Abstract
Definitive radiotherapy for cervical cancer consists of external-beam radiotherapy (EBRT) and brachytherapy. In EBRT, a central shield (CS) reduces the dose to the rectum and bladder. The combination of whole-pelvic (WP)- and CS-EBRT and brachytherapy is the standard radiotherapy protocol in Japan. Despite clinical studies, including multi-institutional clinical trials, showing that the Japanese treatment protocol yields favorable treatment outcomes with low rates of late radiation toxicities, dose–volume parameters for the Japanese treatment protocol remain to be established. We conducted a retrospective dose–volume analysis of 103 patients with uterine cervical cancer treated with the Japanese protocol using computed tomography–based adaptive brachytherapy. The 2-year overall survival and 2-year local control rates according to FIGO stage were 100% and 100% for Stage I, 92% and 94% for Stage II, and 85% and 87% for Stage III–IV, respectively. Late adverse effects in the rectum and bladder were acceptable. Receiver operating characteristic analysis discriminated recurrence within the high-risk clinical target volume (HR-CTV) (n = 5) from no local recurrence (n = 96), with the optimal response obtained at a dose of 36.0 GyEQD2 for HR-CTV D90 and 28.0 GyEQD2 for HR-CTV D98. These values were used as cut-offs in Fisher exact tests to show that high HR-CTV D90 and HR-CTV D98 doses for brachytherapy sessions were significantly associated with tumor control within the HR-CTV. These data suggest a contribution of brachytherapy to local tumor control in WP- and CS-EBRT and brachytherapy combination treatment, warranting validation in multi-institutional prospective studies.
Keywords: uterine cervical cancer; radiotherapy, 3D image-guided brachytherapy; dose–volume parameter
INTRODUCTION
The standard radiotherapy (RT) protocol for uterine cervical cancer is the combination of external-beam radiotherapy (EBRT) to the pelvis and brachytherapy (BT) [1]. BT is a critical component in the treatment of cervical cancer [2]. Three-dimensional image-guided BT (3D-IGBT) using computed tomography (CT) or magnetic resonance imaging (MRI) enables delivery of sufficiently high doses to the cervical tumor while minimizing the doses to organs at risk (OARs), such as the rectum, sigmoid colon, and bladder, contributing to favorable local tumor control with reduced adverse events [3–9]. The use of 3D-IGBT in the treatment of cervical cancer is increasing worldwide [10–13].
The Group Européen de Curiethérapie–European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) working group for gynecologic BT provides recommendations on 3D-IGBT for cervical cancer. The recommendations cover the concepts, terms and implications of 3D-IGBT, including definitions of the high-risk (HR) and intermediate-risk (IR) clinical target volumes (CTVs) of BT, 3D image-based treatment planning, dose–volume parameters of the CTVs and OARs, and recording and reporting of dose–volume parameters, among others [14, 15]. Several investigators have demonstrated a positive relationship between the dose–volume parameters of HR-CTV, such as dose covering 90% and 98% of the HR-CTV (D90 and D98) according to the GEC-ESTRO recommendations, and the probability of tumor control [16–18]. Studies suggest that the dose–volume parameters of the HR-CTV are important factors in local tumor control.
In EBRT for cervical cancer, a central shield (CS) is used to reduce the doses to the rectum and bladder, because these organs may be irradiated with very high doses of BT, potentially causing severe late radiation toxicities. The combination of whole-pelvic (WP)- and CS-EBRT and BT was established in Japan more than four decades ago as the standard RT protocol [19, 20]. In the USA or Europe, CS-EBRT is rarely used, whereas 45–50 Gy of WP-EBRT and 4–5 sessions of BT are commonly used for locally advanced cervical cancer. By contrast, the combination of 30–40 Gy of WP-EBRT, 20–10 Gy of CS-EBRT and 3–4 sessions of BT is commonly used in Japan. Many clinical studies, including several multi-institutional clinical trials, have demonstrated that the Japanese treatment protocol yields favorable treatment outcomes with low rates of late radiation toxicities [19–24]. The GEC-ESTRO working group have recommended that the accumulated dose–volume parameters for HR-CTV D90 and D98 should be estimated by simply adding the biologically equivalent dose of the WP-EBRT to that of every BT session, whereas the dose of the CS-EBRT should be excluded from the estimate [15]. Therefore, the estimated dose–volume parameters in the Japanese protocol are considered to be significantly lower than the actual irradiated dose [25]. Hence, how to accurately estimate the dose–volume parameters of HR-CTV when using CS-EBRT remains controversial [26, 27].
Here, we conducted a retrospective study of patients with cervical cancer treated with definitive RT or concurrent chemoradiotherapy (CCRT) using CT-based 3D-IGBT to determine the effective dose–volume parameters of the HR-CTV and the optimum dose for local tumor control in patients treated with WP- and CS-EBRT and BT.
MATERIALS AND METHODS
Patient selection
Cervical cancer patients who were treated with RT or CCRT at Saitama Medical University International Medical Center between June 2013 and October 2015 were enrolled in the study according to the following criteria: (i) histologically confirmed previously untreated carcinoma of the uterine cervix; (ii) 20–90 years of age; (iii) thoroughly evaluated by CT, MRI and a gynecological examination at pre-treatment time points; (iv) International Federation of Gynecology and Obstetrics (FIGO) Stage IB1–IVA; (v) treated with definitive RT or CCRT using CT-based 3D-IGBT; and (vi) available dose–volume histogram (DVH) data.
Patients who had para-aortic lymph node metastasis at the start of treatment were excluded from the study.
The present study complied with the standards of the Declaration of Helsinki. The study was approved by the institutional review board of Saitama Medical University International Medical Center (Registration number: 17–163).
