Abstract
Herein, we describe a greener protocol for the one-pot synthesis of 3-Se/S-4H-chromen-4-ones. The desired products were obtained in good to excellent yields using 2-hydroxyphenyl enaminones and half equivalents of various odorless diorganyl dichalcogenides (S/Se) in the presence of glycerol (5 molar equiv) and KIO3 (15 mol %) as the catalyst under solvent-free conditions.
1. Introduction
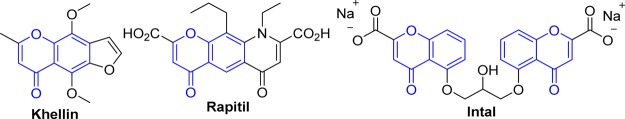
The chromone core is a ubiquitous heterocycle present in many natural bioactive products, and it represents an important “privileged scaffold”.1 Numerous types of biological activities are associated with simple chromones and analogues, including anti-inflammatory, antiplatelet, anticancer, anti-HIV, immune-stimulatory, anti-Alzheimer, and antimicrobial.1,2 Several commercially available drugs have the chromone moiety in their core structure, for example, khelline (used in folk medicines), Rapitil (for asthma and allergic eye reactions), and Intal (for asthma) (Figure 1).3 Several promising radioiodinated styrylchromone derivatives have been used as probes for imaging because of the fluorescence of the core structure.4Hence, considering their structural diversity, biological properties, and synthetic utility, these structures have received considerable attention.1,5
Figure 1.
Chromone-based drugs.
Analogously, the construction of the C–S/Se bond is a very important transformation in organic synthesis, as these compounds exhibit fascinating biological characteristics.6 In the past few decades, these compounds have gained increasing interest, mainly because of their antioxidant, anti-inflammatory, antitumor, and antiviral activities.6,7 They also play a fundamental role in modern organic synthesis and are employed as catalysts, ligands, and ionic liquids in certain reactions and as synthetic intermediates in total synthesis.8,9 In addition, they are applied in asymmetric catalysis and in materials science.10
Despite the biological importance of organochalcogen compounds and the wide spectrum of therapeutic properties of chromones, only a few synthetic methods for the construction of these hybrid structures in a single molecule, 3-chalcogenayl-4H-chromen-4-ones, have been reported.11
Zeni and co-workers have reported the synthesis of selenylated chromones from the reaction of alkynyl aryl ketones and diselenides in the presence of 1.5 equiv of FeCl3 (Scheme 1a).11a Recently, Blond and co-workers have demonstrated the AgOTf-mediated synthesis of chromone containing the phenylselenyl moiety through the reaction of 2-hydroxy-phenyl enaminones and phenylselenyl chloride (Scheme 1b).11e The common approach to access sulfenylated chromones is through the direct sulfenylation of the C–H bond of chromones using different sources of organosulfur and 2–4 molar equiv of NH4I (Scheme 1c).11b−11d During the preparation of this manuscript, a related study using aryl thiols as a sulfenylating agent and 0.3 molar equiv of KIO3 to produce sulfenylated chromones in ethyl lactate as the solvent appeared in the literature (Scheme 1d).11f
Scheme 1. Synthetic Routes for Chalcogenated Chromones.
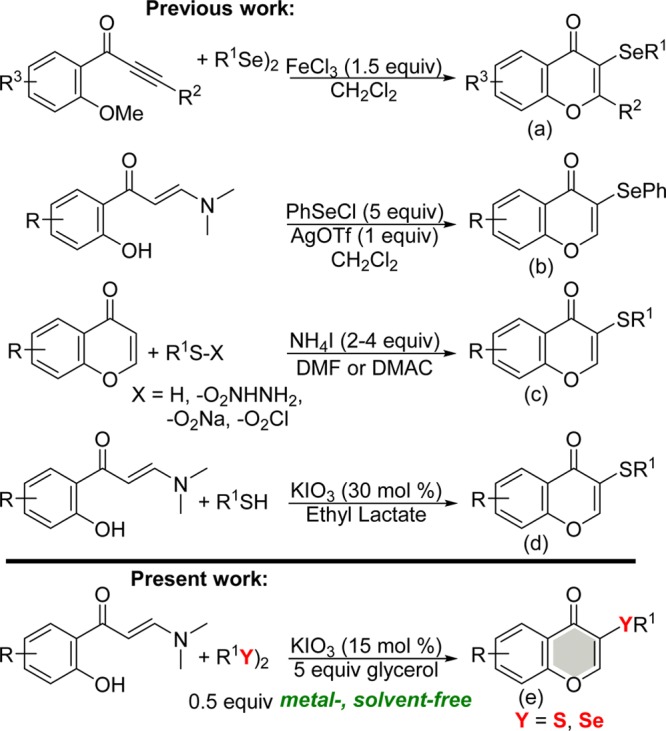
Some of the methods described in the literature are associated with limitations that reduce their synthetic utility. These downsides include the use of nongreen solvents, prefunctionalized coupling partners, low atom economy, narrow substrate scope, the use of transition metal salts, malodorous reagents, and elaborate, multistep processes.
In recent years, various types of organic transformations have been carried out with the application of metal- and solvent-free systems.12
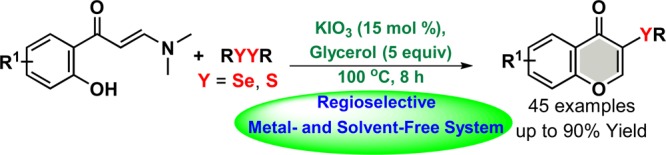
An alternative greener method with a broad scope for the synthesis of these compounds, involving a solvent- and metal-free system that could provide high efficiency, would be advantageous and highly desirable in view of the significance of chalcogenated chromones. As part of our wider research program aimed at designing and developing sustainable processes and solvent-free systems for the chalcogenation of heteroarenes and organochalcogen chemistry,13 herein, we report, for the first time, the KIO3/glycerol catalytic system for the synthesis of C-3 chalcogenated chromones (Scheme 1e). Our new regioselective, broader, metal-, and solvent-free approach worked effectively using enaminones with a half-molar equivalent of diorganyl diselenides or disulfides as a nonmalodorous source of chalcogens, in the presence of catalytic loading of KIO3 (15 mol %).
2. Results and Discussion
To identify the best reaction conditions, enaminone 1a and diphenyl diselenide 2a were selected as model substrates. These were then evaluated under various conditions (Table 1).
Table 1. Optimization of Reaction Conditionsa.
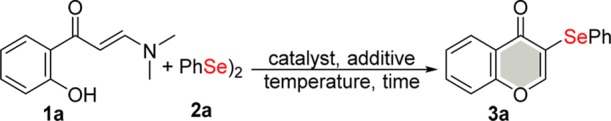
| entry | catalyst (mol %) | additive (equiv) | temp (°C) | time (h) | yieldb (%) |
|---|---|---|---|---|---|
| 1 | PEG-400 (5) | 110 | 10 | NR | |
| 2c | PEG-400 (5) | 110 | 10 | NR | |
| 3 | CuI (20) | PEG-400 (5) | 110 | 10 | traces |
| 4 | ZnI2 (20) | PEG-400 (5) | 110 | 10 | NR |
| 5 | KI (20) | PEG-400 (5) | 110 | 10 | 10 |
| 6 | KIO3 (20) | PEG-400 (5) | 110 | 10 | 61 |
| 7 | NaIO3 (20) | PEG-400 (5) | 110 | 10 | 35 |
| 8 | I2 (20) | PEG-400 (5) | 110 | 10 | 38 |
| 9 | KIO3 (20) | Et. lactate (5) | 110 | 10 | 67 |
| 10 | KIO3 (20) | glycerol (5) | 110 | 10 | 86 |
| 11 | KIO3 (20) | DMSO (5) | 110 | 10 | 52 |
| 12 | KIO3 (20) | toluene (5) | 110 | 10 | traces |
| 13 | KIO3 (15) | glycerol (5) | 110 | 10 | 85 |
| 14 | KIO3 (10) | glycerol (5) | 110 | 10 | 65 |
| 15 | KIO3 (15) | glycerol (5) | 100 | 10 | 86 |
| 16 | KIO3 (15) | glycerol (5) | 90 | 10 | 72 |
| 17 | KIO3 (15) | glycerol (5) | 100 | 8 | 86 |
| 18 | KIO3 (15) | glycerol (5) | 100 | 6 | 70 |
| 19 | KIO3 (15) | glycerol (3) | 100 | 10 | 71 |
| 20d | KIO3 (15) | glycerol | 100 | 10 | 85 |
Reaction conditions: 1a (0.25 mmol), 2a (0.125 mmol), catalyst (mol %), and additive (equivalent).
Isolated yields.
Reaction under argon atmosphere.
2 mL glycerol.
On the basis of our previous experience of solvent-free systems,13a−13d preliminary experiments were carried out in the presence of a stoichiometric amount of poly(ethylene glycol) (PEG-400) as an additive at 110 °C with a reaction time of 10 h and without a catalyst. The reaction under open air (entry 1) and under inert atmosphere (entry 2) conditions was unsuccessful. When the reaction was performed in the presence of 20 mol % of CuI as the transition metal catalyst, 3a was formed in trace amounts (entry 3), whereas the use of ZnI2 was completely ineffective (entry 4). When the reaction was carried out in the presence of KI as the catalyst, the desired product was obtained in 10% yield (entry 5). On switching from KI to KIO3, 3a was isolated with a 61% yield (entry 6) but its sodium analogue gave the selenylated product with a 35% yield (entry 7). Molecular iodine was also not effective and afforded 3a with a 38% yield (entry 8).
After determining the appropriate catalyst, in the next step, the type of additive was screened for this transformation (entries 9–12). Ethyl lactate promoted some improvement in the reaction (entry 9), and this motivated us to test other greener additives. With the use of glycerol, 3a was isolated with an 86% yield (entry 10). An adverse effect on the yield was noted when dimethylsulfoxide (DMSO) was used (entry 11), whereas toluene afforded 3a in traces (entry 12).
Subsequently, the catalyst loading, reaction time, and temperature were screened for this transformation (entries 13–18). Lowering the catalyst loading to 15 mol % did not affect the yield of 3a (entry 13 vs 10). Further decreasing the catalyst quantity to 10 mol % resulted in a lower yield of 3a (entry 14). The reaction temperature and time were screened for this transformation (entries 15–17), and ideal values of 100 °C and 8 h were obtained.
The use of glycerol (2 mL) as the solvent did not provide any further positive influence on the yield of 3a (entry 20 vs 17). Decreasing the quantity of glycerol to 3 molar equiv afforded 3a with a lower yield (entry 19).
