Abstract
For designing energetic materials (EMs), the most challenging issue is to achieve a balance between energetic performance and reliable stability. In this work, we employed an efficient and convenient method to synthesize a new class of EMs: nitrogen-rich tetranuclear metal complexes [M(Hdtim)(H2O)2]4 (M = Zn 1, Mn 2; H3dtim = 1H-imidazol-4,5-tetrazole) with the N content of >46%. The structural analyses illustrate that isomorphous compounds 1 and 2 feature isolated hollow ellipsoid tetranuclear units, which are linked by both π–π interactions and hydrogen-bonding interactions to give a 3D supramolecular architecture. Compounds 1 and 2 exhibit prominent energetic characteristics: excellent detonation performances and reliable thermal, impact, and friction stabilities. Being nitrogen-rich tetrazolate compounds, the enthalpies of combustion of 1 (−11.570 kJ g–1) and 2 (−12.186 kJ g–1) are higher than those of classical EMs, RDX and HMX, and they possess high positive heats of formation. Sensitivity tests demonstrate that 1 and 2 are insensitive to external mechanical action. Excellent energetic performances and low sensitivities promote 1 and 2 to serve as a new class of promising EMs with a desirable level of safety.
Introduction
Energetic materials (EMs), encompassing explosives, propellants, and pyrotechnics, with an irreplaceable role in military affairs and civilian applications, have been extensively used for almost two centuries.1 The desired aim of designing new-generation EMs is to acquire high heat of detonation, low sensitivities including synthesis and handling, good thermal stabilities, low cost, and environmental acceptability.2 However, the traditional EMs, such as 2,4,6-trinitrotoluene (TNT), often encounter the problems regarding detonation properties and sensitivities.3 Recently, nitrogen-rich organic materials and nitrogen-rich energetic salts, as a result of high positive enthalpy of formation derived from the transformation of nitrogen-containing bonds, have attracted immense attention.4 Nevertheless, the conflict of high energy and desired safety cannot be completely resolved. Hence, it is of great importance to develop new-generation EMs to balance the energetic performance and safety.
To deal with the contradiction between the detonation properties and sensitivities, some methods have been developed. One of the valid strategies is to construct structurally stable compounds containing energetic fragments. For example, 2,4,6-trinitrobenzene-1,3,5-triamine holds the typical π-stacked structure, which can effectually buffer against mechanical action to remarkably reduce the sensitivity.5 With the aim of adjusting the structures to achieve the desired properties, the research can mainly be focused on the regulation of components and their spatial arrangements, including synthesis of co-crystals (such as a 1:1 co-crystal of hexanitrohexaazaisowurtzitane (CL-20) and TNT, displaying outstanding explosive power with reduced sensitivity) and exploitation of some energetic salts, which can modulate the relationships between the energetic characteristics and sensitivity by the selection and modification of cationic and anionic components.6 Besides using energetic co-crystals and energetic salts,5a,7 another effective and easy method is to use energetic metal-organic complexes, which have received widespread concern for their structural diversities and have stirred a great deal of interest in the field of EMs in recent years.8 In 2012, the Hope-Weeks group reported two 1D metal-organic frameworks (MOFs) [Co(N2H4)5(ClO4)2]n (CHP) and [Ni(N2H4)5(ClO4)2]n (NHP), which show good detonation properties; however, these two complexes are too sensitive to meet the commercial demand.2a To obtain less sensitive complexes, they synthesized another two 2D MOFs [Co2(N2H4)4(N2H3CO2)2(ClO4)2·H2O]n (CHHP) and [Zn2(N2H4)3(N2H3CO2)2(ClO4)2·H2O]n (ZnHHP), revealing a considerable reduction in the sensitivity with the heat of detonation decreased.9 In 2013, the Pang group reported two 3D energetic MOFs [Cu(atrz)3(NO3)2]n (CuATRZ) and [Ag(atrz)1.5(NO3)]n (AgATRZ) (atrz = 4,4′-azo-1,2,4-triazole), incorporating lots of nitrate anions into the frameworks.10 They obtained large values of the heats of detonation (CuATRZ) with considerable stabilities and acceptable sensitivities. These results suggest that metal-organic complexes with the desired physical and chemical characteristics (such as high density, excellent energetic performance, and high stability), as a newly emerging field, would provide a chance for developing new EMs.11
Despite these advancements, the present energetic metal-organic complexes are mainly restricted to mononuclear metal complexes with low stabilities and MOFs with a permanent porosity, usually giving the negative effects of energetic characteristics. Polynuclear nitrogen-rich metal complexes, as an important type of metal-organic complexes, possess a stable multinuclear structural motif and tightly packed mode, which endow N-rich metal complexes with potential applications in EMs.
As we all know, five-membered nitrogen-rich heterocycles, with excellent chemical stability from the system of aromatic ring, such as imidazole and tetrazole derivatives,12 own high enthalpy of formation derived from the powerful energy release from energetic C–N, N–N, and N=N bonds to provide the main energy source.13 Furthermore, the combustion products of tetrazole derivatives are environmentally friendly.14 Sharpless and Xiong developed an environmentally friendly, efficient, and convenient method to synthesize 5-substituted-1H-tetrazoles by 3 + 2 cycloaddition reactions of azides and nitriles with metal salts as catalysts,15 which has been used to synthesize a variety of tetrazole-based MOFs in our lab,16 some of which exhibit good energetic performance.16b,16c Encouraged by our early success, we try to obtain new EMs assembled by nitrogen-rich tetrazole derivatives.17,18 In this work, the 4,5-di(1H-tetrazol-5-yl)-2H imidazole (H3dtim) ligand (Scheme 1) and Zn(II)/Mn(II) ions were selected on the basis of the following considerations: (i) H3dtim is an aromatic ligand with a high N content of 68.63%, which is inclined to form rigid metal-organic complexes to enhance the structural stability; (ii) the N-containing heterocycles offer the possibility of the π–π stacking interactions to consolidate the product structures; and (iii) H3dtim possesses 10 coordination N atoms and can display diverse coordination modes to form various structures.
Scheme 1. Synthetic Route of 1 and 2.
