Abstract
Small cell carcinoma of the bladder is extremely rare, accounting for <1% of all malignant tumours in the urinary tract. Thus, no standard therapy modality for this malignancy has been established. This study aimed to retrospectively analyse the clinical outcomes associated with definitive radiotherapy for small cell carcinoma of the bladder. A questionnaire-based survey of patients with pathologically proven small cell carcinoma of the bladder treated with definitive radiation therapy between 1990 and 2010 was conducted by the Japanese Radiation Oncology Study Group. The clinical records of 12 eligible patients were collected from nine institutions. The median age of the patients was 70.5 years (range: 44–87 years), and the median follow-up period was 27.3 months (range: 3.3–117.8 months). The median prescribed dose was 60 Gy (range: 50.0–61.0 Gy), and a median of 2.0 Gy (range: 1.2–2.0 Gy) was administered per fraction. Systemic chemotherapy combined with radiotherapy was performed in eight cases (66.7%). The 3- and 5-year overall survival rates were 50.0% and 33.3%, respectively. And the 3- and 5-year local control rates were 66.7% and 55.6%, respectively. Chemotherapy significantly improved overall survival and relapse-free survival (P = 0.006 and 0.001, respectively). No serious adverse events occurred in the observation period. All patients who achieved local control maintained functional bladders. In conclusion, radiotherapy is a potential local treatment option and has an important role in maintaining quality of life. Systemic chemotherapy combined with local radiotherapy seems to be effective in improving survival.
Keywords: small cell carcinoma, bladder, extrapulmonary, radiotherapy, bladder-sparing
INTRODUCTION
Extrapulmonary small cell carcinoma can be diagnosed in almost any organ [1], but small cell carcinoma of the bladder is extremely rare, accounting for only 0.5–0.7% of all malignant tumours of the bladder [2–4]. The Japanese Radiation Oncology Study Group (JROSG) conducted a research survey of all bladder cancers treated with radiotherapy from 2002 to 2006 in Japan, and small cell carcinomas of the bladder accounted for <1% of all bladder cancers [5]. Due to its rarity, no multicentre retrospective analysis has focused on small cell carcinoma of the bladder, and no standard treatment for this cancer has been established.
The origin and histogenesis of small cell carcinoma of the bladder remain unclear, but it is thought that this disease originates from the totipotent stem cells in the bladder submucosa [6–8]. Molecular changes associated with small cell carcinoma of the bladder include deletions at 10q, 4q, 5q and 13q [6]. These molecular biological findings are also commonly observed in small cell lung carcinoma [6, 7, 9], suggesting that the clinical courses of these two diseases are similar [3]. Therefore, we hypothesized that, similar to small cell lung carcinoma, small cell carcinoma of the bladder may be highly sensitive to radiotherapy and chemotherapy.
The purpose of this study was to retrospectively analyse the outcomes associated with radiotherapy for small cell carcinoma of the bladder. In a previous study, we retrospectively analysed the data for patients with small cell carcinoma of the bladder who underwent definitive radiotherapy from 2007 to 2012 in our hospital and related hospitals [10]. The bladder was preserved in all cases, indicating the effectiveness of a bladder-sparing approach to radiotherapy for this disease [10]. However, further investigation was deemed necessary owing to the small sample size in that study. Therefore, we collected clinical data from institutions affiliated with the JROSG, which is a non-profit organisation and the only research organisation to have conducted a nationwide survey on radiation therapy in Japan.
MATERIALS AND METHODS
A questionnaire-based survey of patients with pathologically proven small cell carcinoma of the bladder treated with definitive radiation therapy between 1990 and 2010 was conducted. Patients who had received definitive radiotherapy and had been treated using a bladder-sparing approach were eligible for the survey. The primary end point was overall survival. The secondary end points were local control and progression-free survival. The outcomes were assessed from the first day of radiation to the day of an event. Local control failure was defined as recurrence in the irradiation field. Progression-free survival was defined as the period from the first day of radiation to the day on which local recurrence, distant metastasis, or death occurred. In this study, bladder preservation is defined as: patients maintaining their bladder function without serious dysuria and able to urinate by themselves. This study was approved by the Institutional Review Board at the Yamagata University School of Medicine (Approval Number: 329). Informed consent was waived because the participants’ data were de-identified.