Radiotherapy
Radiotherapy consisted of EBRT and BT. EBRT was delivered to the pelvis with 10 MV X-rays. The CTV for the EBRT included the primary tumor, whole uterus, bilateral parametria, at least the upper half of the vagina, and pelvic lymph nodes (common-, internal-, and external iliac, obturator, and presacral lymph nodes). Three-dimensional conformal RT with anterior–posterior and two lateral portals was used for the treatment. EBRT was administered at a dose of 1.8–2.0 Gy/fraction and 5 fractions/week. After the delivery of 20 Gy (for Stage IB1 or IIA1) or 30–40 Gy (for more advanced stages of disease) of the WP, a 3 cm-wide CS was inserted into the pelvic field, and an additional 30–10 Gy was delivered to the pelvic sidewalls using anterior–posterior parallel-opposed portals, resulting in a total dose of 50 Gy. When a patient had bulky lymph node(s), an additional 6–10 Gy was delivered to the lesion(s) to boost the dose to 56–60 Gy.
BT was initiated after WP-EBRT and was performed weekly in 3–4 sessions in total. A high-dose rate 192Ir source was used for the treatment. Intracavitary (IC) BT or the combination of IC and interstitial (IS) BT was performed to adapt to the tumor volume. Use of the IS technique was considered for the patients with a large tumor and/or asymmetric lateral extension. CT-based 3D-IGBT was performed in every BT session. The HR-CTV and OARs (including the rectum, sigmoid colon, bladder, and small bowel) were delineated on CT images, with reference to MRI images acquired at diagnosis and just before the first session of BT and the findings of the gynecological examination by both gynecological and radiation oncologists. All radiation doses were biologically converted to equivalent doses in 2 Gy (EQD2) by the linear quadratic model using an alpha/beta ratio of 10 Gy for HR-CTV and 3 Gy for OARs. Regarding the dose–volume parameters, the D90 and D98 of the HR-CTV and the D2cm3 of the rectum, sigmoid colon, and bladder were calculated, recorded, and reported at every BT session. BT was planned for delivering the doses according to the following criteria: (i) planning aim dose to the HR-CTV D90 ≥6 Gy at each BT session, and the dose constraints to the OARs of the whole RT; (ii) D2cm3 of the rectum and sigmoid colon ≤70 GyEQD2 and D2cm3 of the bladder ≤ 90 GyEQD2; (iii) when the dose constraints of the HR-CTV and OARs were not achieved simultaneously, the constraints of the OARs were prioritized.
Radiotherapy was withheld if patients developed Grade 4 hematological toxicities or Grade 3–4 non-hematological toxicities as assessed by Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0 [28]. Radiotherapy was resumed when the toxicities recovered to Grade 2.
Chemotherapy
Patients with T1b1N0 or T2a1N0 disease were treated with RT alone. Patients received weekly administration of cisplatin concurrently with EBRT if treatment was considered feasible based on their general conditions and organ functions. Patients who were considered unable to tolerate cisplatin because of old age (basically 75 years or older), and/or insufficient organ function received monthly administration of nedaplatin. Chemotherapy was withheld in patients showing a white blood cell count of <3000/mm3, platelet count <75 000/mm3, fever >38°C, or Grade 3–4 non-hematological toxicities. Chemotherapy was resumed when the toxicities recovered to Grade 1.
Follow-up
Patients were followed up every 1–3 months for the first 2 years and every 3–6 months from the third year after completion of the treatment. Tumor status and adverse events were assessed using patient interviews, physical and gynecological exams, and blood tests. Patients underwent CT and/or MRI at 1 month after treatment to evaluate the therapeutic effects, and every 6–12 months thereafter. Recurrent disease was confirmed by biopsy whenever possible.
Treatment failure was classified into pelvic and extrapelvic failures. Pelvic failure was subclassified into local recurrence and regional lymph node recurrence. Local control (LC) duration was defined as the period from the date of initiation of RT to the date of diagnosis of local recurrence or the date of the most recent follow-up. Overall survival (OS) duration was defined as the period from the date of RT initiation to the date of death from any cause or the date of the most recent follow-up.
Late adverse events were defined as adverse events emerging after the 91st day from completion of RT and were graded according to the CTCAE version 4.0.
Statistics
Statistical analyses were performed using SPSS v. 25.0 (IBM, Illinois, USA). OS and LC rates were calculated by the Kaplan–Meier method and compared by the log-rank test. Significant prognostic factors for local tumor control were identified by univariate analysis of the correlation of LC with various clinicopathological factors (age, T factor, initial tumor size, histology, and the use of chemotherapy) and dose–volume parameters of RT (dose of WP-EBRT and the cumulative HR-CTV D90 and D98 of all BT sessions and those of WP-EBRT + BT) using Fisher’s exact test. The cut-off values for dose–volume parameters related to LC were determined by receiver operating characteristic (ROC) analysis. P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics and treatment
The present study enrolled 103 patients with uterine cervical cancer who met the eligibility criteria. The characteristics of the patients and treatment are summarized in Table 1. Regarding EBRT, a total dose of 39.6–55.0 Gy (median, 50.0 Gy) was delivered. A CS was inserted into the pelvic field after delivery of 19.8–45.0 Gy (median, 30.0 Gy). Regarding BT, a total of 3–5 (median, 4) sessions were performed. Nine patients received combination treatment with IC- and IS-BT. The median HR-CTV D90 and D98 of all BT sessions were estimated at 40.5 GyEQD2 and 31.4 GyEQD2, respectively. The median HR-CTV D90 and D98 of the whole RT, which were calculated by adding the doses of WP-EBRT and those of all BT sessions, were estimated at 74.2 GyEQD2 and 65.1 GyEQD2, respectively.