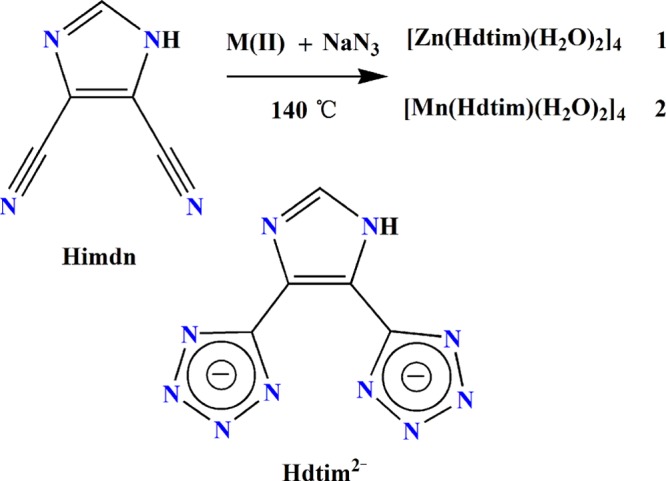
Herein, two isomorphous energetic nitrogen-rich tetranuclear metal complexes, [Zn(Hdtim)(H2O)2]41 and [Mn(Hdtim)(H2O)2]42, were obtained by in situ hydrothermal ligand synthesis (Scheme 1). The structures of 1 and 2 feature rare open ellipsoid tetranuclear units via both π–π stacking interactions and hydrogen-bonding interactions to give a 3D supramolecular network. Both of them exhibit excellent energetic performance and low sensitivity. Notably, the heat of detonation of 2 (3.953 kcal g–1) is comparable to that of Cu(Htztr) (H2tztr = 3-(1H-tetrazol-5-yl)-1H-triazole) (3.958 kcal g–1), the most powerful energetic MOF reported.2c Compounds 1 and 2 are the first examples of the N-rich tetranuclear metal complexes as candidates of EMs.
Results and Discussion
Crystal Structure
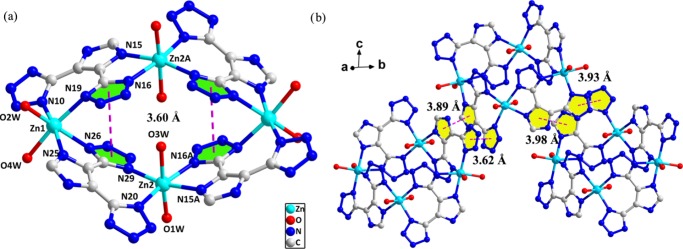
Compounds 1 and 2 are isomorphous and display isolated tetranuclear structures. A full description of 1 is given as a representative example. The asymmetric unit is composed of two Zn(II) atoms, two Hdtim2– groups, and four coordinated H2O molecules, as shown in Figure 1a. The imidazole ring in Hdtim2– retains the original state without deprotonation, and both tetrazole rings are deprotonated after coordination. The two independent Zn(II) atoms have the same coordination pattern to furnish a distorted octahedral geometry. The equatorial bonds in the Zn1 atom are formed through three nitrogen atoms (N10, N25, N26) and one oxygen atom (O2W). The axial bonds are formed by one nitrogen atom (N19) and one oxygen atom (O4W). The Zn2 atom is six-coordinated by four nitrogen atoms (N20, N29, N15A, N16A) and two oxygen atoms (O1W, O3W). The O/N–Zn–O/N angles vary from 76.08(7) to 175.15(8)° and the Zn–N and Zn–O bond lengths vary from 2.104(2) to 2.215(2) Å and 2.0716(19) to 2.195(2) Å, respectively (Table S1). The two Zn(II) atoms are interconnected by the Hdtim2– ligand with a μ2-κN25,N26:κN20,N29 coordination style to result in a dinuclear unit with a Zn1···Zn2 distance of 6.0741(7) Å. Then, the two dinuclear units are further bridged by two μ2-Hdtim2– ligands to produce a centrosymmetric open ellipsoid tetranuclear unit.
Figure 1.
(a) Molecular structure of tetranuclear 1. Symmetry code: (A) 1 – x, 1 – y, 1 – z. The π–π interactions (pink dotted lines) are shown in the tetranuclear unit. (b) The π–π interactions (pink dotted lines) between adjacent tetranuclear units. All hydrogen atoms have been omitted for clarity.
The centroid–centroid distance of the π–π interactions between tetrazole rings in each tetranuclear unit is 3.60 Å (Figure 1a). The π–π interaction distances between adjacent tetranuclear units lie in the range of 3.62–3.98 Å (Figure 1b and Table S2). Because of the presence of a variety of coordinated water molecules, there are abundant hydrogen-bonded donors (coordinated water molecules and protonated imidazole rings) and acceptors (deprotonated tetrazole rings) to construct a hydrogen-bonded supramolecular network (Table S3 and Figure S1). A large number of supramolecular interactions can make contributions to the low sensitivities of energetic compounds 1 and 2.
Thermal Stability
The experimental powdered X-ray diffraction (PXRD) patterns of 1 and 2 are consistent with the simulated data, indicating the phase purities of the samples (Figure S2). The thermogravimetry (TG) and differential scanning calorimetry (DSC) curves (Figures 2 and 3) demonstrate that the weight loss of 12.22% in 1, starting at 112 °C and ending at 236 °C, can be ascribed to the release of two coordinated water molecules (calcd: 11.86%). The weight loss of 11.01%, in the temperature range of 195–251 °C, is in conformity with the removal of two coordinated water molecules for 2 (calcd: 12.28%). Then, no weight loss of both 1 and 2 was observed up to 320 °C. With continuous heating, the compounds continued to break down as a result of the decomposition of organic ligands.
Figure 2.
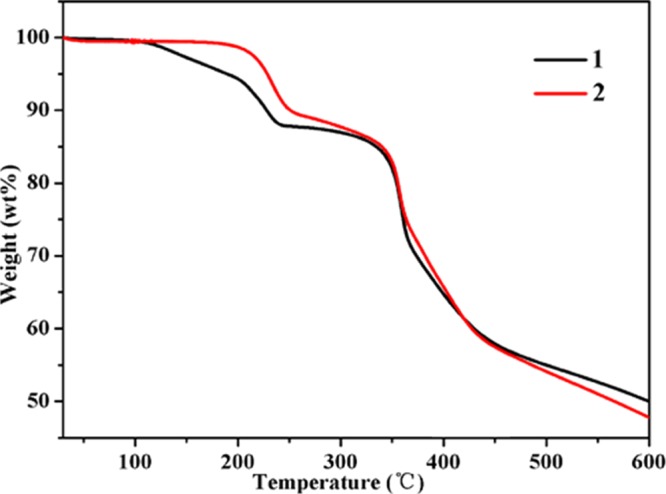
TG curves of 1 and 2.
