Abstract

The ultrathin two-dimensional nanosheets of layered transition-metal dichalcogenides (TMDs) have attracted great interest as an important class of materials for fundamental research and technological applications. Solution-phase processes are highly desirable to produce a large amount of TMD nanosheets for applications in energy conversion and energy storage such as catalysis, electronics, rechargeable batteries, and capacitors. Here, we report a rapid exfoliation by supercritical fluid processing for the production of MoS2 and MoSe2 nanosheets. Atomic-resolution high-angle annular dark-field imaging reveals high-quality exfoliated MoS2 and MoSe2 nanosheets with hexagonal structures, which retain their 2H stacking sequence. The obtained nanosheets were tested for their electrochemical performance in a hybrid Mg–Li-ion battery as a proof of functionality. The MoS2 and MoSe2 nanosheets exhibited the specific capacities of 81 and 55 mA h g–1, respectively, at a current rate of 20 mA g–1.
Introduction
Transition-metal dichalcogenides (TMDs) are an important class of materials, which are expected to be used for hydrogen storage, transistors, lubricants, catalysis, batteries, and photovoltaic devices.1−5 TMDs have the chemical composition MX2, where M is a transition metal (Ti, Nb, Ta, Mo, and W) and X is a chalcogen (e.g., S, Se, and Te). The structure of TMDs consists of repeating layers with strong covalent bonding within layers and weak van der Waals interaction between layers, which are responsible for their two-dimensional (2D) physicochemical properties.6−9 Their outstanding properties have been reported in various applications including photoluminescence, field-effect transistor, and gas sensor.10−15 The catalytic and electronic properties of these materials are strongly dependent on the coordination features of metal and chalcogen as well as the number of layers.16−18 TMDs can be metallic, semimetallic, or semiconductor,17 and their band gap varies in the range of 1.2–1.8 eV.18 Furthermore, TMDs could be intergrown with graphene with excellent electrochemical properties, which make them suitable for versatile electronic applications including battery electrodes.19−24
TMD nanosheets can be synthesized by top-down or bottom-up methods. In bottom-up methods, TMDs can be selectively and epitaxially grown on metal oxide substrates (MoO3 and SiO2) using chemical vapor deposition.25−29 In top-down methods, stacked layers were separated apart to yield single-layer nanosheets. For example, TMDs with high quality and high crystallinity have been synthesized by micromechanical cleavage.30−33 TMD nanosheets can also be synthesized by the exfoliation of Li intercalation compounds.34 However, the disadvantages of these methods are labor consumption, sensitivity to ambient conditions, and structural deformations and alteration of electronic properties.35 The direct liquid-phase exfoliation of bulk TMDs via chemical method, with sonication, has proved to be a suitable method for the industrial-scale production of nanosheets.36−41 Few-layer TMD nanosheets could be prepared by direct exfoliation in certain stabilizing organic solvent or aqueous surfactant solutions under mild sonication38 or high-shear mixing.41
Recently, supercritical fluid (SCF) process has been applied for the production of high-quality, high-yield, and processable graphene and inorganic nanosheets for technological applications.42−44 This rapid and facile one-pot exfoliation method resulted in a highly conductive pristine graphene sheet maintaining its original nature.42 Recently, MoS2 has been exfoliated into nanoscrolls by SCF.45 Here, we extend our method for the exfoliation of TMD-layered materials into high-quality nanosheets. We choose the layered materials including MoS2 and MoSe2 as typical TMDs to demonstrate the ability of our method in the exfoliation of layer compounds. Our procedure is simple and fast; it involves the direct one-pot exfoliation of TMD crystals down to few (1–10)-layer nanosheets by the SCF of dimethylformamide (DMF). The nanosheets were characterized using atomic-resolution high-angle annular dark-field imaging (HAADF), revealing the atomic structure of the monolayer MoS2 and MoSe2 nanosheets with hexagonal structures. The obtained nanosheets showed good electrochemical performance in a hybrid Mg–Li-ion battery. The MoS2 and MoSe2 nanosheets exhibited the specific capacities of 81 and 55 mA h g–1, respectively, at a current rate of 20 mA g–1.
Results and Discussion
Characterization of Exfoliated Nanosheets
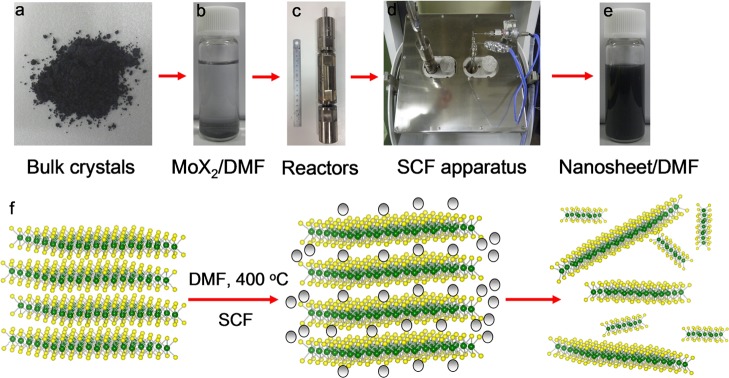
Liquid-phase exfoliation of layer materials in organic solvents with assisted sonication is a very useful approach for the preparation of few-layer graphene and TMDs.36−41 The strong interaction between the solvent and the bulk materials subsidizes the energy requirement for exfoliation and subsequent solvation. For the high-yield exfoliation, the solvent that has high diffusivity and high solvation power is required. The SCF solvent offers advantageous properties including low interfacial tension, excellent wetting of surfaces, and high diffusion coefficients, which makes it a superior medium for diffusion in between the layers of TMDs and its expansion (Figures 1 and S1). To proceed exfoliation, we dispersed bulk crystals into a solvent, namely, DMF, and heated above its critical temperature (377 °C). Figure 1 shows the scheme for the SCF exfoliation of bulk MoS2 and MoSe2 into nanosheets. The exfoliation of TMDs down to a few layers (1–10 layers) was achieved in SCFs in a short reaction time of 1 h. Furthermore, this rapid high-yield exfoliation could produce nanosheets retaining their original pristine structure without any coordination transformation due to an intercalant-free processing.
Figure 1.
Exfoliation of TMDs by SCF processing. The photographs of (a) bulk MoS2 crystals, (b) bulk crystals in DMF, (c) SCF reactor, (d) designed tube furnace (AKICO, Japan) with controlled temperature and pressure, and (e) resultant MoS2 nanosheets dispersed in DMF. (f) Schematic illustration for the mechanism of exfoliation of TMDs by SCFs.