Table 1.
Patient and treatment characteristics
| Characteristics (103 patients) | |
|---|---|
| Age (n) | Median 64 years old (range: 29–85) |
| FIGO stage (n) | |
| IB1 | 7 |
| IB2 | 4 |
| IIB | 50 |
| IIIA | 3 |
| IIIB | 33 |
| IVA | 6 |
| Lymph node metastasis (n) | |
| Positive | 39 |
| Negative | 64 |
| Histologic subtype (n) | |
| SCC | 89 |
| AD | 10 |
| AdSq | 3 |
| SM | 1 |
| Tumor size (n) | |
| <4 cm | 23 |
| 4–6 cm | 50 |
| >6 cm | 30 |
| EBRT | |
| Total dose | Median 50 Gy (range: 39.6–55) |
| WP-EBRT | Median 30 Gy (range: 19.8–45) |
| CS-EBRT | Median 20 Gy (range: 5.4–30) |
| BT | |
| IC-BT (n) | 94 |
| IC+IS-BT (n) | 9 |
| HR-CTV D90 | Median 40.5 GyEQD2 (range: 25.9–55.9) |
| HR-CTV D98 | Median 31.4 GyEQD2 (range: 18.3–45.4) |
| WP-EBRT + BT | |
| HR-CTV D90 | Median 74.2 GyEQD2 (range: 56.4–90.9) |
| HR-CTV D98 | Median 65.1 GyEQD2 (range: 46.8–81.0) |
| Chemotherapy (n) | 76 |
| CDDP | 66 |
| NDP | 10 |
SCC = squamous cell carcinoma, AD = adenocarcinoma, AdSq = adenosquamous cell carcinoma, SM = small-cell carcinoma, EBRT = external beam radiotherapy, WP = whole-pelvic, CS = central shield, BT = brachytherapy, IC-BT = intracavitary brachytherapy, IC+IS-BT = intracavitary and interstitial brachytherapy, CDDP = cisplatin, NDP = nedaplatin.
Seventy-six patients received chemotherapy, of which 66 received weekly cisplatin administration (40 mg/m2 per cycle); 70% (46/66) of the patients received ≥5 cycles (median, 5 cycles; range, 2–6 cycles). Ten patients received monthly nedaplatin administration (80 mg/m2 per cycle; range, 1–2 cycles).
Outcomes
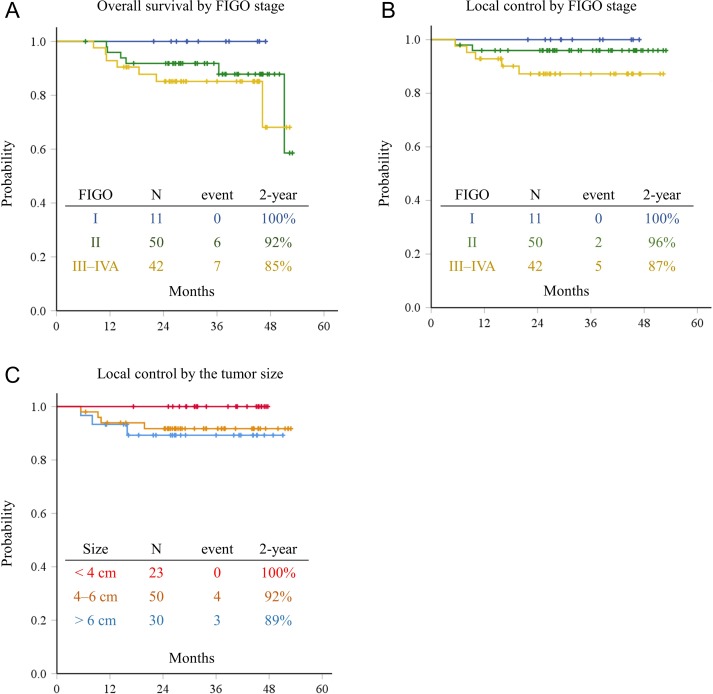
The median follow-up duration was 31.8 months (range, 6.5–52.9 months). The 2-year OS rate according to stage was 100% in Stage I, 92% in Stage II, and 85% in Stages III–IVA patients, respectively (Fig. 1A). There was no significant difference in the 2-year OS according to stage (P = 0.346). The 2-year LC rate according to stage was 100% in Stage I, 96% in Stage II, and 87% in Stages III–IVA patients, respectively, and no significant difference was observed between the groups (P = 0.206) (Fig. 1B). After stratification by tumor size at the time of diagnosis, the 2-year LC rates in patients with tumors <4 cm, 4–6 cm and >6 cm were 100%, 92% and 89%, respectively, and no significant difference was observed between the tumor size groups (P = 0.307) (Fig. 1C).
Figure 1.
Overall survival rates according to FIGO stage (A), local control rates according to FIGO stage (B), and local control rates according to tumor size (C) in 103 eligible patients.