Figure 3.
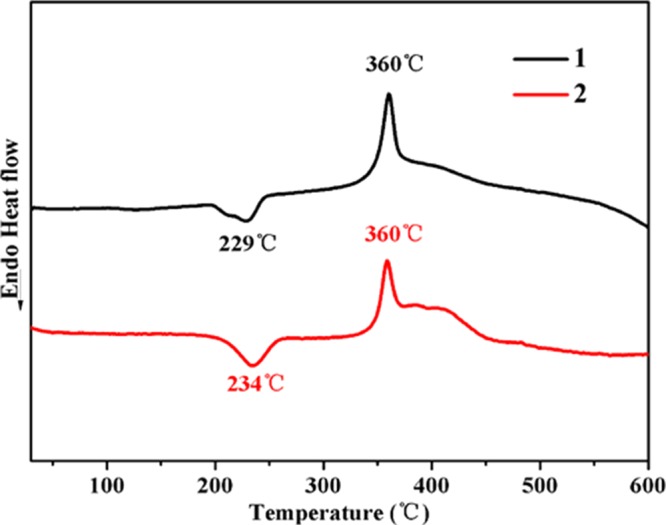
DSC curves of 1 and 2.
The DSC curves show the endothermic peaks of 1 and 2observed at 229 and 234 °C, respectively, on account of the removal of coordinated water molecules. The exothermic process of both 1 and 2 began at 320 °C, peaked at 360 °C to the exothermic peaks, and ended at 372 °C, resulting from the decomposition of organic ligands.
Heat of Combustion
Compared with traditional EMs, such as RDX, with the main energy source being oxidation of the carbon skeletons, nitrogen-rich EMs gain their energy from high positive heats of formation, owing to the presence of a number of energetic N–N and C–N bonds. Both compounds 1 and 2 possess good thermal stability and high nitrogen contents of 46.12 and 47.75%, respectively, to prompt them as prospective EMs. The experimental constant-volume combustion heat (ΔcU) values of 1 and 2 were measured by oxygen bomb calorimetry to be 11.603 and 12.216 kJ g–1, respectively. The enthalpy of combustion (ΔcH) was deduced from ΔcU by the following correction: ΔcH = ΔcU + ΔnRT, where Δn is the change in the total molar quantities of gases during the reaction process, R = 8.314 J mol–1 K–1, and T = 298.15 K. The combustion reactions are given in eqs 1 and 2 as follows
| 1 |
| 2 |
The calculated ΔcH values of 1 and 2 are −11.570 and −12.186 kJ g–1, respectively, which are not only higher than those of the commercial EMs, HMX (−9.44 to −9.88 kJ g–1) and RDX (−9.60 kJ g–1),19 but also higher than those of 3D MOFs CdATZ (−6.672 kJ g–1) (CdATZ = (Et4N)[Cd6Br5(ATZ)8]·H2O, HATZ = 5-aminotetrazolate, Et = ethyl),20 CoBTA (−5.92 kJ g–1) (CoBTA = Co9(BTA)10(HBTA)2(H2O)10, H2BTA = N,N-bis(1H-tetrazole-5-yl)-amine),21 and CuNaMtta (−11.389 kJ g–1) (CuNaMtta = Cu4Na(Mtta)5(CH3CN), Mtta = 5-methyl tetrazole).17 The enthalpies of formation (ΔfH°) of the two compounds were calculated by the Hess thermochemical cycle, as shown in eqs 3 and 4, and deduced as 1.0959 and 0.7697 MJ kg–1, respectively, where ΔfH° (ZnO, s) = −348.00 kJ mol–1, ΔfH° (CO2, g) = −393.51 kJ mol–1, ΔfH° (MnO2, s) = −520.00 kJ mol–1, and ΔfH° (H2O, l) = −285.83 kJ mol–1.
| 3 |
| 4 |
Obviously, compared with traditional EMs and some energetic metal coordination compounds, both 1 (1.0959 MJ kg–1) and 2 (0.7697 MJ kg–1) (Table 1) possess higher ΔfH°, which results in considerable energy from the explosion, making 1 and 2 potential EMs.
Table 1. Physicochemical Properties of 1, 2, TNT, RDX, HMX, and the Related Energetic Metal-Organic Coordination Compounds.
| explosive | ρa (g cm–3) | Nb (%) | Tdecc (°C) | ΔcHd (kJ g–1) | ΔfH°e (MJ kg–1) | ISf (J) | FSg (N) |
|---|---|---|---|---|---|---|---|
| 1 | 2.108 | 46.12 | 229 | –11.570 | 1.0959 | >40 | >360 |
| 2 | 1.968 | 47.75 | 234 | –12.186 | 0.7697 | >40 | >360 |
| HMX2a,19 | 1.950 | 37.80 | 280 | –9.60 | 0.3545 | 7.4 | |
| RDX2a,19 | 1.868 | 37.80 | 210 | –9.61 | 0.4186 | 7.5 | 120 |
| TNT2a,19 | 1.654 | 18.50 | 244 | –16.27 | –0.2952 | 15 | 353 |
| CdATZ20 | 2.530 | 30.31 | 355 | –6.672 | –0.5398 | ||
| CoBTA21 | 1.707 | 59.85 | 253 | –5.92 | 0.3403 | 27 | >360 |
| CuNaMtta17 | 1.975 | 40.08 | 384 | –11.389 | 0.2489 | 36 | >360 |
Density calculated from X-ray diffraction data.
Nitrogen content.
Decomposition temperature.
Enthalpy of combustion.
Enthalpy of formation.
Impact sensitivity.
Friction sensitivity.