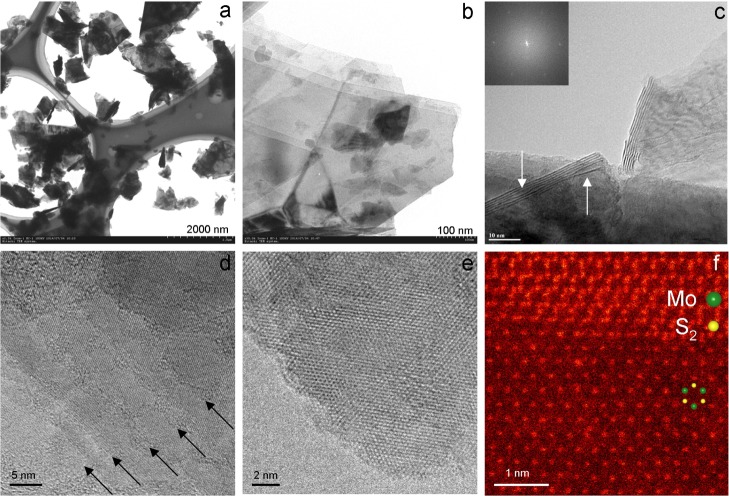
Figure 2 shows the representative electron microscopy images of the MoS2 nanosheets. The low-magnification transmission electron microscopy (TEM) image in Figure 2a indicates the presence of the large number of thin and monodispersed nanosheets with a lateral size of 0.5 to 1 μm (see Figure S1 for more TEM images). The high-magnification image shows the nanosheets with a small number of stacked monolayers (Figure 2b). From TEM images, the bright and transparent contrast on each particle can be observed, which is a typical feature of the thin nanosheet nanostructures. The sample was further studied using high-resolution TEM (HRTEM) to understand the crystal structure of the MoS2 nanosheets. The fast Fourier transform (FFT) pattern taken from a nanosheet is shown in the inset of Figure 2c. The FFT pattern consisting of spots clearly shows hexagonal symmetry. The pattern can be indexed to the [001] zone axis of the hexagonal structure, confirming that MoS2 is exfoliated along the ab planes with exposing large {001} facets. HRTEM analysis of the nanosheet edges reveals that the majority of the nanosheets consists of few layers. The individual layer edges can be observed near the outer region of the particle, indicated by the dark arrows (Figure 2d). The expanse of layer along the c-axis due to SCF treatment can be observed, indicated by the white arrows in Figure 2c. The HRTEM image in Figure 2e clearly shows a well-defined hexagonal symmetry characteristic. The characterization by TEM confirms the high-quality nanosheets produced by SCF exfoliation. The expected hexagonally symmetric structure of MoS2 nanosheets is clearly observed in the atomic-resolution HAADF-STEM image in Figure 2f. In HAADF imaging, the intensity of atomic columns is directly related to the atomic number and the number of atoms in the atomic column. Thus, in the ADF image of monolayer MoS2, Mo columns (Z = 42) are expected to have brighter contrasts, and the dimmer contrasts are sites of (S2) columns (Z = 16), as indicated by the overlaid atomic arrays in Figure 2f.25−27,35,46 In the image of multilayer nanosheets, the 2H phase exhibits the honeycomb pattern, whereas 1T and 3R phases show hexagonal patterns with a reduced lattice spacing of 1.83 Å,35,40 in which a single atomic column locates around each of the 2D Bravais lattice points.40 In the ADF image shown in Figure S2, the honeycomb pattern is readily identified, indicating the presence of 2H phase. The ADF imaging clearly indicates that MoS2 retained its original 2H phase and that the transformation of 2H → 1T phase does not occur during SCF exfoliation.
Figure 2.
Electron microscopy characterization of MoS2 nanosheets. (a) Low-magnification image. (b) Typical TEM image. (c) HRTEM image of nanosheets with six layers. The expanded layers are indicated by the white arrows (the inset corresponding to FFT pattern clearly showing hexagonal symmetry). (d) HRTEM image of a thin MoS2 nanosheet showing the layer edges indicated by the dark arrows. (e) HRTEM image of a thin sheet clearly illustrates the hexagonal structure. (f) Atomic-resolution HAADF-STEM image of the monolayer in the bottom part. The superimposed atomic arrays on the inset indicate the locations of atoms.
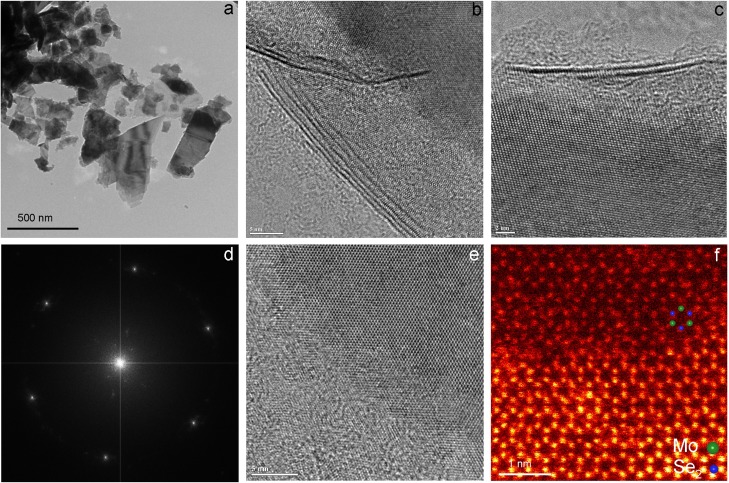
Because of the similarity in the crystal structure, it is expected that the synthesis method can be extended for the exfoliation of other TMDs. Figure 3 shows the results from the characterization using electron microscopies of the MoSe2 nanosheets synthesized by the SCF exfoliation in DMF. The TEM image (Figure 3a) demonstrates the formation of nanosheets with a lateral size of 100–500 nm. The exfoliated nanosheets composed of two to five layers are evidenced from the HRTEM images in Figure 3b,c. Figure 3d shows the selected area electron-diffraction (SAED) pattern of a nanosheet, corresponding to the [001] zone axis of the single-crystal MoSe2 structure. The HRTEM image taken on a portion of the MoSe2 nanosheets displays the crystal planes of {100} and {010} (Figure 3e). Figure 3f shows the typical HAADF-STEM image of the synthesized MoSe2 nanosheets. In the ADF image of the monolayer part of MoSe2, Se2 columns have stronger contrasts and Mo columns have dimmer contrasts, as indicated by the superimposed atomic arrays.28,29,47,48 Neighboring Mo and Se2 sites are separated by 1.98 Å, and the Mo–Mo distance is 5.46 Å. In the image of a multilayer MoSe2, the honeycomb atomic arrangement and the lattice constants as a = b = 0.33 nm can be observed, suggesting the presence of 2H stacking sequence in a multilayer MoSe2 (Figure S3).48 In this image, each column consists of both Mo and Se; thus, every column shows almost equal contrast.28
Figure 3.