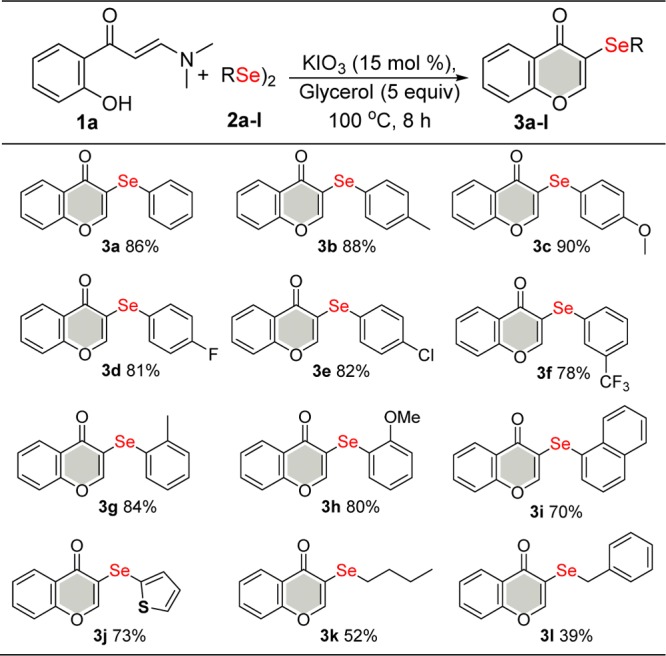
With the optimized conditions in hand (Table 1, entry 17), the applicability of other enaminones 1 and various diorganyl dichalcogenides was investigated (Schemes 2–4). We first evaluated the efficiency and generality of this method with respect to different diorganyl diselenides 2 while keeping enaminone 1a constant (Scheme 2).
Scheme 2. Scope of Diorganyl Diselenide 2.
Isolated yield.
Scheme 4. Thiolation Using Arylsulfonyl Hydrazides 6.
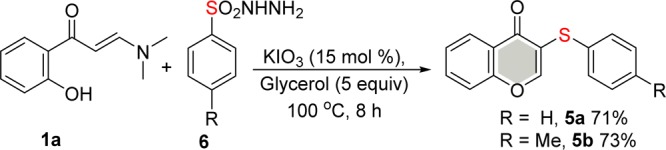
The reaction worked effectively for structurally diverse diselenides 2. Substituents on the aryl moiety, that is, electron-donating (R = F, Cl, and CF3) and electron-withdrawing (R = Me and OMe) groups and a bulky group (naphthyl) successfully afforded the corresponding products 3a–i in good to excellent yields (70–90%; 3a–i). The course of the reaction appears to be influenced by electronic effects. It can be noted that diaryl diselenides with electron-donating groups usually gave the selenylated products (3b–c) in better yields than those achieved with electron-withdrawing groups (3d–f). Furthermore, steric hindrance of the ortho-substituted aryl substrates showed a weaker influence on the yields in relation to the corresponding para-derivatives (3b–c vs 3g–h). However, product 3i was obtained in 70% yield when a fused aromatic substrate (R = naphthyl) was used.
C-2 heteroaryl diselenide 2j afforded the desired product 3j with a 73% yield. Aliphatic diorganyl diselenides 2k and 2l furnished the respective selenylated chromones 3k and 3l in 52 and 39% yields, respectively. 3l was observed when benzylic diselenide was used as the substrate.
The success observed for the KIO3-catalyzed synthesis of selenylated chromones 3 prompted us to expand the scope of this method to access sulfenylated chromones 5 using diorganyl disulfides 4 as the coupling partner (Scheme 3). The desired products 5a–g were obtained in 44–85% yields. Using diphenyl disulfide 4a as the substrate, the sulfenylated product 5a was obtained in 82% yield. It was noted that the method used to prepare the sulfenylated product 5 from disulfides 4 presented electronic and steric effects similar to that used for diorganyl diselenides 2. There was a small decrease in the product yields of sulfenylated compound 5 compared with selenylated analogue 3, which can probably be attributed to the stronger S–S bond of the diaryl disulfides in relation to the respective diselendies 2.
Scheme 3. Scope of Diorganyl Disulfides 4.
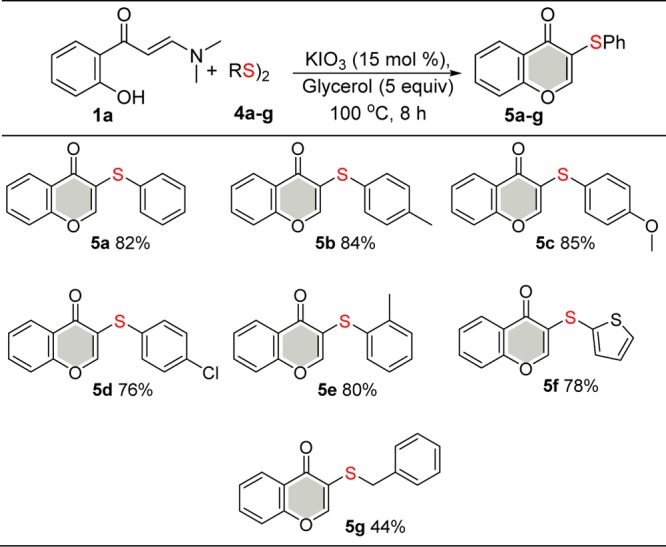
Isolated yield.
We then extended our study to sulfonyl hydrazides 6 (Scheme 4), applying the optimal reaction conditions (Table 1, entry 17), to explore the scope of this new methodology. The protocol described herein is versatile, being applicable to different types of organochalcogen sources. For instance, the reaction of different arylsulfonyl hydrazides 6 with enaminone 1a afforded the corresponding coupled products 5a and 5b in isolated yields of 71 and 73%, respectively (Scheme 4).
To broaden the scope of the optimized reaction in relation to the substrate, the influence of the enaminone 1 moiety was evaluated with 2a and 4a (Scheme 5). Enaminone 1 with different functional groups attached at the aryl moiety, for example, alkyl, alkoxy, halogen, and naphthyl groups, were tested. The system tolerated the electronic effects of the substituents on the phenyl group, and both electron-withdrawing and electron-donating groups are suitable substrates, affording the corresponding products 7a–l and 7aa–al in 70–88% yields. In this case, the electron-donating groups (−Me, −MeO, and −EtO) showed superiority over electron-withdrawing groups (−F, Cl, and Br). Furthermore, the reaction tolerated disubstituted substrates, furnishing the respective product in good to excellent yields. Similarly, a fused aromatic substrate (naphthyl) resulted in the desired products 7m and 7am in 80 and 76% yields, respectively.
Scheme 5. Scope of Enaminones 1.
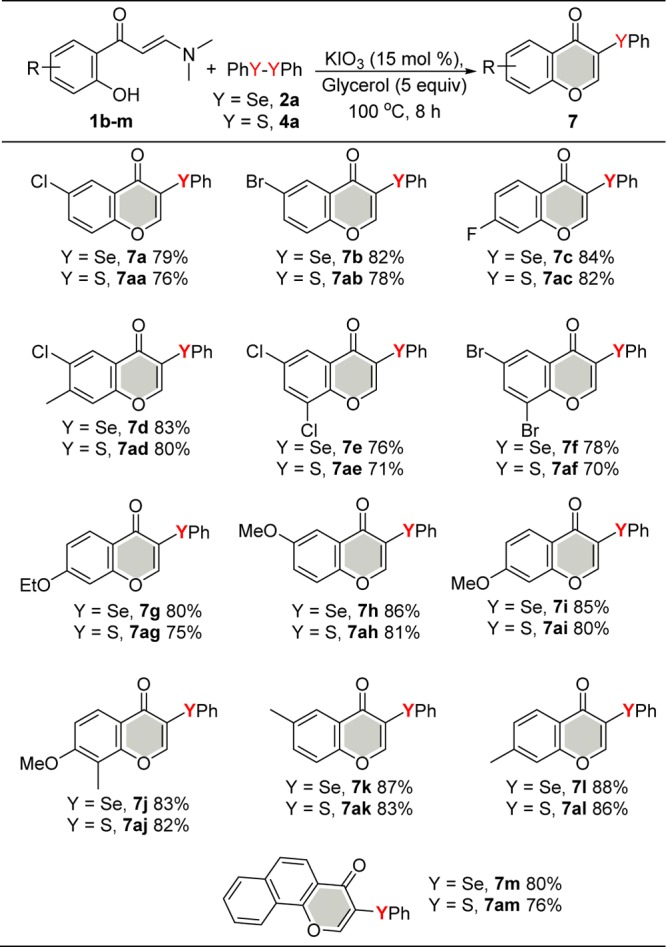
Isolated yield.
In general, we observed that diselenides 2a furnished the targeted selenylated products 7a–m in slightly better yields compared with disulfides 4a.
On the basis of reports in the literature11 and the chemical shifts observed from the nuclear magnetic resonance (NMR) (1H and 13C) spectra of each of the chalcogenated chromone derivatives (3, 8), all products have an organochalcogen moiety bonded at the α-positions of the ketone functions of the chromones.
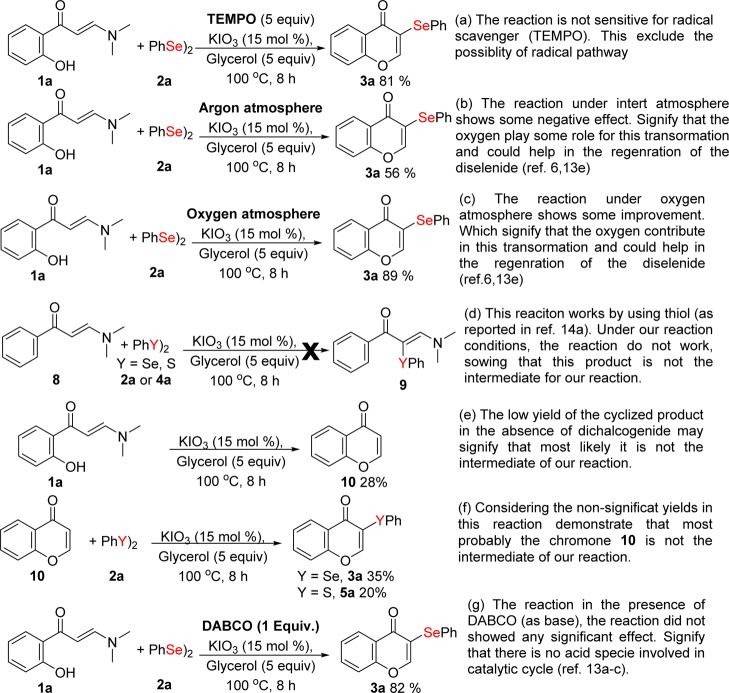
In view of the unique features of the KIO3-catalyzed one-pot cyclization of enaminones and chalcogenation in the presence of dichalcogenide, we decided to investigate the mode of action. Some control experiments were therefore conducted (Scheme 6). The addition of a stoichiometric amount of TEMPO, as a radical inhibitor, did not hamper the reaction, and the selenylated product 3a was obtained in 81% yield (Scheme 6a), which excluded the possibility of a radical pathway. While under an inert atmosphere, the standard reaction shows a decrease in the efficacy (Scheme 6b), indicating that the presence of oxygen is important for this transformation. In agreement with that, the reaction under oxygen atmosphere exhibited some improvement in the isolated yield of 3a (Scheme 6c), indicating the importance of oxygen in the reaction medium. When (E)-3-(dimethylamino)-1-phenylprop-2-en-1-one 8 was used instead of enaminone 1a under standard conditions for 2a and 4a, the expected chalcogenated enaminone 9 was not observed (Scheme 6d). Thus, apparently, the hydroxy group of enaminones plays a key role in the reaction. Subsequently, the reaction in the absence of dichalcogenides furnished the chromone in a low yield (Scheme 6e), signifying that most likely it is not the intermediate in our reaction. Additionally, chromone 10 itself, under optimized conditions, provided the desired products in low yields (Scheme 6f). The presence of DABCO (as base) did not hamper the reaction, and product 3a was obtained in 82% yield (Scheme 6g). This result indicates that most likely the reaction did not involve any active iodine acid species (such as HI) during the catalytic pathway, as previously observed.13a−13c
Scheme 6. Control Experiments.