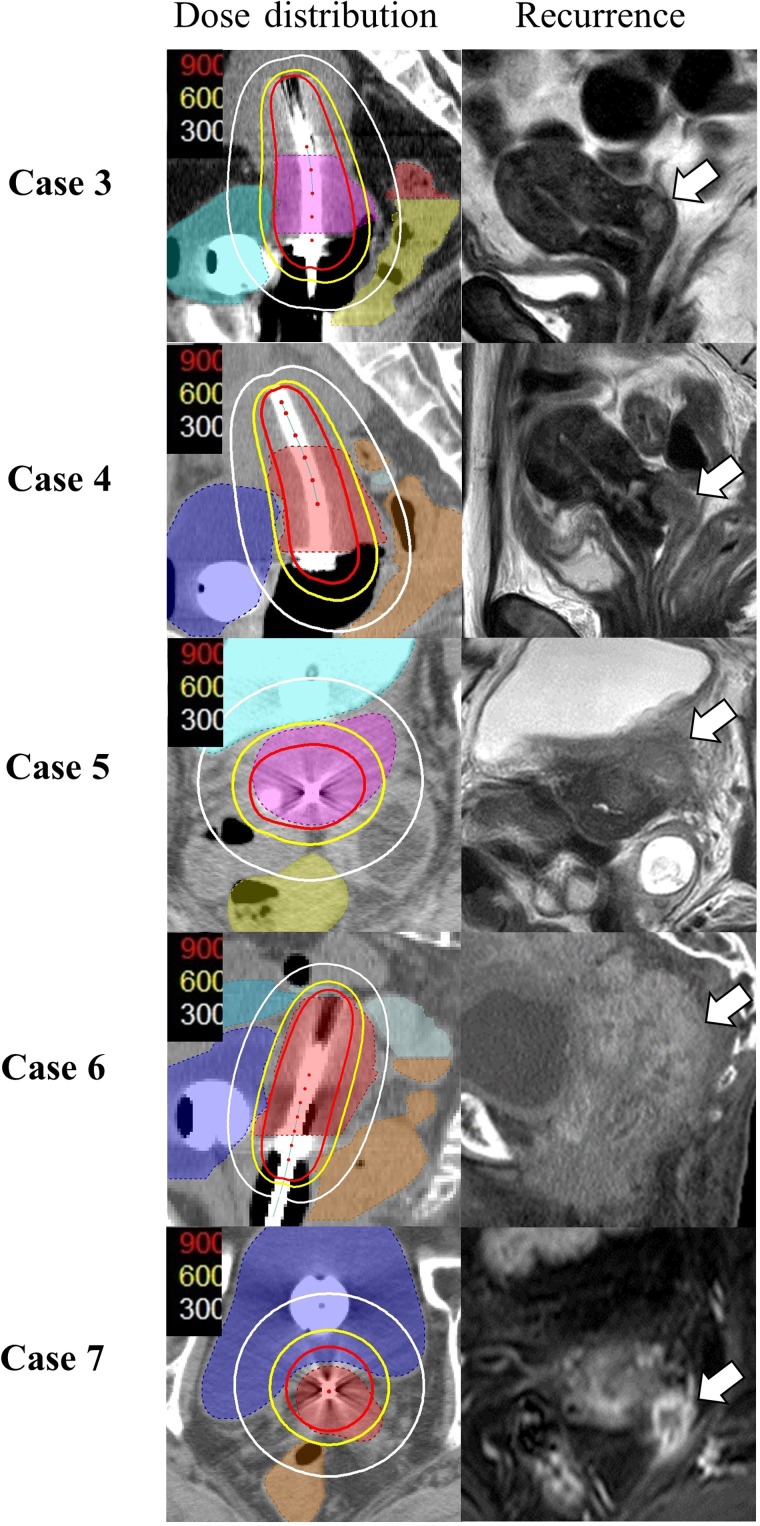
Seven patients developed local recurrence (Table 2). Two of the seven patients (Cases 1 and 2) developed recurrence in the lower vagina or vaginal orifice at 5.4 or 15.9 months after treatment. At the beginning of the treatment, no tumor infiltration in these regions was observed in both MRI and gynecological exams. These regions were included in the field of WP-EBRT and were irradiated with 30 Gy and 40 Gy, respectively. However, they were not included in the HR-CTVs for BT and were not irradiated with high-dose BT. Five patients developed recurrence within the extent of the primary tumor at 5.4–19.8 months after treatment. All tumors were included in the HR-CTVs for BT, and cumulative doses of 31.1–41.7 GyEQD2 were delivered to 90% of the HR-CTVs by BT (Fig. 2).
Table 2.
List of seven patients who developed local recurrence
| Patient no. | Age | FIGO stage | Tumor size (mm) | Histology | EBRT WP/CS | BT | Recurrence interval (months) | Recurrence site | |
|---|---|---|---|---|---|---|---|---|---|
| HR-CTV D90 (GyEQD2) | HR-CTV D98 (GyEQD2) | ||||||||
| 1 | 34 | IIB | 52 | SCC | 30 Gy/20 Gy | 42.4 | 34.3 | 5.4 | Lower vagina |
| 2 | 77 | IIIA | 80 | SCC | 40 Gy/10 Gy | 39.7 | 30.9 | 15.9 | Vaginal orifice |
| 3 | 45 | IIB | 47 | SM | 30 Gy/20 Gy | 41.7 | 32.5 | 9.3 | Cervix |
| 4 | 37 | IIIB | 68 | SCC | 30 Gy/20 Gy | 32.7 | 27.4 | 8.0 | Cervix |
| 5 | 50 | IIIB | 103 | SCC | 40 Gy/10 Gy | 31.1 | 22.6 | 5.4 | Left parametrium |
| 6 | 73 | IIIB | 45 | AD | 40 Gy/10 Gy | 31.9 | 23.5 | 10.0 | Corpus |
| 7 | 74 | IIIB | 44 | SCC | 40 Gy/10 Gy | 35.1 | 24.4 | 19.8 | Left parametrium |
SCC = squamous cell carcinoma, SM = small cell carcinoma, AD = adenocarcinoma, EBRT = external beam radiotherapy, WP = whole-pelvic, CS = central shield, BT = brachytherapy.
Figure 2.
