Abstract
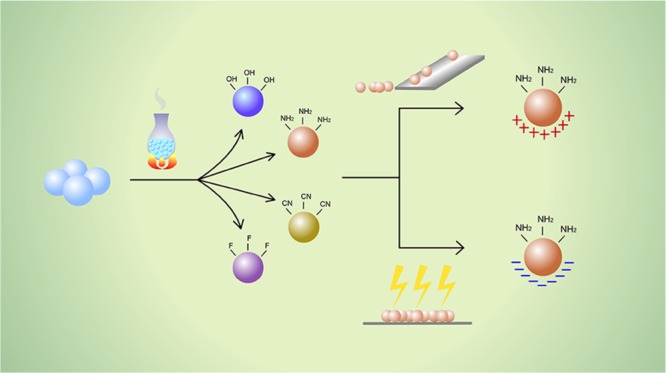
In the present study, the correlation between surface chemical groups and the electrostatic properties of particulate materials was studied. Glass beads were modified to produce OH-, NH2-, CN-, and F-functionalized materials. The materials were charged separately both by friction and by conventional corona charging, and the results were compared. The results obtained from both methods indicated that the electrostatic properties are directly related to the surface functional group chemistry, with hydrophobic groups accumulating greater quantities of charge than hydrophilic groups. The fluorine-rich surface accumulated 5.89 times greater charge upon tribocharging with stainless steel than the hydroxyl-rich surface. However, in contrast to the tribocharging method, the charge polarity could not be determined when corona charging was used. Moreover, discharge profiles at different humidity levels (25% RH, 50% RH, and 75% RH) were obtained for each modified surface, which showed that higher humidity facilitates faster charge decay; however, this enhancement is surface chemistry-dependent. By increasing the humidity from 25% RH to 75% RH, the charge relaxation times can be accelerated 1.6 times for fluorine and 12.2 times for the cyano group. These data confirm that surface functional groups may dictate powder electrostatic behavior and account for observed charge accumulation and discharge phenomena.
1. Introduction
Accumulation of electrostatic charge is frequently observed during powder processing and handling. Operations such as mixing, milling, and conveying involve the movement of the powder against surfaces and therefore can lead to charging via friction, commonly known as tribocharging.1−3 The industrial significance and the importance of the phenomenon itself tends to be underestimated; however, operational problems and safety concerns regarding uncontrolled charge build-up have been identified.2,4 Problems with charged materials are particularly applicable to pharmaceutical powders, as these tend to be organic materials and are thus common insulators. Such materials not only easily acquire static charge but also can retain the charge for prolonged lengths of time because of slow charge decay rates.5 Besides processes where charge build-up is undesirable and should be minimized, controlled charging can be beneficial, for example, during electrostatic separation and in the coating industry.6,7 Even though tribocharging has been recorded since the times of Ancient Greece,8 the scientific theories and principles behind it are still very poorly understood.9
The complexity of powder electrostatics arises from a number of factors that may influence the process of charge generation and charge dissipation. These factors can be divided into three main categories: inherent physico-chemical properties of the powder and contacting surface, interfacial mechanics of the interaction between the particulate material and contacting surfaces, and external environmental conditions.5,10 Consequently, variable and nonstandard experimental conditions and methods reported in studies on powder electrostatics make direct comparisons of experimental results difficult, and as a result, no general database of the chargeability of dry powders currently exists.11−13
Triboelectrification tends to be considered as a surface phenomenon and therefore can be influenced by external parameters such as temperature and relative humidity.12 Typically, higher moisture content in the surrounding environment results in a reduction in the absolute charge generated during the tribocharging process.14,15 However, Elajnaf et al. concluded that this general trend can be further affected by the nature of the contacting surface, as differing extents of charge reduction were observed when the same powder interacted with metal or plastic surfaces.14 The overall reduction in charge by moisture is associated with lowering surface resistivity because of water molecules adsorbing on the surface. Specifically, the existence and adsorption of [OH(H2O)n]− and [H(H2O)n]+ ions on the surface have been identified to contribute toward charge reduction.16,17 In some cases, preferential deposition of either type of ion, depending on the surface type, has been observed.18 In this way, an additional discharge pathway is created compared with the same material under dry conditions.9,19 This could also potentially affect the charge relaxation times; however, no such data have been provided by Elajnaf et al.15 Furthermore, there is evidence that humidity itself can impart electrostatic charge on the isolated surfaces,20 which indicates an important role of water as “a source and sink of ions”18 in the surface electrostatic phenomenon.21,22
The interaction of moisture with surfaces is directly related to surface hydrophobicity and as such can also influence the electrostatic properties of materials. In their study, Jallo and Dave examined the effect of silica and titania coatings on acetaminophen particles and concluded that a sample with hydrophilic silica coating charged less compared with a hydrophobic one.23 Even though the comparison was made between two types of coatings only and additional parameters such as changes in surface area and roughness because of nanoparticle deposition were not taken into account, the findings emphasize that surface composition and therefore properties such as hydrophobicity and surface energy play a crucial role in explaining the electrostatic properties of powder surfaces. Similarly, in the study conducted by Gouveia et al. (2012), the surface acid/base properties of various materials were linked to the adsorption/desorption of OH– and H+ ions upon changes in humidity, which provides an indication of the relationship between the surface properties and its chargeability.24
In addition, most physical properties such as particle size, shape, surface roughness, and purity are known to affect the electrostatic properties of materials.13,25,26 Hence, in total, considering the wide range of parameters associated with powders only, a rather complex picture of powder electrostatics is created, which makes it difficult to study one parameter at a time. Therefore, the dominant parameters governing the charging of powders are still not well understood.