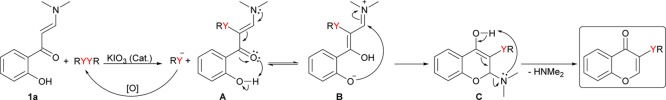
On the basis of these results and of previous reports,13,14a a mechanism for this transformation can be proposed (Scheme 7). Initially, the reaction between diorganyl dichalcogenide and enaminone 1 would form species (A) and organochalcogenyl anion, which would suffer oxidation by air and regenerate the dichalcogenide. The species (A) would afford the cyclic intermediate (C) through the intramolecular cyclization from the tautomeric intermediate (B). Subsequently, the elimination of dimethylamine from the intermediate (C) would furnish the desired chalcogenated chromone.
Scheme 7. Proposed Mechanism for the Reaction.
3. Conclusions
We developed a KIO3-catalyzed, simple, greener, metal-, and solvent-free approach for the preparation of 3-selenyl- and 3-sulfenyl-chromones, a class of compounds of interest for therapeutic applications. Under optimized reaction conditions, the reaction worked well in the presence of KIO3/glycerol, as a nontoxic catalytic system, with 2-hydroxy-phenylenaminones and a half molar equivalent of diorganyl dichalcogenides as an odorless source of chalcogens. This afforded a wide range of chalcogenated (S, Se) chromones at the C3 position in good to excellent yields. The optimized reaction conditions were appropriate for various substituents with different electronic and steric effects. Furthermore, sulfonyl hydrazides were also successfully applied as alternative sulfenylating agents.
The important features of this robust and benign protocol are as follows: (1) it is metal-free and solvent-free; (2) it is performed open air; (3) it is atom-economic, (4) regioselective, and (5) inexpensive; (6) it is a nontoxic catalytic system with (7) a low catalytic loading; and (8) it is applicable to different sources of organochalcogenides and a wide range of enaminones.
4. Experimental Section
4.1. General
Proton nuclear magnetic resonance (1H NMR) spectra were obtained at 200 MHz on a Bruker AC-200 NMR spectrometer. The spectra were recorded in CDCl3 solutions. The chemical shifts are reported in ppm, referenced to the solvent peak of CDCl3 or tetramethylsilane (TMS) as the external reference. Data are reported as follows: chemical shift (δ), multiplicity, coupling constant (J) in hertz, and integrated intensity. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were obtained at 50 MHz on a Bruker AC-200 NMR spectrometer. The spectra were recorded in CDCl3 solutions. The chemical shifts are reported in ppm, referenced to the solvent peak of CDCl3. Abbreviations to denote the multiplicity of a particular signal are s (singlet), d (doublet), t (triplet), q (quartet), quint (quintet), sext (sextet), and m (multiplet). Selenium-77 nuclear magnetic resonance (77Se NMR) spectra were recorded at 38.14 MHz on a Bruker AC-200 NMR spectrometer. The spectra were recorded in CDCl3 solutions. The chemical shifts are reported in ppm, referenced to diphenyl diselenide as the external reference (463.15 ppm). High-resolution mass spectra were recorded on a Bruker micrOTOF-Q II ESI mass spectrometer equipped with an automatic syringe pump for sample injection. The melting points were determined using a Microquimica MQRPF-301 digital model equipment with a heating plate. Column chromatography was performed using silica gel (230–400 mesh). Thin-layer chromatography (TLC) was performed using Merck silica gel GF254 of 0.25 mm thickness. For visualization, TLC plates were either placed under ultraviolet light or stained with iodine vapor and acidic vanillin.
Unless otherwise stated, all reactions were carried out in a Schlenk tube; all reagents and solvents were obtained from commercial sources and used without any further purification. Enaminones 1a–m were synthetized following the procedure reported.14
4.2. General Procedure for the KIO3-Catalyzed Synthesis of 3-Se/S-Chromones from the Cyclization of Enaminones 1a–m Using Diorganyl Chalcogenides
A mixture of appropriate enaminone 1 (0.25 mmol), diorganyl dichalcogenide 2 or 4 (0.125 mmol), KIO3 (15 mol %, 8 mg), and 3 equiv of glycerol (1.25 mmol, 115 mg) was charged in a Schlenck tube. The mixture was heated to 100 °C in an oil bath for 8 h. After this, the reaction mixture was dissolved in ethyl acetate (10 mL) and washed with 2 × 5 mL of an aqueous solution of 10% Na2S2O3. The organic phase was separated, dried over MgSO4, and concentrated under vacuum. The crude product was purified using flash chromatography on silica gel using hexane or a mixture of hexane/ethyl acetate (9:1) as the eluent.
4.3. General Procedure for the KIO3-Catalyzed Reactions of Arylsulfonyl Hydrazides 6 with Enaminones 1a
A mixture of enaminone 1a (0.25 mmol, 48 mg) and appropriate arylsulfonyl hydrazide 6 (0.25 mmol) was used under standard conditions. Yield: 5a, 71% (45 mg) and 5b, 73% (49 mg).
4.4. Control Experiments for the Study of Mechanism
4.4.1. Radical Trapping Study
A mixture of enaminone 1a (0.25 mmol, 48 mg), diphenyl diselenide 2a (0.125 mmol, 39 mg), and TEMPO (1.25 mmol, 195 mg) was used under standard conditions. Yield: 81% (61 mg).
4.4.2. Standard Reaction under Interatmosphere
A mixture of enaminone 1a (0.25 mmol, 48 mg) and diphenyl diselenide 2a (0.125 mmol, 39 mg) was used under standard conditions in the argon atmosphere. Yield: 56% (42 mg).
4.4.3. Standard Reaction under Oxygen Atmosphere
A mixture of enaminone 1a (0.25 mmol, 48 mg) and diphenyl diselenide 2a (0.125 mmol, 39 mg) was used under standard conditions in the oxygen atmosphere. Yield: 89% (67 mg).
4.4.4. Reaction between 8 and 2a or 4a
A mixture of enaminone 8 (0.25 mmol, 44 mg) and diphenyl diselenide 2a (0.125 mmol, 39 mg) or diphengyl disulfide 4a (0.125 mmol, 28 mg) was used under standard conditions.
4.4.5. Cyclization of Enaminone 1a Catalyzed by KIO3
A mixture of enaminone 1a (0.25 mmol, 48 mg), KIO3 (15 mol %, 8 mg), and 3 equiv of glycerol (1.25 mmol, 115 mg) was charged in a Schlenck tube under standard conditions. Yield: 10, 28% (37 mg).
4.4.6. Reaction between 10 and 2a Catalyzed by KIO3
A mixture of 10 (0.25 mmol, 37 mg) and diphenyl diselenide 2a (0.125 mmol, 39 mg) or diphenyl disulfide 4a (0.125 mmol, 28 mg) was used under standard conditions. Yield: 3a, 35% (26 mg) and 5a, 20% (13 mg).
4.4.7. Standard Reaction in the Presence of DABCO (as Base)
A mixture of enaminone 1a (0.25 mmol, 48 mg), diphenyl diselenide 2a (0.125 mmol, 39 mg), and DABCO (0.25 mmol, 28 mg) was used under standard conditions. Yield: 82% (62 mg).
4.5. 3-(Phenylselanyl)-4H-chromen-4-one (3a)11
Yield: 86% (65 mg); beige crystalline solid; mp: 59–60 °C (59 °C);21H NMR (200 MHz, CDCl3): δ = 8.24 (d, J = 8.4 Hz, 1H), 7.89 (s, 1H), 7.72–7.57 (m, 3H), 7.43 (d, J = 7.4 Hz, 2H), 7.34–7.25 (m, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.2, 156.4, 155.8, 133.9, 129.6, 128.2, 128.2, 126.4, 125.6, 123.2, 118.1, 117.9; 77Se NMR (38 MHz, CDCl3): δ = 303.06; HRMS m/z calcd for C15H11O2Se [M + H]+ 302.9919; found, 302.9920.
4.6. 3-(p-Tolylselanyl)-4H-chromen-4-one (3b, New Compound)
Yield: 88% (69 mg); beige solid; mp: 88–89 °C; 1H NMR (200 MHz, CDCl3): δ = 8.22 (dd, J = 8.2, 1.4 Hz, 1H), 7.76 (s, 1H), 7.70–7.60 (m, 1H), 7.52 (d, J = 8.0 Hz, 2H), 7.46–7.34 (m, 2H), 7.12 (d, J = 7.8 Hz, 2H), 2.33 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.2, 156.3, 154.8, 138.5, 134.6, 133.7, 130.4, 126.2, 125.4, 123.9, 123.0, 118.6, 118.0, 21.2; HRMS m/z calcd for C16H13O2Se [M + H]+ 317.0076; found, 317.0079.
4.7. 3-((4-Methoxyphenyl)selanyl)-4H-chromen-4-one (3c, New Compound)
Yield: 90% (74 mg); white crystalline solid; mp: 92–94 °C; 1H NMR (200 MHz, CDCl3): δ = 8.19 (dd, J = 8.2, 1.6 Hz, 1H), 7.71–7.53 (m, 4H), 7.43–7.31 (m, 2H), 6.85 (d, J = 8.8 Hz, 2H), 3.78 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.1, 16.1, 156.2, 153.7, 137.0, 133.6, 126.0, 125.3, 122.8, 119.2, 117.9, 116.9, 115.3, 55.2; HRMS m/z calcd for C16H13O3Se [M + H]+ 333.0025; found, 333.0023.
