Abstract
The Ullmann homocoupling of 2-bromo-perylene diimides (PDIs) gave [2,2′-biperylene]-3,4:9,10:3′,4′:9′,10′-tetrakis(dicarboximide)s, 2,2′-bi(PDI)s, and the Suzuki coupling of a PDI-2-boronic ester and a 1-bromo-PDI gave a [1,2′-biperylene]-3,4:9,10:3′,4′:9′,10′-tetrakis(dicarboximide), 1,2′-bi(PDI). These were compared with [1,1′-biperylene]-3,4:9,10:3′,4′:9′,10′-tetrakis(dicarboximide)s, 1,1′-bi(PDI)s. Solution absorption spectra suggest that the PDIs in 2,2′-bi(PDI)s are more planar and less strongly coupled than those in 1,1′-bi(PDI)s, which is consistent with density functional theory calculations. 2,2′-Bi(PDI)s are less easily reduced than 1,1′- and 1,2′-bi(PDI)s by ca. 70–90 mV. Bulk heterojunction organic solar cells incorporating a 2,2′-bi(PDI) acceptor behaved similarly to those employing its 1,1′-bi(PDI) analogue.
Introduction
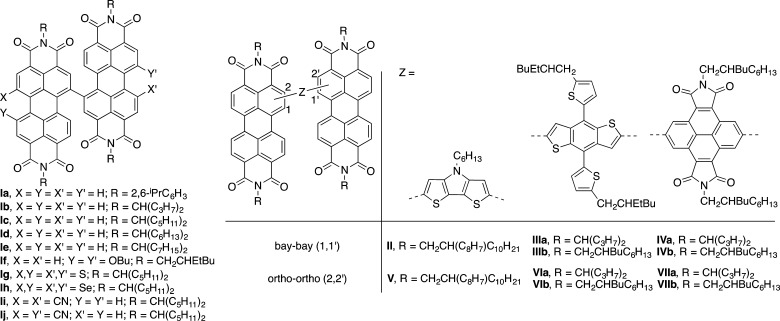
Perylene-3,4:9,10-bis(dicarboximide)s (perylene diimides, PDIs) have attracted interest as dyes and pigments, electron-transporting semiconductors in organic field-effect transistors, two-photon-absorbing chromophores, fluorophores, and acceptors in photoinduced electron-transfer reactions for both fundamental studies and optical limiting.1−4 They have also been extensively used as light-harvesting electron-transporting materials in organic solar cells;2,3,5 indeed, the first donor–acceptor organic solar cell used a PDI-like acceptor in a bilayer heterojunction with copper phthalocyanine.6 A wide range of PDI architectures have been used in bulk-heterojunction (BHJ) organic photovoltaics (OPV), including simple small molecules,7 molecules with bulky 1,7-substituents to hinder aggregation,8 molecules that form columnar discotic liquid–crystal phases,9 and conjugated10 and side-chain11 polymers. Nonfullerene acceptors for solar cells are still an active subject of research,12−18 and PDIs remain one of the major classes under investigation.5 Many of the most efficient examples have three-dimensional (3D) architectures, whereby two or more PDIs are forced out of plane with one another and/or a bridging group.19 One simple approach to such 3D structures is to directly link two PDIs through their “bay” (1,1′) positions (see Figure S1 for the numbering scheme for PDIs), with the PDI units being sterically precluded from achieving coplanarity. A variety of 1,1′-bi(PDI)s, Ia–j, have been reported (Figure 1). Delocalization in Ia•– was investigated,20 and excitonic coupling and electron/energy transfer were studied in Ib,21 but most studies focused on BHJ OPV.22−32a The precursors are generally 1-bromo-PDIs, which are obtained by electrophilic substitution.1,3 2-Functionalized-PDIs have recently become accessible through transition metal catalysis33−37 and been used to synthesize PDI-bridge-PDI species V–VII (Figure 1), whose physical and/or OPV properties have been compared to those of 1-bromo-PDI-derived analogues II–IV.38,39 However, 2,2′-bi(PDI)s have not yet been reported.b Here, we report 2,2′-bi(PDI)s (3a and 3b, Scheme 1) and a 1,2′-bi(PDI) (2a) and compare their absorption spectra and electrochemistry to those of 1,1′-bi(PDI)s (1a and 1b). We also compare the OPV performance of 2,2′-bi(PDI) 3b to that of its 1,1′-linked analogue, 1b.
Figure 1.
Left: reported 1,1′-bi(PDI)s.22−32 Right: PDI-bridge-PDI species for which comparisons have been made between bay- and ortho-linked species.38,39
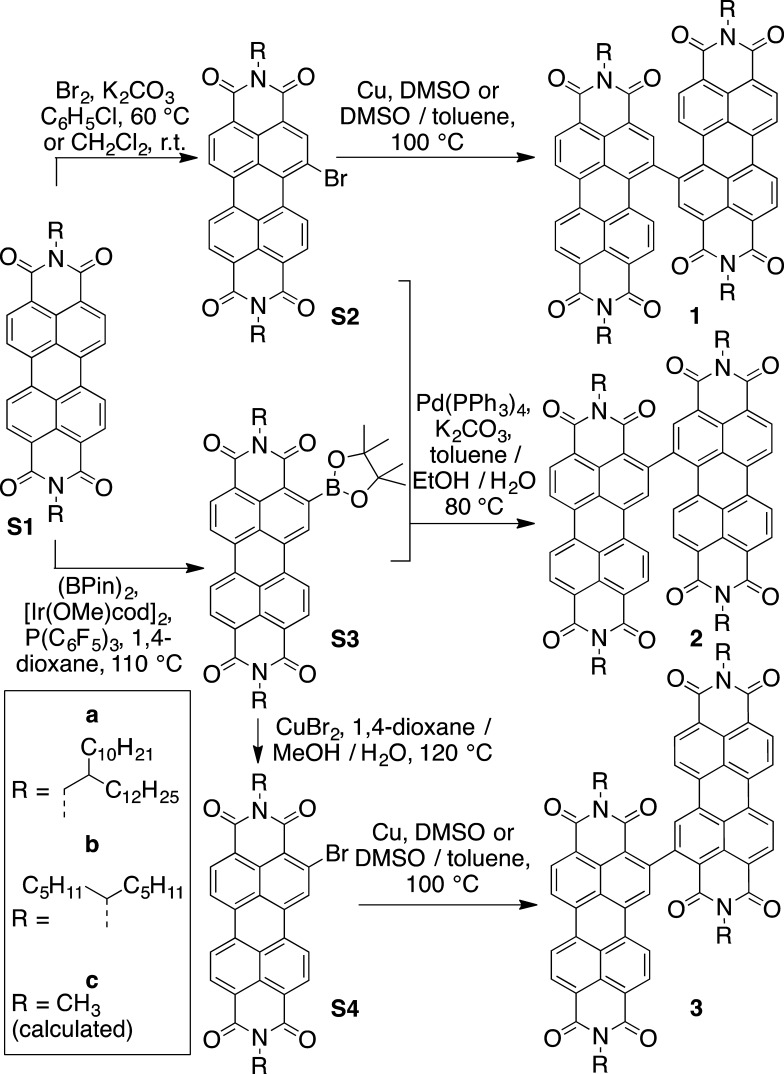
Scheme 1. Synthesis of 1,1′-, 1,2′-, and 2,2′-bi(PDI)s.
Pin = pinacolate; cod = 1,5-cyclooctadiene.
Results and Discussion
Scheme 1 shows the synthesis of 1, 2, and 3. Previously reported 1b (Ic)22 was made in a similar way to other 1,1′-Bi(PDI)s,20−22,25,28,29 that is, by the Ullmann homocoupling of S2b(22,40) in dimethyl sulfoxide (DMSO) (but with added toluene to improve solubility). Boronic esters (S3) were synthesized using the previously described conditions,35,38,41 and, without purification or characterization, they were treated with CuBr2 to yield 2-Br-PDIs (S4),37 which were converted to 2,2′-PDIs (3) using Ullmann conditions similar to those used for 1,1′-PDIs. Boronic ester S3a was also subjected to Suzuki coupling conditions with S2a to obtain 1,2′-bi(PDI) (2a). The new dimers were all characterized by 1H and 13C NMR spectroscopy, mass spectrometry, and combustion analysis. Although several of the derivatives show broadened 1H and/or 13C spectra, likely as a result of restricted rotation around the PDI–PDI bond, especially in the case of 2,2′-isomers, and about the N-alkyl bonds of the derivatives with CH(C5H11)2 chains (as seen for other PDIs with “swallowtail” substituents42,43), the isomers can be readily distinguished by NMR (see Experimental Section and spectra in the Supporting Information).
