user | 1,347 (55.5) | 476 (57.7) | 871 (54.3) |
| Most recent A1C, % | |||
| <7.0 | 929 (39.3) | 432 (53.3) | 497 (32.0) |
| ≥7.0 | 1,436 (60.7) | 379 (46.7) | 1,057 (68.0) |
Age data are expressed as mean (SD, range); all other data are expressed as n (%). CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injection.
Symptoms at Diagnosis
Polydipsia (excessive thirst) (88.1%), polyuria (excessive urination) (82.0%), and fatigue (74.8%) were the most common symptoms at diagnosis for both children and adults with type 1 diabetes. There were notable differences in the prevalence of other symptoms between children and adults (Table 2). Participants diagnosed as adults reported blurred vision, delayed healing of sores, tingling hands and feet, changes in gums, urinary tract infection, and vaginal yeast infection more frequently than respondents diagnosed during childhood. Conversely, participants diagnosed during childhood endorsed polyuria, bedwetting, excessive thirst, stomach pain, nausea or vomiting, and flu-like symptoms more frequently.
TABLE 2.
Symptoms Present at Diagnosis
| Adult Diagnosis (n = 856) | Pediatric Diagnosis (n = 1,670) | |
|---|---|---|
| Excessive thirst** | 84.3% | 90.0% |
| Fatigue* | 77.9% | 73.2% |
| Polyuria** | 77.0% | 84.6% |
| Weight loss | 73.9% | 71.4% |
| Blurred vision** | 49.3% | 20.0% |
| Increased appetite | 39.1% | 35.4% |
| Headache | 26.6% | 25.8% |
| Vaginal yeast infection** | 21.3% | 7.4% |
| Nausea or vomiting** | 17.5% | 31.9% |
| Flu-like symptoms** | 17.4% | 29.9% |
| Other | 15.0% | 12.3% |
| Tingling hands and feet** | 12.4% | 3.7% |
| Slow-healing of sores** | 11.8% | 5.9% |
| Stomach pain** | 11.1% | 21.6% |
| Urinary tract infection** | 9.0% | 4.1% |
| Bedwetting** | 4.4% | 1.7% |
| Changes in gums** | 4.0% | 39.7% |
| Unsure/don’t remember* | 1.1% | 2.3% |
P <0.05.
P <0.001. Boldface type indicates statistical significance.
Misdiagnosis
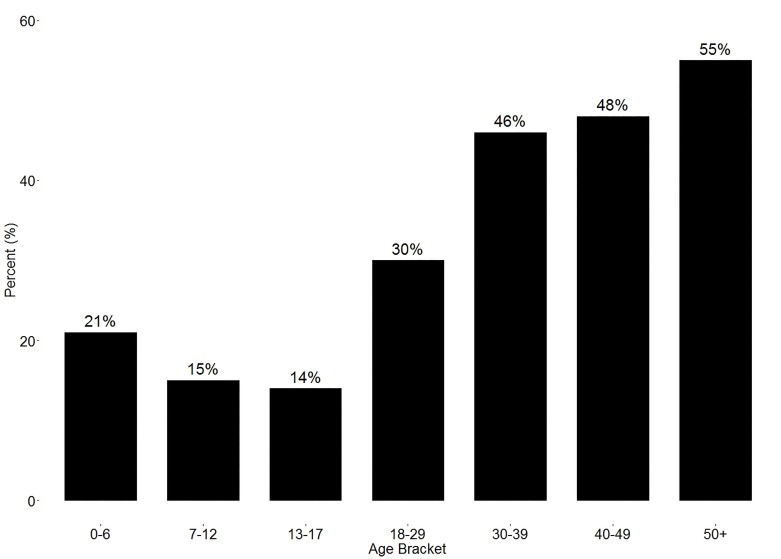
Twenty-four percent of participants reported being misdiagnosed with another condition before being diagnosed with type 1 diabetes. The diagnosis was missed in 16% of those who were diagnosed at <18 years of age and in 38.6% of those who were diagnosed at ≥18 years of age (χ2[1] = 137.2, P <0.001). Across seven age-groups (ages 0–6, 7–12, 13–17, 18–29, 30–39, 40–49, and ≥50 years), rates of missed diagnosis of type 1 diabetes rose as individuals got older, with the exception of the 7–17 years age-group (χ2[6] = 183.78, P <0.001) (Figure 1). Fifteen percent of those diagnosed before 1985 reported being misdiagnosed, compared to 20.4% of people diagnosed between 1985 and 1999, and 29.5% of people who were diagnosed after 2000 (χ2[2] = 47.7, P <0.001).