Electron microscopy characterization of MoSe2 nanosheets. (a) Low-magnification image. (b,c) HRTEM images of nanosheets with four layers and two layers. HRTEM images of a thin MoSe2 nanosheet showing layer edges indicated by the white arrows showing layer edges. (d) HRTEM image of a thin nanosheet clearly illustrates the hexagonal structure. (e) Typical electron-diffraction pattern clearly showing hexagonal symmetry. (f) HAADF-STEM image of the monolayer in the top part with a structural model overlaid.
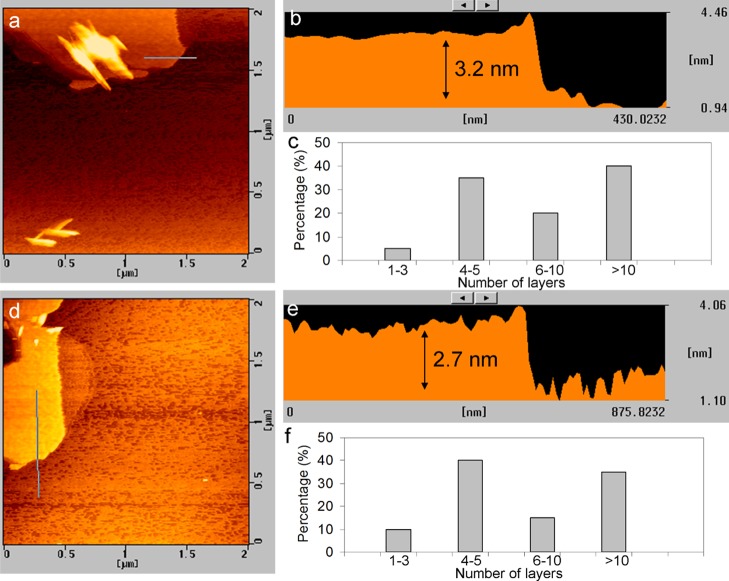
The thickness of the exfoliated sheets is characterized using atomic force microscopy (AFM). Figure 4 shows the typical tapping-mode AFM images of the exfoliated MoS2 and MoSe2 nanosheets deposited on a Si substrate by drop casting. Generally, the thicknesses of a single-layer MoS2 and MoSe2 are between 0.7 and 1.0 nm.29,34 The average topographic heights are around 2.7 to 3.2 nm, corresponding to the thickness of four- or five-layer nanosheets. Statistical analysis of the thin nanosheets (excluded the unexfoliated particles) using AFM reveals that 35–40% of the nanosheets comprised four to five layers (see the size histograms in Figure 4e,f). Additional AFM images with a height profile can be found in Figure S4.
Figure 4.
(a,d) AFM images of MoS2 and MoSe2 nanosheets. (b,e) Corresponding height profiles obtained along the lines indicated in the AFM images. (c,f) Thickness distribution histograms of the nanosheets, as estimated from AFM analysis.
Figure 5 shows the Mo 3d, S 2s, S 2p, and Se 3d spectra of the bulk and exfoliated MoS2 and MoSe2. The Mo 3d peak positions are at the binding energies of 229.6 and 232.8 eV. MoS2 shows two characteristic peaks that were fitted with the two distinct doublets (2p3/2 and 2p1/2) at the binding energies of 162.4 and 163.6 eV. These peaks arise from the 2H phase, and there are no peaks derived from the 1T phase at a lower binding energy in both Mo 3d and S 2p spectra.35 In addition, the peaks observed at the binding energies of 54.7 and 55.5 eV in the Se 3d spectra can be assigned to the Se 3d5/2 and Se 3d3/2 orbitals of divalent selenide ions (Se2–), indicating that the exfoliated MoSe2 is also derived from the 2H phase.
Figure 5.
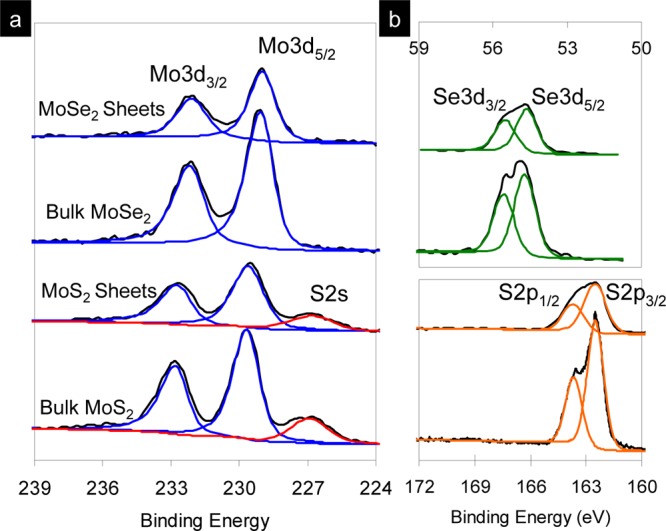
XPS spectra of the bulk and exfoliated MoS2 and MoSe2 showing (a) Mo 3d, S 2s and (b) S 2p and Se 3d core level peak regions.