Differential scanning calorimetry (DSC) (10 °C min–1, Figure S2) gave melting points of 167, 188, and 275 °C for 1a, 2a, and 3a, respectively, all considerably higher than that of monomeric S1a (108 °C) under the same conditions. In contrast, the bi(PDI)s with secondary alkyl substituents, 1b and 3b, showed no evidence of crystallinity, consistent with GIWAXS measurements on 1b,30 but exhibited glass transitions at 132 and 85 °C, respectively (Figure S3). The decomposition temperatures (5 wt % loss, thermogravimetric analysis, 10 °C min–1), which vary slightly with linkage motif, are similar to those of monomeric PDIs and are slightly lower for compounds with secondary alkyl imide substituents than for those with branched primary substituents (432, 432, 440, 420, 383, and 381 °C for 1a, 2a, 3a, S1a, 1b, and 3b, respectively).
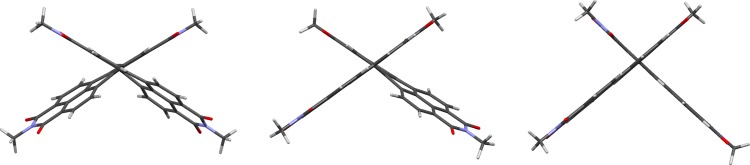
Density functional theory (DFT) (B3LYP/6-31G**) calculations for 1c, 2c, and 3c (in which the N,N′-dialkyl groups are simplified to CH3, Scheme 1) provide insight into their conformations and energetics. The two PDI units are severely twisted relative to one another, which is consistent with the previous calculations for 1,1′-bi(PDI)s;20,25,28 ψ, the CH–C–C–CH torsion angle across the PDI–PDI bond, increases from 1,1′- to 1,2′- and 2,2′-linkages (Figure 2, Table 1). On the other hand, the two naphthalene subunits of the bay-substituted PDIs of 1c and 2c are somewhat twisted from coplanarity (as quantified by ϕ), whereas the ortho-functionalized PDIs of 2c and 3c are virtually planar. Twisted cores are often found for bay-substituted PDIs, even with medium-sized substituents, and they help relieve steric interactions between the 1-substituent and the hydrogen or other substituent in the 12-position.44−46 However, steric interaction between 2-substituents and the 3-oxo-group cannot be relieved by an intra-PDI twist, ϕ, and hence results in a larger inter-PDI twist, ψ. Similar differences in bridge/PDI twists and intra-PDI distortion were seen between bay- and ortho-linked II and V.38
Figure 2.
Molecular conformations of isolated molecules of, from left to right, 1,1′-, 1,2′-, and 2,2′-Bi(PDI)s (1c–3c) according to DFT calculations (B3LYP/6-31G**).
Table 1. Geometric and Energetic Characteristics of 1,1′-, 1,2′-, and 2,2′-Bi(PDI)s (Isolated Molecules) from DFT Calculations (B3LYP/6-31G**).
Frontier molecular orbitals for 1c–3c were also calculated (DFT, B3LYP/6-31**); the wavefunctions and energies for 1c are similar to those previously reported for Ia.20 The LUMO and LUMO + 1 of the symmetric bi(PDI)s (1c and 3c) are bonding and antibonding combinations, respectively, of two PDI LUMOs (see Table S2). The LUMO/LUMO + 1 energetic separation (Table 1) is much smaller for 3c than that for 1c; this can be attributed to the larger ψ in 3c, which reduces the inter-PDI π-overlap, and to the ortho positions of PDIs having smaller LUMO coefficients than the bay positions. Similarly, HOMO and HOMO – 1 resemble, respectively, antibonding and bonding combinations of two PDI HOMOs; again, a larger separation is seen for 1c. The large LUMO/LUMO + 1 and HOMO/HOMO – 1 separations seen for 2c are partly due to its inherent asymmetry; the LUMO and HOMO – 1 are localized on the ortho-substituted PDI, whereas the LUMO + 1 and HOMO are localized on the bay-substituted PDI.
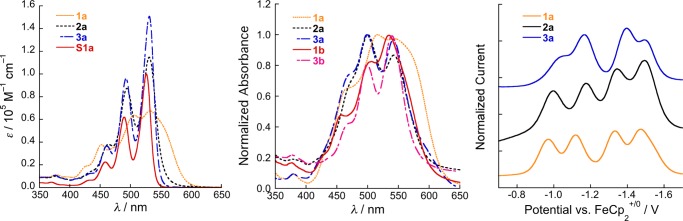
Figure 3 compares solution absorption spectra of 1a–3a and S1a (Figure S4 shows spectra of 1b and 3b, which are very similar to those of their analogues). Spectra for 1a,b are consistent with previous reports on 1,1′-bi(PDI)s20−22 and are considerably broader and more complex than those of monomeric PDIs, such as S1a, with lower peak absorptivities (εmax). The broadening and consequent lowering of εmax are partially attributed to the nonplanarity of the PDI units;44,45 however, inter-PDI electronic coupling helps to determine the vibrational structure.21 Spectra of 3a,b more closely resemble those of monomeric PDIs, suggesting the PDI units of 2,2′-bi(PDI)s are more planar and less strongly coupled than those of 1,1′-bi(PDI)s, consistent with DFT results (see above). The differences in the linewidth and absorptivity seen here between 1,1′- and 2,2′-PDIs are reminiscent of those previously seen for the PDI-based transitions of II and V, although the additional complexity attributable to PDI–PDI coupling for 1a,b is not seen for II.38c Finally, the spectrum of 2a is quite similar to that of 3a, but with a lower εmax and broadened vibronic sub-bands, consistent with the superposition of the spectra of a planar (ortho-substituted) and less planar (bay-substituted) PDI and with small coupling. Despite a large variation in εmax, the oscillator strengths of the main PDI-like bands are similar for all three linkages and only a little less than twice those of S1a (Table 2).
Figure 3.
Left: Absorption spectra of bi(PDI)s with different linkages and the corresponding monomeric PDI in CHCl3. Center: Absorption spectra of films of bi(PDI)s with different linkages and alkyl substituents. Right: Reductive differential pulse voltammograms of bi(PDI)s in THF/0.1 Bu4NPF6.
Table 2. Electrochemicala and Solution Optical Propertiesb of a PDI and 1,1′-, 1,2′-, and 2,2′-Bi(PDI)s.
| redox
potentials versus FeCp2+/0 (V) |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0/– | –/2– | 2–/3– | 3–/4– | EAestc (eV) | λmax (nm) | εmax (105 M–1 cm–1) | fd | |
| S1a | –1.07 | –1.38 | 3.7 | 525 | 1.0 | 0.73 | ||
| 1a | –0.97 | –1.12 | –1.34 | –1.48 | 3.8 | 532 | 0.69 | 1.30 |
| 2a | –0.99 | –1.18 | –1.34 | –1.49 | 3.8 | 532 | 1.1 | 1.27 |
| 3a | –1.06 | –1.17 | –1.40 | –1.50 | 3.7 | 532 | 1.5 | 1.25 |
Peak reduction potentials from differential pulse voltammetry in tetrahydrofuran (THF)/0.1 M Bu4NPF6.
In chloroform.
Estimated solid-state electron affinity obtained from EA = eE0/– + 4.8 eV.
Oscillator strength obtained as f = 4.32 × 10–9 ∫ε dν̅ (ε and ν̅ are in M–1 cm–1 and cm–1, respectively) over the PDI-like absorption band (ν̅ > 25 000 cm–1).