4.8. 3-((4-Fluorophenyl)selanyl)-4H-chromen-4-one (3d, New Compound)
Yield: 81% (65 mg); yellow crystalline solid; mp: 84–85 °C; 1H NMR (200 MHz, CDCl3): δ = 8.21 (dd, J = 8.3, 1.5 Hz, 1H), 7.89 (s, 1H), 7.71–7.57 (m, 3H), 7.46–7.36 (m, 2H), 7.05–6.91 (m, 2H); 13C NMR (50 MHz, CDCl3): δ = 175.1, 163.0 (d, JC–F = 248.6 Hz), 156.3, 155.6, 136.4 (d, JC–F = 8.1 Hz), 133.9, 126.3, 125.6, 123.1, 122.6 (d, JC–F = 3.6 Hz), 118.1, 118.0, 116.8 (d, JC–F = 21.6 Hz); HRMS m/z calcd for C15H10O2SeF [M + H]+ 320.9825; found, 320.9827.
4.9. 3-((4-Chlorophenyl)selanyl)-4H-chromen-4-one (3e, New Compound)
Yield: 82% (69 mg); beige crystalline solid; mp: 118–119 °C; 1H NMR (200 MHz, CDCl3): δ = 8.26–8.18 (m, 1H), 8.00 (s, 1H), 7.72–7.62 (m, 1H), 7.54–7.38 (m, 4H), 7.23 (d, J = 8.4 Hz, 2H); 13C NMR (50 MHz, CDCl3): δ = 175.0, 156.5, 156.3, 134.8, 134.3, 134.0, 129.6, 126.7, 126.4, 125.7, 123.2, 118.1, 117.2; HRMS m/z calcd for C15H10O2SeCl [M + H]+ 336.9532; found, 336.9535.
4.10. 3-((3-(Trifluoromethyl)phenyl)selanyl)-4H-chromen-4-one (3f, New Compound)
Yield: 78% (72 mg); beige crystalline sold; mp: 110–111 °C; 1H NMR (200 MHz, CDCl3): δ = 8 8.21 (d, J = 7.9 Hz, 1H), 8.14 (s, 1H), 7.83–7.79 (m, 1H), 7.75–7.63 (m, 2H), 7.53–7.32 (m, 4H); 13C NMR (50 MHz, CDCl3): δ = 174.9, 157.5, 156.4, 136.1, 134.1, 131.63 (q, JC–F = 32.5 Hz), 130.2, 129.7, 129.30 (q, JC–F = 3.3 Hz), 126.4, 125.8, 124.58 (q, JC–F = 3.5 Hz), 126.3 (q, JC–F = 271 Hz), 123.4, 118.2, 116.3; HRMS m/z calcd for C16H10O2SeF3 [M + H]+ 370.9793; found, 370.9795.
4.11. 3-(o-Tolylselanyl)-4H-chromen-4-one (3g, New Compound)
Yield: 84% (66 mg); red crystalline solid; mp: 118 °C; 1H NMR (200 MHz, CDCl3): δ = 8.22 (dd, J = 8.3, 1.5 Hz, 1H), 7.75–7.58 (m, 2H), 7.51–7.34 (m, 3H), 7.27–7.03 (m, 3H), 2.48 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.2, 156.3, 154.6, 140.5, 134.5, 133.7, 130.4, 128.5, 128.4, 127.0, 126.2, 125.4, 122.9, 118.0, 117.3, 22.3; HRMS m/z calcd for C16H13O2Se [M + H]+ 317.0076; found, 317.0072.
4.12. 3-((2-Methoxyphenyl)selanyl)-4H-chromen-4-one (3h, New Compound)
Yield: 80% (67 mg); white solid; mp: 110–111 °C; 1H NMR (200 MHz, CDCl3): δ = 8.27 (dd, J = 7.9, 1.8 Hz, 1H), 8.08 (s, 1H), 7.75–7.63 (m, 1H), 7.50–7.39 (m, 2H), 7.28–7.16 (m, 2H), 6.91–6.79 (m, 2H), 3.89 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.5, 157.8, 157.5, 156.5, 133.9, 131.8, 128.7, 126.6, 125.7, 123.4, 121.8, 118.5, 118.2, 114.9, 110.8, 56.0; HRMS m/z calcd for C16H13O3Se [M + H]+ 333.0025; found, 333.0024.
4.13. 3-(Naphthalen-1-ylselanyl)-4H-chromen-4-one (3i, New Compound)
Yield: 70% (61 mg); yellow solid; mp: 67–68 °C; 1H NMR (200 MHz, CDCl3): δ = 8.22 (dd, J = 8.2, 1.6 Hz, 1H), 8.13–8.05 (m, 1H), 7.86 (s, 1H), 7.79–7.58 (m, 5H), 7.50–7.34 (m, 4H); 13C NMR (50 MHz, CDCl3): δ = 175.2, 156.3, 155.7, 134.0, 133.8, 133.3, 132.7, 130.8, 129.1, 127.8, 127.5, 126.6, 126.6, 126.3, 125.5, 125.4, 123.1, 118.1, 118.0; HRMS m/z calcd for C19H13O2Se [M + H]+ 353.0076; found, 353.0074.
4.14. 3-(Thiophen-2-ylselanyl)-4H-chromen-4-one (3j, New Compound)
Yield: 73% (56 mg); yellow solid; mp: 114–116 °C; 1H NMR (200 MHz, CDCl3): δ = 8.20 (dd, J = 8.2, 1.6 Hz, 1H), 7.64 (ddd, J = 8.8, 7.1, 1.7 Hz, 1H), 7.56 (s, 1H), 7.51–7.47 (m, 1H), 7.44–7.35 (m, 3H), 7.09–7.03 (m, 1H); 13C NMR (50 MHz, CDCl3): δ = 175.0, 156.3, 153.3, 137.9, 133.8, 132.8, 128.6, 126.0, 125.5, 122.7, 120.0, 119.9, 118.1; HRMS m/z calcd for C13H9O2SeS [M + H]+ 308.9483; found, 308.9485.
4.15. 3-(Butylselanyl)-4H-chromen-4-one (3k, New Compound)
Yield: 52% (37 mg); yellow solid; mp: 54–55 °C; 1H NMR (200 MHz, CDCl3): δ = 8.38–8.13 (m, 2H), 7.68 (ddd, J = 8.5, 7.0, 1.7 Hz, 1H), 7.56–7.38 (m, 2H), 2.89 (t, J = 6.7 Hz, 2H), 1.66 (dt, J = 15.0, 7.1 Hz, 2H), 1.43 (dq, J = 14.8, 6.9 Hz, 2H), 0.90 (t, J = 7.2 Hz, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.9, 156.4, 156.3, 133.8, 126.4, 125.5, 123.3, 118.1, 114.8, 32.2, 26.1, 22.9, 13.6; HRMS m/z calcd for C13H15O2Se [M + H]+ 283.0232; found, 283.0228.
4.16. 3-(Benzylselanyl)-4H-chromen-4-one (3l, New Compound)
Yield: 39% (31 mg); white solid; mp: 95–96 °C; 1H NMR (200 MHz, CDCl3): δ = 8.28–8.24 (m, 1H), 7.87 (s, 1H), 7.87–7.62 (m, 1H), 7.47–7.38 (m, 2H), 7.27–7.14 (m, 5H), 4.10 (s, 2H); 13C NMR (50 MHz, CDCl3): δ = 176.0, 157.4, 156.4, 137.8, 133.9, 129.1, 128.5, 127.2, 126.3, 125.7, 123.6, 118.5, 118.2, 37.2; HRMS m/z calcd for C16H13O2Se [M + H]+ 317.0076; found, 317.0077.
4.17. 3-(Phenylthio)-4H-chromen-4-one (5a)11
Yield: 82% (52 mg); white crystalline solid; mp: 99–101 °C (98–101 °C);21H NMR (200 MHz, CDCl3): δ = 8.24 (dd, J = 7.8, 1.4 Hz, 1H), 8.14 (s, 1H), 7.75–7.63 (m, 1H), 7.49–7.37 (m, 4H), 7.32–7.19 (m, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.09, 157.43, 156.35, 134.05, 129.86, 129.22, 127.14, 126.42, 125.78, 123.66, 119.92, 118.20; HRMS m/z calcd for C15H11O2S [M + H]+ 255.0474; found, 255.0474.
4.18. 3-(p-Tolylthio)-4H-chromen-4-one (5b)11
Yield: 84% (56 mg); white crystalline solid; mp: 107–108 °C (108–110 °C);21H NMR (200 MHz, CDCl3): δ = 8.28–8.17 (m, 1H), 8.03 (s, 1H), 7.76–7.60 (m, 1H), 7.48–7.32 (m, 4H), 7.14–7.04 (m, 2H), 2.30 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.1, 156.3, 137.6, 133.9, 131.0, 130.1, 129.8, 126.4, 125.6, 123.6, 121.1, 118.1, 21.1; HRMS m/z calcd for C16H13O2S [M + H]+ 269.0631; found, 269.0631.
4.19. 3-((4-Methoxyphenyl)thio)-4H-chromen-4-one (5c)11
Yield: 85% (60 mg); white solid; mp: 116–118 °C (117–119 °C);21H NMR (200 MHz, CDCl3): δ = 8.23 (dd, J = 7.8, 1.8 Hz, 1H), 7.90 (s, 1H), 7.73–7.60 (m, 1H), 7.52–7.36 (m, 4H), 6.86 (d, J = 8.9 Hz, 2H), 3.78 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.2, 159.9, 156.3, 155.0, 134.3, 133.9, 126.3, 125.5, 123.4, 123.1, 122.6, 118.1, 115.0, 55.4; HRMS m/z calcd for C16H13O2S [M + H]+ 285.0580; found, 285.0578.
4.20. 3-((4-Chlorophenyl)thio)-4H-chromen-4-one (5d)11
Yield: 76% (55 mg); beige solid; mp: 49–50 °C (169–170 °C);21H NMR (200 MHz, CDCl3): δ = 8.23 (s, 2H), 7.80–7.08 (m, 7H); 13C NMR (50 MHz, CDCl3): δ = 174.9, 157.9, 156.4, 134.2, 133.1, 132.9, 131.0, 129.3, 126.4, 125.9, 123.8, 119.3, 118.2; HRMS m/z calcd for C15H10O2SCl [M + H]+ 289.0085; found, 289.0086.
4.21. 3-(o-Tolylthio)-4H-chromen-4-one (5e, New Compound)
Yield: 80% (54 mg); beige crystalline solid; mp: 154–156 °C; 1H NMR (200 MHz, CDCl3): δ = 0.34–8.20 (m, 1H), 7.86 (s, 1H), 7.76–7.62 (m, 1H), 7.51–7.38 (m, 2H), 7.28–7.09 (m, 4H), 2.49 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.1, 156.4, 155.6, 138.9, 134.0, 132.2, 130.9, 130.7, 127.7, 126.9, 126.4, 125.7, 123.4, 120.4, 118.2, 20.4; HRMS m/z calcd for C15H13O2S [M + H]+ 269.0631; found, 269.0630.