Electrochemical Performance in a Rechargeable Hybrid Mg–Li-Ion Battery
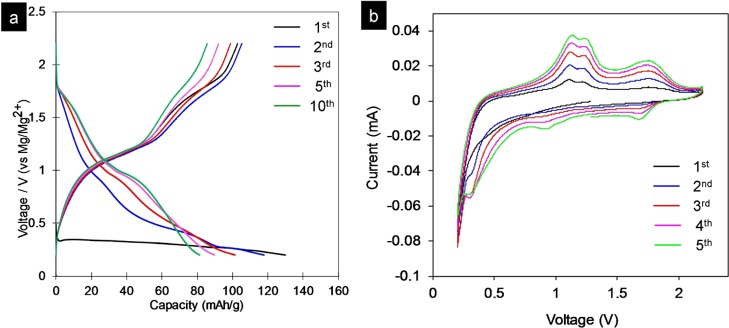
Recently, magnesium-ion batteries have attracted great attention as a prospective candidate for an energy storage system because of the abundance, stability, and high volumetric capacity of Mg (3832 mA h cm–3).49−55 Because of the slow diffusion of divalent Mg ions into the intercalation host, most of the cathode materials show low specific capacities or rapid capacity fade.55−58 The hybrid Mg–Li-ion battery, which involves lithiation/delithiation at the cathode and magnesiation/demagnesiation at the anode, has improved the rate capacity and discharge voltage.59,60 At the same time, 2D materials with remarkable electrochemical properties have emerged as novel electrode materials with high energy and power densities.61 Thus, several intercalation compounds showed high specific capacity and good rate performance, in which the layered TMDs such as TiS2 and MoS2 are promising because of their structural flexibility with a large layer spacing.62−64 In our previous report, MoS clusters have been found to be good electrode materials for the Mg–Li-ion battery.51 In this study, the synthesized exfoliated MoS2 and MoSe2 nanosheets were used as cathodes with Mg plate anodes for hybrid Mg–Li-ion batteries as a proof of the functional properties. When the synthesized exfoliated MoS2 was used for an Mg-ion battery in all-phenyl complex (APC) electrolytes, the electrode showed a capacity of 25 mA h g–1 (Figure S5). However, the capacity was much higher in hybrid Mg–Li-ion electrolytes. Figure 6a shows the typical charge/discharge voltage profiles of the MoS2 nanosheets at a current rate of 20 mA g–1 in the voltage window of 0.2–2.2 V versus Mg/Mg2+. During the first lithiation process (discharge), the discharge voltage fades rapidly with a discharge capacity of up to 135 mA h g–1. However, from the second lithiation process, the electrodes show a capacity of 118 mA h g–1 and remain as high as 81 mA h g–1 after 10 cycles. The electrochemical performances of MoS2 and MoSe2 nanosheets are medium among other materials such as MoS2/graphene synthesized by Hsu et al.62 This is because the electrode materials are pristine nanosheets, without any modification such as graphene coating. The further work on carbon coating on the exfoliated nanosheets is required for an enhanced electrode performance. Figure 6b presents the cyclic voltammogram (CV) curves of MoS2 nanosheets at a sweeping rate of 0.1 mV s–1 in the voltage window of 0.2–2.2 V versus Mg/Mg2+. In the first cycle, the electrode exhibits no clear cathodic peaks, two anodic peaks at 1.15 and 1.23 V, and one broadened anodic peak at 1.78 V. From the second cycle, the cathodic peaks were observed at 0.94 and 1.70 V. The first cathodic peak can be attributed to the lithiation of MoS2 into LixMoS2 by either intercalation65 or surface reaction (Figure S6).66 In the anodic scans, the oxidation peak can be ascribed to the conversion reaction: Mo + 2Li2S → MoS2 + 4 Li.
Figure 6.
Electrochemical performances of the synthesized MoS2 nanosheets in hybrid Mg–Li-ion batteries tested in the potential range of 0.2–2.2 V vs Mg/Mg2+. (a) Typical charge/discharge profiles at a current rate of 20 mA g–1 and (b) CV curves of the cell containing MoS2 nanosheets.
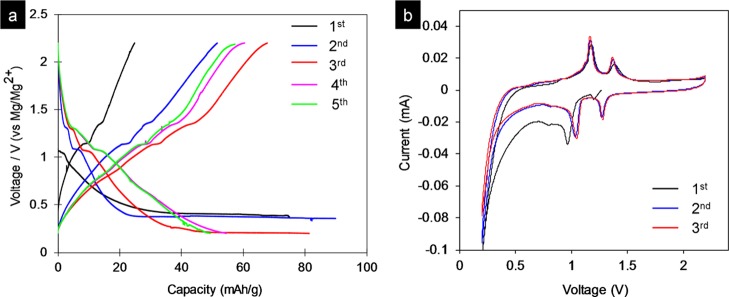
There have been many reports on the electrochemical performance of MoS2; however, the electrochemical properties of MoSe2 are less known.67,68 In this report, the electrochemical properties of MoSe2 nanosheets for the Mg–Li hybrid battery were investigated for the first time. The first five charge/discharge curves of the MoSe2 nanosheets at a current rate of 20 mA g–1 with a cutoff voltage window of 0.2 to 2.2 V versus Mg/Mg2+ are shown in Figure 7a. During the first lithiation process (discharge), the MoSe2 nanosheet electrode possesses one voltage plateau at 1.03 V versus Mg/Mg2+. In the subsequent lithiation process (charge), the MoSe2 nanosheets exhibited two voltage plateaus at 1.30 and 1.10 V versus Mg/Mg2+, indicating two-step lithium intercalation. The first CV cycle of the cell containing MoSe2 nanosheets shows one cathodic peak at 0.97 V versus Mg/Mg2+ and two anodic peaks at 1.17 and 1.39 V (Figure 7b). The CV curve of the second cathodic sweep shows two reduction peaks at 1.27 and 1.04 V.
Figure 7.
Electrochemical performances of the synthesized MoSe2 nanosheets in hybrid Mg–Li-ion batteries tested in the potential range of 0.2–2.2 V vs Mg/Mg2+. (a) Typical first charge/discharge profiles at a current rate of 20 mA g–1 and (b) CV curves of the cell containing MoS2 nanosheets.
The MoSe2 nanosheets exhibited an initial discharge and a charge capacity of 75 and 25 mA h g–1 at a current rate of 20 mA g–1, respectively. The irreversible capacity with a low Coulombic efficiency in the first three cycles may be due to the lithium trapping inside of the lattice and the formation of a solid electrolyte interface layer. The discharge capacities increased up to 82 mA h g–1 after three cycles. As shown in Figure 7a, the discharge capacity of nanosheets could be maintained at 55 mA h g–1 after several cycles.
Figure S7 shows the typical charge/discharge profiles of the MoS2 bulk materials at a current rate of 20 mA g–1 as a control sample. The bulk materials exhibited higher discharge/charge capacities with a capacity of 210 mA h g–1 compared to 81 mA h g–1 of the nanosheet materials. Figure S8 presents the charge/discharge curves of the cells containing MoSe2 nanosheets at a discharge rate of 20 mA g–1. Notably, the nanosheets delivered lower capacity; they exhibited a discharge capacity of 55 mA h g–1, whereas the bulk material delivered the capacity of 93 mA h g–1. The performance of MoS2 nanosheets is worse than that of the bulk material because of the formation of various kinds of intrinsic defects under SCF, which may trap Li-ions and inhibit the intercalation reaction. The investigation using atomic-resolved ADF imaging reveals that the obtained exfoliated nanosheets contain vacancies and antisite defects and undergo edge reconstruction. The work is now in progress.