PDI–PDI π–π interactions typically result in a retained vibronic structure but a decreased ratio of the absorbances of 0,0 to 0,1 sub-bands.47 Thus, spectra of bi(PDI) thin films (Figure 3) can be understood as resulting from the effects of the planarity and through-bond electronic coupling responsible for the solution spectra and these intermolecular interactions. The variation in π–π interactions between bi(PDI)s with different connectivities cannot be easily gauged because of the variation of the solution spectra. However, these effects are more pronounced for the branched primary alkyl derivatives, 1a and 3a, than for their secondary alkyl analogues, 1b and 3b, consistent with the trends for monomeric PDIs7 and indicating a more disrupted π-stacking when the alkyl branching is immediately adjacent to the nitrogen atoms. The effects of π–π interactions appear to be less severe for the bi(PDI)s than for monomeric PDIs (see Figure S4 and spectra of secondary alkyl PDIs in ref (7)).
Monomeric PDIs show two successive reversible one-electron reductions. The bi(PDI)s show four solution reductions (Table 2, Figures 3 and S5), consistent with other 1,1′-bi(PDI)s.20−22,26,28,32 Splittings between redox potentials in compounds with linked equivalent redox centers result from an interplay of effects, including electronic coupling in the mixed-valence species, solvent-mediated Coulombic effects, and through-bond inductive effects.48 Here, electronic coupling is likely weak: the absorption spectrum of Ia•– indicates that the excess electron is localized on one PDI unit and the coupling is small,20 and the DFT LUMO/LUMO + 1 splittings (Table 1) suggest an even weaker coupling in 2,2′-bi(PDI)s.d For 1,2′-bi(PDI) (2a), the inequivalence of the two PDIs may also contribute to the electrochemical splitting.
The first reduction potentials of the bi(PDI)s are similar to one another and to that of S1a; however, both the 1,1′- and 1,2′-bi(PDI)s (1a,b and 2a, respectively) are somewhat more readily reduced than the 2,2′ dimers (3a,b), consistent with the DFT LUMO energies (Table 1), or than S1a. The differences between 1a,b and 3a,b may be due to the effects of not only planarity but also differences in the inductive and electronic coupling effects of the linkage motifs. The potential of 2a is close to that of 1a, which might suggest its bay-linked PDI that is reduced first; however, DFT indicates that the LUMO of 2a is localized on the ortho-linked PDI (Table S2), perhaps suggesting that PDI is a more effective inductively electron-withdrawing group through its bay position.
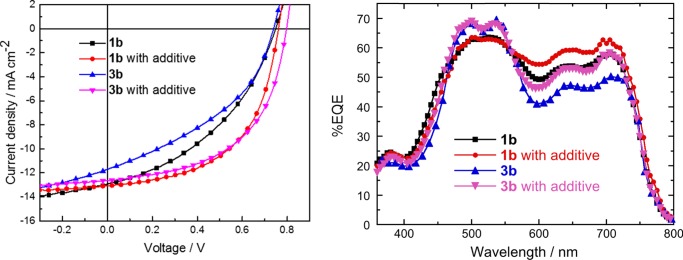
The feasibility of using 2,2′-bi(PDI)s as nonfullerene acceptors in BHJ OPVs was investigated using “inverted” devices with the structure of indium tin oxide (ITO)/ZnO/PTB7-Th:bi(PDI)/MoO3/Ag. We compared 1b and 3b because (a) 1b (Ic) has been used as a nonfullerene acceptor in previous studies22−24,26,27,30 and (b) monomeric PDIs with secondary alkyl substituents often give more favorable morphologies and better performance than those with linear or branched primary alkyl groups.7 The device structure and the donor polymer, PTB7-Th (also known as PCE10 and PBDTT-F-TT),49 were selected on the basis of their previous use with 1b (Ic), which gave an optimized power conversion efficiency (PCE) of 5.3% (even higher with a surface modifier applied to ZnO);24 the same PTB7-Th:1b ratio and additives afforded very similar device parameters in our experiment (Table 3, Figure 4). With 3b as acceptor, the same ratios, additives, and processing gave PCE = 4.8%. However, varying the donor/acceptor ratios and additives led to PCE values close to those for PTB7-Th:1b devices (Table 3; see Supporting Information for additional characterization of blends and devices). The similarity in the behavior of the optimized blends is consistent with similar EQE spectra (Figure 4), where, at least for the additive-containing films, the acceptor and donor contributions increase and decrease, respectively, for 3b versus 1b films, consistent with a higher acceptor content of the films. The similarity in behavior is also consistent with a fairly small difference in the electron mobility of the blend films (5.0 × 10–5 and 2.2 × 10–5 cm2 V–1 s–1, respectively, for the 1b and 3b optimized blends; see Figure S8). AFM also suggests similar surface morphologies, although phase images indicate somewhat larger feature sizes for optimized 3b blends (Figure S7). Overall, at least with PTB7-Th, the 2,2′-bi(PDI) offers no clear advantage over its 1,1′ analogue. However, different optimum donor/acceptor/additive compositions found for the two bi(PDI)s indicates they are not interchangeable and suggests that which performs best may vary from system to system.
Table 3. Performance of ITO/ZnO/PTB7-Th:biBPI/MoO3/Ag Solar Cellsa.
| acceptor | D/A wt ratio | additivesb | VOC (V) | JSC (mA cm–2)e | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| 1b | 1:1 | 0.74 ± 0.01 | 12.63 ± 0.35 | 40.78 ± 1.03 | 3.81 ± 0.19 (3.99) | |
| 1b | 1:1 | 1 wt % DIO + 2 wt % CN | 0.76 ± 0.00 | 12.57 ± 0.29 | 55.02± 0.75 | 5.24 ± 0.14 (5.44) |
| 3b | 1:1.5 | 0.74 ± 0.01 | 11.38 ± 0.30 | 39.85 ± 0.56 | 3.34 ± 0.15 (3.49) | |
| 3b | 1:1.5 | 2 wt % DIO | 0.78 ± 0.01 | 12.57 ± 0.17 | 52.36 ± 1.07 | 5.16 ± 0.19 (5.43) |
The values are averages from over eight devices; the error bars are standard deviations, and the value of PCE in parentheses is the highest value obtained.
DIO = 1,8-diiodooctane; CN = 1-chloronaphthalene.
Figure 4.
J–V curves (left) and EQE (right) spectra for ITO/ZnO/PTB7-Th:biBPI/MoO3/Ag solar cells (1:1 and 1:1.5 wt ratios for 1b and 3b, respectively) with and without additives (1 wt % DIO + 2 wt % CN for 1b; 2 wt % DIO for 3b).e See Figure S6 for the absorption spectra of blend films.
Conclusions
1,1′-, 1,2′-, and 2,2′-bi(PDI)s differ subtly in their solution absorption spectra and electrochemistry; these changes are consistent with DFT geometries and frontier orbital energies. However, solid-state spectra are more strongly dependent on the nature of the N,N′-substituents. A 2,2′-bi(PDI) has been shown to act as a nonfullerene acceptor in BHJ solar cells, and it exhibits a similar performance to its 1,1′ analogue, albeit with a different optimum active-layer composition, indicating the 2,2′-bi(PDI) moiety is also a viable building block for nonfullerene acceptors.
Experimental Section
General Synthesis and Characterization
Chromatographic separations were performed with standard flash column chromatography methods using silica gel purchased from Sorbent Technologies (60 Å, 40–63 μm). Electrochemical measurements were carried out under nitrogen in dry deoxygenated 0.1 M tetra-n-butylammonium hexafluorophosphate in THF using a conventional three-electrode cell with a glassy carbon working electrode, a platinum wire counter electrode, and a Ag wire coated with AgCl as the pseudoreference electrode. Potentials were referenced by using ferrocenium/ferrocene as an internal reference. Cyclic voltammograms were recorded at a scan rate of 50 mV s–1.