Statistical analysis
The cumulative rates of overall survival, local control, and progression-free survival were calculated using the Kaplan–Meier method, and survival curves were created. Tumour response to radiotherapy was evaluated using Response Evaluation Criteria in Solid Tumors ver. 1.1 based on the different imaging modalities available in each hospital. Patients were classified into subgroups according to characteristics of patients, modality of treatment, and prescribed dose, and the survival of the patients in each subgroup was tested via a log-rank test. Meanwhile, adverse events were evaluated according to the Common Terminology Criteria for Adverse Events ver. 4.03 [11]. The χ2 test was conducted to evaluate significant differences in the frequency of early or late adverse events of Grade 3 or higher in patients who did and did not undergo chemotherapy. The Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer late radiation morbidity scoring system was used to evaluate the occurrence of late adverse events [12]. All statistical analyses were performed using SPSS 20 software (SPSS Inc., Chicago, IL), and a P-value of ≤0.05 was considered significant.
RESULTS
Patient characteristics
The clinical records of 12 eligible patients were collected from nine institutions. The patients’ characteristics and treatment characteristics are shown in Tables 1 and 2, respectively. The median age of the 12 patients was 70.5 years (range: 44–87 years), and most patients (91.7%) were men. Localized disease (TXN0–1M0), according to the clinical stage of the small cell lung carcinoma, accounted for 83.3% of the cases. Chemotherapy was used in eight cases (66.7%): the regimen most frequently used was the platinum-based regimen for small cell lung carcinoma in Table 2.
Table 1.
Patient characteristics (n = 12)
| Characteristics | Value, n (%) |
|---|---|
| Age (years), median (range) | 70.5 (44–87) |
| Sex | |
| Male | 11 (91.7%) |
| Female | 1 (8.3%) |
| TNM classification (UICC 7th) | |
| Limited disease (TXN0–1M0) | 10 (83.3%) |
| Extensive disease (TXN2–3M0–1) | 2 (16.7%) |
| Clinical stage | |
| I | 1 (8.3%) |
| II | 4 (33.3%) |
| III | 2 (16.7%) |
| IV | 5 (41.7%) |
| ECOG performance status | |
| 0 | 5 (41.7%) |
| 1 | 5 (41.7%) |
| 2 | 0 (0.0%) |
| 3 | 2 (16.6%) |
| 4 | 0 (0.0%) |
| Pathology | |
| Pure | 5 (41.7%) |
| Mixed | 3 (25.0%) |
| Unknown | 4 (33.3%) |
ECOG = Eastern Cooperative Oncology Group, UICC = Union for International Cancer Control.
Table 2.
Treatment characteristics (n = 12)
| Adjuvant therapy with radiotherapy | |||
|---|---|---|---|
| Number of patients (%) | |||
| Chemotherapy and TURBT | 4 (33.3%) | ||
| Chemotherapy | 4 (33.3%) | ||
| Radiotherapy | 3 (25.0%) | ||
| TURBT | 1 (8.4%) | ||
| Total | 12 | ||
| Radiotherapy | |||
| Total dose, median (range) | 60.0 (50.0–61.0) Gy | ||
| Dose per fraction, median (range) | 2.0 (1.2–2.0) Gy | ||
| Number of fractions, median (range) | 30 (25–50) Fr. | ||
| Duration of treatment (days), median (range) | 40.5 (29–57) | ||
| Clinical target volume | Number of patients (%) | ||
| Whole pelvis | 6 (50.0%) | ||
| Whole bladder | 3 (25.0%) | ||
| Whole pelvis + tumour (boost) | 2 (16.7%) | ||
| Small pelvis | 1 (8.3%) | ||
| Total | 12 | ||
| Chemotherapy | |||
| Regimen and number of cycles | Neoadjuvant | Concurrent | Sequential |
| CDDP/VP-16 | 4 | 1 | 2 |
| CBDCA/VP-16 | 1 | ||
| CDDP/VP-16/CPT-11 | 1 | ||
| CDDP/GEM | 1 | ||
TURBT = transurethral resection of the bladder tumour, CDDP = cisplatin,VP-16 = etoposide, CBDCA = carboplatin, CPT-11 = irinotecan, GEM = gemcitabine.