Conclusions
We have demonstrated an SCF process for the exfoliation of MoS2 and MoSe2. This rapid and facile one-pot exfoliation method resulted in the high-quality pristine nanosheets maintaining their original stacking nature with a thickness of three to six layers and a lateral size of 500–1000 nm. Atomic-resolution HAADF imaging reveals the atomic structure of monolayer and few-layer MoS2 and MoSe2 nanosheets with hexagonal structures. As a proof of functionality, the obtained nanosheets were tested for their electrochemical performance in a hybrid Mg–Li-ion battery. The MoS2 and MoSe2 nanosheets exhibited the specific capacities of 81 and 55 mA h g–1, respectively, at a current rate of 20 mA g–1. This SCF processing can be readily extended to the exfoliation of TMDs and controlled synthesis of lateral heterojunctions within monolayers or vertical heterostructures of the TMDs.
Experimental Section
Synthesis Method
MoS2 and MoSe2 nanosheets were directly exfoliated by one-pot supercritical solvent method at 400 °C with a reaction time of 1 h. All SCF exfoliations were performed in a hastelloy reactor with a maximum volume of 10 mL. Typically, 20 mg of bulk crystals (MoS2, Aldrich, USA and MoSe2, Alfa Aesar, USA) was dispersed into 5 mL of DMF by low-power sonication (AS ONE US cleaner, US-4R, 40 kHz, 160 W) for 5 min. The obtained suspension was transferred to a batch reactor vessel, and the sealed reactor was heated at 400 °C for 1 h in a specially designed tube furnace (AKICO, Japan) (Figure 1). Then, the reactors were allowed to cool to room temperature by cool water quenching. The exfoliated solution was centrifuged at 2000 rpm for 30 min using a AS ONE centrifuge (Hsiangtai Co., Ltd.). The supernatant was collected, and the sediment containing unexfoliated, thick flakes was removed. Then, the supernatant was centrifuged at 30 000 rpm for 1 h using a Himac CP85β (Hitachi) instrument. The nanosheets were collected, and the supernatant was discarded. The fresh DMF was added to the obtained powder, and the solution was sonicated and centrifuged again to obtain clean exfoliated nanosheets. Finally, the obtained specimen was dried at 60 °C in vacuum for 1 day.
Material Characterization
The morphology of the particles was observed using a field emission scanning electron microscope (FESEM, Hitachi S-4800 with energy-dispersive X-ray spectroscopy, EDS) at an accelerating voltage of 5 kV. TEM (Hitachi H7650, 100 kV) and HRTEM (TOPCOM EM-002B, 200 kV) analyses were conducted. The samples for TEM and STEM analyses were prepared by placing the nanosheet materials onto holey carbon TEM grids. The materials were dispersed into ethanol and then dropped onto a Cu microgrid coated with a holey carbon film, followed by the evaporation of ethanol at 60 °C. HAADF imaging was performed on an aberration-corrected JEM-ARM200F, equipped with a cold field emission electron gun, operated with 200 kV. The camera length is 6 cm. The convergence semiangle for the incident probe was set at 29 mrad, and a probe current of about 34 pA was used. Most of the ADF images were collected for a half-angle range of 90–370 mrad.
The AFM measurements were recorded with SPA400 (SII Nanotechnology Inc). The nanosheet suspensions were applied directly on a thin native oxide on the Si(100) substrate. After drying the substrate in a vacuum, the measurements were recorded in air at ambient temperature and ambient pressure. XPS measurements were recorded using a ULVAC PHI 500 (Versa Probe II) equipped with a monochromatic Al Kα (1486.6 eV) X-ray source.
Electrochemical Measurement
The electrochemical performance of MoS2 and MoSe2 was investigated using coin-type cells (CR2032). The working electrodes were composed of 80 wt % active materials, 10 wt % PTFE [poly(tetrafluoroethylene)] as a binder, and 10 wt % acetylene black. These materials were ground by conventional agar mortar to make an electrode paste. The prepared paste was spread uniformly, rolling into a sheet and then dried in a vacuum oven for 4 h at 160 °C. The cathode sheet was punched into circular discs and cut into wafers (7 mm in diameter, 0.025 mm in thickness, and 5–6 mg). The tested cell was assembled inside of an argon-filled glovebox. For electrochemical measurements, the cell was composed of a magnesium metal counter, reference electrodes, and an MoX2 positive electrode. The anode and reference electrodes were separated by a microporous polypropylene film.
APC electrolyte was prepared in an Ar-filled glovebox by the addition of anhydrous AlCl3 (Sigma-Aldrich, 99.99%) to anhydrous tetrahydrofuran (THF, Kanto Chemical Inc., 99.9%), followed by mixing with 2 M PhMgCl solution in THF (Sigma-Aldrich, 100%) and extra THF to obtain 0.4 M APC [Mg2Cl3]+[AlPh2Cl2]−/THF.51 The addition of anhydrous LiCl salts (Kanto Chemical Inc., 99%) into the 0.4 M APC electrolyte under stirring was carried out to make 0.4 M LiCl. The charge/discharge cycling was performed galvanostatically between 0.2 and 2.2 V versus Mg/Mg2+ for MoS2 on multichannel battery testers (Hokuto Denko, Japan) at various charge/discharge rates of 20 mA g–1. Current densities and specific capacities were calculated based on the weight of MoX2 in the electrode.
Acknowledgments
This research work was financially supported by Japan Society for Promotion of Science (JSPS, grant no. PU15903), Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST), and Core Technology Consortium for Advanced Energy Devices, Tohoku University, Japan. The work was partially supported by ALCA-SPRING (ALCA-Specially Promoted Research for Innovative Next Generation Batteries) from Japan Science and Technology Agency (JST). The authors thank Dr. Taniki for fruitful discussion.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00379.