1-Bromo-N,N′-di(2-decyltetradecyl)perylene-3,4:9,10-bis(dicarboximide), S2a
This compound was previously reported in ref (50) but its synthesis and characterization were not. An alternative preparation involving imidization of a mixture of brominated perylene dianhydrides was reported in ref (51); the characterizing data in ref (51) are consistent with those reported below. N,N′-Di(2-decyltetradecyl)perylene-3,4:9,10-bis(dicarboximide), S1a,52 (6.0 g, 5.6 mmol), K2CO3 (4.0 g, 29 mmol), and chlorobenzene (80 mL) were mixed in a 200 mL round-bottom flask equipped with a condenser. Bromine (4.8 mL, 93 mmol) in chlorobenzene (10 mL) was added dropwise. The reaction mixture was then heated to 60 °C and kept overnight before it was cooled to room temperature and poured into a saturated Na2S2O3 solution (500 mL). The mixture was extracted with CHCl3 (2 × 200 mL), and the organic phase was washed with water (2 × 100 mL) and dried over Na2SO4. After the solvent was removed, the residue was purified by column chromatography on silica gel, with CHCl3/hexane (1:1) as the eluent. After the solvent was removed under reduced pressure, S2a was obtained as a red solid (2.7 g, 42%). 1H NMR (500 MHz, CDCl3): δ 9.62 (d, J = 8.0 Hz, 1H), 8.72 (s, 1H), 8.51 (m, 3H), 8.36 (m, 2H), 4.09 (d, J = 7.0 Hz, 2H), 4.06 (d, J = 7.0 Hz, 2H), 1.95 (m, 2H), 1.5–1.1 (m, 80H), 0.82 (m, 12H). 13C{1H} NMR (125 MHz, CDCl3): δ 163.5, 163.2, 163.1, 162.3, 138.9, 133.5, 133.1, 133.0, 130.8, 130.3, 128.5, 128.3, 127.9, 127.6, 126.6, 123.5, 123.4, 123.3, 122.92, 122.7, 122.5, 120.9, 44.79, 44.72, 36.63, 36.58, 31.9, 31.7, 30.1, 30.0, 29.7, 29.6 (2 close peaks), 29.6, 29.4, 26.5 (2 close peaks), 22.7, 14.1 (2 aromatic carbon peaks and 31 alkyl carbon peaks were not observed, presumably due to overlap). HRMS (MALDI-TOF): calcd for C72H105BrN2O (M+), 1140.728; found, 1140.734. Anal. Calcd for C72H105BrN2O: C, 75.69; H, 9.26; N, 2.45. Found: C, 75.77; H, 9.26; N, 2.50.
N,N′,N″,N‴-Tetra(2-decyltetradecyl)-[1,1′-biperylene]-3,4:9,10:3′,4′:9′,10′-tetrakis(dicarboximide), 1a
A mixture of S2a (0.25 g, 0.22 mmol), dry toluene (8 mL), and dry DMSO (23 mL) was loaded into a pressure vessel and deoxygenated with a flow of nitrogen; copper powder (40–60 nm particle size, 0.14 g, 2.2 mmol) was then added, and the reaction vessel was sealed and heated to 100 °C for 5 h. The reaction mixture was cooled to room temperature and diluted with dichloromethane (50 mL); the resulting solution was washed with brine and then dried over MgSO4. After filtration and evaporation of the solvents, the crude product was purified by column chromatography (silica gel; 3:2 dichloromethane/hexane), followed by size-exclusion chromatography (SX-1 BioRad; THF) to afford a red solid (0.146 g, 63%). Mp (DSC): 167 °C. 1H NMR (500 MHz, CDCl3): δ 8.84–8.79 (m, 8H), 8.47 (d, J = 8.0 Hz, 2H), 8.24 (s, 2H), 8.16 (d, J = 8.0 Hz, 2H), 4.08–3.99 (m, 8H), 1.93–1.91 (m, 4H), 1.34–1.17 (m, 160H), 0.87–0.81 (m, 24H). 13C{1H} NMR (125 MHz, CDCl3): δ 163.85, 163.72, 163.45, 163.36, 142.01, 135.05, 134.49, 134.33, 133.27, 131.92, 131.88, 130.97, 129.53, 129.05, 128.91, 127.81, 127.67, 124.47, 124.28, 123.82, 123.70, 123.65, 123.52, 44.96, 44.84, 36.81, 32.13, 32.09, 31.89, 31.82, 31.80, 31.77, 30.26, 30.22, 30.21, 30.18, 29.90, 29.87, 29.86, 29.84, 29.82, 29.58, 29.56, 29.53, 26.64, 26.46, 22.89, 22.86, 14.32 (1 aromatic carbon peak and 22 alkyl carbon peaks were not observed, presumably due to overlap). HRMS (MALDI-TOF): calcd for C144H211N4O8 (MH+), 2124.6227; found, 2124.6271. Anal. Calcd for C144H210N4O8: C, 81.38; H, 9.96; N, 2.64. Found: C, 81.47; H, 10.03; N, 2.66.
2-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-N,N′-di(2-decyltetradecyl)perylene-3,4:9,10-bis(dicarboximide), S3a, and 2-Bromo-N,N′-di(2-decyltetradecyl)perylene-3,4:9,10-bis(dicarboximide), S4a
A solution of S1a(52) (2.0 g, 1.9 mmol), tris(pentafluorophenyl)phosphine (0.12 g, 0.23 mmol), and bis(pinacolato)diboron (0.57 g, 1.9 mmol) in anhydrous 1,4-dioxane (50 mL) was deoxygenated with argon for 30 min, and then (Ir(OMe)cod)2 {cod = 1,5-cyclooctadiene} (0.037 g, 0.056 mmol) was added to a reaction vessel. The resulting mixture was heated to reflux for 24 h, allowed to cool to room temperature, and evaporated under reduced pressure to yield crude S3a. For use in the Suzuki coupling with S2a to afford 2a (see below), the crude product was purified by column chromatography (silica gel; 2:1 to 10:1 dichloromethane/hexane) to afford S2a containing minor impurities (0.23 g, 19% yield if pure), which was used in the subsequent Suzuki coupling reactions without additional purification. For conversion to S4a, crude S3a (1.8 g) (including some unreacted starting material and catalyst) was dissolved in 1,4-dioxane (35 mL); to the resulting red solution, methanol (5 mL) and aqueous CuBr2 (1.68 g in 2 mL of water) were added, and the resulting mixture was heated at 120 °C in a sealed tube. After the conversion was complete (ca. 12 h, monitored using thin-layer chromatography (TLC)), the product was extracted with dichloromethane. The extracts were washed with water, dried over MgSO4, filtered, and evaporated under reduced pressure. The resulting residue was purified by column chromatography (silica gel; 3:2 to 5:1 dichloromethane/hexane) to give S4a as a red solid (0.626 g, 78% over two steps from S1a). 1H NMR (500 MHz, CDCl3): δ 8.34 (d, J = 8 Hz, 2H), 8.29 (d, J = 8 Hz, 1H), 8.07–8.02 (m, 3H), 7.93 (d, J = 8 Hz, 1H), 4.07 (d, J = 7.5 Hz, 2H), 4.00 (d, J = 7.5 Hz, 2H), 2.05–1.86 (m, 2H), 1.43–1.21 (m, 80H), 0.87–0.83 (m, 12H). 13C{1H} NMR (125 MHz, CDCl3): δ 162.73, 161.76, 160.60, 132.77, 132.72, 131.59, 130.67, 130.38, 130.16, 129.76, 128.33, 128.12, 124.97, 124.30, 123.17, 122.55, 122.15, 119.86, 44.76, 44.62, 36.60, 36.32, 31.77, 31.75, 31.55, 31.41, 29.98, 29.57, 29.44, 29.22, 29.20, 26.37, 26.23, 22.51 (6 sp2 and 32 alkyl resonances were not observed, presumably due to overlap). HRMS (MALDI-TOF): calcd for C72H106BrN2O4 (MH+), 1141.7305; found, 1141.7333. Anal. Calcd for C72H105BrN2O4: C, 75.69; H, 9.26; N, 2.45. Found: C, 75.80; H, 9.29; N, 2.52.