Dose distributions at brachytherapy (BT) and patterns of local recurrence within the primary tumor. HR-CTV of BT sessions only are shown. The white, yellow and red lines represent the 3-, 6- and 9-Gy isodose lines, respectively. White arrows show the sites of local recurrence. Note that the regions of local recurrence were outside the 6-Gy isodose lines in all cases.
Late adverse events in the lower gastrointestinal and genitourinary tracts were observed in 30 and 12 patients, respectively, and most were classified as Grade 1 or 2. Two patients developed a Grade 4 rectovaginal fistula, of which one had Stage IVA disease with rectal invasion. Two patients developed a Grade 4 vesicovaginal fistula, of which one had Stage IVA bladder invasion.
Correlation of clinicopathological factors and DVH parameters with local control
The correlation of LC with various clinicopathological factors and dose–volume parameters was analyzed. The two patients who developed recurrence outside the CTV (Cases 1 and 2) were excluded from the analyses. The factors, including age, T factor, initial tumor size, histology, dose of WP-EBRT, and the use of chemotherapy, were not significantly correlated with LC in univariate analysis using Fisher’s exact test (Table 3).
Table 3.
Univariate analyses of local recurrence using Fisher’s exact test: recurrence within the HR-CTV (n = 5) vs no local recurrence (n = 96)
| Factors | Local recurrence within the HR-CTV (n) | Total (n) | P-values | ||
|---|---|---|---|---|---|
| Presence | Absence | ||||
| Age | <50 | 2 | 23 | 25 | P = 0.595 |
| ≥50 | 3 | 73 | 76 | ||
| Tumor size | <60 mm | 3 | 69 | 72 | P = 0.623 |
| ≥60 mm | 2 | 27 | 29 | ||
| T factor | T1–2 | 1 | 59 | 60 | P = 0.155 |
| T3–4 | 4 | 37 | 41 | ||
| Histology | Non SCC | 2 | 12 | 14 | P = 0.140 |
| SCC | 3 | 84 | 87 | ||
| Use of chemotherapy | Presence | 5 | 69 | 74 | P = 0.321 |
| Absence | 0 | 27 | 27 | ||
| WP-EBRT | <35 GyEQD2 | 2 | 56 | 58 | P = 0.648 |
| ≥35 GyEQD2 | 3 | 40 | 43 | ||
| HR-CTV D90WP-EBRT + BT | <72 GyEQD2 | 4 | 41 | 45 | P = 0.169 |
| ≥72 GyEQD2 | 1 | 55 | 56 | ||
| HT-CTV D98WP-EBRT + BT | <63.5 GyEQD2 | 4 | 39 | 43 | P = 0.160 |
| ≥63.5 GyEQD2 | 1 | 57 | 58 | ||
| HR-CTV D90 at BT | <36 GyEQD2 | 4 | 10 | 14 | P = 0.001 |
| ≥36 GyEQD2 | 1 | 86 | 87 | ||
| HR-CTV D98 at BT | <28 GyEQD2 | 4 | 12 | 16 | P = 0.002 |
| ≥28 GyEQD2 | 1 | 84 | 85 | ||
SCC = squamous cell carcinoma, WP = whole-pelvic, EBRT = external beam radiotherapy, BT = brachytherapy.
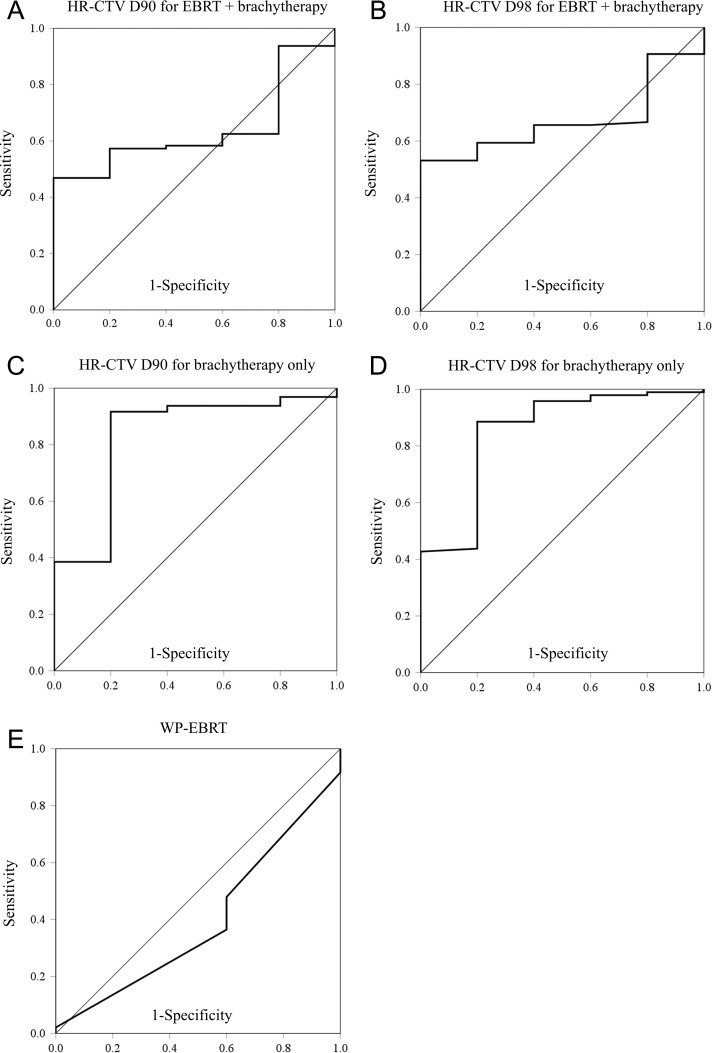
Regarding dose–volume parameters, we first analyzed the correlation between HR-CTV D90/D98 of the whole WP-EBRT + BT and LC. ROC analyses indicated that the closest points to the ideal coordinates for the HR-CTV D90 and D98 were 72.0 GyEQD2 and 63.5 GyEQD2, respectively (Fig. 3A and B). Fisher’s exact tests using these cut-off values showed that the HR-CTV D90/D98 of WP-EBRT + BT were not significantly correlated with LC (P > 0.05) (Table 3). Next, the correlation of the HR-CTV D90/D98 of BT only and LC was analyzed to assess the contribution of BT to LC. ROC analyses showed that the cut-off values for the HR-CTV D90 and D98 at BT were 36.0 GyEQD2 and 28.0 GyEQD2, respectively (Fig. 3C and D). Fisher’s exact tests using these cut-off values showed that the HR-CTV D90/D98 at BT only were significantly correlated with LC (P < 0.05) (Table 3).
