Abstract
Progressive supranuclear palsy with predominant cerebellar ataxia (PSP-C) has been reported as a rare clinical subtype, but the underlying pathology of its cerebellar ataxia remains unclear. Here, we report a patient with the coexistence of PSP with pontocerebellar atrophy and myotonic dystrophy type 1 (DM1). A 73-year-old man who was an asymptomatic carrier of DM1 (66 CTG repeats) started developing ataxic gait with multiple falls, visual blurring, double vision, and word finding difficulty at age 62 and was initially diagnosed with multiple system atrophy (MSA). Subsequently, the diagnosis was changed to PSP due to hypometric downward gaze, reduced blink frequency, symmetric bradykinesia, rigidity, and the absence of autonomic dysfunction. He eventually developed delayed grip opening with percussion myotonia at age 72. At autopsy, severe neuronal degeneration and astrogliosis in the pontocerebellar structures suggested MSA, but immunohistochemistry for α-synuclein did not reveal neuronal or glial cytoplasmic inclusions. Immunohistochemistry for phospho-tau and 4-repeat tau confirmed a neuropathological diagnosis of PSP with exceptionally numerous coiled bodies and threads in the pontine base and cerebellar white matter. This unusual distribution of 4-repeat tau pathology and neuronal degeneration with astrogliosis is a plausible clinicopathological substrate of PSP-C.
Keywords: Inferior olivary hypertrophy, Myotonic dystrophy type 1, Palatal myoclonus, Palatal tremor, Pontocerebellar atrophy, Progressive supranuclear palsy, Progressive supranuclear palsy with predominant cerebellar ataxia (PSP-C)
INTRODUCTION
Progressive supranuclear palsy (PSP) is a 4-repeat tauopathy characterized by neuronal and glial tau aggregates: pretangles, neurofibrillary tangles (NFTs), neuropil threads, tufted astrocytes, and coiled bodies (1). These tau pathologies, accompanied by neuronal loss and gliosis, affect predominantly the globus pallidus, subthalamic nucleus, substantia nigra, and cerebellar dentate nucleus (1). Clinically, PSP is an atypical parkinsonian disorder typically presenting with levodopa-unresponsive parkinsonism, postural instability, frequent falls, vertical supranuclear gaze palsy, and cognitive impairment (2). In addition to this classical phenotype, also referred to as Richardson syndrome, PSP shows various clinical presentations, such as corticobasal syndrome, behavioral variant frontal dementia, and progressive nonfluent aphasia (2). Although the cerebellar efferent pathways (i.e. the dentate nucleus, red nucleus, and thalamus) are vulnerable to tau pathology and neurodegeneration, cerebellar ataxia is not a principal symptom in PSP. Several studies have described PSP with predominant cerebellar ataxia (PSP-C), but the underlying pathology of its cerebellar ataxia remains unclear (3, 4); therefore, PSP-C is not considered a distinct clinicopathological subtype of PSP in the Movement Disorder Society criteria (2).
Myotonic dystrophy type 1 (DM1) is an autosomal dominant inherited muscular dystrophy, caused by abnormal expansion of an unstable CTG trinucleotide repeat in a gene encoding for DM1 protein kinase (DMPK) (5). The severity of the disease is correlated to the length of this repeat expansion; affected individuals who carry CTG repeat greater than 50 present a highly variable phenotype, ranging from asymptomatic or mild (50–80) to a severe congenital form (100–4000) of the disease (6). DM1 patients show not only muscle weakness and myotonia but also arrhythmia, cataracts, and central nervous system symptoms, such as personality changes and cognitive impairment (7). Although the underlying brain pathology of cognitive impairment in DM1 remains unclear, the brain involvement has been examined by both in vivo neuroimaging and autopsy-based studies. DM1 is a progressive white matter disease with the process of myelin degeneration or axonal loss (8, 9). The presence of NFTs in the amygdala, hippocampus, entorhinal cortex, and temporal cortex has also been reported (9, 10). Although the distribution of NFTs in DM1 is limited compared with AD, the distribution and expression of NFTs are higher than that in age-matched unaffected individuals; thus, DM1 can be considered a tauopathy (8–11).
In this study, we describe a patient with PSP with pontocerebellar atrophy who also had DM1 based on genetic testing. To our knowledge, this combination of two tauopathies has not been reported previously.