N,N′,N″,N‴-Tetra(2-decyltetradecyl)-[1,2′-biperylene]-3,4:9,10;3′,4′:9′,10′-tetrakis(dicarboximide), 2a
A solution of K2CO3 (0.087 g, 0.63 mmol) in water (6 mL) was added to a solution of S3a (0.32 g, 0.27mmol) and S2a (0.24 g, 0.21 mmol) in a mixture of toluene (60 mL) and EtOH (0.8 mL); after deoxygenation by bubbling with argon for 30 min, Pd(PPh3)4 (0.026 g, 0.022 mmol) was added, and the resulting mixture was heated to 80 °C under argon overnight. After the conversion of the starting material (according to TLC) was complete, the product was extracted with dichloromethane. The organic phase was washed with water, dried over MgSO4, filtered, and evaporated under reduced pressure. The residue was purified by column chromatography (silica gel; 3:1 dichloromethane/hexane), followed by size-exclusion chromatography (SX-1 BioRad; THF) to give 2a as a red solid (0.13 g, 29%). Mp (DSC): 188 °C. 1H NMR (500 MHz, CDCl3, 325 K): δ 8.76 (d, J = 8.0, 1H), 8.71–8.52 (m, 7H), 8.46 (d, J = 7.0, 1H), 8.41(s, 1H), 8.37 (d, J = 7.0, 1H), 8.30–8.20 (m, 1H), 8.02 (d, J = 8.5, 1H), 7.83 (d, J = 8.5, 1H), 4.14 (d, J = 6.5, 2H), 3.96 (d, J = 6.5, 2H), 3.91–3.60 (m, 4H), 2.03–1.78 (m, 4H), 1.41–1.01 (m, 160H), 0.87–0.78 (m, 24H). 13C NMR (125 MHz, CDCl3): δ 163.79, 163.73, 163.56, 163.40, 163.29, 148.82, 141.25, 135.84, 134.84, 134.66, 134.66, 134.09, 133.39, 131.71, 131.47, 131.09, 130.99, 130.80, 129.43, 129.32, 128.99, 128.27, 127.87, 127.39, 126.24, 125.97, 124.26, 123.96, 123.58, 123.27, 122.90, 122.12, 120.07, 45.08, 44.83, 44.72, 37.01, 36.77, 36.65, 36.43, 32.13, 32.11, 32.10, 31.95, 31.75, 31.70, 31.61, 30.31, 30.27, 30.22, 30.19, 29.90, 29.86, 29.81, 29.75, 29.67, 29.51, 26.76, 26.73, 26.68, 26.58, 26.50, 26.41, 26.34, 22.88, 14.31, 14.30, 14.25 (16 sp2 and 61 alkyl resonances were not observed, presumably due to overlap). HRMS (MALDI-TOF): calcd for C144H211N4O8 (MH+), 2124.6227; found, 2124.6300. Anal. Calcd for C144H210N4O8: C, 81.38; H, 9.96; N, 2.64. Found: C, 81.24; H, 9.78; N, 2.74.
N,N′,N″,N‴-Tetra(2-decyltetradecyl)-[2,2′-biperylene]-3,4:9,10;3′,4′:9′,10′-tetrakis(dicarboximide), 3a
Compound 3a was synthesized in a similar manner to 1a, using S4a (0.734 g, 0.64 mmol), copper (0.41 g, 6.4 mmol), toluene (17 mL), and DMSO (23 mL), and refluxed overnight to give a red solid (0.232 g, 34%). Mp (DSC): 275 °C. 1H NMR (500 MHz, CDCl3, 325 K): δ 8.75–8.40 (m, 14H), 4.12–4.04 (m, 4H), 3.92–3.72 (m, 4H), 2.05–1.93 (m, 2H), 1.89–1.78 (m, 2H), 1.41–1.04 (m, 160H), 0.86–0.08 (m, 24H). 13C{1H} NMR (125 MHz, CDCl3): δ 165.33, 163.69, 163.51, 148.44, 134.36, 134.34, 134.05, 131.63, 130.26, 129.32, 126.30, 123.45, 44.86, 44.41, 36.92, 36.77, 32.13, 32.12, 31.93, 31.71, 30.33, 30.26, 29.91, 29.88, 29.84, 29.77, 29.73, 29.57, 29.55, 29.48, 26.69, 26.38, 22.89, 22.86, 22.83, 14.31, 14.29 (12 sp2 and 23 alkyl resonances were not observed, presumably due to overlap). HRMS (MALDI-TOF): calcd for C144H211N4O8 (MH+), 2124.6227; found, 2124.6232. Anal. Calcd for C144H210N4O8: C, 81.38; H, 9.96; N, 2.64. Found: C, 81.54; H, 9.84; N, 2.71.
N,N′,N″,N‴-Tetra(undecan-6-yl)-[1,1′-biperylene]-3,4:9,10;3′,4′:9′,10′-tetrakis(dicarboximide), 1b
This compound was synthesized from S2b(22,40) according to the literature.22Tg (DSC): 132 °C. 1H NMR (400 MHz, CDCl3): δ 8.81–8.77 (m, 8H), 8.49 (d, J = 8 Hz, 2H), 8.19–8.16 (m, 4H), 5.13–5.00 (m, 4H), 2.22–2.14 (m, 8H), 1.80–1.75 (m, 8H), 1.23 (s, 48H), 0.78 (s, 24H). 13C{1H} NMR (100 MHz, CDCl3): δ 164.70, 164.61, 164.41, 164.32, 164.15, 164.01, 163.68, 163.49, 141.89, 134.23, 131.49, 131.37, 130.62, 130.60, 129.60, 128.86, 128.77, 127.50, 127.38, 124.01, 123.41, 54.94, 54.77, 32.35, 32.30, 31.71, 31.64, 31.62, 26.54, 22.49, 22.47, 13.98 (one aromatic and one alkyl resonance were not observed, presumably due to overlap, whereas the doubling of the carbonyl resonances relative to what is expected for the most symmetric possible conformer is consistent with other studies of PDIs with “swallowtail” substituents and attributable to the effects of restricted rotation about the N–CHR2 bonds28). HRMS (MALDI-TOF): calcd for C92H106N4O8 (M+): 1394.8011; found, 1394.8073. Anal. Calcd for C92H106N4O8: C, 79.16; H, 7.65; N, 4.01. Found: C, 78.90; H, 7.68; N, 3.93.
2-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)-N,N′-di(undecan-6-yl)perylene-3,4:9,10-bis(dicarboximide), S3b, and 2-Bromo-N,N′-di(undecan-6-yl)perylene-3,4:9,10-bis(dicarboximide), S4b
A mixture of S1b(53) (2.10 g, 3.00 mmol), (Ir(OMe)cod)2 (0.06 g, 0.09 mmol), bis(pinacolato)diboron (0.84 g, 3.30 mmol), tris(pentafluorophenyl)phosphine (0.19 g, 0.36 mmol), and 1,4-dioxane (60 mL) was deoxygenated by bubbling with argon for 30 min. The mixture was then stirred under argon at 110 °C for 24 h. The solvent was removed under reduced pressure after filtering through Celite. The residue (crude S3b) was dissolved in dioxane (200 mL), and aqueous CuBr2 (2.70 g, 12.1 mmol) and methanol (90 mL) were added. The mixture was heated at 120 °C for 12 h. After cooling to room temperature, the mixture was poured into water and extracted with dichloromethane. The organic phase was dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. The residue was purified by column chromatography (silica gel; 1:1 dichloromethane/hexane) to afford S4b as a red solid (0.72 g, 31%). 1H NMR (400 MHz, CDCl3): δ 8.73–8.69 (m, 4H), 8.59 (dd, J = 4 Hz, 2H), 8.54 (d, J = 8 Hz, 1H), 5.23–5.16 (m, 2H), 2.29–2.21 (m, 4H), 1.93–1.85 (m, 4H), 1.31–1.24 (m, 24H), 0.86–0.82 (m, 12H). 13C{1H} NMR (100 MHz, CDCl3): δ 164.47, 163.48, 163.27, 134.55, 134.21, 134.07, 134.03, 132.92, 131.31, 130.70, 129.38, 126.25, 125.63, 123.49, 123.32, 122.91, 55.29, 54.86, 32.23, 32.24, 31.75, 29.71, 26.69, 26.64, 22.57, 14.06 (8 sp2 and 2 alkyl resonances were not observed, presumably due to overlap). HRMS (MALDI-TOF): calcd for C46H54N2O4Br (MH+), 777.3267; found, 777.3239. Anal. Calcd for C46H53N2O4Br: C, 71.03; H, 6.87; N, 3.60. Found: C, 71.10; H, 7.08; N, 3.45.