Figure 3.
Receiver operator characteristic curve related to local control (LC) based on the sensitivity and specificity of the HR-CTV D90 for external beam radiotherapy (EBRT) and brachytherapy combined (A), HR-CTV D98 for EBRT and brachytherapy combined (B), HR-CTV D90 for brachytherapy sessions only (C), and HR-CTV D98 for brachytherapy sessions only (D).
DISCUSSION
In the present study, cervical cancer patients were treated with RT or CCRT using CT-based 3D-IGBT. Regarding EBRT, most patients were treated with a combination of 30–40 Gy of WP-EBRT and 20–10 Gy of CS-EBRT. The 2-year OS and LC rates in the present series were favorable, and mostly consistent with those of other series using MRI- or CT-based 3D-IGBT (Table 4). LC rates according to stage or initial tumor size did not differ significantly, suggesting that cervical cancer can be locally controlled irrespective of the stage or tumor size (Fig. 1B and C). Late adverse effects in the rectum and bladder were acceptable. These results suggested that radiotherapy combined with CS-EBRT and 3D-IGBT using the IC or IC + IS technique is an effective treatment modality to control cervical tumors while minimizing late toxicities.
Table 4.
Reported clinical outcomes of radiotherapy for uterine cervical cancer
| Author | Year | Number of patients | Stage (III/IV) | Imaging modality for 3D planning | Local control | Overall survival | Late toxicity ≥Grade 3 for rectum | Late toxicity ≥Grade 3 for bladder |
|---|---|---|---|---|---|---|---|---|
| Tan [3] | 2005–2007 | 28 | 14%/0% | CT |
|
|
1 patient | 0% |
| Pötter [6] | 2001–2008 | 156 | 24%/4% | MRI |
|
|
4% (5 years) | 3% (5 years) |
| Murakami [32] | 2008–2010 | 51 | 37%/14% | CT |
|
|
1 patient | 0% |
| Ohno [31] | 2008–2011 | 80 | 34%/3% | CT |
|
|
0% | 1% |
| Zolciak-Siwinska [8] | 2010–2011 | 216 | 30%/0.4% | CT |
|
|
4% | 3% |
| Sturdza [7] (retroEMBRACE) |
1998–2012 | 731 | 23%/3% | MRI |
|
|
5% (5 years) | 7% (5 years) |
| Gill [9] | 2007–2013 | 128 | 16%/0% | CT and MRI |
|
|
1 % (GU/GI toxicity) | |
| Current study | 2013–2015 | 103 | 35%/6% | CT |
|
|
2% (2 years) | 2% (2 years) |
GI = gastrointestinal, GU = genitourinary.
To evaluate the treatment of cervical tumors, the GEC-ESTRO working group and ICRU Report 89 provides recommendations regarding several dose–volume parameters, including the HR-CTV D90 and D98 values [14, 15, 29]. There have been several reports on the relationship between dose–volume parameters and local tumor control. Researchers from the Medical University of Vienna demonstrated a positive correlation between HR-CTV D90 and local tumor control [16, 17], and showed that an increased HR-CTV D90 contributed significantly to improving LC in patients with tumors >5 cm in diameter [5, 6]. More recently, a multi-institutional retrospective clinical study demonstrated that HR-CTV D90 was a significant factor for LC of large tumors [7, 18, 30]. These results suggested that dose–volume parameters of HR-CTV were effective predictive factors with respect to local tumor control, and a total HR-CTV D90 dose of >85–87 Gy might be necessary for adequate local tumor control. In these studies, however, patients were treated with a combination of WP-EBRT and BT, and CS-EBRT was not used.
It was recommended that the total dose–volume parameters to the HR-CTV should be estimated by simply adding the biologically equivalent dose of the WP-EBRT and that of every BT session [15]. The CS-EBRT dose is excluded from the estimation. However, phantom studies showed that the contribution of CS-EBRT to the HR-CTV was not negligible, and varied significantly according to tumor size, shape and extent [26, 27]. Furthermore, it is difficult to precisely estimate the contribution of CS-EBRT to the HR-CTV, because the tumor size, shape and position may vary during the treatment course. Therefore, in the present study, we did not estimate dose contribution from CS-EBRT, but estimated the total HR-CTV D90/98 by simply adding those of WP-EBRT and BT using the conventional method. The HR-CTV D90/D98 of WP-EBRT + BT was not significantly correlated with LC in univariate analysis (Table 3). One possible reason for this result is that the cumulative dose of WP-EBRT + BT while omitting the CS-EBRT dose may not represent the dose actually delivered to the HR-CTV when CS-EBRT is used during the treatment course. Another possible reason might be statistical uncertainties due to the small number of local recurrences.