Case Presentation
This patient was a 73-year-old Caucasian man who had an 11-year history of an unsteady gait, frequent falls, and cognitive dysfunction. He had a past medical history of a right frontal meningioma, which was surgically removed when he was 60 years of age. His 2 granddaughters, who had muscular weakness and cognitive difficulties, were diagnosed with DM1 when the patient was at age 61. He was found to be positive for the CTG repeat with 66 repeats (mild expansion); however, he had no clinical evidence of myotonic dystrophy, including normal electromyography at that time. Two out of three daughters also had DM1. His sister also had the CTG expansion (the repeat number was unknown) and was clinically diagnosed with amyotrophic lateral sclerosis. His mother developed personality changes and cognitive impairment, but she did not have any motor symptoms suggestive of myotonic dystrophy.
At age 62, the patient started developing imbalance with multiple falls, visual blurring, double vision, and cognitive impairment with language disorder, characterized by paraphasias and word finding difficulties, as well as visuospatial problems. He had several episodes of syncope at age 64. He underwent Holter electrocardiogram and echocardiogram, which showed normal findings. He was evaluated by a movement disorder neurologist at age 64 and found to have mild ataxia and palatal tremor. This raised a question of whether the patient might have multiple system atrophy (MSA); therefore, the patient was further referred to a tertiary center (Mayo Clinic, Rochester, MN). Neurological examination revealed hypometric downward gaze, reduced blinking, palatal tremor, marginal bradykinesia, and marginal rigidity of the limbs, neck, and trunk. His gait was ataxic and cautious and he had mildly decreased arm swing bilaterally. He was unable to tandem walk at all. Neither appendicular ataxia nor pyramidal signs were observed. He had no difficulties with bladder or bowel and no problems with impotence. Thermoregulatory sweat test and autonomic reflex screen test were normal. A sleep study revealed severe obstructive sleep apnea and periodic limb movements. Neuropsychological test findings were consistent with dementia, particularly subcortical or frontal type. Mild word retrieval difficulty and moderate-to-severe difficulty in word fluency were also noted. A brain MRI revealed symmetrical hyperintense lesions in both inferior olivary nuclei, which was consistent with hypertrophic olivary degeneration. Atrophy of the brainstem and cerebellum was not observed. The genetic evaluation for hereditary ataxia was negative for SCA1-8, SCA10, SCA17, dentatorubral-pallidoluysian atrophy, and Friedreich’s ataxia. Based on these findings, he was given a diagnosis of possible PSP.
Neurological examinations at age 68 revealed progression of gaze palsy in all directions, severe postural instability, marked bilateral bradykinesia, axial rigidity, palatal tremor, as well as ocular, chin, and tongue tremor. He eventually developed delayed grip opening with percussion myotonia, which was suggestive of myotonic dystrophy, at age 72.
MATERIALS AND METHODS
The present study was performed at the brain bank for neurodegenerative disorders at the Mayo Clinic Florida. The brain bank operates under procedures approved by the Mayo Clinic Institutional Review Board. The autopsy on this patient was performed after informed consent from his caregiver who had legal power-of-attorney for this patient.
The left hemibrain was fixed in formalin and embedded in paraffin; the right was frozen for biochemical and genetic studies. The areas sampled for histology were middle frontal gyrus, superior temporal gyrus, inferior parietal gyrus, pre-and post-central gyri, visual cortex, cingulate gyrus, anterior and posterior hippocampus, amygdala, basal nucleus of Meynert, caudate nucleus, putamen, thalamus, midbrain, pons, and cerebellum. Paraffin-embedded 5-μm-thick sections mounted on glass slides were stained with hematoxylin and eosin (H&E), Luxol fast blue (LFB), and thioflavin S stains. Immunohistochemistry was performed using antibodies against phospho-tau (CP13, 1:1000, mouse monoclonal, from Dr Peter Davies, Feinstein Institute, North Shore Hospital, NY), glial fibrillary acidic protein (GFAP, 1:5000, mouse monoclonal GA-5, BioGenex, Fremont, CA), SV2A (1:500, mouse monoclonal 15E11, Abcam, Cambridge, MA), α-synuclein (NACP, 1:3000, rabbit polyclonal, Mayo Clinic antibody), 3-repeat tau (RD3, mouse monoclonal, 1:5000; Millipore, Temecula, CA), 4-repeat tau (RD4, mouse monoclonal, 1:5000; Millipore, Temecula, CA), or phospho-TDP-43 (409/410, mouse monoclonal, 1:5000, Cosmo Bio, Tokyo, Japan) on a DAKO Autostainer (Universal Staining System). After immunostaining, the sections were briefly counterstained with hematoxylin.