Radiotherapy
The median prescribed dose was 60.0 Gy (range: 50.0–61.0 Gy), and the median dose per fraction was 2.0 Gy (range: 1.2–2.0 Gy). Hyperfractionation was performed in only two cases (16.7%). The initial clinical target volume was the whole pelvis in eight cases (66.7%). The clinical target volume was decreased to only the tumour or lymph nodes as a boost after initial radiation in two of the eight cases. The other four patients received irradiation to the whole bladder without the pelvic lymph nodes or small pelvis. Prophylactic cranial irradiation was not performed.
Survival
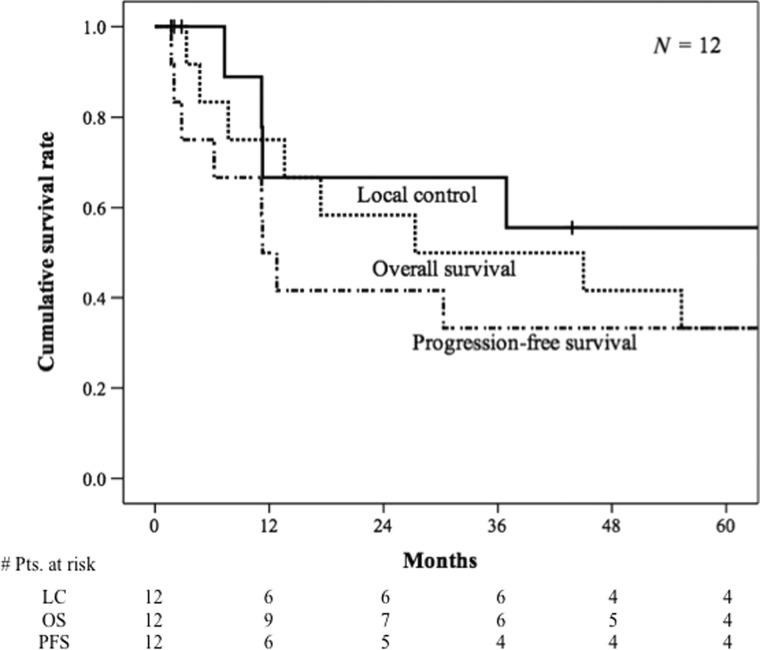
The posttreatment clinical course of each patient is shown in Table 3. The median follow-up period was 27.3 months (range: 3.3–117.8 months), and the 3- and 5-year survival rates were 50.0% (95% confidence interval: 0.22–0.78) and 33.3% (95% confidence interval: 0.07–0.60), respectively (Fig. 1). All patients showed an initial complete or partial response within the irradiation field. However, four cases (33.3%) showed relapse within the irradiation field during the follow-up period. Additionally, no patients required salvage surgery. Of all patients, eight maintained bladder functionality, thus the bladder preservation rate was 83.3%. The median local control period was incalculable (range: 1.7–128.7 months), and the 3- and 5-year local control rates were 66.7% (95% confidence interval: 0.36–0.97) and 55.6% (95% confidence interval: 0.23–0.88), respectively (Fig. 1). The median progression-free survival period was 11.3 months (range: 1.7–114.5 months), and the 3- and 5-year progression-free survival rates were both 33.3% (95% confidence interval: 0.07–0.60) (Fig. 1). The most frequently observed site of recurrence was distant metastases (n = 7, 58.3%) followed by regional lymph nodes (n = 4, 33.3%). Distant metastases were observed in the liver, bones, para-aortic lymph nodes, and lungs. Two cases (16.7%) showed metastasis in the brain.
Table 3.