N,N′,N″,N‴-Tetra(undecan-6-yl)-[2,2′-biperylene]-3,4:9,10;3′,4′:9′,10′-tetrakis(dicarboximide), 3b
This compound was synthesized in a similar manner to 1a, using S4b (0.39 g, 0.50 mmol), copper (0.32 g, 5.00 mmol), DMSO (8 mL), and toluene (5 mL), and heated to 100 °C for 5 h. The product was purified by column chromatography (silica gel; 1:1 dichloromethane/hexane) to give a red solid (0.22 g, 64%). Tg (DSC): 85 °C. 1H NMR (400 MHz, CDCl3): δ 8.80–8.71 (m, 8H), 8.56–8.47 (m, 4H), 8.37 (s, 2H), 5.18–4.86 (m, 4H), 2.25–1.74 (m, 16H), 1.27–1.19 (m, 48H), 0.84–0.76 (m, 24H). 13C{1H} NMR (100 MHz, CDCl3): δ 164.5 (br), 164.4 (br), 163.5 (b), 148.7 (br), 134.52, 134.15, 133.8 (br), 133.6 (br), 132.3 (br), 131.9 (br), 131.6 (br), 131.2 (br), 130.8 (br), 130.53, 129.42, 126.36, 126.13, 124.9 (br), 124.3 (br), 124.0 (br), 123.17, 122.99, 119.31 (br), 54.75, 54.56, 32.31, 32.28, 32.11, 31.73, 31.57, 26.63, 26.54, 22.60, 22.55, 22.45, 14.03, 13.98. HRMS (MALDI-TOF): calcd for C92H106N4O8 (M+), 1394.8011; found, 1394.8122. Anal. Calcd for C92H106N4O8: C, 79.16; H, 7.65; N, 4.01. Found: C, 79.28; H, 7.63; N, 3.95.
Solar-Cell Fabrication and Testing
ITO-coated glass substrates were ultrasonically cleaned in detergent (sodium dodecyl sulfate), deionized water, acetone, and isopropanol in successive steps. The substrates were treated with UV–ozone for 10 min before use. A thin layer of sol–gel ZnO (ca. 10 nm) was spin-coated onto precleaned ITO-coated glass substrates at 4000 rpm and then annealed at 150 °C for 10 min in air. The ZnO precursor solution was prepared by dissolving Zn(OAc)2·2H2O and ethanolamine in 2-methoxyethanol and then stirred overnight. A 24 mg/mL total concentration solution of PTB7-Th and the relevant bi(PDI) in chlorobenzene with different mass ratios and additive concentrations was spin-cast on ZnO layer in a nitrogen-filled glovebox. At the final stage, the substrates were transferred to high vacuum, and MoO3 (10 nm) topped with silver (100 nm) was thermally evaporated onto the active layer. The active area defined by shadow masks was 0.1 cm2. The current density–voltage characteristics of the photovoltaic devices were measured in the glovebox using a Keithley 2400 source meter. A xenon arc lamp (300 W) was used as the light source, the intensity of which was calibrated using a KG5-filtered Si cell. The solar-cell performance was measured using an Air Mass 1.5 G solar simulator with an irradiation intensity of 100 mW/cm2. The thickness of films was measured by a KLA-Tencor P-15 profiler.
DFT Calculations
DFT calculations were carried out using the B3LYP functional,54 which combines Becke’s three-parameter hybrid-exchange functional55 with the Lee–Yang–Parr correlation functional,56 and the 6-31G** basis set as implemented within the Spartan’14 package.57 Frequency calculations on the optimized geometries indicated no imaginary frequencies.
Acknowledgments
This work was supported by the Department of the Navy, Office of Naval Research Award No. N00014-14-1-0580 (CAOP MURI). Y.F. thanks the State-Sponsored Scholarship for Graduate Students from China Scholarship Council. The authors thank Yuting Gao for help with some of the synthetic work and Timothy Parker for help with the DFT calculations.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00537.
PDI atom numbering and definitions of torsion angles; DSC data; additional electrochemical and optical data; additional data characterizing OPV devices and blends; NMR spectra for the bi(PDI)s and new intermediates; additional data and plots from DFT calculations; and Cartesian coordinates for minimized structures (PDF)
Author Present Address
§ Department of Radiology, Optical Radiology Laboratory, Washington University in St. Louis, School of Medicine, 4515 McKinley Avenue, St. Louis, Missouri 63011, United States (K.Z.).
Author Present Address
∥ National Institute of Standards and Technology, 100 Bureau Drive, Gaithersburg, Maryland 20899, United States (S.Z.).
The authors declare no competing financial interest.
Footnotes
In addition to the compounds shown in Figure 1, halo-substituted derivatives have been reported as precursors to Ig–i but have not been studied extensively spectroscopically and electrochemically or in devices.28,32
1,1′-Bi(PDIs) have, however, been compared as OPV acceptors with analogues with direct N,N′-links.26,27
Comparisons between the spectra of III, IV and VI, VII are complicated by the effects of aggregation, even in a dilute solution.39
Expected JSC values obtained from convolution of the four EQE spectra shown in Figure 4 with the AM1.5 spectrum are 12.4, 13.1, 11.4, and 12.4 mA cm–2, roughly consistent with the values obtained by J–V measurements (note that the values given in Table 3 are averages over eight devices).
Supplementary Material
References
- Würthner F. Perylene Bisimide Dyes as Versatile Building Blocks for Functional Supramolecular Architectures. Chem. Commun. 2004, 1564–1579. 10.1039/B401630K. [DOI] [PubMed] [Google Scholar]
- Zhan X.; Facchetti A.; Barlow S.; Marks T. J.; Ratner M. A.; Wasielewski M. R.; Marder S. R. Rylene and Related Diimides for Organic Electronics. Adv. Mater. 2011, 23, 268–284. 10.1002/adma.201001402. [DOI] [PubMed] [Google Scholar]
- Huang C.; Barlow S.; Marder S. R. Perylene-3,4,9,10-Tetracarboxylic Acid Diimides: Synthesis, Physical Properties, and Use in Organic Electronics. J. Org. Chem. 2011, 76, 2386–2407. 10.1021/jo2001963. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Zhang G.; Cai Z.; Chen X.; Luo H.; Li Y.; Wang J.; Zhang D. New Organic Semiconductors with Imide/Amide-Containing Molecular Systems. Adv. Mater. 2014, 26, 6965–6977. 10.1002/adma.201305718. [DOI] [PubMed] [Google Scholar]
- Fernández-Lázaro F.; Zink-Lorre N.; Sastre-Santos Á. Perylenediimides as Non-Fullerene Acceptors in Bulk-Heterojunction Solar Cells (BHJSCs). J. Mater. Chem. A 2016, 4, 9336–9346. 10.1039/C6TA02045C. [DOI] [Google Scholar]
- Tang C. W. Two-Layer Organic Photovoltaic Cell. Appl. Phys. Lett. 1986, 48, 183–185. 10.1063/1.96937. [DOI] [Google Scholar]