To determine the CTG trinucleotide repeat length in DMPK, we performed fluorescent-based fragment analysis. Genomic DNA was extracted from frozen cerebellum tissue from the patient and from a neuropathologically normal control subject using standard procedures. The trinucleotide repeat was amplified in all samples by PCR using a FAM-labeled primer set designed to span the triplet repeat. The amplicons were analyzed on an ABI 3730 and fragment length determined using Genemapper software (Thermo Fisher Scientific, Waltham, MA). Genotyping for MAPT H1/H2 (SNP rs1052553 A/G, A = H1, G = H2) and APOE alleles (SNP rs429358 C/T and rs7412 C/T) was assessed with TaqMan SNP genotyping assays (Applied Biosystems, Foster City, CA) as previously reported (12).
For biochemical analysis, frozen brain tissues from the middle frontal gyrus were homogenized (w/v) in 5 volumes of 1× TBS (50-mM Tris, 150-mM Nacl, and 1-mM phenylmethylsulfonyl fluoride (PMSF), pH 7.6 supplemented with protease (Thermo Fisher Scientific) and phosphatase (Biotool, Houston, TX) inhibitor cocktails), snap frozen on dry ice, and stored at −80°C. Aliquots of 400-μl homogenates were spun at 47 000 rpm for 60 minutes in a Beckman TLA55 rotor (Beckman Coulter, Brea, CA). Pellets were suspended in 400 μl of high salt buffer (10-mM Tris-HCl, 800-mM NaCl, 10% sucrose, 1-mM ethylene glycol tetraacetic acid, and 1-mM PMSF, pH 7.5) and clarified at 20 000 rpm for 20 minutes in a TLA55 rotor. The resulting supernatant was supplemented with 1% sarkosyl for 1 hour at 37°C and the P3 fraction was collected at 47 000 rpm for 2 hours in a TLA55. The sarkosyl-insoluble P3 fractions were resuspended in 160 μl of 1× Laemmli sample buffer (Thermo Fisher Scientific) containing 2% sodium dodecyl sulfate and 10% β-mercaptoethanol. For Western blotting, insoluble tau samples were separated on 10% Tris-glycine gels (Thermo Fisher Scientific), transferred to nitrocellulose membranes (Millipore, Burlington, MA), and probed using the primary antibody E1 against exon 1 of human tau (1:1000, from Leonard Petrucelli, Mayo Clinic Florida, Jacksonville, FL).
RESULTS
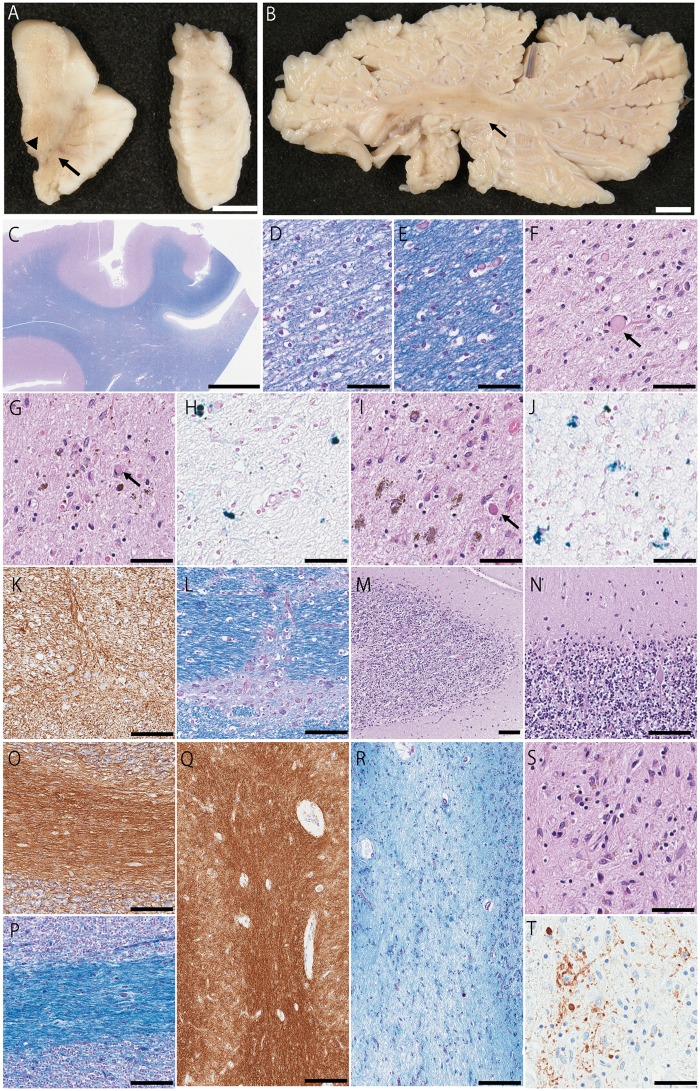