Detonation Properties
The heat of detonation (ΔHdet) is a critical parameter to evaluate energetic performance of EMs, which is difficult to evaluate through experiments. To compare the ΔHdet values of 1 and 2 with those of other reported energetic MOFs, we employed the DFT method adopted by Hope-Weeks to evaluate ΔHdet,2a,9 which has been widely applied to estimate the relative energetic performance of MOFs (Tables S4 and S5).2c,8b,8c,8e,10,17,21 Owing to the shortage of oxygen, metal oxides, carbon, nitrogen, water, and ammonia are presumed to be the decomposition products of 1 and 2. The detonation reactions considered for 1 and 2 are described by eqs 5 and 6.
| 5 |
| 6 |
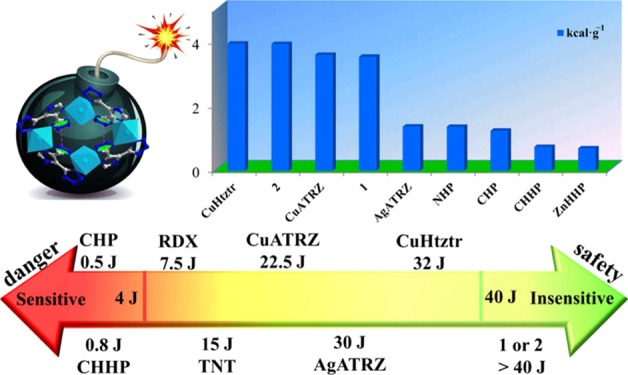
The ΔHdet values are calculated to be 3.558 kcal g–1 for 1 and 3.953 kcal g–1 for 2. Both of them show prominent detonation performance, and their heats of detonation are superior to those of the majority of the previously reported energetic metal-organic complexes (e.g., NHP,2a CHHP,9 and AgATRZ10). To the best of our knowledge, the ΔHdet of 2 (3.953 kcal g–1) is close to the largest value reported for energetic MOF CuHtztr2c (3.958 kcal g–1) (Figure 4 and Table S5). The high heats of detonation of 1 and 2 are perhaps ascribed to the enhanced structural reinforcement and high nitrogen contents.
Figure 4.
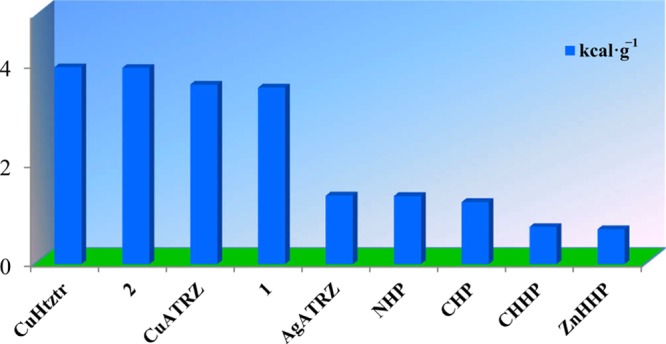
Bar diagram representing the ΔHdet values for some previously reported energetic MOFs including CuHtztr,2c CuATRZ,10 AgATRZ,10 NHP,2a CHP,2a CHHP,9 and ZnHHP.9 The predicted ΔHdet values for 1 and 2 are also shown for comparison.
To further study the detonation feature, the detonation pressure (P) and detonation velocity (D) of 1 and 2 were estimated by Kamlet–Jacbos equations.22 Thermochemical properties P and D of 1, 2, and the selected energetic metal coordination compounds are displayed in Figure 5 and Table S5. The calculated values of P and D of 1 were 44.57 GPa and 9.610 km s–1, respectively, which have more prominent features than those of most of the reported energetic MOFs.
Figure 5.
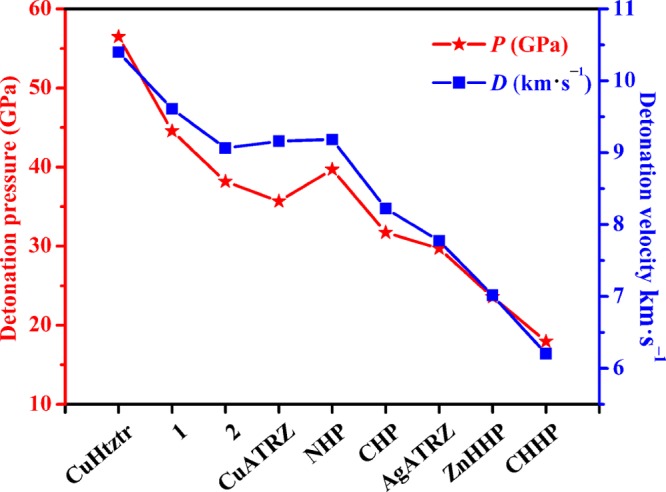
Comparison of the P and D values of 1 and 2 with those of some reported energetic MOFs.
Sensitivity Test
In both the civilian applications and military energetic devices, one of the most vital indicators to assess the safety of EMs is the sensitivity, which was classified according to the U.N. Recommendations on the Transport of Dangerous Goods.a The impact sensitivity values of 1 and 2 are greater than 40 J, implying insensitivity to impact. No explosion was observed up to 360 N for 1 and 2, which reveals insensitivity to friction. The collected data of 1, 2, and other explosives are also summarized in Table 1. Compounds 1 and 2 exhibit low sensitivities, which is probably attributed to the advantage of the structures with a large number of solid tetranuclear structural motifs, π–π interactions, and hydrogen bonds to increase the stability.5
Conclusions
In summary, we employed an environmentally friendly and convenient method to synthesize the first examples of two energetic nitrogen-rich tetranuclear metal complexes 1 and 2 by in situ hydrothermal ligand synthesis. Compounds 1 and 2 feature unusual open ellipsoid tetranuclear units, which are connected via both π–π interactions and hydrogen bonds to give a 3D supramolecular architecture. In contrast to other reported metal-organic EMs, 1 and 2 exhibit a combination of powerful detonation performances and low sensitivities because of the high nitrogen contents (>46%) and close-packed structures. It is worth pointing out that 1 and 2 possess high heats of detonation, which are comparable to the largest value reported for energetic MOF CuHtztr, indicating that the two compounds are potentially valuable EMs with a desirable level of safety. This work not only first reports rare nitrogen-rich tetranuclear metal complexes as a new class of EMs but also enhances the future prospects for a reasonable structural design of EMs.