FIGURE 1.
Percentage of patients misdiagnosed, by age-group.
People ≥18 years of age whose type 1 diabetes diagnosis was missed were most likely to be initially diagnosed with type 2 diabetes (76.8%), whereas those <18 years of age were more likely to be diagnosed with the flu or other viral infection (53.7%) (Table 3).
TABLE 3.
Misdiagnosed Conditions
| Adult Diagnosis (n = 856) | Pediatric Diagnosis (n = 1,670) | |
|---|---|---|
| Type 2 diabetes** | 76.8% | 4.1% |
| Other | 18.4% | 38.2% |
| Flu/viral infection** | 8.6% | 53.7% |
| Urinary tract infection* | 6.3% | 7.7% |
| Dehydration | 3.5% | 10.6% |
| Strep/bacterial infection* | 2.5% | 19.1% |
| Psychiatric condition | 2.9% | 5.3% |
| Mononucleosis | 0.3% | 4.5% |
P <0.05.
P <0.001. Boldface type indicates statistical significance.
DKA at Diagnosis
At diagnosis, 66.1% were hospitalized, and 40.9% experienced DKA. Of the participants who were in DKA, 38.1% reported vomiting, and 89.2% required intravenous (IV) fluids. Not surprisingly, those in DKA at diagnosis also reported higher blood glucose levels compared to those who did not experience DKA at diagnosis (t[1411.1] = 16.86, P <0.001).
Pediatric patients were more likely to be in DKA (48.0 vs. 28.1%; relative risk [RR] 1.71, 95% CI 1.51–1.93, P <0.001) and more likely to need IV fluids (92.9 vs. 78.4%; RR 1.18, 95% CI 1.10–1.28, P <0.001).
A two-way ANOVA was conducted to assess the impact of age at diagnosis and the influence of DKA on blood glucose values at diagnosis. There was a main effect of age (F[6, 1,772] = 24.78, P <0.001), a main effect of DKA (F[1, 1,772] = 242.23, P <0.001), but no interaction effect of these variables on blood glucose (F[6, 1,772] = 1.2, P >0.05).
Patients with a missed diagnosis of type 1 diabetes were at a 17.6% increased risk for DKA compared to those who were correctly diagnosed at onset (45.2 vs. 38.4%; RR 1.176, 95% CI 1.05–1.32, P <0.05). However, this relationship was only found for those <18 years of age at diagnosis. Sixty-eight percent of children with a missed type 1 diabetes diagnosis experienced DKA compared to 42.8% of children whose diagnosis was not missed (RR 1.58, 95% CI 1.41–1.77, P <0.001). There was no difference in the rate of DKA between those whose type 1 diabetes diagnosis was missed and those who were correctly diagnosed with type 1 diabetes as adults.
Twenty-one percent of all study participants reported being admitted to an intensive care unit (ICU) while in the hospital. The majority of those admitted to the ICU were in DKA (83.4%). One-fourth (25.0%) of those admitted to the ICU were unconscious.
Discussion
This study evaluated self-reported data from 2,526 individuals with type 1 diabetes or caregivers of children with type 1 diabetes surrounding their experience at the time of type 1 diabetes diagnosis. Initial symptoms differed between the adult and pediatric groups and were consistent with previous reports (11,15,23,29,31).