The calculated brain weight was 1060 g. The cortical gray mantle was normal in thickness and distribution. The putamen was unremarkable, while the globus pallidus had subtle discoloration. The thalamus was well preserved, but the subthalamic nucleus had very severe atrophy and dark discoloration. Horizontal sections of the midbrain and pons were remarkable for marked pontine atrophy and loss of pigment in the substantia nigra, especially in lateral part, and in the locus coeruleus (Fig. 1A). The cerebellar section showed atrophy of the dentate nucleus and gray discoloration of the dentate hilus. The cerebellar white matter was markedly atrophic with a dusky gray appearance (Fig. 1B). The medulla was not available for study.
FIGURE 1.
Macroscopic and histopathologic features. (A) The midbrain and pons are atrophic. Loss of pigment in the substantia nigra is observed. (B) The cerebellar white matter and dentate nucleus are markedly atrophic with a dusky gray appearance. (C, D) Patchy myelin pallor is observed in the periventricular white matter. (E) For comparison, an image of well-myelinated region is shown. (F) The subthalamic nucleus has severe neuronal loss and gliosis with spheroid (arrow). (G) The substantia nigra has severe neuronal loss and gliosis with extraneuronal neuromelanin, granular foamy axonal spheroids (arrow), and iron type pigment (H). (I) The red nucleus has severe neuronal loss and gliosis with spheroids (arrow) and iron type pigment (J). (K) The pontine base has reactive astrocytes, although the myelination is relatively preserved (L). (M) The cerebellum shows extensive neuronal loss in Purkinje and internal granular cell layers with many axonal torpedoes (N) and diffuse Bergmann gliosis. (O) The cerebellar white matter has severe astrogliosis with relatively intact myelinated fiber (P). (Q) The hilus and superior cerebellar peduncle shows dense fibrillary gliosis and almost complete loss of myelinated fibers (R). (S) The cerebellar dentate nucleus has an almost total neuronal loss with dense fibrillary gliosis and coarse granular synaptic vesicles around the remaining neurons (T). (C–E, L, P, R) LFB staining; (F, G, I, M, N, S) H&E staining; (H, J) Berlin blue staining; (K, O, Q) GFAP immunostaining; and (T) SV2A immunostaining. Scale bars: (A, B) 1 cm; (C) 5 mm; (D–J, S, T) 50 μm; and (K–R) 100 μm.