Treatment outcomes after radiotherapy
| Pt. | Stage | Treatment | Chemotherapy | Relapse site | PFS (months) | OS (months) | Prognosis |
|---|---|---|---|---|---|---|---|
| 1 | LD | TURBT + CRT | CDDP +VP-16 | None | 77.2 | 77.2 | NED |
| 2 | LD | TURBT + CRT | CDDP + VP16 | None | 114.5 | 114.5 | NED |
| 3 | LD | TURBT + CRT | CDDP + CPT-11 | None | 79.4 | 117.8 | NED |
| 4 | ED | TURBT + CRT | CDDP/CBDCA + VP16* | Bladder, common iliac node, obturator node | 12.8 | 55.3 | AWD |
| 5 | LD | CRT | CDDP + VP-16 | Brain | 30.3 | 45.0 | DOD |
| 6 | LD | CRT | CDDP + VP-16 | Bladder, liver, lesser curvature of stomach LN | 6.2 | 7.7 | DOD |
| 7 | LD | CRT | GEM + CDDP | Bladder, internal iliac node | 11.3 | 17.4 | DOD |
| 8 | ED | CRT | CDDP + VP-16 + CPT-11 | None | 78.8 | 78.8 | NED |
| 9 | LD | TURBT + RT | - | Supraclavicular node, PALN | 2.8 | 3.3 | DOD |
| 10 | LD | RT | - | Common iliac node, external iliac node, liver | 11.2 | 27.3 | DOD |
| 11 | LD | RT | - | Common iliac node, supraclavicular node | 1.7 | 13.6 | DOD |
| 12 | LD | RT | - | PALN | 2.0 | 4.7 | DOD |
*Both transcatheter arterial embolisation and systemic chemotherapy were included.
LD = localised disease, ED = extensive disease, TURBT = transurethral resection of the bladder tumour, CRT = chemoradiotherapy, RT = radiotherapy, CDDP = cisplatin, CBDCA = carboplatin, GEM = gemcitabine, LN = lymph node, PALN = para-aortic lymph node, NED = no evidence of disease, AWD = alive with disease, DOD = dead of disease.
Fig. 1.
Overall survival, local control and progression-free survival of 12 patients with small cell carcinoma of the bladder. Kaplan–Meier survival curves for patients with small cell carcinoma of the bladder show overall survival (dashed line), local control (solid line) and progression-free survival (another dashed line).
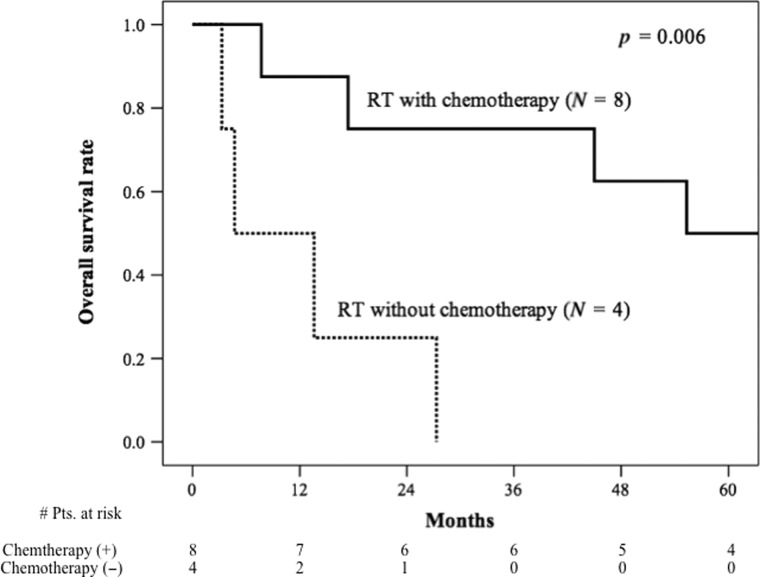
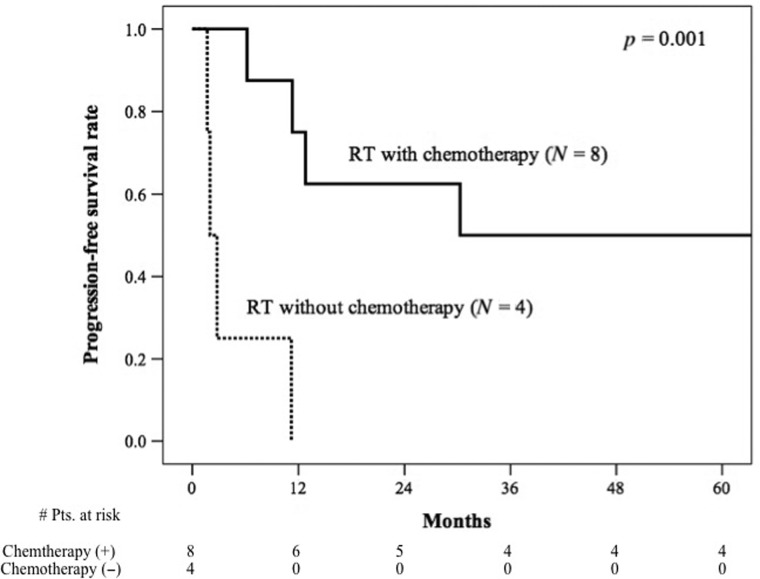
The median overall survival period was 55.3 months (range: 7.7–117.8 months) for patients who received chemotherapy and 4.7 months (range: 3.3–27.3 months) for those who did not receive chemotherapy (Fig. 2). Meanwhile, the median progression-free survival period for those who did and did not receive chemotherapy was 30.3 months (range: 6.2–114.5 months) and 2.0 months (range: 1.7–11.2 months), respectively (Fig. 3). Overall survival and progression-free survival were significantly different between the patients who did and did not receive chemotherapy (P = 0.006 and P = 0.001, respectively).