Experimental Section
General Caution
The sodium azide and tetrazolate compounds are hazardous materials, which may detonate in some cases and should be used only in small quantities. Proper protective measures, such as safety glasses, face shield, leather coat, and plastic spatulas, should be taken.
Materials and Measurements
All reagents purchased commercially were used without further purification. PXRD patterns were recorded on a Rigaku Miniflex II diffractometer using Cu Kα radiation (λ = 1.5406 Å). The FT-IR spectrums were recorded on a Perkin-Elmer spectrometer using KBr pellets from 4000–400 cm–1. DSC and thermogravimetric analysis (TGA) measurements were performed on METTLER TOLEDO instrument in N2 at a heating rate of 5 K min–1. The combustion energies were tested by oxygen bomb calorimetry. First, the certified benzoic acid (about 1.0 g, pellet) was adopted by combustion in an oxygen atmosphere at a pressure of 2.30 MPa to calibrate the calorimeter. Second, 300 mg of the pure sample was well-mixed with the certified benzoic acid (900 mg), which were pressed into a tablet to guarantee full combustion. Finally, the pellet was sufficiently burned in a 2.30 MPa atmosphere of pure oxygen in combustion pots. The impact sensitivities were performed on a BAM fall hammer BFH-12 produced by OZM Research according to STANAG 4489. A 5 kg weight of the sample was dropped from a set height onto a 20 mg sample placed on a copper cap. No explosion occurred at the point of 80 cm corresponding to an impact energy of 40 J. The friction sensitivity was determined using a BAM friction apparatus FSKM-10 produced by OZM Research on the basis of STANAG 4487. No explosion happened up to 360 N for the sample, which implies insensitivity to friction.
Synthesis of [Zn(Hdtim)(H2O)2]4, 1
The reactants Zn(ClO4)2·6H2O (0.400 mmol), NaN3 (0.600 mmol), Himdn (0.200 mmol), and a drop of HBF4 in H2O (8 mL) were sealed in a 20 mL Teflon-lined stainless steel autoclave, heated at 140 °C, kept for 3 days, and then cooled to room temperature over 1 day. Colorless block crystals of 1 were collected (yield: 71% based on Himdn). Anal. Calcd for C20H24N40O8Zn4: C, 19.78; H, 2.00; N, 46.12%. Found: C, 19.74; H, 2.01; N, 46.03%. IR (KBr pellet, cm–1): 3583 m, 3138 m, 3060 w, 2856 w, 1615 s, 1570 s, 1481 s, 1454 s, 1391 s, 1249 m, 1204 s, 1120 s, 1043 m, 985 s, 844 w, 760 s, 697 m, 640 s, 587 s, 490 s.
Synthesis of [Mn(Hdtim)(H2O)2]4, 2
The ratio of reactants and the synthesis process for 2 were similar to those for 1, except that Mn(ClO4)2·6H2O replaced Zn(ClO4)2·6H2O. Yield: 58% (based on Himdn). Anal. Calcd for C20H24N40O8Mn4: C, 20.49; H, 2.05; N, 47.75%. Found: C, 20.91; H, 2.02; N, 47.54%. IR (KBr pellet, cm–1): 3550 m, 3420 m, 3133 m, 2860 w, 1622 s, 1560 s, 1487 s, 1454 s, 1390 s, 1345 w, 1242 m, 1208 s, 1118 s, 1053 w, 985 s, 850 s, 757 s, 700 m, 644 s, 590 s, 488 s. The two compounds are stable in common solvents, such as water, methanol, and ethanol, maintaining their crystallinities for at least several months.
Crystal Structure Determination
Diffraction data for 1 and 2 were recorded by a Rigaku PILATUS CCD diffractometer (Mo Kα, λ = 0.71073 Å) at 293 K. The intensity data sets were collected using a ω-scan technique and reduced using CrystalClear software.23 The structures were solved by direct methods, and the subsequent successive difference Fourier syntheses yielded other nonhydrogen atoms. The hydrogen atoms were calculated in idealized positions and allowed to ride on their parent atoms. The final structures were refined using a full-matrix least-squares refinement on F2 with the SHELXTL-2014 program.24 Pertinent crystal data and structural refinement results are listed in Table 2.
Table 2. Crystal Data and Structure Refinements for 1 and 2.
| compound | 1 | 2 |
|---|---|---|
| CCDC | 1463790 | 1463791 |
| empirical formula | C20H24N40O8Zn4 | C20H24N40O8Mn4 |
| Mr (g mol–1) | 1214.27 | 1172.55 |
| crystal system | monoclinic | monoclinic |
| space group | P21/n | P21/n |
| Z | 2 | 2 |
| a (Å) | 7.5445(8) | 7.581(2) |
| b (Å) | 16.0615(18) | 16.404(4) |
| c (Å) | 16.153(2) | 16.285(5) |
| α (deg) | 90 | 90 |
| β (deg) | 102.219(2) | 102.321(6) |
| γ (deg) | 90 | 90 |
| V (Å3) | 1913.0(4) | 1978.5(9) |
| Dc (g cm–3) | 2.108 | 1.968 |
| temperature (K) | 293(2) | 293(2) |
| F(000) | 1216 | 1176 |
| reflns | 19734 | 20714 |
| Rint | 0.0336 | 0.1099 |
| params | 369 | 325 |
| S on F2 | 0.999 | 1.065 |
| R1 (I > 2σ(I))a | 0.0367 | 0.0699 |
| wR2 (I > 2σ(I))b | 0.1146 | 0.1248 |
R1 = ∑(Fo – Fc)/∑Fo.
wR2 = [∑w(Fo2 – Fc2)2/∑w(Fo2)2]1/2.
Acknowledgments
This work was financially supported by the National Nature Science Foundation of China (21371170 and 21601186) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000). We acknowledge Prof. Rui-Hu Wang (State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China) for his kind help and discussions on energetic performances.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00431.
The authors declare no competing financial interest.
Footnotes
Impact: insensitive > 40 J, less sensitive ≥ 35 J, sensitive ≥ 4 J, very sensitive ≤ 3 J; friction: insensitive > 360 N, less sensitive = 360 N, 80 N < sensitive < 360 N, very sensitive ≤ 80 N, extreme sensitive ≤ 10 N.