The neocortex had mild neuronal loss and gliosis in the frontal lobe sections, especially the superior frontal gyrus. The centrum semiovale, especially in the frontal lobe, and the periventricular white matter had myelin pallor, dilated perivascular spaces, and perivascular collagenosis (Fig. 1C–E). The thalamus had moderate gliosis in the ventrolateral region, and the subthalamic nucleus had severe atrophy with neuronal loss and gliosis (Fig. 1F). The substantia nigra had severe neuronal loss and gliosis with extraneuronal neuromelanin, iron type pigment and granular foamy axonal spheroids (Fig. 1G, H). Cytoplasmic eosinophilic inclusions, which were frequently observed in DM1 (13), were not detected in the thalamus and substantia nigra. The red nucleus had severe neuronal loss and gliosis with iron type pigment and spheroids (Fig. 1I, J). The midbrain tectum had severe atrophy and gliosis. The locus coeruleus was relatively well populated. The pontine base had severe neuronal loss and gliosis; immunohistochemistry for GFAP revealed reactive astrocytes, although the myelination was relatively preserved (Fig. 1K, L). The cerebellum showed the extensive neuronal loss in Purkinje and internal granular cell layers with many axonal torpedoes and diffuse Bergmann gliosis, especially in the cerebellar vermis (Fig. 1M, N). The cerebellar white matter had severe astrogliosis with relatively intact myelinated fiber (Fig. 1O, P). The hilus and superior cerebellar peduncle showed almost complete loss of myelinated fibers and dense fibrillary gliosis (Fig. 1Q, R). The cerebellar dentate nucleus had almost total neuronal loss with dense fibrillary gliosis, and immunohistochemistry for SV2A showed large, coarse granular synaptic vesicles around the remaining neurons (Fig. 1S, T).
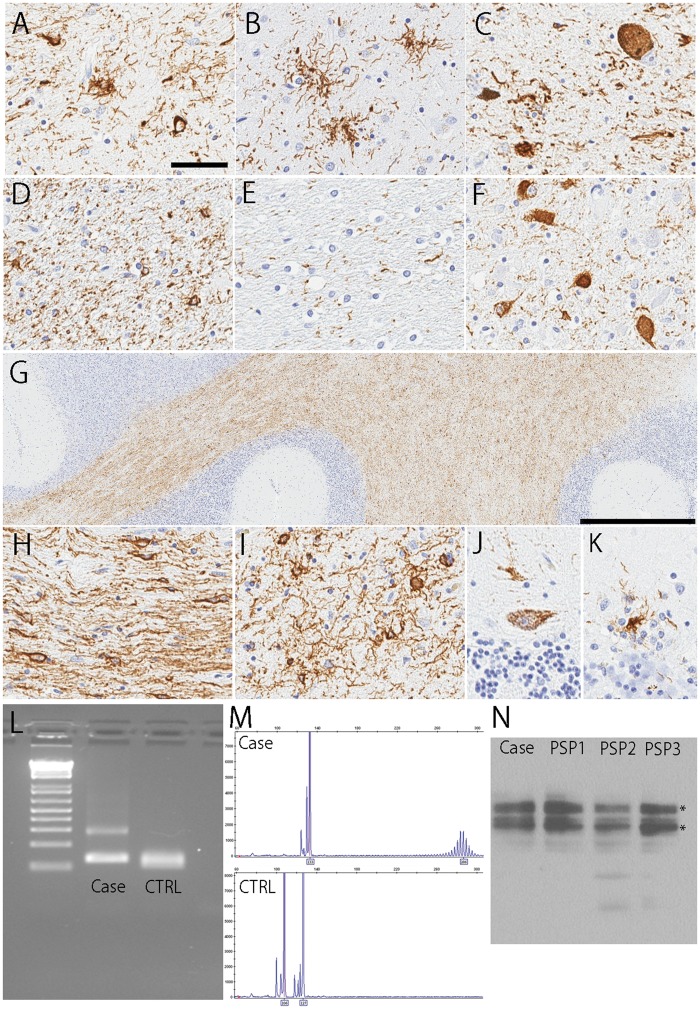
Immunohistochemistry for phospho-tau revealed extensive neuronal and glial tau pathology in the substantia nigra, subthalamic nucleus, globus pallidus, motor and premotor cortex, ventral thalamus, corpus striatum, and the olivopontocerebellar system, which was consistent with a neuropathological diagnosis of PSP (Fig. 2A–C). Intriguingly, tau pathology in the pontocerebellar region was strikingly severe compared with typical PSP. The pontine base had many pretangles, threads and glial lesions, with particularly notable threads and many coiled bodies in longitudinal fiber tracts (Fig. 2D–F). The cerebellar white matter had numerous coiled bodies and threads (Fig. 2G–I). Some pretangles and tau-positive glial cells were observed in the Purkinje cell layer (Fig. 2J, K). The distribution and severity of each tau lesion are summarized in Table. These tau pathologies were labeled by RD4, but not RD3, indicating 4-repeat tau aggregates (Supplementary DataFig. S1).