Type 1 diabetes was initially missed in a staggering 39% of patients diagnosed as adults, with more than three-fourths of those adults receiving an incorrect diagnosis of type 2 diabetes. Although type 1 diabetes and type 2 diabetes share some similarities, the phenotypes and treatments are different. People with type 1 diabetes must take insulin, whereas people with type 2 diabetes can often be treated successfully by other means. Misdiagnosis of type 1 diabetes as type 2 diabetes therefore often triggers a treatment algorithm without insulin, resulting in prolonged hyperglycemia and the risk of unfavorable outcomes, including DKA. Fortunately, missed diagnoses of type 1 diabetes in adults in this study were not associated with an increased risk of DKA. The fact that adults typically have a slower decline in insulin production than children may be a protective factor because this slower decline may result in longer symptomatic periods before the onset of DKA and overall lower rates of DKA at onset (19–24). Genetic screening and monitoring for β-cell autoimmunity at diagnosis, which has demonstrated decreased severity of illness and frequency of DKA, may be beneficial (29,31,32).
Because type 2 diabetes is nearly 20 times more prevalent than type 1 diabetes in adults, there is frequently a diagnosis bias in which type 1 diabetes is misdiagnosed as type 2 diabetes. Type 1 diabetes should be considered as a possible diagnosis in adults presenting with hyperglycemia, especially in those who do not match the phenotype of coexistent obesity, hyperlipidemia, and signs of insulin resistance (12,22,33–35).
The diagnosis of type 1 diabetes was missed in fewer patients with type 1 diabetes onset during childhood or adolescence than in their adult counterparts, but unlike the adults, there was a strong association between misdiagnosis and the likelihood of DKA in this group. In addition to the dangers and hospital costs of DKA, this condition is also associated with a lower frequency of partial remission and poorer long-term glycemic management (36). As previously noted, DKA is the leading cause of death in youth with type 1 diabetes (5,31,35). In a recent analysis from the Virginia Health Information database, it was noted that the cost of DKA was >$12,000 per episode (37). Therefore, a timely diagnosis of type 1 diabetes among youth is vital to improve outcomes.
The most common alternate diagnoses in children and adolescents were infectious diseases. Viral and bacterial infections are common in these age-groups and may be the family’s chief concern for seeking medical attention. Many symptoms of viral illnesses are nonspecific and may overlap with new-onset type 1 diabetes. Awareness campaigns focusing on symptoms more specific to type 1 diabetes, including polyuria, polydipsia, and recent onset of bedwetting have proved successful in significantly reducing the DKA rate in new-onset type 1 diabetes (17,27,38). Global campaigns that target HCPs, educators, and families and focus on the most common early symptom of type 1 diabetes (i.e., polyuria) through clear and concise communication may be one way to reduce the incidence of incorrect or delayed diagnosis and to reduce DKA during this period (17,27,39).
Based on the information reported by study participants, adult HCPs should consider type 1 diabetes when diagnosing type 2 diabetes, and pediatric HCPs should rule out type 1 diabetes as a possible diagnosis when a patient reports nonspecific symptoms. Improved diagnostics that will result in timely and accurate type 1 diabetes diagnoses and clinical classification of diabetes subtype are crucial. It is currently unknown whether electronic health record functionalities and interoperability between institutions could offer emergency and primary care clinicians decision support to increase the likelihood of identifying new-onset type 1 diabetes. For example, weight loss in a growing child could trigger alerts suggesting inexpensive glucose testing, and a diagnosis of type 2 diabetes in a nonobese adult could similarly offer the option to check diabetes antibodies.
Analogous to recent work to raise awareness of missed diagnosis or misdiagnosis of heart disease in women, it is important to raise awareness among HCPs regarding the frequency of type 1 diabetes being misdiagnosed as type 2 diabetes in adults. Education and quality improvement efforts to increase HCP awareness and highlight differences between adult and pediatric symptom presentation is key. Furthermore, it is important to build public awareness programs to increase awareness and promote early diagnosis for type 1 diabetes and prevent DKA. Collaboration with medical communities, health organizations, local hospitals, and community clinics, as well as school-based health centers, will be crucial to maximize the implementation, coordination, and success of these public health efforts to save lives and health care dollars.
Strengths
Although type 1 diabetes accounts for a small percentage of all people with diabetes, recent evidence suggests that >40% of patients with autoimmune diabetes are diagnosed after 30 years of age (35). Our results emphasize the importance of improving diagnostic pathways for adults with new-onset diabetes.