4.22. 3-(Thiophen-2-ylthio)-4H-chromen-4-one (5f, New Compound)
Yield: 78% (51 mg); beige solid; mp: 99–102 °C; 1H NMR (200 MHz, CDCl3): δ = 8.22 (d, J = 7.6 Hz, 1H), 7.86 (s, 1H), 7.65 (t, J = 7.7 Hz, 1H), 7.46–7.35 (m, 4H), 7.07–6.98 (m, 1H); 13C NMR (50 MHz, CDCl3): δ = 174.7, 156.2, 154.5, 135.8, 133.9, 131.1, 130.0, 127.9, 126.2, 125.6, 123.3, 123.1, 118.1; HRMS m/z calcd for C13H9O2S2 [M + H]+ 261.00375; found, 261.00386.
4.23. 3-(Benzylthio)-4H-chromen-4-one (5g, New Compound)
Yield: 44% (30 mg); white solid; mp: 112–114 °C; 1H NMR (200 MHz, CDCl3): δ = 8.28 (dd, J = 7.9, 1.7 Hz, 1H), 7.82 (s, 1H), 7.67 (ddd, J = 8.7, 7.7, 1.6 Hz, 1H), 7.46–7.17 (m, 7H), 4.06 (s, 2H); 13C NMR (50 MHz, CDCl3): δ = 176.0, 157.3, 156.3, 137.8, 133.9, 129.5, 129.1, 128.5, 127.2, 126.3, 125.7, 123.6, 37.2; HRMS m/z calcd for C16H13O2S [M + H]+ 269.0631; found, 269.0628.
4.24. 6-Chloro-3-(phenylselanyl)-4H-chromen-4-one (7a)11
Yield: 79% (66 mg); yellow crystalline solid; mp: 105–106 °C (105 °C);21H NMR (200 MHz, CDCl3): δ = 8.15 (d, J = 2.6 Hz, 1H), 7.82 (s, 1H), 7.67–7.53 (m, 3H), 7.40–7.27 (m, 4H); 13C NMR (50 MHz, CDCl3): δ = 174.0, 155.4, 154.6, 134.1, 134.0, 131.4, 129.6, 128.4, 127.6, 125.6, 123.9, 119.9, 118.2; HRMS m/z calcd for C15H10O2SeCl [M + H]+ 336.9527; found, 336.9529.
4.25. 6-Chloro-3-(phenylthio)-4H-chromen-4-one (7aa)11
Yield: 76% (55 mg); white crystalline solid; mp: 124–126 °C; 1H NMR (200 MHz, CDCl3): δ = 8.16 (d, J = 2.5 Hz, 1H), 8.08 (s, 1H), 7.59 (dd, J = 8.9, 2.6 Hz, 1H), 7.43–7.22 (m, 6H); 13C NMR (50 MHz, CDCl3): δ = 151.5, 148.0, 137.5, 173.9, 156.9, 154.6, 134.2, 133.4, 131.6, 130.3, 129.3, 127.4, 125.6, 124.4, 120.54, 120.01; HRMS m/z calcd for C15H10O2SCl [M + H]+ 289.0085; found, 289.0086.
4.26. 6-Bromo-3-(phenylselanyl)-4H-chromen-4-one (7b, New Compound)
Yield: 82% (78 mg); beige crystalline solid; mp: 104–105 °C; 1H NMR (200 MHz, CDCl3): δ = 8.31 (s, 1H), 7.86–7.48 (m, 4H), 7.37–7.14 (m, 4H); 13C NMR (50 MHz, CDCl3): δ = 173.9, 155.4, 155.1, 136.8, 134.2, 129.6, 128.8, 128.4, 127.6, 124.3, 120.1, 118.9, 118.3; HRMS m/z calcd for C15H10O2SeBr [M + H]+ 380.9021; found, 380.9023.
4.27. 6-Bromo-3-(phenylthio)-4H-chromen-4-one (7ab)11
Yield: 78% (65 mg); white crystalline solid; mp: 119–120 °C; 1H NMR (200 MHz, CDCl3): δ = 8.33 (d, J = 2.1 Hz, 1H), 8.08 (s, 1H), 7.74 (dd, J = 8.9, 2.2 Hz, 1H), 7.43–7.24 (m, 6H); 13C NMR (50 MHz, CDCl3): δ = 173.8, 156.9, 155.1, 137.0, 133.4, 130.4, 129.3, 128.9, 127.5, 124.8, 120.7, 120.2, 119.2; HRMS m/z calcd for C15H10O2SBr [M + H]+ 332.9579; found, 332.9578.
4.28. 7-Fluoro-3-(phenylselanyl)-4H-chromen-4-one (7c, New Compound)
Yield: 84% (65 mg); white crystalline solid; mp: 105–107 °C; 1H NMR (200 MHz, CDCl3): δ = 8.23 (dd, J = 8.7, 6.2 Hz, 1H), 7.79 (s, 1H), 7.60 (dd, J = 6.5, 3.0 Hz, 2H), 7.36–7.27 (m, 3H), 7.19–7.05 (m, 2H); 13C NMR (50 MHz, CDCl3): δ = 174.2, 165.65 (d, JC–F = 255.7 Hz), 157.36 (d, J = 13.2 Hz), 155.35, 134.21, 129.67, 128.98 (d, JC–F = 10.7 Hz), 128.39, 127.73, 119.99, 118.52, 114.46 (d, JC–F = 23.0 Hz), 104.73 (d, JC–F = 25.3 Hz); HRMS m/z calcd for C15H10O2SeF [M + H]+ 320.9825; found, 320.9828.
4.29. 7-Fluoro-3-(phenylthio)-4H-chromen-4-one (7ac, New Compound)
Yield: 82% (56 mg); white crystalline solid; mp: 114–117 °C; 1H NMR (200 MHz, CDCl3): δ = 8.27 (dd, J = 9.6, 6.2 Hz, 1H), 8.06 (s, 1H), 7.43–7.10 (m, 7H); 13C NMR (50 MHz, CDCl3): δ = 174.13, 165.78 (d, JC–F = 256.0 Hz), 157.39 (d, JC–F = 13.3 Hz), 156.93, 133.59, 130.38, 129.34, 29.10 (d, JC–F = 10.7 Hz), 127.47, 120.82, 120.57 (d, JC–F = 2.4 Hz), 114.62 (d, JC–F = 22.8 Hz), 104.90 (d, JC–F = 25.4 Hz); HRMS m/z calcd for C15H10O2SF [M + H]+ 273.0380; found, 273.0383.
4.30. 6-Chloro-7-methyl-3-(phenylselanyl)-4H-chromen-4-one (7d, New Compound)
Yield: 83% (72 mg); white crystalline solid; mp: 138–140 °C; 1H NMR (200 MHz, CDCl3): δ = 8.14 (s, 1H), 7.85–7.75 (m, 1H), 7.63–7.52 (m, 2H), 7.33–7.24 (m, 4H), 2.46 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.0, 155.4, 154.6, 143.2, 134.0, 132.2, 129.6, 128.3, 127.9, 125.8, 122.1, 119.9, 117.9, 20.8; HRMS m/z calcd for C16H12O2SeCl [M + H]+ 350.9684; found, 350.9681.
4.31. 6-Chloro-7-methyl-3-(phenylthio)-4H-chromen-4-one (7ad, New Compound)
Yield: 80% (60 mg); white crystalline solid; mp: 140–142 °C; 1H NMR (200 MHz, CDCl3): δ = 8.15 (s, 1H), 8.06 (s, 1H), 7.45–7.19 (m, 6H), 2.48 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 173.9, 157.0, 154.6, 143.4, 133.8, 132.4, 130.1, 129.3, 127.3, 126.0, 122.7, 120.2, 120.0, 20.8; HRMS m/z calcd for C16H12O2SCl [M + H]+ 303.0241; found, 303.0242.
4.32. 6,8-Dichloro-3-(phenylselanyl)-4H-chromen-4-one (7e, New Compound)
Yield: 76% (70 mg); white crystalline solid; mp: 113–115 °C; 1H NMR (200 MHz, CDCl3): δ = 8.08 (d, J = 2.5 Hz, 1H), 7.77 (s, 1H), 7.69 (d, J = 2.5 Hz, 1H), 7.66–7.57 (m, 2H), 7.38–7.30 (m, 3H); 13C NMR (50 MHz, CDCl3): δ = 173.5, 154.3, 150.7, 134.9, 134.0, 131.2, 129.9, 128.8, 126.8, 124.5, 124.4, 119.4; HRMS m/z calcd for C15H9O2SeCl2 [M + H]+ 370.9135; found, 370.9133.
4.33. 6,8-Dichloro-3-(phenylthio)-4H-chromen-4-one (7ae, New Compound)
Yield: 71% (57 mg); white crystalline solid; mp: 129–130 °C; 1H NMR (200 MHz, CDCl3): δ = 8.08 (d, J = 2.5 Hz, 1H), 8.04 (s, 1H), 7.70 (d, J = 2.5 Hz, 1H), 7.48–7.28 (m, 5H); 13C NMR (50 MHz, CDCl3): δ = 173.2, 155.7, 150.7, 134.1, 132.4, 131.4, 131.2, 129.5, 128.0, 125.1, 124.5, 122.0; HRMS m/z calcd for C15H9O2SCl2 [M + H]+ 322.9695; found, 322.9683.
4.34. 6,8-Dibromo-3-(phenylselanyl)-4H-chromen-4-one (7f, New Compound)
Yield: 78% (89 mg); beige crystalline solid; mp: 116–118 °C; 1H NMR (200 MHz, CDCl3): δ = 8.28 (d, J = 2.3 Hz, 1H), 7.98 (d, J = 2.3 Hz, 1H), 7.76 (s, 1H), 7.67–7.58 (m, 2H), 7.40–7.30 (m, 3H); 13C NMR (50 MHz, CDCl3): δ = 173.4, 154.3, 152.0, 139.5, 134.9, 129.9, 129.8, 128.8, 128.3, 126.8, 124.8, 119.4, 118.8, 112.9; HRMS m/z calcd for C15H9O2SeBr2 [M + H]+ 458.8128; found, 458.8125.
4.35. 6,8-Dibromo-3-(phenylthio)-4H-chromen-4-one (7af, New Compound)
Yield: 70% (72 mg); brown sold, solid; mp: 126–127 °C; 1H NMR (200 MHz, CDCl3): δ = 8.30 (d, J = 2.3 Hz, 1H), 8.04 (s, 1H), 8.02 (d, J = 2.3 Hz, 1H), 7.47–7.40 (m, 2H), 7.36–7.27 (m, 3H); 13C NMR (50 MHz, CDCl3): δ = 173.2, 155.8, 152.1, 139.7, 132.4, 131.3, 129.5, 128.4, 128.1, 125.4, 122.1, 119.0, 113.0; HRMS m/z calcd for C15H9O2SBr2 [M + H]+ 410.8664; found, 412.86678.