Fig. 2.
Overall survival curves for small cell carcinoma of the bladder patients who received local radiotherapy with chemotherapy and local radiotherapy without chemotherapy. Kaplan–Meier survival curves are shown for patients with small cell carcinoma of the bladder stratified according to radiotherapy with chemotherapy (solid line), or without chemotherapy (dashed line).
Fig. 3.
Progression-free survival curves for small cell carcinoma of the bladder patients who received local radiotherapy with chemotherapy and local radiotherapy without chemotherapy. Kaplan–Meier survival curves are shown for patients with small cell carcinoma of the bladder stratified according to radiotherapy with chemotherapy (solid line), or without chemotherapy (dashed line).
Adverse events
Regarding early adverse events, Grade 1 urinary frequency and Grade 1 haematuria occurred in four cases each (41.7%), and haematotoxicities of Grade 1–3 were frequently observed. No toxicities of Grade 4 or higher occurred during the follow-up period. One patient developed symptoms of ileus in the 20 days after the start of radiotherapy, but the symptoms improved with conservative treatment. This patient was diagnosed with radiation enteritis. No late adverse events of Grade 3 or higher occurred. The bladder was preserved in all patients during the follow-up period, and none of the cases required urinary tract construction or urinary diversion. No significant differences were observed in the frequencies of early or late adverse events between the patients who did and did not receive chemotherapy.
DISCUSSION
This study is the first to analyse the results of a nationwide survey in Japan on small cell carcinoma of the bladder treated with definitive radiotherapy. Although the efficacy of transurethral resection of the bladder tumour or radiotherapy combined with systemic chemotherapy has been shown in several recent retrospective analyses [13–15], few studies has focused on the bladder-sparing approach.