Self-reports allowed for inclusion of the patient voice on a first-person basis. The inclusion of first-person reports allowed the research team and readers to become familiar with and appreciate the diagnosis experience of >2,500 people who are navigating the daily demands of type 1 diabetes. Social media and computer-based methods provided a low-cost opportunity to recruit a large number of individuals relatively quickly.
Limitations
Although self-reported data provide an important, first-person account of the type 1 diabetes experience by patients or their caregivers, the retrospective nature of the study introduced the possibilities of ascertainment and recall biases. The consent process and surveys were completed online; therefore, individuals who participated in this study may represent a more highly educated or technology-savvy sample than the general population of people with type 1 diabetes. In addition, our sample did not include an ethnically diverse population, possibly reflecting a self-selection bias for Caucasian ethnicity.
Future Directions
Future studies should collect additional data to obtain a more comprehensive understanding of the diagnosis and DKA experience of individuals with type 1 diabetes. For example, information about treatment-seeking behaviors and the psychological impact of the DKA experience resulting from delayed diagnosis or misdiagnosis could be insightful regarding the impact of patient factors on the diagnosis and treatment process. Given known disparities in diabetes, assessing the DKA experience among a diverse group of individuals is important (38,40). In addition, gathering data on health insurance information would be beneficial given previous findings that lack of adequate health insurance coverage can negatively affect health-seeking behaviors (38).
Acknowledgments
Duality of Interest
D.J. has received institutional grants from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk for center participation in multicenter pharmacology trials. G.T.A. has been a paid speaker for Roche Diabetes Care. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
C.M. wrote the manuscript; A.F. wrote the survey for data collection and reviewed/edited the manuscript; C.G. analyzed data and reviewed/edited the manuscript; and T.K., D.J., G.T.A., and A.M.-F. contributed to the discussion and reviewed/edited the manuscript. A.M.-F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017;376:1419–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39:481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imperatore G, Mayer-Davis EJ, Orchard TJ, Zhong VW, Mayer-Davis EJ. Prevalence and incidence of type 1 diabetes among children and adults in the United States and comparison with non-U.S. countries. In Diabetes in America. 3rd ed. Cowie CC, Casagrande SS, Menke A, et al., Eds. NIH Publ. No. 17-1468. Bethesda, Md, National Institutes of Health, 2018, p. 2.1–2.17 [Google Scholar]
- 4.Gorham ED, Barrett-Connor E, Highfill-Mcroy RM, et al. Incidence of insulin-requiring diabetes in the US military. Diabetologia 2009;52:2087–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingensmith GJ, Tamborlane WV, Wood J, et al. Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr 2013;162:330–334 [DOI] [PubMed] [Google Scholar]
- 6.Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998–2012. JAMA 2015;313:1570–1572 [DOI] [PubMed] [Google Scholar]
- 7.Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes 2018;19:314–319 [DOI] [PubMed] [Google Scholar]
- 8.Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 2012;55:2878–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewers A, Klingensmith G, Davis C, et al. Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the SEARCH for Diabetes in Youth study. Pediatrics 2008;121:e1258–e1266 [DOI] [PubMed] [Google Scholar]
- 10.Choleau C, Maitre J, Filipovic Pierucci A, et al. Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes Metab 2014;40:137–142 [DOI] [PubMed] [Google Scholar]
- 11.Baldelli L, Flitter B, Pyle L, et al. A survey of youth with new onset type 1 diabetes: opportunities to reduce diabetic ketoacidosis. Pediatr Diabetes 2016;18:547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for Diabetes in Youth study. Pediatrics 2014;133:e938–e945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ 2011;343:d4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hekkala A, Reunanen A, Koski M, Knip M, Veijola R. Age-related differences in the frequency of ketoacidosis at diagnosis of type 1 diabetes in children and adolescents. Diabetes Care 2010;33:1500–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr 2016;171:104–110 [DOI] [PubMed] [Google Scholar]