By contrast, the HR-CTV D90 and D98 at BT only were significantly correlated with LC in the present study. Patients who received a cumulative HR-CTV D90 of >36.0 GyEQD2 or a HR-CTV D98 of >28.0 GyEQD2 in the BT sessions had significantly better LC in the univariate analysis (Table 3). The results suggested the importance of BT for local tumor control, and indicated that 90% or 98% of the HR-CTV should be covered with at least 6.5 Gy (~9 GyEQD2) or 5.5 Gy (~7 GyEQD2), respectively, at each BT session. In the five patients who developed local recurrence, the sites of recurrence were outside the 6 Gy isodose line at BT (Fig. 2). Murakami et al. and Ohno et al. reported similar treatment results using the combination of WP- and CS-EBRT and BT. These authors also indicated that a 6 Gy isodose line should cover the HR-CTV to achieve a HR-CTV D90 of >6 Gy at each BT session [31, 32]. Similarly, sufficient coverage with a high dose to HR-CTV was the most significant prognostic factor in the present study (Table 3, Fig. 2), but the dose of the WP-EBRT was not a significant prognostic factor for local tumor control. Tamaki et al. compared the dose distributions of WP- plus CS-EBRT and BT with those of WP-EBRT and BT in their phantom studies, and they also suggested that the low dose volumes in EBRT caused by inserting a CS could be covered with a very high dose of BT, when the BT sources were adequately placed [26, 27]. Therefore, it was suggested from the present study that a HR-CTV D90 of >36.0 GyEQD2 or a HR-CTV D98 of >28 GyEQD2 for all BT sessions might be important factors for local tumor control, when the patients received either 30 or 40 Gy of WP-EBRT.
The optimum method for accurately estimating the dose–volume parameters of the HR-CTV when using CS-EBRT remains to be established. One possible method is to generate composite dose distributions of WP- and CS-EBRT and all BT sessions projected onto one CT series using deformable image registration, and to estimate the cumulative dose–volume parameters for the HR-CTV [33]. However, changes in the uterus and cervix positions and significant regression of the tumor volume may occur during the treatment course, and these positional uncertainties may cause difficulty in generating composite dose distributions. Therefore, further studies are needed to establish the optimum method.
In the present series, two patients developed recurrence in their lower vagina or vaginal orifice. The regions were not included in the respective HR-CTVs at BT because no tumor infiltration was detected in those regions at the beginning of the treatment. Repetitive careful gynecological examinations by both gynecological and radiation oncologists are necessary to check vaginal infiltration [14, 34]. These vaginal recurrences may have reflected vaginal skip metastasis because they were an atypical pattern of recurrence. Positron emission tomography using 18F-fluorodeoxyglucose (FDG) in combination with MRI/CT reportedly improve the detection of metastatic lesions, and prognosis in uterine cancer [35, 36], which may help achieve more accurate pretreatment evaluation of cervical tumors.
The present study had several limitations. First, this was a retrospective single-institution study. Second, the small number of cases of local recurrence limited the statistical reliability. However, it is difficult to design a study including a higher number of recurrent cases because of the high LC probability for RT or CCRT in cervical cancer [31, 32].
In conclusion, the present study demonstrated that the dose to the HR-CTV was significantly correlated with local tumor control in RT for cervical cancer, and suggested that optimum dose distribution to cervical tumors by BT particularly contributed to LC. Further multi-institutional clinical studies are necessary to determine the optimum dose–volume parameters of the HR-CTV to sufficiently control the local tumor when using CS-EBRT.
ACKNOWLEDGEMENTS
We would like to express our deepest appreciation to Takahiro Oike, MD PhD, for editing a draft of this manuscript and providing insightful comments and suggestions.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
FUNDING
This research was supported by the program for Leading Graduate Schools, Cultivating Global Leaders in Heavy Ion Therapeutics and Engineering of the Ministry of Education, Culture, Sports, Science and Technology of Japan granted to Gunma University.
REFERENCES
- 1. Monk BJ, Tewari KS, Koh W-J. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol 2007;25:2952–65. [DOI] [PubMed] [Google Scholar]
- 2. Han K, Milosevic M, Fyles A et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013;87:111–9. [DOI] [PubMed] [Google Scholar]
- 3. Tan LT, Coles CE, Hart C et al. Clinical impact of computed tomography–based image-guided brachytherapy for cervix cancer using the tandem-ring applicator—the Addenbrooke’s experience. Clin Oncol 2009;21:175–82. [DOI] [PubMed] [Google Scholar]
- 4. Pötter R, Dimopoulos J, Bachtiary B et al. 3D conformal HDR-brachy- and external beam therapy plus simultaneous cisplatin for high-risk cervical cancer: clinical experience with 3 year follow-up. Radiother Oncol 2006;79:80–6. [DOI] [PubMed] [Google Scholar]
- 5. Pötter R, Dimopoulos J, Georg P et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol 2007;83:148–55. [DOI] [PubMed] [Google Scholar]
- 6. Pötter R, Georg P, Dimopoulos JCA et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sturdza A, Pötter R, Fokdal LU et al. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016;120:428–33. [DOI] [PubMed] [Google Scholar]
- 8. Zolciak-Siwinska A, Gruszczynska E, Bijok M et al. Computed tomography–planned high-dose-rate brachytherapy for treating uterine cervical cancer. Int J Radiat Oncol Biol Phys 2016;96:87–92. [DOI] [PubMed] [Google Scholar]
- 9. Gill BS, Kim H, Houser CJ et al. MRI-guided high-dose-rate intracavitary brachytherapy for treatment of cervical cancer: the University of Pittsburgh experience. Int J Radiat Oncol Biol Phys 2015;91:540–7. [DOI] [PubMed] [Google Scholar]