The treatment and outcomes of small cell carcinoma of the bladder reported in the current and prior studies are shown in Table 4. Lohrisch et al. [16] analysed 10 patients treated with multi-agent chemotherapy and local irradiation and reported overall survival rates of 70% at 2 years and 44% at 5 years. In another retrospective analysis of 17 patients with localised small cell carcinoma of the bladder who were treated with sequential chemoradiation, the median overall survival period was 32.5 months, and the estimated 5-year survival rate was 36% (confidence interval: 0.14–0.61) [17]. In a similar retrospective analysis of 17 patients with localized small cell carcinoma of the bladder, the median overall survival period was 26 months, and the 5-year overall survival rate was 22.2% [18]. In those studies, although the sample size was small, sequential chemoradiation for small cell carcinoma of the bladder was shown to be beneficial. No clinical trial on radiotherapy for small cell carcinoma of the bladder has compared the results of radiotherapy and cystectomy as local treatments. However, some retrospective studies reported no significant difference in survival between patients who received cystectomy and those who did not receive cystectomy (Table 4) [19, 20]. Especially in patients with non-metastatic disease, a radical cystectomy did not significantly influence the overall survival or the disease-free survival [20]. Our results are comparable with or better than those of previous studies regarding bladder-sparing treatment. However, the presence of selection bias cannot be ruled out, as only elderly people and patients with comorbidities were included.
Table 4.
Literature review of treatment for small cell carcinoma of the bladder
| Author | Year | Pt. (n) | Stage (n) | Local treatment | Chemotherapy | OS | P-value | |
|---|---|---|---|---|---|---|---|---|
| 5-year OS | Median (months) | |||||||
| Cheng et al. [19] | 2004 | 64 | N/A | Cystectomy +/Cystectomy - | 36% | 16%/18% | 21 | 0.65 |
| Pasquier et al. [20] | 2015 | 90 | T2-4N0-3M0 | Cystectomy +/Cystectomy - | 62% | N/A | 12.9 | 0.3 |
| Lohrisch et al. [16] | 1999 | 10 | N/A | TURBT ± RT | 100% | 44% | 41 | |
| Bex et al. [17] | 2009 | 17 | LD (17) | TURBT + RT | 100% | 36% | 32.5 | |
| Meijer et al. [18] | 2013 | 27 | LD (27) | TURBT + RT | 100% | 22% | 26 | |
| Bryant et al. [21] | 2016 | 11 | T2-4N0-3M0 | TURBT + RT | 100% | (3-year OS 24%) | 13 | |
| This study | 2019 | 12 | LD (10), ED (2) | TURBT ± RT | 67% | 33% | 27 | |
LD = local disease, ED (TXN2–3M0–1) = extensive disease, N/A = not available, TURBT = transurethral resection of the bladder tumour, RT = radiotherapy, OS = overall survival, N.S. = no significant difference.
Previous studies have reported that the percentage of patients in whom the bladder is preserved during the observation period of local treatment is as high as 82–100%, with no serious adverse events [17, 21–23]. In this study, the bladder preservation rate was 83.3%, because two patients with local relapse required conservative treatment for urinary obstruction (Table 4, Patients 4 and 10). However, the bladder function of four patients experiencing a local relapse was maintained within a range of 7–54 months. Patients who are treated with total cystectomy lose their bladder function immediately after surgery and are forced to undergo urinary diversion, which impairs their quality of life. Given its potential in improving the quality of life of patients with small cell carcinoma of the bladder, radiotherapy should be considered as a local treatment option for patients without a surgical indication and for those seeking an alternative to cystectomy.
Relatively few studies on the dose and irradiation field for small cell carcinoma of the bladder have been conducted. The median dose we used was similar to that reported in other studies [17, 20, 21, 23–25]. In patients with limited-stage small cell carcinoma of the bladder, those receiving a total radiation dose of ≥54 Gy had significant improvements in overall survival compared with those receiving a dose <54 Gy [26]. The present study showed no significant differences in survival according to the total dose (P = 0.799). The optimal irradiation field for small cell carcinoma of the bladder patients is difficult to define because of the rarity of the disease. Irradiation of the whole bladder and regional lymph nodes is considered to be important, considering the tendency of rapid progression and microinvasion of small cell carcinoma of the bladder. At present, it is difficult to recommend prophylactic cranial irradiation owing to the lower incidence of brain metastasis in patients with small cell carcinoma of the bladder than that in patients with small cell lung carcinoma [27–29]. Further studies are needed to identify the optimal dose and irradiation field for small cell carcinoma of the bladder.