- 16.Szypowska A, Skórka A. The risk factors of ketoacidosis in children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes 2011;12:302–306 [DOI] [PubMed] [Google Scholar]
- 17.Vanelli M, Chiari G, Lacava S, Iovane B. Campaign for diabetic ketoacidosis prevention still effective 8 years later. Diabetes Care 2007;30:e12. [DOI] [PubMed] [Google Scholar]
- 18.Lokulo-Sodipe K, Moon RJ, Edge JA, Davies JH. Identifying targets to reduce the incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in the UK. Arch Dis Child 2014;99:438–442 [DOI] [PubMed] [Google Scholar]
- 19.Pilla SJ, Maruthur NM, Schweitzer MA, et al. The role of laboratory testing in differentiating type 1 diabetes from type 2 diabetes in patients undergoing bariatric surgery. Obes Surg 2018;28:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebenthal Y, Fisch Shvalb N, Gozlan Y, et al. The unique clinical spectrum of maturity onset diabetes of the young type 3. Diabetes Res Clin Pract 2018;135:18–22 [DOI] [PubMed] [Google Scholar]
- 21.Gunn ER, Albert BB, Hofman PL, et al. Pathways to reduce diabetic ketoacidosis with new onset type 1 diabetes: evidence from a regional pediatric diabetes center: Auckland, New Zealand, 2010 to 2014. Pediatr Diabetes 2016;18:553–558 [DOI] [PubMed] [Google Scholar]
- 22.Merger SR, Leslie RD, Boehm BO. The broad clinical phenotype of type 1 diabetes at presentation. Diabet Med 2013;30:170–178 [DOI] [PubMed] [Google Scholar]
- 23.Laugesen E, Østergaard JA, Leslie RDG. Latent autoimmune diabetes of the adult: current knowledge and uncertainty. Diabet Med 2015;32:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab 2009;94:4635–4644 [DOI] [PubMed] [Google Scholar]
- 25.Marino KR, Lundberg RL, Jasrotia A, et al. A predictive model for lack of partial clinical remission in new-onset pediatric type 1 diabetes. PLoS One 2017;12:e0176860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jefferies CA, Nakhla M, Derraik JGB, Gunn AJ, Daneman D, Cutfield WS. Preventing diabetic ketoacidosis. Pediatr Clin North Am 2015;62:857–871 [DOI] [PubMed] [Google Scholar]
- 27.Garibaldi L, Becker D. Is the risk of diabetic ketoacidosis modifiable? J Pediatr 2016;171:10–12 [DOI] [PubMed] [Google Scholar]
- 28.Cameron FJ, Scratch SE, Nadebaum C, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 2014;37:1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 2000;23:1326–1332 [DOI] [PubMed] [Google Scholar]
- 30.R Foundation for Statistical Computing R: a language and environment for statistical computing. Available from www.r-project.org.
- 31.Karjalainen J, Salmela P, Ilonen J, Surcel HM, Knip M. A comparison of childhood and adult type I diabetes mellitus. N Engl J Med 1989;320:881–886 [DOI] [PubMed] [Google Scholar]
- 32.Larsson HE, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care 2011;34:2347–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maahs DM, Hermann JM, Holman N, et al. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care 2015;38:1876–1882 [DOI] [PubMed] [Google Scholar]
- 34.King BR, Howard NJ, Verge CF, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes 2012;13:647–651 [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S1–S153 [DOI] [PubMed] [Google Scholar]
- 36.Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 2017;401249–1255 [DOI] [PubMed] [Google Scholar]
- 37.Ballinger G, Ullal J. Cost and characteristics of diabetic ketoacidosis in eastern Virginia. Endocr Pract 2016;22:334–335 [Google Scholar]
- 38.Maniatis AK, Goehrig SH, Gao D, Rewers A, Walravens P, Klingensmith GJ. Increased incidence and severity of diabetic ketoacidosis among uninsured children with newly diagnosed type 1 diabetes mellitus. Pediatr Diabetes 2005;6:79–83 [DOI] [PubMed] [Google Scholar]
- 39.Jelley DH, Marra CG, Paul J. Primary prevention of DKA: a campaign to raise awareness of diabetes symptoms in the community. Pediatr Diabetes 2010;11:3819496970 [Google Scholar]
- 40.Weisman A, Lovblom L, Keenan H, et al. Diabetes care disparities in long-standing type 1 diabetes in Canada and the U.S.: a cross-sectional comparison. Diabetes Care 2018;41:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]