FIGURE 2.
Representative images of immunohistochemistry for CP13. The coiled bodies (A), tufted astrocytes (A, B), and pretangles (C) are observed in the globus pallidus (A), thalamus (B), and substantia nigra (C). Numerous threads and coiled bodies are seen in the longitudinal fibers (D), and less severe in the pontocerebellar fibers (E). Pontine nucleus shows many pretangles (F). Numerous threads and coiled bodies are seen in the cerebellar white matter (G–I). Some pretangles and tau-positive glial cells are seen in the Purkinje cell layer (J, K). Scale bars: (A–F, H–K) 50 μm and (G) 1 mm. (L)DMPK triplet spanning PCR of the present case (shown as Case) and wildtype (shown as CTRL) visualized on 2% agarose gel and by FAM fluorescent fragment analysis. (M) The expanded allele is estimated to be >60 repeats. (N) Immunoblotting shows PSP- prominent tau-immunoreactive bands at ∼64 and 68 kDa (*) in the present case (shown in Case) as well as control PSP cases (shown in PSP1, PSP2, and PSP3).
TABLE.
The Distribution and Severity of Tau Pathology
| Region | NFT & Pre-NFT | Coiled Bodies | Tufted Astrocytes | Threads |
|---|---|---|---|---|
| Superior frontal gyrus | +++ | +++ | +++ | +++ |
| Motor cortex | ++ | +++ | +++ | ++ |
| Caudate/putamen | ++ | +++ | +++ | ++ |
| Globus pallidus | +++ | +++ | ++ | +++ |
| Basal nucleus | +++ | ++ | + | +++ |
| Hypothalamus | +++ | + | − | ++ |
| Ventral thalamus | +++ | +++ | +++ | +++ |
| Subthalamic nucleus | ++ | +++ | ++ | +++ |
| Thalamic fasciculus | − | +++ | − | +++ |
| Red nucleus | ++ | +++ | +++ | +++ |
| Substantia nigra | ++ | +++ | ++ | +++ |
| Midbrain tectum | +++ | ++ | ++ | +++ |
| Locus coeruleus | +++ | − | − | +++ |
| Pontine tegmentum | +++ | +++ | − | +++ |
| Pontine base | +++ | ++ | − | +++ |
| Dentate nucleus | ++ | + | + | + |
| Cerebellar white matter | − | +++ | − | +++ |
The severity of each tau lesion type is shown in 4-point scale: −, none; +, mild; ++, moderate; and +++, severe.
The severe atrophy in the pontocerebellar structures suggested the possibility of MSA, especially olivopontocerebellar atrophy (OPCA), but immunohistochemistry for α-synuclein did not reveal neuronal or glial cytoplasmic inclusions in the basal ganglia or pons (data not shown), excluding a diagnosis of MSA. Since corticobasal degeneration, another primary 4-repeat tauopathy, rarely presents with OPCA and TDP-43 pathology in these regions (14), we also immunostained phospho-TDP-43. Scattered neuronal cytoplasmic inclusions were observed in the midbrain and amygdala, but not in the pons and cerebellum. Thioflavin S microscopy revealed only a few NFTs in the entorhinal cortex and hippocampus CA1, and no senile plaques were found in the neocortex. Immunohistochemistry for 3-repeat tau (RD3) also demonstrated a few NFTs in the entorhinal cortex as well as hippocampal CA2 and CA1, but not in the dentate gyrus or inferior temporal gyrus (Supplementary DataFig. S1). These findings indicate Braak NFT stage III and Thal amyloid phase 0.
To confirm whether the patient had CTG trinucleotide repeat expansion, we performed the fluorescent-based fragment analysis using postmortem brain tissue. The expanded allele was estimated to be >60 repeats, which was consistent with the result of antemortem genetic analysis (Fig. 2L, M). The MAPT haplotype of the patient was H1/H2, and APOE genotype was E2/E3.
Finally, to confirm the pathological diagnosis of PSP, we performed immunoblot analysis using sarkosyl-insoluble fractions from the midfrontal cortex. The sample from the patient as well as control PSP patients showed PSP- prominent tau-immunoreactive bands at approximately 64 and 68 kDa in all samples, consistent with the pattern expected in PSP (Fig. 2N).