More TEM images of MoS2, HAADF-STEM images of multilayer MoS2 and MoSe2, AFM images of MoS2 nanosheets, schematic illustration of surface reaction and intercalation model of Li-ion with MoS2, and more charge/discharge profiles of MoS2 and MoSe2 materials (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chhowalla M.; Shin H. S.; Eda G.; Li L.-J.; Loh K. P.; Zhang H. The Chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. 10.1038/nchem.1589. [DOI] [PubMed] [Google Scholar]
- Geim A. K.; Grigorieva I. V. van der Waals heterostructures. Nature 2013, 499, 419–425. 10.1038/nature12385. [DOI] [PubMed] [Google Scholar]
- Wang Q. H.; Kalantar-Zadeh K.; Kis A.; Coleman J. N.; Strano M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- Georgiou T.; Jalil R.; Belle B. D.; Britnell L.; Gorbachev R. V.; Morozov S. V.; Kim Y.-J.; Gholinia A.; Haigh S. J.; Makarovsky O.; et al. Vertical field-effect transistor based on graphene–WS2 heterostructures for flexible and transparent electronics. Nat. Nanotechnol. 2013, 8, 100–103. 10.1038/nnano.2012.224. [DOI] [PubMed] [Google Scholar]
- Jariwala D.; Sangwan V. K.; Lauhon L. J.; Marks T. J.; Hersam M. C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1120. 10.1021/nn500064s. [DOI] [PubMed] [Google Scholar]
- Lee C.; Yan H.; Brus L. E.; Heinz T. F.; Hone J.; Ryu S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. 10.1021/nn1003937. [DOI] [PubMed] [Google Scholar]
- Gong Y.; Lin J.; Wang X.; Shi G.; Lei S.; Lin Z.; Zou X.; Ye G.; Vajtai R.; Yakobson B. I.; Terrones H.; Terrones M.; Tay B. K.; Lou J.; Pantelides S. T.; Liu Z.; Zhou W.; Ajayan P. M. Vertical and in-plane heterostructures from WS2/MoS2 monolayers. Nat. Mater. 2014, 13, 1135–1142. 10.1038/nmat4091. [DOI] [PubMed] [Google Scholar]
- Matte H. S. S. R.; Gomathi A.; Manna A. K.; Late D. J.; Datta R.; Pati S. K.; Rao C. N. R. MoS2 and WS2 analogues of graphene. Angew. Chem., Int. Ed. 2010, 49, 4059–4062. 10.1002/anie.201000009. [DOI] [PubMed] [Google Scholar]
- Yin Z.; Li H.; Li H.; Jiang L.; Shi Y.; Sun Y.; Lu G.; Zhang Q.; Chen X.; Zhang H. Single-layer MoS2 phototransistors. ACS Nano 2012, 6, 74–80. 10.1021/nn2024557. [DOI] [PubMed] [Google Scholar]
- Mak K. F.; Lee C.; Hone J.; Shan J.; Heinz T. F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. 10.1103/physrevlett.105.136805. [DOI] [PubMed] [Google Scholar]
- Splendiani A.; Sun L.; Zhang Y.; Li T.; Kim J.; Chim C.-Y.; Galli G.; Wang F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. 10.1021/nl903868w. [DOI] [PubMed] [Google Scholar]
- Radisavljevic B.; Radenovic A.; Brivio J.; Giacometti V.; Kis A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. 10.1038/nnano.2010.279. [DOI] [PubMed] [Google Scholar]
- Li H.; Yin Z.; He Q.; Li H.; Huang X.; Lu G.; Fam D. W. H.; Tok A. I. Y.; Zhang Q.; Zhang H. Fabrication of single- and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. 10.1002/smll.201101016. [DOI] [PubMed] [Google Scholar]
- Radisavljevic B.; Whitwick M. B.; Kis A. Integrated circuits and logic operations based on single-layer MoS2. ACS Nano 2011, 5, 9934–9938. 10.1021/nn203715c. [DOI] [PubMed] [Google Scholar]
- Bertolazzi S.; Brivio J.; Kis A. Stretching and breaking of ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. 10.1021/nn203879f. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Huang S.-Y.; Li Y.; Steinmann S. N.; Yang W.; Cao L. Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett. 2014, 14, 553–558. 10.1021/nl403620g. [DOI] [PubMed] [Google Scholar]
- Allan D. R.; Kelsey A. A.; Clark S. J.; Angel R. J.; Ackland G. J. High-pressure semiconductor-semimetal transition in TiS2. Phys. Rev. B: Condens. Matter Mater. Phys. 1998, 57, 5106–5110. 10.1103/physrevb.57.5106. [DOI] [Google Scholar]
- Mak K. F.; Lee C.; Hone J.; Shan J.; Heinz T. F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. 10.1103/physrevlett.105.136805. [DOI] [PubMed] [Google Scholar]
- Chang H.-Y.; Yang S.; Lee J.; Tao L.; Hwang W.-S.; Jena D.; Lu N.; Akinwande D. High-performance, highly bendable MoS2 transistors with high-K dielectrics for flexible low-power systems. ACS Nano 2013, 7, 5446–5452. 10.1021/nn401429w. [DOI] [PubMed] [Google Scholar]
- He Q.; Zeng Z.; Yin Z.; Li H.; Wu S.; Huang X.; Zhang H. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small 2012, 8, 2994–2999. 10.1002/smll.201201224. [DOI] [PubMed] [Google Scholar]
- Hong X.; Kim J.; Shi S.-F.; Zhang Y.; Jin C.; Sun Y.; Tongay S.; Wu J.; Zhang Y.; Wang F. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. 10.1038/nnano.2014.167. [DOI] [PubMed] [Google Scholar]
- Chang K.; Chen W. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. 10.1039/c1cc10631g. [DOI] [PubMed] [Google Scholar]
- Chang K.; Chen W. X. l-cysteine-assisted synthesis of layered MoS2/graphene Composites with excellent electrochemical performances for lithium ion batteries. ACS Nano 2011, 28, 4720–4728. 10.1021/nn200659w. [DOI] [PubMed] [Google Scholar]
- Wang H.; Feng H.; Li J. Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 2014, 10, 2165–2181. 10.1002/smll.201303711. [DOI] [PubMed] [Google Scholar]
- Najmaei S.; Liu Z.; Zhou W.; Zou X.; Shi G.; Lei S.; Yakobson B. I.; Idrobo J.-C.; Ajayan P. M.; Lou J. Vapour phase growth and grain boundary structure of molybdenum disulphide atomic layers. Nat. Mater. 2013, 12, 754–759. 10.1038/nmat3673. [DOI] [PubMed] [Google Scholar]