We found that systemic chemotherapy combined with radiotherapy improved the patients’ overall survival and progression-free survival. Systemic chemotherapy is an important treatment modality for small cell carcinoma of the bladder because of the possibility of micrometastasis, even in local disease. Platinum-based chemotherapy has been frequently used for patients with small cell carcinoma of the bladder according to the regimen accepted for small cell lung carcinoma [18, 24, 25]. A platinum-based agent plus etoposide was used in many patients in this study, and the treatment was well tolerated. Similar results associated with systemic chemotherapy for small cell carcinoma of the bladder have been reported previously [24, 30, 31].
We acknowledge that due to the retrospective nature of our analysis, no definitive conclusions regarding treatment can be provided. However, because the bladders were preserved well in this study and adverse events were acceptable, as has been previously reported, our results provide important information on bladder-sparing radiotherapy for this rare disease.
Preservation of the bladder can be achieved using radiotherapy in patients with small cell carcinoma of the bladder. Radiotherapy has an important role with regard to the patient’s quality of life and is likely to become an option for local treatment. In addition, the findings from this study suggest that chemotherapy combined with local radiotherapy improves survival.
ACKNOWLEDGEMENTS
The authors are grateful for the support received from all institutions and researchers involved in the study, all members of the JROSG Working Subgroup of Urologic Oncology, and Professor Norihiko Tsuchiya, M.D., Yamagata University School of Medicine, for his fruitful discussion.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
None.
REFERENCES
- 1. Walenkamp AM, Sonke GS, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev 2009;35:228–36. [DOI] [PubMed] [Google Scholar]
- 2. Koay EJ, Teh BS, Paulino AC et al. A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer 2011;117:5325–33. [DOI] [PubMed] [Google Scholar]
- 3. Blomjous CE, Vos W, De Voogt HJ et al. Small cell carcinoma of the urinary bladder. A clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer 1989;64:1347–57. [DOI] [PubMed] [Google Scholar]
- 4. Holmang S, Borghede G, Johansson SL. Primary small cell carcinoma of the bladder: a report of 25 cases. J Urol 1995;153:1820–2. [PubMed] [Google Scholar]
- 5. Maebayashi T, Ishikawa H, Yorozu A et al. Patterns of practice in the radiation therapy for bladder cancer: survey of the Japanese Radiation Oncology Study Group (JROSG). Jpn J Clin Oncol 2014;44:1109–15. [DOI] [PubMed] [Google Scholar]
- 6. Brenner B, Tang LH, Shia J et al. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol 2007;34:43–50. [DOI] [PubMed] [Google Scholar]
- 7. Lau SK, Zhang Y. Pathologic quiz case. A 73-year-old man with a bladder mass. Combined primary small cell neuroendocrine carcinoma and high-grade papillary urothelial carcinoma of the urinary bladder. Arch Pathol Lab Med 2004;128:1055–6. [DOI] [PubMed] [Google Scholar]
- 8. Devesa SS, Bray F, Vizcaino AP et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005;117:294–9. [DOI] [PubMed] [Google Scholar]
- 9. Fahed E, Hansel DE, Raghavan D et al. Small cell bladder cancer: biology and management. Semin Oncol 2012;39:615–8. [DOI] [PubMed] [Google Scholar]
- 10. Akamatsu H, Nomiya T, Harada M et al. Bladder-sparing approach with radiotherapy in patients with small cell carcinoma of the bladder. J Cancer Ther 2014;5:797–805. [Google Scholar]
- 11. National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 (CTCAE). 14 June 2010. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (29 January 2019, date last accessed).