DISCUSSION
We report a PSP patient with severe rubro-pontocerebellar pathology with severe pontocerebellar gliosis and tau pathology who also had genetically confirmed DM1. The macroscopic features of this patient resemble MSA, but immunohistochemistry for α-synuclein failed to show neuronal and glial cytoplasmic inclusions. Instead, severe neuronal and glial tau pathology was detected in cardinal nuclei, such as the subthalamic nucleus and globus pallidus, confirming the neuropathologic diagnosis of PSP. On the other hand, antemortem and postmortem genetic analysis consistent with DM1, with 66 CTG repeats in DMPK, a repeat length that can be associated with a mild clinical phenotype. He had blurred vision suggestive of cataracts, percussion myotonia, and cognitive impairment.
The most striking pathologic feature of this patient is rubro-pontocerebellar atrophy with astrogliosis and tau pathology. In addition to the cerebellar dentate nucleus and hilus, which are typically affected in PSP, he also had numerous glial tau inclusions and severe astrogliosis in the cerebellar white matter. This unusual distribution of tau pathology is similar to that in PSP-C (3, 15, 16). Kanazawa et al reported 3 PSP-C patients who had a severe neuronal loss with gliosis in the dentate nucleus and pretangles in the Purkinje cell layers (15). Subsequently, Iwasaki et al described a patient with PSP with olivopontocerebellar involvement, who also had severe neuronal loss with gliosis in the dentate nucleus, numerous coiled bodies in the cerebellar white matter, pretangles and tufted astrocytelike glial cells in the Purkinje cell layer, numerous neuronal and glial tau aggregates in the pontine nucleus, and inferior olivary hypertrophy (16). Our previous study reported 5 Caucasian PSP-C patients who were selected by their clinical presentations. Given the fact that there was no pathological difference other than Purkinje cell loss compared to PSP without cerebellar ataxia, we concluded that there might be a genetic or ethnic difference in cerebellar pathology in PSP (3). The present study, however, demonstrated that a subset of PSP patients in the Caucasian population may also develop severe neurodegeneration and tau pathology in the pontocerebellar structures.
Patients with PSP-C have usually been misdiagnosed as the MSA cerebellar type (3, 4, 17). In fact, the present patient was initially diagnosed with MSA. The lack of autonomic failure and the absence of the “hot cross bun” sign on MRI also met the proposed clinical criteria for PSP-C (4). Hereditary cerebellar ataxias were adequately excluded. The presence of palatal tremor is consistent with the findings of Shimohata et al who reported that 2 of 10 PSP-C patients developed palatal tremor (4). A clinicoradiologic study reported that ataxia was the most frequent clinical finding (82%) among clinically symptomatic patients with bilateral hypertrophic olivary degeneration, followed by palatal tremor (50%) (18). These studies indicate that palatal tremor could be a characteristic feature of PSP-C.
In the present case, comorbid DM1 necessitated exclusion of clinicopathological features consistent with DM1 rather than PSP-C. DM1 is associated with NFTs in limbic structures, even in the relatively young individuals (i.e. in their 30s–50s), while senile plaques are usually not observed (9, 10). The NFTs in DM1 are composed of 3-repeat and 4-repeat tau and immunostained by both RD3 and RD4. In this study, RD3 immunohistochemistry did not reveal 3-repeat pathology in the cerebellum indicating that the severe cerebellar glial tau pathology was that of PSP, rather than DM1. Itoh et al also described that no apparent neuronal loss in the pontine base, inferior olivary nucleus, Purkinje cells, and granular cells in the cerebellum in any of 11 patients with DM1 (9), indicating pontocerebellar atrophy in our present patient was unlikely caused by DM1.
The limitation of this study is that the medulla was unavailable to be assessed at autopsy. Although the pontocerebellar atrophy with astrogliosis is similar to those in MSA, we could not assess the medulla histologically. A brain MRI, however, revealed bilateral hypertrophic olivary degeneration.