4.36. 7-Ethoxy-3-(phenylselanyl)-4H-chromen-4-one (7g, New Compound)
Yield: 80% (69 mg); beige crystalline solid; mp: 119–120 °C; 1H NMR (200 MHz, CDCl3): δ = 8.10 (d, J = 8.9 Hz, 1H), 7.79 (s, 1H), 7.63–7.52 (m, 2H), 7.32–7.22 (m, 3H), 6.94 (dd, J = 8.9, 2.3 Hz, 1H), 6.76 (d, J = 2.2 Hz, 1H), 4.08 (q, J = 7.0 Hz, 2H), 1.45 (t, J = 7.0 Hz, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.5, 163.5, 158.1, 155.3, 133.7, 129.5, 128.4, 128.0, 127.6, 117.7, 116.9, 115.2, 100.6, 64.3, 14.5; HRMS m/z calcd for C17H15O3Se [M + H]+ 347.0182; found, 347.0178.
4.37. 7-Ethoxy-3-(phenylthio)-4H-chromen-4-one (7ag, New Compound)
Yield: 75% (56 mg); white crystalline solid; mp: 119–120 °C; 1H NMR (200 MHz, CDCl3): δ = 8.13 (d, J = 8.9 Hz, 1H), 8.06 (s, 1H), 7.46–7.14 (m, 5H), 6.96 (dd, J = 8.9, 2.0 Hz, 1H), 6.81 (d, J = 2.0 Hz, 1H), 4.11 (q, J = 6.9 Hz, 2H), 1.46 (t, J = 7.0 Hz, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.4, 163.7, 158.2, 157.0, 134.4, 129.7, 129.2, 127.8, 127.0, 119.8, 117.5, 115.3, 100.9, 64.4, 14.6; HRMS m/z calcd for C17H15O3S [M + H]+ 299.0736; found, 299.0738.
4.38. 6-Methoxy-3-(phenylselanyl)-4H-chromen-4-one (7h, New Compound)
Yield: 86% (71 mg); white crystalline solid; mp: 99–101 °C; 1H NMR (200 MHz, CDCl3): δ = 7.90 (s, 1H), 7.59 (dd, J = 6.6, 2.9 Hz, 3H), 7.38–7.20 (m, 5H), 3.87 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.0, 157.2, 155.8, 151.2, 133.7, 129.5, 128.4, 128.0, 124.0, 123.8, 116.8, 56.0; HRMS m/z calcd for C16H13O3Se [M + H]+ 333.0025; found, 333.0022.
4.39. 6-Methoxy-3-(phenylthio)-4H-chromen-4-one (7ah, New Compound)
Yield: 81% (58 mg); white crystalline solid: 108–109 °C; 1H NMR (200 MHz, CDCl3): δ = 7.87 (s, 1H), 7.30 (d, J = 3.0 Hz, 1H), 7.14–7.07 (m, 3H), 7.03–6.91 (m, 4H), 3.59 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.0, 157.4, 151.3, 134.4, 129.8, 129.2, 127.1, 124.5, 124.1, 119.6, 119.0, 105.5, 56.0; HRMS m/z calcd for C16H13O3S [M + H]+ 285.0580; found, 285.0584.
4.40. 7-Methoxy-3-(phenylselanyl)-4H-chromen-4-one (7i, New Compound)
Yield: 85% (70 mg); yellow solid; mp: 90–92 °C; 1H NMR (200 MHz, CDCl3): δ = 8.11 (d, J = 8.9 Hz, 1H), 7.80 (s, 1H), 7.61–7.54 (m, 2H), 7.31–7.25 (m, 3H), 6.96 (dd, J = 8.9, 2.3 Hz, 1H), 6.78 (d, J = 2.2 Hz, 1H), 3.87 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.5, 164.2, 158.1, 155.3, 133.7, 129.5, 128.4, 128.0, 127.7, 117.8, 117.1, 114.9, 100.2, 55.9; HRMS m/z calcd for C16H13O3Se [M + H]+ 333.0125; found, 333.0029.
4.41. 7-Methoxy-3-(phenylthio)-4H-chromen-4-one (7ai, New Compound)
Yield: 80% (57 mg); yellow crystalline solid; mp: 98–100 °C; 1H NMR (200 MHz, CDCl3): δ = 8.12 (d, J = 8.9 Hz, 1H), 8.05 (s, 1H), 7.43–7.17 (m, 5H), 6.97 (dd, J = 8.9, 2.3 Hz, 1H), 6.82 (d, J = 2.2 Hz, 1H), 3.88 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.3, 164.3, 158.1, 157.0, 134.3, 129.7, 129.2, 127.8, 127.0, 119.8, 117.6, 115.0, 100.4, 55.9; HRMS m/z calcd for C16H13O3S [M + H]+ 285.0579; found, 285.0579.
4.42. 7-Methoxy-8-methyl-3-(phenylselanyl)-4H-chromen-4-one (7j, New Compound)
Yield: 83% (72 mg); yellow crystalline solid; mp: 128–130 °C; 1H NMR (200 MHz, CDCl3): δ = 8.05 (d, J = 8.7 Hz, 1H), 7.87 (s, 1H), 7.58 (s, 2H), 7.27 (s, 3H), 6.95 (d, J = 8.6 Hz, 1H), 3.91 (s, 3H), 2.22 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.0, 161.3, 155.7, 155.4, 133.5, 129.4, 128.5, 127.8, 124.8, 117.0, 116.8, 113.9, 108.9, 56.1, 8.0; HRMS m/z calcd for C17H15O3Se [M + H]+ 347.0182; found, 347.0180.
4.43. 7-Methoxy-8-methyl-3-(phenylthio)-4H-chromen-4-one (7aj, New Compound)
Yield: 82% (61 mg); yellow crystalline solid; mp: 134–136 °C; 1H NMR (200 MHz, CDCl3): δ = 8.28–7.94 (m, 2H), 7.60–7.13 (m, 5H), 7.10–6.85 (m, 1H), 3.92 (s, 3H), 2.26 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.9, 161.5, 157.4, 155.4, 134.5, 129.5, 129.0, 126.8, 124.8, 118.7, 117.5, 114.1, 109.0, 56.1, 8.0; HRMS m/z calcd for C17H15O3S [M + H]+ 299.0736; found, 299.0738.
4.44. 6-Methyl-3-(phenylselanyl)-4H-chromen-4-one (7k, New Compound)
Yield: 87% (69 mg); beige crystalline solid; mp: 100–101 °C; 1H NMR (200 MHz, CDCl3): δ = 7.98 (s, 1H), 7.87 (s, 1H), 7.63–7.53 (m, 2H), 7.44 (dd, J = 8.6, 2.0 Hz, 1H), 7.33–7.22 (m, 4H), 2.42 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.1, 155.8, 154.5, 135.5, 135.0, 133.6, 129.4, 128.4, 127.9, 125.5, 122.8, 117.8, 117.4, 20.9; HRMS m/z calcd for C16H13O2Se [M + H]+ 317.0076; found, 317.0078.
4.45. 6-Methyl-3-(phenylthio)-4H-chromen-4-one (7ak)11
Yield: 83% (57 mg); white crystalline solid; mp: 119–120 °C; 1H NMR (200 MHz, CDCl3): δ = 8.11 (s, 1H), 8.07–7.96 (m, 1H), 7.48–7.14 (m, 7H), 2.42 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 175.0, 157.4, 154.5, 135.7, 135.2, 134.30, 129.6, 129.1, 126.9, 125.6, 123.3, 119.4, 117.9, 20.9; HRMS m/z calcd for C16H13O2S [M + H]+ 269.0631; found, 269.0629.
4.46. 7-Methyl-3-(phenylselanyl)-4H-chromen-4-one (7l, New Compound)
Yield: 88% (69 mg); white solid; mp: 110–112 °C; 1H NMR (200 MHz, CDCl3): δ = 8.08 (d, J = 8.6 Hz, 1H), 7.82 (s, 1H), 7.64–7.51 (m, 2H), 7.31–7.15 (m, 5H), 2.44 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.9, 156.4, 155.5, 145.2, 133.6, 129.4, 128.3, 127.9, 127.0, 125.9, 120.9, 117.7, 117.6, 21.8; HRMS m/z calcd for C16H13O2Se [M + H]+ 317.0076; found, 317.0073.
4.47. 7-Methyl-3-(phenylthio)-4H-chromen-4-one (7al, New Compound)
Yield: 86% (58 mg); white solid; mp: 115–116 °C; 1H NMR (200 MHz, CDCl3): δ = 8.18–8.03 (m, 2H), 7.42–7.17 (m, 7H), 2.46 (s, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.8, 157.2, 156.4, 145.4, 134.3, 129.7, 129.1, 127.2, 127.0, 126.1, 121.4, 119.6, 117.8, 21.8; HRMS m/z calcd for C16H13O2S [M + H]+ 269.0631; found, 269.0633.
4.48. 3-(Phenylselanyl)-4H-benzo[h]chromen-4-one (7m, New Compound)
Yield: 80% (70 mg); brown solid; mp: 111–113 °C; 1H NMR (200 MHz, CDCl3): δ = 8.41 (d, J = 7.0 Hz, 1H), 8.28–8.06 (m, 2H), 7.98–7.86 (m, 1H), 7.81–7.61 (m, 3H), 7.51–7.22 (m, 5H); 13C NMR (50 MHz, CDCl3): δ = 174.8, 155.5, 153.8, 135.9, 133.5, 130.6, 129.6, 129.3, 128.1, 127.5, 127.4, 125.8, 123.8, 122.3, 122.2, 121.1, 119.8; HRMS m/z calcd for C19H13O2Se [M + H]+ 379.0709; found, 379.0707.
4.49. 3-(Phenylthio)-4H-benzo[h]chromen-4-one (7am, New Compound)
Yield: 76% (58 mg); brown solid; mp: 117–119 °C; 1H NMR (200 MHz, CDCl3): δ = 8.45 (d, J = 8.7 Hz, 1H), 8.27–8.12 (m, 2H), 7.99–7.89 (m, 1H), 7.81–7.64 (m, 3H), 7.53–7.43 (m, 2H), 7.36–7.25 (m, 3H); 13C NMR (50 MHz, CDCl3): δ = 174.9, 153.8, 135.8, 132.2, 129.5, 128.4, 128.1, 127.6, 127.3, 125.7, 123.8, 122.2, 121.1, 120.2, 119.2; HRMS m/z calcd for C19H13O2S [M + H]+ 305.0631; found, 305.0633.