- 12. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31:1341–6. [DOI] [PubMed] [Google Scholar]
- 13. Patel SG, Stimson CJ, Zaid HB et al. Locoregional small cell carcinoma of the bladder: clinical characteristics and treatment patterns. J Urol 2014;191:329–34. [DOI] [PubMed] [Google Scholar]
- 14. Geynisman DM, Handorf E, Wong YN et al. Advanced small cell carcinoma of the bladder: clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. Cancer Med 2016;5:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koay EJ, Teh BS, Paulino AC et al. Treatment trends and outcomes of small-cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys 2012;83:64–70. [DOI] [PubMed] [Google Scholar]
- 16. Lohrisch C, Murray N, Pickles T et al. Small cell carcinoma of the bladder: long term outcome with integrated chemoradiation. Cancer 1999;86:2346–52. [DOI] [PubMed] [Google Scholar]
- 17. Bex A, de Vries R, Pos F et al. Long-term survival after sequential chemoradiation for limited disease small cell carcinoma of the bladder. World J Urol 2009;27:101–6. [DOI] [PubMed] [Google Scholar]
- 18. Meijer RP, Meinhardt W, van der Poel HG et al. Local control rate and prognosis after sequential chemoradiation for small cell carcinoma of the bladder. Int J Urol 2013;20:778–84. [DOI] [PubMed] [Google Scholar]
- 19. Cheng L, Pan CX, Yang XJ et al. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer 2004;101:957–62. [DOI] [PubMed] [Google Scholar]
- 20. Pasquier D, Barney B, Sundar S et al. Small cell carcinoma of the urinary bladder: a retrospective, multicenter Rare Cancer Network Study of 107 patients. Int J Radiat Oncol Biol Phys 2015;92:904–10. [DOI] [PubMed] [Google Scholar]
- 21. Bryant CM, Dang LH, Stechmiller BK et al. Treatment of small cell carcinoma of the bladder with chemotherapy and radiation after transurethral resection of a bladder tumor. Am J Clin Oncol 2016;39:69–75. [DOI] [PubMed] [Google Scholar]
- 22. Karpman E, Goldberg Z, Saffarian A et al. Analysis of treatment for small cell cancer of the bladder and report of three cases. Urology 2004;64:494–8. [DOI] [PubMed] [Google Scholar]
- 23. Mattes MD, Kan CC, Dalbagni G et al. External beam radiation therapy for small cell carcinoma of the urinary bladder. Pract Radiat Oncol 2015;5:e17–22. [DOI] [PubMed] [Google Scholar]
- 24. Bex A, Nieuwenhuijzen JA, Kerst M et al. Small cell carcinoma of bladder: a single-center prospective study of 25 cases treated in analogy to small cell lung cancer. Urology 2005;65:295–9. [DOI] [PubMed] [Google Scholar]
- 25. Eswara JR, Heney NM, Wu C-L et al. Long-term outcomes of organ preservation in patients with small cell carcinoma of the bladder. Urol Int 2015;94:401–5. [DOI] [PubMed] [Google Scholar]
- 26. Germino E, Fischer-Valuck BW, Rudra S et al. Radiation therapy as definitive local treatment in patients with limited-stage small cell carcinoma of the bladder: does total dose matter? Bladder Cancer 2018;4:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aupérin A, Arriagada R, Pignon JP et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476–84. [DOI] [PubMed] [Google Scholar]
- 28. Naidoo J, Teo MY, Deady S et al. Should patients with extrapulmonary small-cell carcinoma receive prophylactic cranial irradiation? J Thorac Oncol 2013;8:1215–21. [DOI] [PubMed] [Google Scholar]
- 29. Yazici O, Ozdemir NY, Sendur MA et al. Current approaches for prophylactic cranial irradiation in extrapulmonary small cell carcinoma. Curr Med Res Opin 2014;30:1327–36. [DOI] [PubMed] [Google Scholar]
- 30. Mukesh M, Cook N, Hollingdale AE et al. Small cell carcinoma of the urinary bladder: a 15-year retrospective review of treatment and survival in the Anglian Cancer Network. BJU Int 2009;103:747–52. [DOI] [PubMed] [Google Scholar]
- 31. Kaushik D, Frank I, Boorjian SA et al. Long-term results of radical cystectomy and role of adjuvant chemotherapy for small cell carcinoma of the bladder. Int J Urol 2015;22:549–54. [DOI] [PubMed] [Google Scholar]