- van der Zande A. M.; Huang P. Y.; Chenet D. A.; Berkelbach T. C.; You Y.; Lee G.-H.; Heinz T. F.; Reichman D. R.; Muller D. A.; Hone J. C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. 10.1038/nmat3633. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Zou X.; Najmaei S.; Liu Z.; Shi Y.; Kong J.; Lou J.; Ajayan P. M.; Yakobson B. I.; Idrobo J.-C. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 2013, 13, 2615–2622. 10.1021/nl4007479. [DOI] [PubMed] [Google Scholar]
- Wang X.; Gong Y.; Shi G.; Chow W. L.; Keyshar K.; Ye G.; Vajtai R.; Lou J.; Liu Z.; Ringe E.; Tay B. K.; Ajayan P. M. Chemical vapor deposition growth of crystalline monolayer MoSe2. ACS Nano 2014, 8, 5125–5131. 10.1021/nn501175k. [DOI] [PubMed] [Google Scholar]
- Lu X.; Utama M. I. B.; Lin J.; Gong X.; Zhang J.; Zhao Y.; Pantelides S. T.; Wang J.; Dong Z.; Liu Z.; Zhou W.; Xiong Q. Large-area synthesis of monolayer and few-layer MoSe2 films on SiO2 substrates. Nano Lett. 2014, 14, 2419–2425. 10.1021/nl5000906. [DOI] [PubMed] [Google Scholar]
- Desai S. B.; Madhvapathy S. R.; Sachid A. B.; Llinas J. P.; Wang Q.; Ahn G. H.; Pitner G.; Kim M. J.; Bokor J.; Hu C.; Wong H.-S. P.; Javey A. MoS2 transistors with 1-nanometer gate lengths. Science 2016, 354, 99–102. 10.1126/science.aah4698. [DOI] [PubMed] [Google Scholar]
- Lopez-Sanchez O.; Lembke D.; Kayci M.; Radenovic A.; Kis A. Ultrasensitive photodetectors based on monolayer MoS2. Nat. Nanotechnol. 2013, 8, 497–501. 10.1038/nnano.2013.100. [DOI] [PubMed] [Google Scholar]
- Lee C.; Yan H.; Brus L. E.; Heinz T. F.; Hone J.; Ryu S. Anomalous lattice vibrations of single- and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. 10.1021/nn1003937. [DOI] [PubMed] [Google Scholar]
- Li H.; Wu J.; Yin Z.; Zhang H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. 10.1021/ar4002312. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Yin Z.; Huang X.; Li H.; He Q.; Lu G.; Boey F.; Zhang H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Angew. Chem., Int. Ed. 2011, 50, 11093–11097. 10.1002/anie.201106004. [DOI] [PubMed] [Google Scholar]
- Eda G.; Yamaguchi H.; Voiry D.; Fujita T.; Chen M.; Chhowalla M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. 10.1021/nl201874w. [DOI] [PubMed] [Google Scholar]
- Winchester A.; Ghosh S.; Feng S.; Elias A. L.; Mallouk T.; Terrones M.; Talapatra S. Electrochemical characterization of liquid phase exfoliated two-dimensional layers of molybdenum disulfide. ACS Appl. Mater. Interfaces 2014, 6, 2125–2130. 10.1021/am4051316. [DOI] [PubMed] [Google Scholar]
- Bang G. S.; Nam K. W.; Kim J. Y.; Shin J.; Choi J. W.; Choi S.-Y. Effective liquid-phase exfoliation and sodium ion battery application of MoS2 nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 7084–7089. 10.1021/am4060222. [DOI] [PubMed] [Google Scholar]
- Coleman J. N.; Lotya M.; O’Neill A.; Bergin S. D.; King P. J.; Khan U.; Young K.; Gaucher A.; De S.; Smith R. J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. 10.1126/science.1194975. [DOI] [PubMed] [Google Scholar]
- Smith R. J.; King P. J.; Lotya M.; Wirtz C.; Khan U.; De S.; O’Neill A.; Duesberg G. S.; Grunlan J. C.; Moriarty G.; et al. Large-scale exfoliation of inorganic layered compounds in aqueous surfactant solutions. Adv. Mater. 2011, 23, 3944–3948. 10.1002/adma.201102584. [DOI] [PubMed] [Google Scholar]
- Shmeliov A.; Shannon M.; Wang P.; Kim J. S.; Okunishi E.; Nellist P. D.; Dolui K.; Sanvito S.; Nicolosi V. Unusual stacking variations in liquid-phase exfoliated transition metal dichalcogenides. ACS Nano 2014, 8, 3690–3699. 10.1021/nn5003387. [DOI] [PubMed] [Google Scholar]
- Paton K. R.; Varrla E.; Backes C.; Smith R. J.; Khan U.; O’Neill A.; Boland C.; Lotya M.; Istrate O. M.; King P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. 10.1038/nmat3944. [DOI] [PubMed] [Google Scholar]
- Rangappa D.; Sone K.; Wang M.; Gautam U. K.; Golberg D.; Itoh H.; Ichihara M.; Honma I. Rapid and direct conversion of graphite crystals into high-yielding, good-quality graphene by supercritical fluid exfoliation. Chem.—Eur. J. 2010, 16, 6488–6494. 10.1002/chem.201000199. [DOI] [PubMed] [Google Scholar]
- Dinesh R.; Murukanahally K. D.; Tomai T.; Unemoto A.; Honma I. Ultrathin nanosheets of Li2MSiO4 (M = Fe, Mn) as high-capacity Li-ion battery electrode. Nano Lett. 2012, 12, 1146–1151. 10.1021/nl202681b. [DOI] [PubMed] [Google Scholar]
- Truong Q. D.; Devaraju M. K.; Honma I. Benzylamine-directed growth of olivine-type LiMPO4 nanoplates by a supercritical ethanol process for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 17400–17407. 10.1039/c4ta03566f. [DOI] [Google Scholar]
- Thangasamy P.; Sathish M. Rapid, One-Pot Synthesis of luminescent MoS2 nanoscrolls using supercritical fluid processing. J. Mater. Chem. C 2016, 4, 1165–1169. 10.1039/c5tc03630e. [DOI] [Google Scholar]
- Lin Y.-C.; Dumcenco D. O.; Huang Y.-S.; Suenaga K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2. Nat. Nanotechnol. 2014, 9, 391–396. 10.1038/nnano.2014.64. [DOI] [PubMed] [Google Scholar]
- Gong Y.; Liu Z.; Lupini A. R.; Shi G.; Lin J.; Najmaei S.; Lin Z.; Elías A. L.; Berkdemir A.; You G.; et al. Band gap engineering and layer-by-layer mapping of selenium-doped molybdenum disulfide. Nano Lett. 2014, 14, 442–449. 10.1021/nl4032296. [DOI] [PubMed] [Google Scholar]