- Sun J.-P.; Hendsbee A. D.; Dobson A. J.; Welch G. C.; Hill I. G. Perylene Diimide Based All Small-Molecule Organic Solar Cells: Impact of Branched-Alkyl Side Chains on Solubility, Photophysics, Self- Assembly, and Photovoltaic Parameters. Org. Electron. 2016, 35, 151–157. 10.1016/j.orgel.2016.05.012. [DOI] [Google Scholar]
- Sharma G. D.; Balraju P.; Mikroyannidis J. A.; Stylianakis M. M. Bulk Heterojunction Organic Photovoltaic Devices Based on Low Band Gap Small Molecule BTD-TNP and Perylene–Anthracene Diimide. Sol. Energy Mater. Sol. Cells 2009, 93, 2025–2028. 10.1016/j.solmat.2009.08.003. [DOI] [Google Scholar]
- Schmidt-Mende L.; Fechtenkötter A.; Müllen K.; Moons E.; Friend R. H.; MacKenzie J. D. Self-Organized Discotic Liquid Crystals for High-Efficiency Organic Photovoltaics. Science 2001, 293, 1119–1122. 10.1126/science.293.5532.1119. [DOI] [PubMed] [Google Scholar]
- Zhan X.; Tan Z.; Domercq B.; An Z.; Zhang X.; Barlow S.; Li Y.; Zhu D.; Kippelen B.; Marder S. R. A High-Mobility Electron-Transport Polymer with Broad Absorption and Its Use in Field-Effect Transistors and All-Polymer Solar Cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. 10.1021/ja071760d. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Cirpan A.; Russell T. P.; Emrick T. Donor–Acceptor Poly(Thiophene-Block-Perylene Diimide) Copolymers: Synthesis and Solar Cell Fabrication. Macromolecules 2009, 42, 1079–1082. 10.1021/ma801504e. [DOI] [Google Scholar]
- Lin Y.; Zhan X. Non-Fullerene Acceptors for Organic Photovoltaics: An Emerging Horizon. Mater. Horiz. 2014, 1, 470–488. 10.1039/C4MH00042K. [DOI] [Google Scholar]
- Eftaiha F. E.; Sun J.-P.; Hill I. G.; Welch G. C. Recent Advances of Non-Fullerene, Small Molecular Acceptors for Solution Processed Bulk Heterojunction Solar Cells. J. Mater. Chem. A 2014, 2, 1201–1213. 10.1039/C3TA14236A. [DOI] [Google Scholar]
- Nielsen C. B.; Holliday S.; Chen H.-Y.; Cryer S. J.; McCulloch I. Non-Fullerene Electron Acceptors for Use in Organic Solar Cells. Acc. Chem. Res. 2015, 48, 2803–2812. 10.1021/acs.accounts.5b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvé G.; Fernando R. Beyond Fullerenes: Designing Alternative Molecular Electron Acceptors for Solution-Processable Bulk Heterojunction Organic Photovoltaics. J. Phys. Chem. Lett. 2015, 6, 3770–3780. 10.1021/acs.jpclett.5b01471. [DOI] [PubMed] [Google Scholar]
- Zhan C.; Zhang X.; Yao J. New Advances in Non-Fullerene Acceptor Based Organic Solar Cells. RSC Adv. 2015, 5, 93002–93026. 10.1039/C5RA17715D. [DOI] [Google Scholar]
- Zhan C.; Yao J. More Than Conformational “Twisting” or “Coplanarity”: Molecular Strategies for Designing High-Efficiency Nonfullerene Organic Solar Cells. Chem. Mater. 2016, 28, 1948–1964. 10.1021/acs.chemmater.5b04339. [DOI] [Google Scholar]
- Lin Y.; Zhan X. Oligomer Molecules for Efficient Organic Photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. 10.1021/acs.accounts.5b00363. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Wang Y.; Wang J.; Hou J.; Li Y.; Zhu D.; Zhan X. A Star-Shaped Perylene Diimide Electron Acceptor for High-Performance Organic Solar Cells. Adv. Mater. 2014, 26, 5137–5142. 10.1002/adma.201400525. [DOI] [PubMed] [Google Scholar]
- Jiang W.; Xiao C.; Hao L.; Wang Z.; Ceymann H.; Lambert C.; Di Motta S.; Negri F. Localization/Delocalization of Charges in Bay-Linked Perylene Bisimides. Chem. Eur. J. 2012, 18, 6764–6775. 10.1002/chem.201103954. [DOI] [PubMed] [Google Scholar]
- Horinouchi H.; Sakai H.; Araki Y.; Sakanoue T.; Takenobu T.; Wada T.; Tkachenko N. V.; Hasobe T. Controllable Electronic Structures and Photoinduced Processes of Bay-Linked Perylenediimide Dimers and a Ferrocene-Linked Triad. Chem. Eur. J. 2016, 22, 9631–9641. 10.1002/chem.201601058. [DOI] [PubMed] [Google Scholar]
- Jiang W.; Ye L.; Li X.; Xiao C.; Tan F.; Zhao W.; Hou J.; Wang Z. Bay-Linked Perylene Bisimides as Promising Non-Fullerene Acceptors for Organic Solar Cells. Chem. Commun. 2014, 50, 1024–1026. 10.1039/C3CC47204C. [DOI] [PubMed] [Google Scholar]
- Ye L.; Jiang W.; Zhao W.; Zhang S.; Qian D.; Wang Z.; Hou J. Selecting a Donor Polymer for Realizing Favorable Morphology in Efficient Non-Fullerene Acceptor-Based Solar Cells. Small 2014, 10, 4658–4663. 10.1002/smll.201401082. [DOI] [PubMed] [Google Scholar]
- Zang Y.; Li C. Z.; Chueh C. C.; Williams S. T.; Jiang W.; Wang Z. H.; Yu J. S.; Jen A. K. Y. Integrated Molecular, Interfacial, and Device Engineering Towards High-Performance Non-Fullerene Based Organic Solar Cells. Adv. Mater. 2014, 26, 5708–5714. 10.1002/adma.201401992. [DOI] [PubMed] [Google Scholar]
- Wang J.; Yao Y.; Dai S.; Zhang X.; Wang W.; He Q.; Han L.; Lin Y.; Zhan X. Oligothiophene-Bridged Perylene Diimide Dimers for Fullerene-Free Polymer Solar Cells: Effect of Bridge Length. J. Mater. Chem. A 2015, 3, 13000–13010. 10.1039/C5TA02589C. [DOI] [Google Scholar]
- Ye L.; Sun K.; Jiang W.; Zhang S.; Zhao W.; Yao H.; Wang Z.; Hou J. Enhanced Efficiency in Fullerene-Free Polymer Solar Cell by Incorporating Fine-Designed Donor and Acceptor Materials. ACS Appl. Mater. Interfaces 2015, 7, 9274–9280. 10.1021/acsami.5b02012. [DOI] [PubMed] [Google Scholar]
- Wu C.-H.; Chueh C.-C.; Xi Y.-Y.; Zhong H.-L.; Gao G.-P.; Wang Z.-H.; Pozzo L. D.; Wen T.-C.; Jen A. K.-Y. Influence of Molecular Geometry of Perylene Diimide Dimers and Polymers on Bulk Heterojunction Morphology toward High-Performance Nonfullerene Polymer Solar Cells. Adv. Funct. Mater. 2015, 25, 5326–5332. 10.1002/adfm.201501971. [DOI] [Google Scholar]
- Sun D.; Meng D.; Cai Y.; Fan B.; Li Y.; Jiang W.; Huo L.; Sun Y.; Wang Z. Non-Fullerene-Acceptor-Based Bulk-Heterojunction Organic Solar Cells with Efficiency over 7%. J. Am. Chem. Soc. 2015, 137, 11156–11162. 10.1021/jacs.5b06414. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Li Y.; Lin H.; Liu Y.; Jiang K.; Mu C.; Ma T.; Lai J. Y. L.; Hu H.; Yu D.; Yan H. High-Efficiency Non-Fullerene Organic Solar Cells Enabled by a Difluorobenzothiadiazole-Based Donor Polymer Combined with a Properly Matched Small Molecule Acceptor. Energy Environ. Sci. 2015, 8, 520–525. 10.1039/C4EE02990A. [DOI] [Google Scholar]
- Feng G.; Xu Y.; Zhang J.; Wang Z.; Zhou Y.; Li Y.; Wei Z.; Li C.; Li W. All-Small-Molecule Organic Solar Cells Based on an Electron Donor Incorporating Binary Electron Deficient Units. J. Mater. Chem. A 2016, 4, 6056–6063. 10.1039/C5TA10430K. [DOI] [Google Scholar]