Acknowledgments
We gratefully acknowledge CAPES, CNPq, CERSusChem GSK/FAPESP (grant 2014/50249-8), INCT-Catálise, HEC-SRGP-161, and FAPESC-Pronex for financial support. J.R. (postdoctoral fellow) is grateful to CAPES for fellowships. J.R. and S.S. contributed equally to this work. The authors also acknowledge CEBIME for the HRMS analysis.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b00445.
Spectra data for all compounds (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Gaspar A.; Matos M. J.; Garrido J.; Uriate E.; Borges F. Chromone: A valid scaffold in medicinal chemistry. Chem. Rev. 2014, 114, 4960–4992. 10.1021/cr400265z. [DOI] [PubMed] [Google Scholar]
- a Emami S.; Ghanbarimasir Z. Recent advances of chroman-4-one derivatives: Synthetic approaches and bioactivities. Eur. J. Med. Chem. 2015, 93, 539–563. 10.1016/j.ejmech.2015.02.048. [DOI] [PubMed] [Google Scholar]; b Fletcher A. C.; Porter L. J.; Haslam E.; Gupta R. K. Plant proanthocyanidins. Part 3. Conformational and configurational studies of natural procyanidins. J. Chem. Soc., Perkin Trans. 1 1977, 1628–1637. 10.1039/p19770001628. [DOI] [Google Scholar]; c Yamashita A. Total synthesis of khellin via a chromium carbene complex. J. Am. Chem. Soc. 1985, 107, 5823–5824. 10.1021/ja00306a052. [DOI] [Google Scholar]; d Cagide F.; Silva T.; Reis J.; Gaspar A.; Borges F.; Gomes L. R.; Low J. N. Discovery of two new classes of potent monoamine oxidase-B inhibitors by tricky chemistry. Chem. Commun. 2015, 51, 2832–2835. 10.1039/c4cc08798d. [DOI] [PubMed] [Google Scholar]; e Reis J.; Cagide F.; Chavarria D.; Silva T.; Fernandes C.; Gasper A.; Uriarte E.; Remião F.; Alcaro S.; Ortuso F.; Borges F. Discovery of new chemical entities for old targets: Insights on the lead optimization of chromone-based monoamine oxidase B (MAO-B) inhibitors. J. Med. Chem. 2016, 59, 5879–5893. 10.1021/acs.jmedchem.6b00527. [DOI] [PubMed] [Google Scholar]
- a Edwards A. M.; Howell J. B. L. The chromones: History, chemistry and clinical development. A tribute to the work of Dr R. E. C. Altounyan. Clin. Exp. Allergy 2000, 30, 756–774. 10.1046/j.1365-2222.2000.00879.x. [DOI] [PubMed] [Google Scholar]; b Vanachayangkul P.; Chow N.; Khan S. R.; Butterweck V. Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urol. Res. 2010, 39, 189–195. 10.1007/s00240-010-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cook N.; Mushtaq F.; Leitner C.; Ilchyshyn A.; Smith G. T.; Cree I. A. Chronic tarsal conjunctivitis. BMC Ophthalmol. 2016, 16, 130. 10.1186/s12886-016-0294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Schönberg A.; Sina A. On Visnagin and Khellin and related compounds. A simple synthesis of chromone. J. Am. Chem. Soc. 1950, 72, 3396–3399. 10.1021/ja01164a022. [DOI] [Google Scholar]
- a Ma H.; Reyes-Gutierrez P.; Pederson T. Visualization of repetitive DNA sequences in human chromosomes with transcription activator-like effectors. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 21048–21053. 10.1073/pnas.1319097110. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tehfe M.-A.; Dumur F.; Xiao P.; Graff B.; Morlet-Savary F.; Fouassier J.-P.; Gigmes D.; Lalevée J. New chromone based photoinitiators for polymerization reactions under visible light. Polym. Chem. 2013, 4, 4234–4244. 10.1039/c3py00536d. [DOI] [Google Scholar]; c Miao J.; Cui H.; Jin J.; Lai F.; Wen H.; Zhang X.; Ruda G. F.; Chen X.; Yin D. Development of 3-alkyl-6-methoxy-7-hydroxy-chromones (AMHCs) from natural isoflavones, a new class of fluorescent scaffolds for biological imaging. Chem. Commun. 2015, 51, 881–884. 10.1039/c4cc06762b. [DOI] [PubMed] [Google Scholar]; d Zimmerman J. R.; Johntony O.; Steigerwald D.; Criss C.; Myers B. J.; Kindler D. H. The synthesis of a new class of highly fluorescent chromones via an inverse-demand hetero-Diels–Alder reaction. Org. Lett. 2015, 17, 3256–3259. 10.1021/acs.orglett.5b01417. [DOI] [PubMed] [Google Scholar]; e Ono M.; Saji H. Recent advances in molecular imaging probes for β-amyloid plaques. Med. Chem. Commun. 2015, 6, 391–402. 10.1039/c4md00365a. [DOI] [Google Scholar]
- a Yang J.; Yoshikai N. Cobalt-catalyzed annulation of salicylaldehydes and alkynes to form chromones and 4-chromanones. Angew. Chem., Int. Ed. 2016, 55, 2870–2874. 10.1002/anie.201510999. [DOI] [PubMed] [Google Scholar]; b Chavan S. P.; Varadwaj G. B. B.; Parida K. M.; Bhanage B. M. Solvent-switchable regioselective synthesis of aurones and flavones using palladium-supported amine-functionalized montmorillonite as a heterogeneous catalyst. ChemCatChem 2016, 8, 2649–2658. 10.1002/cctc.201600549. [DOI] [Google Scholar]; c Wang B.; Xu C.; Xie J.; Yang Z.; Sun S. pH controlled release of chromone from chromone-Fe3O4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 14436–14437. 10.1021/ja806519m. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chaoi H.; Min M.; Peng Q.; Kang D.; Paton R. S.; Hong S. Unraveling innate substrate control in site-selective palladium-catalyzed C–H heterocycle functionalization. Chem. Sci. 2016, 7, 3900–3909. 10.1039/c5sc04590h. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Sun P.; Gao S.; Yang C.; Guo S.; Lin A.; Yao H. Controllable Rh(iii)-catalyzed annulation between salicylaldehydes and diazo compounds: Divergent synthesis of chromones and benzofurans. Org. Lett. 2016, 18, 6464–6467. 10.1021/acs.orglett.6b03355. [DOI] [PubMed] [Google Scholar]; f Matsui J. K.; Molander G. A. Direct α-arylation/heteroarylation of 2-trifluoroboratochromanones via photoredox/nickel dual catalysis. Org. Lett. 2017, 19, 436–439. 10.1021/acs.orglett.6b03448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Organic Selenium Compounds: Their Chemistry and Biology (Chemistry of Organometallic Compounds); Klayman D. L., Gunthe W. H. H., Eds; John Wiley & Sons Inc: New York, 1973. [Google Scholar]; b Schwarz K.; Foltz C. M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292. 10.1021/ja01569a087. [DOI] [PubMed] [Google Scholar]; c Braga A. L.; Rafique J.. Synthesis of biologically relevant small molecules containing selenium. Part A. Antioxidant compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport Z., Ed.; Wiley & Sons, Ltd.: Chichester, 2014; Vol. 4, Chapter 13, pp 989–1052. [Google Scholar]; d Braga A. L.; Rafique J.. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport Z., Ed.; Wiley & Sons, Ltd.: Chichester, 2014; Vol. 4, Chapter 14, pp 1053–1118. [Google Scholar]; e Braga A. L.; Rafique J.. Synthesis of biologically relevant small molecules containing selenium. Part C. Miscellaneous biological activities. In The Chemistry of Organic Selenium and Tellurium Compounds; Rappoport Z., Ed.; Wiley & Sons, Ltd.: Chichester, 2014; Vol. 4, Chapter 15, pp 1119–1174. [Google Scholar]; f Reich H. J.; Hondal R. J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. 10.1021/acschembio.6b00031. [DOI] [PubMed] [Google Scholar]; g Rafique J.; Saba S.; Canto R. F. S.; Frizon T. E. A.; Hassan W.; Waczuk E. P.; Jan M.; Back D. F.; Rocha J. B. T. D.; Braga A. L. Synthesis and biological evaluation of 2-picolylamide-based diselenides with non-bonded interactions. Molecules 2015, 20, 10095–10109. 10.3390/molecules200610095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Manna D.; Roy G.; Mugesh G. Antithyroid drugs and their analogues: Synthesis, structure, and mechanism of action. Acc. Chem. Res. 2013, 46, 2706–2715. 10.1021/ar4001229. [DOI] [PubMed] [Google Scholar]; b Kumar S.; Yan J.; Poon J.-f.; Singh V. P.; Lu X.; Ott M. K.; Engman L.; Kumar S. Multifunctional antioxidants: Regenerable radical-trapping and hydroperoxide-decomposing ebselenols. Angew. Chem., Int. Ed. 2016, 55, 3729–3733. 10.1002/anie.201510947. [DOI] [PubMed] [Google Scholar]; c Rafique J.; Canto R. F. S.; Saba S.; Barbosa F. A. R.; Braga A. L. Recent advances in the synthesis of biologically relevant selenium-containing 5-membered heterocycles. Curr. Org. Chem. 2016, 20, 166–188. 10.2174/1385272819666150810222057. [DOI] [Google Scholar]; d Press D. J.; Back T. G. Enhanced glutathione peroxidase activity of conformationally restricted naphthalene peri-dichalcogenides. Org. Lett. 2011, 13, 4104–4107. 10.1021/ol201617t. [DOI] [PubMed] [Google Scholar]; e Manjare S. T.; Kim Y.; Churchill D. G. Selenium- and tellurium-containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. 10.1021/ar500187v. [DOI] [PubMed] [Google Scholar]; f Barbosa F. A. R.; Canto R. F. S.; Saba S.; Rafique J.; Braga A. L. Synthesis and evaluation of dihydropyrimidinone-derived selenoesters as multi-targeted directed compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2016, 24, 5762–5770. 10.1016/j.bmc.2016.09.031. [DOI] [PubMed] [Google Scholar]; g Alberto E. E.; do Nascimento V.; Braga A. L. Catalytic application of selenium and tellurium compounds as glutathione peroxidase enzyme mimetics. J. Braz. Chem. Soc. 2010, 21, 2032–2041. 10.1590/s0103-50532010001100004. [DOI] [Google Scholar]