- Huang C.; Wu S.; Sanchez A. M.; Peters J. J. P.; Beanland R.; Ross J. S.; Rivera P.; Yao W.; Cobden D. H.; Xu X. Lateral heterojunctions within monolayer MoSe2–WSe2 semiconductors. Nat. Mater. 2014, 13, 1096–1101. 10.1038/nmat4064. [DOI] [PubMed] [Google Scholar]
- Wan L. F.; Prendergast D. The solvation structure of Mg Ions in dichloro complex solutions from first-principles molecular dynamics and simulated X-ray absorption spectra. J. Am. Chem. Soc. 2014, 136, 14456–14464. 10.1021/ja505967u. [DOI] [PubMed] [Google Scholar]
- Carter T. J.; Mohtadi R.; Arthur T. S.; Mizuno F.; Zhang R.; Shirai S.; Kampf J. W. Boron clusters as highly stable magnesium-battery electrolytes. Angew. Chem., Int. Ed. 2014, 53, 3173–3177. 10.1002/anie.201310317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong Q. D.; Devaraju M. K.; Nguyen D. N.; Gambe Y.; Nayuki K.; Sasaki Y.; Tran P. D.; Honma I. Disulfide-bridged (Mo3S11) cluster polymer: Molecular dynamics and application as electrode material for a rechargeable magnesium battery. Nano Lett. 2016, 16, 5829–5835. 10.1021/acs.nanolett.6b02593. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Chen X.; Qiao L.; Lipson A. L.; Fister T. T.; Zeng L.; Kim C.; Yi T.; Sa N.; Proffit D. L.; Burrell A. K.; Cabana J.; Ingram B. J.; Biegalski M. D.; Bedzyk M. J.; Fenter P. Phase-controlled electrochemical activity of epitaxial Mg-spinel thin films. ACS Appl. Mater. Interfaces 2015, 7, 28438–28443. 10.1021/acsami.5b09346. [DOI] [PubMed] [Google Scholar]
- Larcher D.; Tarascon J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. 10.1038/nchem.2085. [DOI] [PubMed] [Google Scholar]
- Yoo H. D.; Shterenberg I.; Gofer Y.; Gershinsky G.; Pour N.; Aurbach D. Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 2013, 6, 2265–2279. 10.1039/c3ee40871j. [DOI] [Google Scholar]
- Muldoon J.; Bucur C. B.; Gregory T. Quest for nonaqueous multivalent secondary batteries: magnesium and beyond. Chem. Rev. 2014, 114, 11683–11720. 10.1021/cr500049y. [DOI] [PubMed] [Google Scholar]
- Levi E.; Gofer Y.; Aurbach D. On the way to rechargeable Mg batteries: The challenge of new cathode materials. Chem. Mater. 2010, 22, 860–868. 10.1021/cm9016497. [DOI] [Google Scholar]
- Kim H. S.; Arthur T. S.; Allred G. D.; Zajicek J.; Newman J. G.; Rodnyansky A. E.; Oliver A. G.; Boggess W. C.; Muldoon J. Structure and compatibility of a magnesium electrolyte with a sulphur cathode. Nat. Commun. 2011, 2, 427. 10.1038/ncomms1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C.; Mizuno F. Phase stability of post-spinel compound AMn2O4 (A = Li, Na, or Mg) and its application as a rechargeable battery cathode. Chem. Mater. 2013, 25, 3062–3071. 10.1021/cm401250c. [DOI] [Google Scholar]
- Yagi S.; Ichitsubo T.; Shirai Y.; Yanai S.; Doi T.; Murase K.; Matsubara E. A. A concept of dual-salt polyvalent-metal storage battery. J. Mater. Chem. A 2014, 2, 1144–1149. 10.1039/c3ta13668j. [DOI] [Google Scholar]
- Cho J.-H.; Aykol M.; Kim S.; Ha J.-H.; Wolverton C.; Chung K. Y.; Kim K.-B.; Cho B.-W. Controlling the intercalation chemistry to design high-performance dual-salt hybrid rechargeable batteries. J. Am. Chem. Soc. 2014, 136, 16116–16119. 10.1021/ja508463z. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Hou L.; Ciesielski A.; Samori P. 2D materials beyond graphene for high performance energy storage applications. Adv. Energy Mater. 2016, 6, 1600671. 10.1002/aenm.201600671. [DOI] [Google Scholar]
- Hsu C.-J.; Chou C.-Y.; Yang C.-H.; Lee T.-C.; Chang J.-K. MoS2/graphene cathodes for reversibly storing Mg2+ and Mg2+/Li+ in rechargeable magnesium-anode batteries. Chem. Commun. 2016, 52, 1701–1704. 10.1039/c5cc09407k. [DOI] [PubMed] [Google Scholar]
- Yoo H. D.; Liang Y.; Li Y.; Yao Y. High areal capacity hybrid magnesium–lithium-ion battery with 99.9% Coulombic efficiency for large-scale energy storage. ACS Appl. Mater. Interfaces 2015, 7, 7001–7007. 10.1021/acsami.5b01206. [DOI] [PubMed] [Google Scholar]
- Gao T.; Han F.; Zhu Y.; Suo L.; Luo C.; Xu K.; Wang C. Hybrid Mg2+/Li+ battery with long cycle life and high rate capability. Adv. Energy Mater. 2015, 5, 1401507. 10.1002/aenm.201401507. [DOI] [Google Scholar]
- Wang H.; Lu Z.; Xu S.; Kong D.; Cha J. J.; Zheng G.; Hsu P.-C.; Yan K.; Bradshaw D.; Prinz F. B.; et al. Electrochemical tuning of vertically aligned MoS2 nanofilms and its application in improving hydrogen evolution reaction. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 19701–19706. 10.1073/pnas.1316792110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.; Lin B.; Li X.; Song X.; Xia H.; Li L.; Zeng H. Monolayer MoS2–graphene hybrid aerogels with controllable porosity for lithium-ion batteries with high reversible capacity. ACS Appl. Mater. Interfaces 2016, 8, 2680–2687. 10.1021/acsami.5b10692. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Hua C.; Li B.; Fang X.; Yao C.; Zhang Y.; Hu Y.-S.; Wang Z.; Chen L.; Zhao D.; Stucky G. D. Highly ordered mesoporous crystalline MoSe2 material with efficient visible-light-driven photocatalytic activity and enhanced lithium storage performance. Adv. Funct. Mater. 2013, 23, 1832–1838. 10.1002/adfm.201202144. [DOI] [Google Scholar]
- Tang Y.; Zhao Z.; Wang Y.; Dong Y.; Liu Y.; Wang X.; Qiu J. Carbon-stabilized interlayer-expanded few-layer MoSe2 nanosheets for sodium ion batteries with enhanced rate capability and cycling performance. ACS Appl. Mater. Interfaces 2016, 8, 32324–32332. 10.1021/acsami.6b11230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.