Supplementary Material
References
- a Zhang M.-X.; Eaton P. E.; Gilardi R. Hepta- and Otanitrocubanes. Angew. Chem., Int. Ed. 2000, 39, 401–404. . [DOI] [PubMed] [Google Scholar]; b Klapötke T. M.; Stierstorfer J. The CN7– Anion. J. Am. Chem. Soc. 2009, 131, 1122–1134. 10.1021/ja8077522. [DOI] [PubMed] [Google Scholar]; c Gao H.; Shreeve J. M. Azole-Based Energetic Salts. Chem. Rev. 2011, 111, 7377–7436. 10.1021/cr200039c. [DOI] [PubMed] [Google Scholar]; d Wang R.; Xu H.; Guo Y.; Sa R.; Shreeve J. M. Bis[3-(5-nitroimino-1,2,4-triazolate)]-Based Energetic Salts: Synthesis and Promising Properties of a New Family of High-Density Insensitive Materials. J. Am. Chem. Soc. 2010, 132, 11904–11905. 10.1021/ja1055033. [DOI] [PubMed] [Google Scholar]; e Badgujar D. M.; Talawar M. B.; Asthana S. N.; Mahulikar P. P. Advances in Science and Technology of Modern Energetic Materials: An Overview. J. Hazard. Mater. 2008, 151, 289–305. 10.1016/j.jhazmat.2007.10.039. [DOI] [PubMed] [Google Scholar]; f Göbel M.; Klapötke T. M. Development and Testing of Energetic Materials: The Concept of High Densities Based on the Trinitroethyl Functionality. Adv. Funct. Mater. 2009, 19, 347–365. 10.1002/adfm.200801389. [DOI] [Google Scholar]; g Tao G.-H.; Twamley B.; Shreeve J. M. A Thermally Stable Nitrogen-Rich Energetic Material–3,4,5-triamino-1-tetrazolyl-1,2,4-triazole (TATT). J. Mater. Chem. 2009, 19, 5850–5854. 10.1039/b908214j. [DOI] [Google Scholar]
- a Bushuyev O. S.; Brown P.; Maiti A.; Gee R. H.; Peterson G. R.; Weeks B. L.; Hope-Weeks L. J. Ionic Polymers as a New Structural Motif for High-Energy-Density Materials. J. Am. Chem. Soc. 2012, 134, 1422–1425. 10.1021/ja209640k. [DOI] [PubMed] [Google Scholar]; b Qin J.-S.; Zhang J.-C.; Zhang M.; Du D.-Y.; Li J.; Su Z.-M.; Wang Y.-Y.; Pang S.-P.; Li S.-H.; Lan Y.-Q. A Highly Energetic N-Rich Zeolite-Like Metal-Organic Framework with Excellent Air Stability and Insensitivity. Adv. Sci. 2015, 2, 1500150 10.1002/advs.201500150. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Liu X.; Gao W.; Sun P.; Su Z.; Chen S.; Wei Q.; Xie G.; Gao S. Environmentally Friendly High-Energy MOFs: Crystal Structures, Thermostability, Insensitivity and Remarkable Detonation Performances. Green Chem. 2015, 17, 831–836. 10.1039/C4GC02184C. [DOI] [Google Scholar]; d Steinhauser G.; Klapotke T. M. “Green” Pyrotechnics: A Chemists’ Challenge. Angew. Chem., Int. Ed. 2008, 47, 3330–3347. 10.1002/anie.200704510. [DOI] [PubMed] [Google Scholar]; e Joo Y.-H.; Shreeve J. M. Nitroimino-tetrazolates and Oxy-nitroimino-tetrazolates. J. Am. Chem. Soc. 2010, 132, 15081–15090. 10.1021/ja107729c. [DOI] [PubMed] [Google Scholar]; f Li Y.-C.; Qi C.; Li S.-H.; Zhang H.-J.; Sun C.-H.; Yu Y.-Z.; Pang S.-P. 1,1′-Azobis-1,2,3-triazole: A High-Nitrogen Compound with Stable N8 Structure and Photochromism. J. Am. Chem. Soc. 2010, 132, 12172–12173. 10.1021/ja103525v. [DOI] [PubMed] [Google Scholar]
- Talawar M. B.; Agrawal A. P.; Anniyappan M.; Wani D. S.; Bansode M. K.; Gore G. M. Primary Explosives: Electrostatic Discharge Initiation, Additive Effect and Its Relation to Thermal and Explosive Characteristics. J. Hazard. Mater. 2006, 137, 1074–1078. 10.1016/j.jhazmat.2006.03.043. [DOI] [PubMed] [Google Scholar]
- a Yin P.; Zhang Q.; Shreeve J. M. Dancing with Energetic Nitrogen Atoms: Versatile N-Functionalization Strategies for N-Heterocyclic Frameworks in High Energy Density Materials. Acc. Chem. Res. 2016, 49, 4–16. 10.1021/acs.accounts.5b00477. [DOI] [PubMed] [Google Scholar]; b Qi C.; Li S.-H.; Li Y.-C.; Wang Y.; Chen X.-K.; Pang S.-P. A Novel Stable High-Nitrogen Energetic Material: 4,4′-Azobis(1,2,4-triazole). J. Mater. Chem. 2011, 21, 3221–3225. 10.1039/c0jm02970j. [DOI] [Google Scholar]; c Chavez D. E.; Bottaro J. C.; Petrie M.; Parrish D. A. Synthesis and Thermal Behavior of a Fused, Tricyclic 1,2,3,4-Tetrazine Ring System. Angew. Chem., Int. Ed. 2015, 54, 1–4. 10.1002/anie.201506744. [DOI] [PubMed] [Google Scholar]; d Jones C. B.; Haiges R.; Schroer T.; Christe K. O. Oxygen-Balanced Energetic Ionic Liquid. Angew. Chem., Int. Ed. 2006, 118, 5103–5106. 10.1002/ange.200600735. [DOI] [PubMed] [Google Scholar]; e Liang L.; Cao D.; Song J.; Huang H.; Wang K.; Bian C.; Dong X.; Zhou Z. Synthesis and Characteristics of Novel Energetic Salts Based on Bis(N-dinitroethyl)aminofurazan. J. Mater. Chem. A 2013, 1, 8857–8865. 10.1039/c3ta11784g. [DOI] [Google Scholar]; f Klapötke T. M.; Mayer P.; Stierstorfer J.; Weigand J. J. Bistetrazolylamines—Synthesis and Characterization. J. Mater. Chem. 2008, 18, 5248–5258. 10.1039/b811273h. [DOI] [Google Scholar]; g Zhang Y.; Gao H.; Joo Y.-H.; Shreeve J. M. Ionic Liquids as Hypergolic Fuels. Angew. Chem., Int. Ed. 2011, 50, 9554–9562. 10.1002/anie.201101954. [DOI] [PubMed] [Google Scholar]