- Meng D.; Sun D.; Zhong C.; Liu T.; Fan B.; Huo L.; Li Y.; Jiang W.; Choi H.; Kim T.; Kim J. Y.; Sun Y.; Wang Z.; Heeger A. J. High-Performance Solution-Processed Non-Fullerene Organic Solar Cells Based on Selenophene-Containing Perylene Bisimide Acceptor. J. Am. Chem. Soc. 2016, 138, 375–380. 10.1021/jacs.5b11149. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Yang F.; Ji Y.; Wu Y.; Zhang A.; Li C.; Li W. A Perylene Bisimide Derivative with a LUMO Level of −4.56 eV for Non-Fullerene Solar Cells. J. Mater. Chem. C 2016, 4, 4134–4137. 10.1039/C6TC01045H. [DOI] [Google Scholar]
- Nakazono S.; Easwaramoorthi S.; Kim D.; Shinokubo H.; Osuka A. Synthesis of Arylated Perylene Bisimides through C–H Bond Cleavage under Ruthenium Catalysis. Org. Lett. 2009, 11, 5426–5429. 10.1021/ol902271b. [DOI] [PubMed] [Google Scholar]
- Bullock J. E.; Vagnini M. T.; Ramanan C.; Co D. T.; Wilson T. M.; Dicke J. W.; Marks T. J.; Wasielewski M. R. Photophysics and Redox Properties of Rylene Imide and Diimide Dyes Alkylated Ortho to the Imide Groups. J. Phys. Chem. B 2010, 114, 1794–1802. 10.1021/jp908679c. [DOI] [PubMed] [Google Scholar]
- Teraoka T.; Hiroto S.; Shinokubo H. Iridium-Catalyzed Direct Tetraborylation of Perylene Bisimides. Org. Lett. 2011, 13, 2532–2535. 10.1021/ol2004534. [DOI] [PubMed] [Google Scholar]
- Battagliarin G.; Li C.; Enkelmann V.; Müllen K. 2,5,8,11-Tetraboronic Ester Perylenediimides: A Next Generation Building Block for Dye-Stuff Synthesis. Org. Lett. 2011, 13, 3012–3015. 10.1021/ol2008457. [DOI] [PubMed] [Google Scholar]
- Battagliarin G.; Zhao Y.; Li C.; Müllen K. Efficient Tuning of Lumo Levels of 2,5,8,11-Substituted Perylenediimides Via Copper Catalyzed Reactions. Org. Lett. 2011, 13, 3399–3401. 10.1021/ol201144w. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Singh S.; Hwang D. K.; Barlow S.; Kippelen B.; Marder S. R. 2-Bromo Perylene Diimide: Synthesis Using C–H Activation and Use in the Synthesis of Bis(Perylene Diimide)-Donor Electron-Transport Materials. J. Mater. Chem. C 2013, 1, 5093–5100. 10.1039/c3tc30918e. [DOI] [Google Scholar]
- Zhao D.; Wu Q.; Cai Z.; Zheng T.; Chen W.; Lu J.; Yu L. Electron Acceptors Based on A-Substituted Perylene Diimide (PDI) for Organic Solar Cells. Chem. Mater. 2016, 28, 1139–1146. 10.1021/acs.chemmater.5b04570. [DOI] [Google Scholar]
- Schmidt C. D.; Lang N.; Jux N.; Hirsch A. A Facile Route to Water-Soluble Coronenes and Benzo[ghi]Perylenes. Chem. Eur. J. 2011, 17, 5289–5299. 10.1002/chem.201003232. [DOI] [PubMed] [Google Scholar]
- Ito S.; Hiroto S.; Shinokubo H. Synthesis of Pyridine-Fused Perylene Imides with an Amidine Moiety for Hydrogen Bonding. Org. Lett. 2013, 15, 3110–3113. 10.1021/ol401316q. [DOI] [PubMed] [Google Scholar]
- Wescott L. D.; Mattern D. L. Donor-σ-Acceptor Molecules Incorporating a Nonadecyl-Swallowtailed Perylenediimide Acceptor. J. Org. Chem. 2003, 68, 10058–10066. 10.1021/jo035409w. [DOI] [PubMed] [Google Scholar]
- Huang C.; Potscavage W. J.; Tiwari S. P.; Sutcu S.; Barlow S.; Kippelen B.; Marder S. R. Polynorbornenes with Pendant Perylene Diimides for Organic Electronic Applications. Polym. Chem. 2012, 3, 2996–3006. 10.1039/c2py20401k. [DOI] [Google Scholar]
- Würthner F.; Stepanenko V.; Chen Z.; Saha-Möller C. R.; Kocher N.; Stalke D. Preparation and Characterization of Regioisomerically Pure 1,7-Disubstituted Perylene Bisimide Dyes. J. Org. Chem. 2004, 69, 7933–7939. 10.1021/jo048880d. [DOI] [PubMed] [Google Scholar]
- Chao C.-C.; Leung M.-K.; Su Y. O.; Chiu K.-Y.; Lin T.-H.; Shieh S.-J.; Lin S.-C. Photophysical and Electrochemical Properties of 1,7-Diaryl-Substituted Perylene Diimides. J. Org. Chem. 2005, 70, 4323–4331. 10.1021/jo050001f. [DOI] [PubMed] [Google Scholar]
- Schmidt R.; Oh J. H.; Sun Y.-S.; Deppisch M.; Krause A.-M.; Radacki K.; Braunschweig H.; Könemann M.; Erk P.; Bao Z.; Würthner F. High-Performance Air-Stable n-Channel Organic Thin Film Transistors Based on Halogenated Perylene Bisimide Semiconductors. J. Am. Chem. Soc. 2009, 131, 6215–6228. 10.1021/ja901077a. [DOI] [PubMed] [Google Scholar]
- Li A. D. Q.; Wang W.; Wang L.-Q. Folding versus Self-Assembling. Chem. Eur. J. 2003, 9, 4594–4601. 10.1002/chem.200305025. [DOI] [PubMed] [Google Scholar]
- Winter R. F. Half-Wave Potential Splittings ΔE1/2 as a Measure of Electronic Coupling in Mixed-Valent Systems: Triumphs and Defeats. Organometallics 2014, 33, 4517–4536. 10.1021/om500029x. [DOI] [Google Scholar]
- Liao S.-H.; Jhuo H.-J.; Cheng Y.-S.; Chen S.-A. Fullerene Derivative-Doped Zinc Oxide Nanofi Lm as the Cathode of Inverted Polymer Solar Cells with Low-Bandgap Polymer (PTB7-Th) for High Performance. Adv. Mater. 2013, 25, 4766–4771. 10.1002/adma.201301476. [DOI] [PubMed] [Google Scholar]
- Shoaee S.; Clarke T. M.; Huang C.; Barlow S.; Marder S. R.; Heeney M.; McCulloch I.; Durrant J. R. Acceptor Energy Level Control of Charge Photogeneration in Organic Donor/Acceptor Blends. J. Am. Chem. Soc. 2010, 132, 12919–12926. 10.1021/ja1042726. [DOI] [PubMed] [Google Scholar]
- Zhan X.; Zhang J.; Tang S.; Lin Y.; Zhao M.; Yang J.; Zhang H.-L.; Peng Q.; Yu G.; Li Z. Pyrene Fused Perylene Diimides: Synthesis, Characterization and Applications in Organic Field-Effect Transistors and Optical Limiting with High Performance. Chem. Commun. 2015, 51, 7156–7159. 10.1039/C5CC00966A. [DOI] [PubMed] [Google Scholar]
- Odom S. A.; Kelley R. F.; Ohira S.; Ensley T. R.; Huang C.; Padilha L. A.; Webster S.; Coropceanu V.; Barlow S.; Hagan D. J.; Van Stryland E. W.; Brédas J. L.; Anderson H. L.; Wasielewski M. R.; Marder S. R. Photophysical Properties of an Alkyne-Bridged Bis(Zinc Porphyrin)-Perylene Bis(Dicarboximide) Derivative. J. Phys. Chem. A 2009, 113, 10826–10832. 10.1021/jp905214g. [DOI] [PubMed] [Google Scholar]
- Demmig S.; Langhals H. Leichtlösliche, Lichtechte Perylen-Fluoreszenzfarbstoffe. Chem. Ber. 1988, 121, 225–230. 10.1002/cber.19881210205. [DOI] [Google Scholar]
- Stephens P. J.; Devlin F. J.; Chabalowski C. F.; Frisch M. J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. 10.1021/j100096a001. [DOI] [Google Scholar]
- Becke A. D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Spartan’14; Wavefunction Inc.: Irvine, CA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.