- a Freudendahl D. M.; Santoro S.; Shahzad S. A.; Santi C.; Wirth T. Green chemistry with selenium reagents: Development of efficient catalytic reactions. Angew. Chem., Int. Ed. 2009, 48, 8409–8411. 10.1002/anie.200903893. [DOI] [PubMed] [Google Scholar]; Godoi M.; Paixão M. W.; Braga A. L. Chiral organoselenium-transition-metal catalysts in asymmetric transformations. Dalton Trans. 2011, 40, 11347–11355. 10.1039/c1dt11022e. [DOI] [PubMed] [Google Scholar]; Trenner J.; Depken C.; Weber T.; Breder A. Direct oxidative allylic and vinylic amination of alkenes through selenium catalysis. Angew. Chem., Int. Ed. 2013, 52, 8952–8956. 10.1002/anie.201303662. [DOI] [PubMed] [Google Scholar]; d Modha S. G.; Mehta V. P.; Van der Eycken E. V. Transition metal-catalyzed C–C bond formation via C–S bond cleavage: An overview. Chem. Soc. Rev. 2013, 42, 5042–5055. 10.1039/c3cs60041f. [DOI] [PubMed] [Google Scholar]; e Godoi B.; Schumacher R. F.; Zeni G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom. Chem. Rev. 2011, 111, 2937–2980. 10.1021/cr100214d. [DOI] [PubMed] [Google Scholar]; f Nicolaou K. C.; Edmonds D. J.; Bulger P. G. Cascade reactions in total synthesis. Angew. Chem., Int. Ed. 2006, 45, 7134–7186. 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]; g Rocha M. S. T.; Rafique J.; Saba S.; Azeredo J. B.; Back D.; Godoi M.; Braga A. L. Regioselective hydrothiolation of terminal acetylene catalyzed by magnetite (Fe3O4) nanoparticles. Synth. Commun. 2017, 47, 291–298. 10.1080/00397911.2016.1262421. [DOI] [Google Scholar]
- a Cresswell A. J.; Eey S. T.-C.; Denmark S. E. Catalytic, stereospecific syn-dichlorination of alkenes. Nat. Chem. 2015, 7, 146–152. 10.1038/nchem.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Luo J.; Zhu Z.; Liu Y.; Zhao X. Diaryl selenide catalyzed vicinal trifluoromethylthioamination of alkenes. Org. Lett. 2015, 17, 3620–3623. 10.1021/acs.orglett.5b01727. [DOI] [PubMed] [Google Scholar]; c Seixas J. D.; Chaves-Ferreira M.; Montes-Grajales D.; Gonçalves A. M.; Marques A. R.; Saraiva L. M.; Olivero-Verbel J.; Romão C. C.; Bernardes G. J. L. An N-acetyl cysteine ruthenium tricarbonyl conjugate enables simultaneous release of CO and ablation of reactive oxygen species. Angew. Chem., Int. Ed. 2015, 21, 14708–14712. 10.1002/chem.201502474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Frizon T. E.; Rafique J.; Saba S.; Bechtold I. H.; Gallardo H.; Braga A. L. Synthesis of functionalized organoselenium materials: selenides and diselenides containing cholesterol. Eur. J. Org. Chem. 2015, 3470–3476. 10.1002/ejoc.201500124. [DOI] [Google Scholar]; b Gu J.; Zhao Z.-Q.; Ding Y.; Chen H.-L.; Zhang Y.-W.; Yan C.-H. Liquid-phase syntheses and material properties of two-dimensional nanocrystals of rare earth–selenium compound containing planar se layers: RESe2 nanosheets and RE4O4Se3 nanoplates. J. Am. Chem. Soc. 2013, 135, 8363–8371. 10.1021/ja4028583. [DOI] [PubMed] [Google Scholar]; c Patra A.; Wijsboom Y. H.; Leitus G.; Bendikov M. Tuning the band gap of low-band-gap polyselenophenes and polythiophenes: The effect of the heteroatom. Chem. Mater. 2011, 23, 896–906. 10.1021/cm102395v. [DOI] [Google Scholar]; d Brutchey R. L. Diorganyl dichalcogenides as useful synthons for colloidal semiconductor nanocrystals. Acc. Chem. Res. 2015, 48, 2918–2926. 10.1021/acs.accounts.5b00362. [DOI] [PubMed] [Google Scholar]; e Ho P. C.; Rafique J.; Lee J.; Lee L. M.; Jenkins H. A.; Britten J. F.; Braga A. L.; Vargas-Baca I. Synthesis and structural characterisation of the aggregates of benzo-1,2-chalcogenazole 2-oxides. Dalton Trans. 2017, 46, 6570–6579. 10.1039/C7DT00612H. [DOI] [PubMed] [Google Scholar]
- a Godoi B.; Sperança A.; Bruning C. A.; Back D. F.; Menezes P. H.; Nogueira C. W.; Zeni G. Iron(Iii) chloride/diorganyl diselenides-promoted regioselective cyclization of alkynyl aryl ketones: Synthesis of 3-organoselenyl chromenones under ambient atmosphere. Adv. Synth. Catal. 2011, 353, 2042–2050. 10.1002/adsc.201100189. [DOI] [Google Scholar]; b Zhao W.; Zhou A. Regioselective C–S bond formation between flavones and arylsulfonyl chlorides through the use of ammonium iodide. ChemCatChem 2015, 7, 3464–3467. 10.1002/cctc.201500673. [DOI] [Google Scholar]; c Zhao W.; Xie P.; Bian Z.; Zhou A.; Ge H.; Zhang M.; Ding Y.; Zeng L. Ammonium iodide induced nonradical regioselective sulfenylation of flavones via a C–H functionalization process. J. Org. Chem. 2015, 80, 9167–9175. 10.1021/acs.joc.5b01602. [DOI] [PubMed] [Google Scholar]; d Ding Y.; Wu W.; Zhao W.; Li Y.; Xei P.; Huang Y.; Liu Y.; Zhou A. Generation of thioethers via direct C–H functionalization with sodium benzenesulfinate as a sulfur source. Org. Biomol. Chem. 2016, 14, 1428–1431. 10.1039/c5ob02073e. [DOI] [PubMed] [Google Scholar]; e Suffert J.; Blond G.; Joussot J.; Schoenfelder A.; Larquetoux L.; Nicolas M. Synthesis of 3-substituted chromones and quinolones from enaminones. Synthesis 2016, 48, 3364–3372. 10.1055/s-0035-1562513. [DOI] [Google Scholar]; f Zhao W.; Xie P.; Bian Z.; Zhou A.; Ge H.; Niu B.; Ding Y. Generation of ArS-substituted flavone derivatives using aryl thiols as sulfenylating agents. RSC Adv. 2015, 5, 59861–59864. 10.1039/c5ra10763f. [DOI] [Google Scholar]; g Zhong S.; Liu Y.; Cao X.; Wan J.-P. KIO3-catalyzed domino C(sp2)–H bond sulfenylation and C–N bond oxygenation of enaminones toward the synthesis of 3-sulfenylated chromones. ChemCatChem 2017, 9, 465–458. 10.1002/cctc.201601273. [DOI] [Google Scholar]
- a Sarkar A.; Santra S.; Kundu S. K.; Hajra A.; Zyryanov G. V.; Chupakhin O. N.; Charushin V. N.; Majee A. A decade update on solvent and catalyst-free neat organic reactions: A step forward towards sustainability. Green Chem. 2016, 18, 4475–4525. 10.1039/c6gc01279e. [DOI] [Google Scholar]; b Martins M. A. P.; Frizzo C. P.; Moreira D. N.; Buriol L.; Machado P. Solvent-free heterocyclic synthesis. Chem. Rev. 2009, 109, 4140–4182. 10.1021/cr9001098. [DOI] [PubMed] [Google Scholar]; c Borisova N. E.; Reshetova M. D.; Ustynyuk Y. A. Metal-free methods in the synthesis of macrocyclic Schiff bases. Chem. Rev. 2007, 107, 46–79. 10.1021/cr0683616. [DOI] [PubMed] [Google Scholar]; d Tanaka K.; Toda F. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025–1074. 10.1021/cr940089p. [DOI] [PubMed] [Google Scholar]
- a Rafique J.; Saba S.; Rosário A. R.; Braga A. L. Regioselective, solvent- and metal-free chalcogenation of imidazo[1,2-a]pyridines by employing I2/DMSO as the catalytic oxidation system. Chem.—Eur. J. 2016, 22, 11854–11862. 10.1002/chem.201600800. [DOI] [PubMed] [Google Scholar]; b Saba S.; Rafique J.; Braga A. L. DMSO/iodine-catalyzed oxidative C–Se/C–S bond formation: A regioselective synthesis of unsymmetrical chalcogenides with nitrogen- or oxygen-containing arenes. Catal. Sci. Technol. 2016, 6, 3087–3098. 10.1039/c5cy01503k. [DOI] [Google Scholar]; c Saba S.; Rafique J.; Braga A. L. Synthesis of unsymmetrical diorganyl chalcogenides under greener conditions: Use of an iodine/DMSO system, solvent- and metal-free approach. Adv. Synth. Catal. 2015, 357, 1446–1452. 10.1002/adsc.201500024. [DOI] [Google Scholar]; d Godoi M.; Botteselle G. V.; Rafique J.; Rocha M. S. T.; Pena J. M.; Braga A. L. Solvent-free Fmoc protection of amines under microwave irradiation. Asian J. Org. Chem. 2013, 2, 746–749. 10.1002/ajoc.201300092. [DOI] [Google Scholar]; e Rafique J.; Saba S.; Rosário A. R.; Zeni G.; Braga A. L. K2CO3-mediated, direct C–H bond selenation and thiolation of 1,3,4-oxadiazoles in the absence of metal catalyst: An eco-friendly approach. RSC Adv. 2014, 4, 51648–51652. 10.1039/c4ra10490k. [DOI] [Google Scholar]
- a Wan J.-P.; Zhong S.; Xie L.; Cao X.; Liu Y.; Wei L. KIO3-catalyzed aerobic cross-coupling reactions of enaminones and thiophenols: Synthesis of polyfunctionalized alkenes by metal-free C–H sulfenylation. Org. Lett. 2016, 18, 584–587. 10.1021/acs.orglett.5b03608. [DOI] [PubMed] [Google Scholar]; b Xiang H.; Yang C. A facile and general approach to 3-((trifluoromethyl)thio)-4H-chromen-4-one. Org. Lett. 2014, 16, 5686–5689. 10.1021/ol502751k. [DOI] [PubMed] [Google Scholar]; c Mutai P.; Pavadai E.; Wiid I.; Ngwane A.; Baker B.; Chibale K. Synthesis, antimycobacterial evaluation and pharmacophore modeling of analogues of the natural product formononetin. Bioorg. Med. Chem. Lett. 2015, 25, 2510–2513. 10.1016/j.bmcl.2015.04.064. [DOI] [PubMed] [Google Scholar]; d Levchenko K. S.; Semenova I. S.; Yarovenko V. N.; Shmelin P. S.; Krayushkin M. M. Facile syntheses of 2-substituted 3-cyanochromones. Tetrahedron Lett. 2012, 53, 3630–3632. 10.1016/j.tetlet.2012.05.031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.