- a Zhang J.; Zhang Q.; Vo T. T.; Parrish D. A.; Shreeve J. M. Energetic Salts with π-Stacking and Hydrogen-Bonding Interactions Lead the Way to Future Energetic Materials. J. Am. Chem. Soc. 2015, 137, 1697–1704. 10.1021/ja5126275. [DOI] [PubMed] [Google Scholar]; b Zhang C.; Wang X.; Huang H. π-Stacked Interactions in Explosive Crystals: Buffers against External Mechanical Stimuli. J. Am. Chem. Soc. 2008, 130, 8359–8365. 10.1021/ja800712e. [DOI] [PubMed] [Google Scholar]
- a Bolton O.; Matzger A. J. Improved Stability and Smart-Material Functionality Realized in an Energetic Cocrystal. Angew. Chem., Int. Ed. 2011, 50, 8960–8963. 10.1002/anie.201104164. [DOI] [PubMed] [Google Scholar]; b Millar D. I. A.; Maynard-Casely H. E.; Allan D. R.; Cumming A. S.; Lennie A. R.; Mackay A. J.; Oswald I. D. H.; Tang C. C.; Pulham C. R. Crystal Engineering of Energetic Materials: Co-crystals of CL-20. CrystEngComm 2012, 14, 3742–3749. 10.1039/c2ce05796d. [DOI] [Google Scholar]
- Landenberger K. B.; Bolton O.; Matzger A. J. Energetic–Energetic Cocrystals of Diacetone Diperoxide (DADP): Dramatic and Divergent Sensitivity Modifications via Cocrystallization. J. Am. Chem. Soc. 2015, 137, 5074–5079. 10.1021/jacs.5b00661. [DOI] [PubMed] [Google Scholar]
- a Zhang H.; Zhang M.; Lin P.; Malgras V.; Tang J.; Alshehri S. M.; Yamauchi Y.; Du S.; Zhang J. A Highly Energetic N-Rich Metal–Organic Framework as a New High-Energy-Density Material. Chem. – Eur. J. 2016, 22, 1141–1145. 10.1002/chem.201503561. [DOI] [PubMed] [Google Scholar]; b Gao W.; Liu X.; Su Z.; Zhang S.; Yang Q.; Wei Q.; Chen S.; Xie G.; Yang X.; Gao S. High-energy-density Materials with Remarkable Thermostability and Insensitivity: Syntheses, Structures and Physicochemical Properties of Pb(II) Compounds with 3-(tetrazol-5-yl) triazole. J. Mater. Chem. A 2014, 2, 11958–11965. 10.1039/C4TA01746C. [DOI] [Google Scholar]; c Zhang S.; Yang Q.; Liu X.; Qu X.; Wei Q.; Xie G.; Chen S.; Gao S. High-Energy Metal–Organic Frameworks (HE-MOFs): Synthesis, Structure and Energetic Performance. Coord. Chem. Rev. 2016, 307, 292–312. 10.1016/j.ccr.2015.08.006. [DOI] [Google Scholar]; d Cui Y.; Zhang J.; Zhang T.; Yang L.; Zhang J.; Hu X. Synthesis, Structural Investigation, Thermal Decomposition Mechanism and Sensitivity Properties of an Energetic Compound [Cd(DAT)6](ClO4)2 (DAT = 1,5-diaminotetrazole). J. Hazard. Mater. 2008, 160, 45–50. 10.1016/j.jhazmat.2008.02.078. [DOI] [PubMed] [Google Scholar]; e Feng Y.; Bi Y.; Zhao W.; Zhang T. Anionic Metal–Organic Frameworks Lead The Way to Eco-Friendly High-Energy-Density Materials. J. Mater. Chem. A 2016, 4, 7596–7600. 10.1039/C6TA02340A. [DOI] [Google Scholar]
- Bushuyev O. S.; Peterson G. R.; Brown P.; Maiti A.; Gee R. H.; Weeks B. L.; Hope-Weeks L. J. Metal–Organic Frameworks (MOFs) as Safer, Structurally Reinforced Energetics. Chem. – Eur. J. 2013, 19, 1706–1711. 10.1002/chem.201203610. [DOI] [PubMed] [Google Scholar]
- Li S.; Wang Y.; Qi C.; Zhao X.; Zhang J.; Zhang S.; Pang S. 3D Energetic Metal–Organic Frameworks: Synthesis and Properties of High Energy Materials. Angew. Chem., Int. Ed. 2013, 52, 14031–14035. 10.1002/anie.201307118. [DOI] [PubMed] [Google Scholar]
- a Zhang Q.; Shreeve J. M. Metal–Organic Frameworks as High Explosives: A New Concept for Energetic Materials. Angew. Chem., Int. Ed. 2014, 53, 2540–2542. 10.1002/anie.201310014. [DOI] [PubMed] [Google Scholar]; b Zhang J.; Shreeve J. M. 3D Nitrogen-Rich Metal–Organic Frameworks: Opportunities for Safer Energetics. Dalton Trans. 2016, 45, 2363–2368. 10.1039/C5DT04456A. [DOI] [PubMed] [Google Scholar]
- a Ye Y.; Zhang L.; Peng Q.; Wang G.; Shen Y.; Li Z.; Wang L.; Ma X.; Chen Q.-H.; Zhang Z.; Xiang S. High Anhydrous Proton Conductivity of Imidazole-Loaded Mesoporous Polyimides over a Wide Range from Subzero to Moderate Temperature. J. Am. Chem. Soc. 2015, 137, 913–918. 10.1021/ja511389q. [DOI] [PubMed] [Google Scholar]; b Li J.-R.; Tao Y.; Yu Q.; Bu X.-H.; Sakamoto H.; Kitagawa S. Selective Gas Adsorption and Unique Structural Topology of a Highly Stable Guest-Free Zeolite-Type MOF Material with N-rich Chiral Open Channels. Chem. – Eur. J. 2008, 14, 2771–2776. 10.1002/chem.200701447. [DOI] [PubMed] [Google Scholar]; c Hu T.; Bi W.; Hu X.; Zhao X.; Sun D. Construction of Metal - Organic Frameworks with Novel {Zn8O13} SBU or Chiral Channels through in Situ Ligand Reaction. Cryst. Growth Des. 2010, 10, 3324–3326. 10.1021/cg100725j. [DOI] [Google Scholar]