In conclusion, we report clinical, pathologic, biochemical and genetic studies of a patient with both PSP and DM1. Based on the severe pontocerebellar tau pathology with astrogliosis and his clinical presentations, this patient is considered to have a rare variant of PSP, that is, PSP-C. PSP-C is not included in the latest clinical criteria for PSP; however, this case indicates that a subset of PSP can present with cerebellar ataxia associated with severe tau pathology in the pons and cerebellum. Further studies on cerebellar pathology in PSP are needed to expand our understanding of this unusual subtype of PSP.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the patients and their families who donated brains to help further the scientific understanding of neurodegeneration. The authors would also like to acknowledge Linda Rousseau, Virginia Phillips, and Ariston L. Librero for histologic support, and Monica Castanedes-Casey (Mayo Clinic, Jacksonville) for immunohistochemistry support.
This work is supported by NIH grants P50 NS072187 and U54-NS100693 to Dennis Dickson. Shunsuke Koga receives support from CBD Solutions Research grant and Betty F. Dyer Foundation Fellowship in progressive supranuclear palsy research.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at academic.oup.com/jnen.
REFERENCES
- 1. Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 1994;44:2015–9 [DOI] [PubMed] [Google Scholar]
- 2. Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The Movement Disorder Society criteria. Mov Disord 2017;32:853–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koga S, Josephs KA, Ogaki K, et al. Cerebellar ataxia in progressive supranuclear palsy: An autopsy study of PSP-C. Mov Disord 2016;31:653–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shimohata T, Kanazawa M, Yoshida M, et al. Clinical and imaging findings of progressive supranuclear palsy with predominant cerebellar ataxia. Mov Disord 2016;31:760–2 [DOI] [PubMed] [Google Scholar]
- 5. Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992;69:385. [DOI] [PubMed] [Google Scholar]
- 6. Hunter A, Tsilfidis C, Mettler G, et al. The correlation of age of onset with CTG trinucleotide repeat amplification in myotonic dystrophy. J Med Genet 1992;29:774–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Machuca-Tzili L, Brook D, Hilton-Jones D.. Clinical and molecular aspects of the myotonic dystrophies: A review. Muscle Nerve 2005;32:1–18 [DOI] [PubMed] [Google Scholar]
- 8. Minnerop M, Weber B, Schoene-Bake JC, et al. The brain in myotonic dystrophy 1 and 2: Evidence for a predominant white matter disease. Brain 2011;134:3530–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Itoh K, Mitani M, Kawamoto K, et al. Neuropathology does not correlate with regional differences in the extent of expansion of CTG repeats in the brain with myotonic dystrophy type 1. Acta Histochem Cytochem 2010;43:149–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiuchi A, Otsuka N, Namba Y, et al. Presenile appearance of abundant Alzheimer’s neurofibrillary tangles without senile plaques in the brain in myotonic dystrophy. Acta Neuropathol 1991;82:1–5 [DOI] [PubMed] [Google Scholar]
- 11. Caillet-Boudin ML, Fernandez-Gomez FJ, Tran H, et al. Brain pathology in myotonic dystrophy: When tauopathy meets spliceopathy and RNAopathy. Front Mol Neurosci 2014;6:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koga S, Sanchez-Contreras M, Josephs KA, et al. Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 2017;32:246–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono S, Takahashi K, Kanda F, et al. Immunohistochemical study of intracytoplasmic inclusion bodies of the thalamus in myotonic dystrophy. J Neurol Sci 1996;140:96–100 [DOI] [PubMed] [Google Scholar]
- 14. Kouri N, Oshima K, Takahashi M, et al. Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: An unusual clinicopathologic variant of CBD. Acta Neuropathol 2013;125:741–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanazawa M, Shimohata T, Toyoshima Y, et al. Cerebellar involvement in progressive supranuclear palsy: A clinicopathological study. Mov Disord 2009;24:1312–8 [DOI] [PubMed] [Google Scholar]
- 16. Iwasaki Y, Mori K, Ito M, et al. An autopsied case of progressive supranuclear palsy presenting with cerebellar ataxia and severe cerebellar involvement. Neuropathology 2013;33:561–7 [DOI] [PubMed] [Google Scholar]
- 17. Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology 2015;85:404–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konno T, Broderick DF, Tacik P, et al. Hypertrophic olivary degeneration: a clinico-radiologic study. Parkinsonism Relat Disord 2016;28:36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.