- 10. Ohno T, Toita T, Tsujino K et al. A questionnaire-based survey on 3D image-guided brachytherapy for cervical cancer in Japan: advances and obstacles. J Radiat Res 2015;56:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan LT. Implementation of image-guided brachytherapy for cervix cancer in the UK: progress update. Clin Oncol 2011;23:681–4. [DOI] [PubMed] [Google Scholar]
- 12. Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2010;76:104–9. [DOI] [PubMed] [Google Scholar]
- 13. Grover S, Harkenrider MM, Cho LP et al. Image Guided Cervical Brachytherapy: 2014 Survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2016;94:598–604. [DOI] [PubMed] [Google Scholar]
- 14. Haie-Meder C, Pötter R, Van Limbergen E et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol 2005;74:235–45. [DOI] [PubMed] [Google Scholar]
- 15. Pötter R, Haie-Meder C, Van Limbergen E et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 16. Dimopoulos JCA, Lang S, Kirisits C et al. Dose–volume histogram parameters and local tumor control in magnetic resonance image–guided cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys 2009;75:56–63. [DOI] [PubMed] [Google Scholar]
- 17. Schmid MP, Kirisits C, Nesvacil N et al. Local recurrences in cervical cancer patients in the setting of image-guided brachytherapy: a comparison of spatial dose distribution within a matched-pair analysis. Radiother Oncol 2011;100:468–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanderup K, Fokdal LU, Sturdza A et al. Effect of tumor dose, volume and overall treatment time on local control after radiochemotherapy including MRI guided brachytherapy of locally advanced cervical cancer. Radiother Oncol 2016;120:441–6. [DOI] [PubMed] [Google Scholar]
- 19. Arai T, Nakano T, Morita S et al. High‐dose‐rate remote afterloading intracavitary radiation therapy for cancer of the uterine cervix. A 20‐year experience. Cancer 1992;69:175–80. [DOI] [PubMed] [Google Scholar]
- 20. Nakano T, Kato S, Ohno T et al. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer 2005;103:92–101. [DOI] [PubMed] [Google Scholar]
- 21. Toita T, Kitagawa R, Hamano T et al. Phase II study of concurrent chemoradiotherapy with high-dose-rate intracavitary brachytherapy in patients with locally advanced uterine cervical cancer: efficacy and toxicity of a low cumulative radiation dose schedule. Gynecol Oncol 2012;126:211–6. [DOI] [PubMed] [Google Scholar]
- 22. Toita T, Kato S, Niibe Y et al. Prospective multi-institutional study of definitive radiotherapy with high-dose-rate intracavitary brachytherapy in patients with nonbulky (<4-cm) stage I and II uterine cervical cancer (JAROG0401/JROSG04-2). Int J Radiat Oncol Biol Phys 2012;82:e49–56. [DOI] [PubMed] [Google Scholar]
- 23. Kato S, Ohno T, Thephamongkhol K et al. Multi-institutional phase II clinical study of concurrent chemoradiotherapy for locally advanced cervical cancer in East and Southeast Asia. Int J Radiat Oncol Biol Phys 2010;77:751–7. [DOI] [PubMed] [Google Scholar]
- 24. Kato S, Ohno T, Thephamongkhol K et al. Long-term follow-up results of a multi-institutional phase 2 study of concurrent chemoradiation therapy for locally advanced cervical cancer in east and southeast Asia. Int J Radiat Oncol Biol Phys 2013;87:100–5. [DOI] [PubMed] [Google Scholar]
- 25. Viswanathan AN, Creutzberg CL, Craighead P et al. International brachytherapy practice patterns: a survey of the Gynecologic Cancer Intergroup (GCIG). Int J Radiat Oncol Biol Phys 2012;82:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamaki T, Ohno T, Noda SE et al. Filling the gap in central shielding: three-dimensional analysis of the EQD2 dose in radiotherapy for cervical cancer with the central shielding technique. J Radiat Res 2015;56:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamaki T, Noda SE, Ohno T et al. Dose–volume histogram analysis of composite EQD2 dose distributions using the central shielding technique in cervical cancer radiotherapy. Brachytherapy 2016;15:598–606. [DOI] [PubMed] [Google Scholar]
- 28.Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. National Cancer Institute. 2010. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (21 March 2019, date last accessed).
- 29.International Commission on Radiation Units and Measurements (ICRU) Report 89: Prescribing, recording, and reporting brachytherapy for cancer of the cervix. J ICRU 2013;13:1–258. [DOI] [PubMed] [Google Scholar]
- 30. Fokdal L, Sturdza A, Mazeron R et al. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol 2016;120:434–40. [DOI] [PubMed] [Google Scholar]
- 31. Ohno T, Noda SE, Okonogi N et al. In-room computed tomography–based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res 2017;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murakami N, Kasamatsu T, Wakita A et al. CT based three dimensional dose–volume evaluations for high-dose rate intracavitary brachytherapy for cervical cancer. BMC Cancer 2014;14:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abe T, Tamaki T, Makino S et al. Assessing cumulative dose distributions in combined radiotherapy for cervical cancer using deformable image registration with pre-imaging preparations. Radiat Oncol 2014;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colombo N, Carinelli S, Colombo A et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl. 7):vii27–32. 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 35. Han S, Kim H, Kim YJ et al. Prognostic value of volume-based metabolic parameters of 18F-FDG PET/CT in uterine cervical cancer: a systematic review and meta-analysis. Am J Roentgenol 2018;211:1112–21. [DOI] [PubMed] [Google Scholar]
- 36. Nogami Y, Iida M, Banno K et al. Application of FDG-PET in cervical cancer and endometrial cancer: utility and future prospects. Anticancer Res 2014;34:585–92. [PubMed] [Google Scholar]