- Talawar M. B.; Sivabalan R.; Mukundan T.; Muthurajan H.; Sikder A. K.; Gandhe B. R.; Rao A. S. Environmentally Compatible Next Generation Green Energetic Materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. 10.1016/j.jhazmat.2008.04.011. [DOI] [PubMed] [Google Scholar]
- a Drukenmüller I. E.; Klapötke T. M.; Morgenstern Y.; Rusan M.; Stierstorfer J. Metal Salts of Dinitro-, Trinitropyrazole, and Trinitroimidazole. Z. Anorg. Allg. Chem. 2014, 640, 2139–2148. 10.1002/zaac.201400233. [DOI] [Google Scholar]; b Rahm M.; Brinck T. Kinetic Stability and Propellant Performance of Green Energetic Materials. Chem. – Eur. J. 2010, 16, 6590–6600. 10.1002/chem.201000413. [DOI] [PubMed] [Google Scholar]
- a Himo F.; Demko Z. P.; Noodleman L.; Sharpless K. B. Mechanisms of Tetrazole Formation by Addition of Azide to Nitriles. J. Am. Chem. Soc. 2002, 124, 12210–12216. 10.1021/ja0206644. [DOI] [PubMed] [Google Scholar]; b Xiong R. G.; Xue X.; Zhao H.; You X. Z.; Abrahams B. F.; Xue Z. L. Novel, Acentric Metal–Organic Coordination Polymers from Hydrothermal Reactions Involving In Situ Ligand Synthesis. Angew. Chem., Int. Ed. 2002, 41, 3800–3803. . [DOI] [PubMed] [Google Scholar]
- a Liu Z.-F.; Wu M.-F.; Zheng F.-K.; Wang S.-H.; Zhang M.-J.; Chen J.; Xiao Y.; Guo G.-C.; Wu A.-Q. Zinc(II) Coordination Compounds Based on In Situ Generated 3-(5H-tetrazol)benzaldehyde with Diverse Modes: Hydrothermal Syntheses, Crystal Structures and Photoluminescent Properties. CrystEngComm 2013, 15, 7038–7047. 10.1039/c3ce27111k. [DOI] [Google Scholar]; b Wang S.-H.; Zheng F.-K.; Zhang M.-J.; Liu Z.-F.; Chen J.; Xiao Y.; Wu A.-Q.; Guo G.-C.; Huang J.-S. Homochiral Zinc(II) Coordination Compounds Based on In-Situ-Generated Chiral Amino Acid–Tetrazole Ligands: Circular Dichroism, Excitation Light-Induced Tunable Photoluminescence, and Energetic Performance. Inorg. Chem. 2013, 52, 10096–10104. 10.1021/ic401409b. [DOI] [PubMed] [Google Scholar]; c Xiao Y.; Wang S.-H.; Xu J.-G.; Sun C.; Li R.; Zhao Y.-P.; Yan Y.; Zheng F.-K.; Guo G.-C. An Enhanced Extended Hook Method to Realize Tetranuclear Metal Clusters Embedded in Energetic Metal–Organic Framework Channels. CrystEngComm 2016, 18, 5803–5806. 10.1039/C6CE00892E. [DOI] [Google Scholar]
- Feng Y.; Liu X.; Duan L.; Yang Q.; Wei Q.; Xie G.; Chen S.; Yang X.; Gao S. In Situ Synthesized 3D Heterometallic Metal–Organic Framework (MOF) as a High-Energy-Density Material Shows High Heat of Detonation, Good Thermostability and Insensitivity. Dalton Trans. 2015, 44, 2333–2339. 10.1039/C4DT03131H. [DOI] [PubMed] [Google Scholar]
- Karaghiosoff K.; Klapötke T. M.; Michailovski A. N.; Nöth M. S. H.; Holl G. 1,4-Diformyl-2,3,5,6-Tetranitratopiperazine: A New Primary Explosive Based on Glyoxal. Propellants, Explos., Pyrotech. 2003, 28, 1–6. 10.1002/prep.200390002. [DOI] [Google Scholar]
- Ou Y. X.Explosives; Beijing Institute of Technology Press: China, 2006; p 143. [Google Scholar]
- Lin J.-D.; Wang S.-H.; Cai L.-Z.; Zheng F.-K.; Guo G.-C.; Huang J.-S. Tetraalkylammonium Cations as Templates in the Construction of Two Cadmium(II) Metal–Organic Frameworks. CrystEngComm 2013, 15, 903–910. 10.1039/C2CE26213D. [DOI] [Google Scholar]
- Zhang S.; Liu X.; Yang Q.; Su Z.; Gao W.; Wei Q.; Xie G.; Chen S.; Gao S. A New Strategy for Storage and Transportation of Sensitive High-Energy Materials: Guest-Dependent Energy and Sensitivity of 3D Metal–Organic-Framework-Based Energetic Compounds. Chem. – Eur. J. 2014, 20, 7906–7910. 10.1002/chem.201402783. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang J.; Su H.; Li S.; Zhang S.; Pang S. A Simple Method for the Prediction of the Detonation Performances of Metal-Containing Explosives. J. Phys. Chem. A 2014, 118, 4575–4581. 10.1021/jp502857d. [DOI] [PubMed] [Google Scholar]
- CrystalClear, version 1.3.5, Software User’s Guide for the Rigaku R-Axis, and Mercury and Jupiter CCD Automated X-ray Imaging System; Rigaku Molecular Structure Corporation: UT, 2002.
- Sheldrick G. M. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.