In this study, the emphasis is placed on investigating the parameters associated with powder surfaces only, and, apart from humidity, all other factors such as surfaces in contact and mechanics of interaction, are kept constant. There are several publications27−30 covering these aspects in detail and reviews on other factors affecting powder electrostatics in general.9,12,31,32
Therefore, in terms of powder properties the objective of this study is to evaluate the influence of surface chemistry, as a single variable, on the propensity for particulate materials to acquire and dissipate electrostatic charge. The main hypothesis to be tested is that the functional groups present at the contact surfaces of particles, and therefore the surface composition, can be considered to be one of the dominant factors in determining powder electrostatic properties. It has been previously shown that the composition of the tribocharging surface can affect the charging of a powder;33 however, to the best of our knowledge, no such relationship has been determined for the reverse scenario. In addition, as surface chemistry determines properties such as the degree of hydrophobicity, it is reasonable to expect a change in electrostatic properties when the surfaces are exposed to differing humidity conditions. These could potentially influence not only the maximum charge accumulated, as shown by Elajnaf et al.,15 but also changes in the charge relaxation behavior. The main aim of the experimental design is to prepare and study the materials in such a way that only the surface functionality is changed exclusively across the samples studied while all other factors are kept the same to allow direct comparison with the electrostatic behavior. The results obtained from tribocharging experiments are compared and contrasted with the results where a more conventional corona discharge was used to charge the materials to determine the capability of the two methods to provide information on particle electrostatics.
2. Results and Discussion
The aim of this study is to limit the number of variables that could lead to inconsistent results when measuring the electrostatic properties of the particulate system. The use of spherical glass beads as a model material ensured that the size, morphology, and surface area were kept constant and that only the surface chemistry modifications resulted in changes to the electrostatic properties of the samples.
This section is divided into two parts. First, the functionalization of particles is discussed in section 2.1, followed by the results of the electrostatic measurements (section 2.2), with maximum charging abilities being covered in section 2.2.1 and charge decay profiles being presented in section 2.2.2.
2.1. Surface Modification
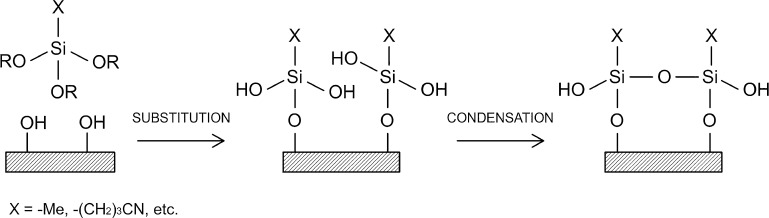
The surface chemistry of the glass beads was modified via a series of silanization reactions. The general reaction involves substitution and condensation reactions, as schematically depicted in Figure 1.
Figure 1.
Schematic diagram of silanization reaction.34
Three different silane solutions were used to produce fluoro-functionalized glass beads (GB-F), cyano-functionalized glass beads (GB-CN), and amino-functionalized glass beads (GB-NH2). In addition, cleaned unmodified glass beads with hydroxyl groups (GB-OH) were studied.
The wetting behavior of all samples was characterized using contact angle measurements to assess whether the surfaces were chemically modified. The measurements also provide information on the degree of surface hydrophobicity achieved. The results obtained for each surface are presented in Table 1.
Table 1. Equilibrium Contact Angle of Probe Liquid Water on Studied Surfaces (n = 5, Mean ± SD).
| sample | contact angle (deg) |
|---|---|
| GB-OH | 28.3 ± 0.8 |
| GB-NH2 | 63.3 ± 6.6 |
| GB-CN | 87.8 ± 6.4 |
| GB-F | 115.7 ± 4.0 |
The values in Table 1 are characteristic of the surface chemistries induced as a result of silanization35,36 and indicate that the glass bead surfaces have been successfully functionalized. Although the data reported here could be affected by surface roughness of the prepared slides, the data spread is reasonable to prove that distinct surface chemistries were prepared. Based on the contact angle data, the samples can be ranked in the order of increasing hydrophobicity
2.2. Characterization of Electrostatic Behavior
2.2.1. Ability to Accumulate Electrostatic Charge
2.2.1.1. Charge Accumulation by Tribocharging
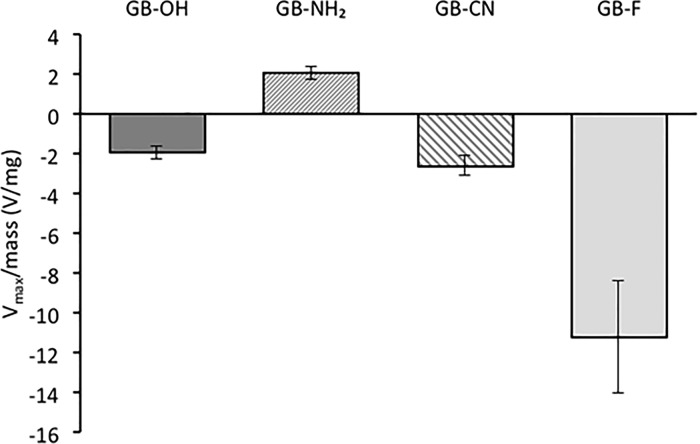
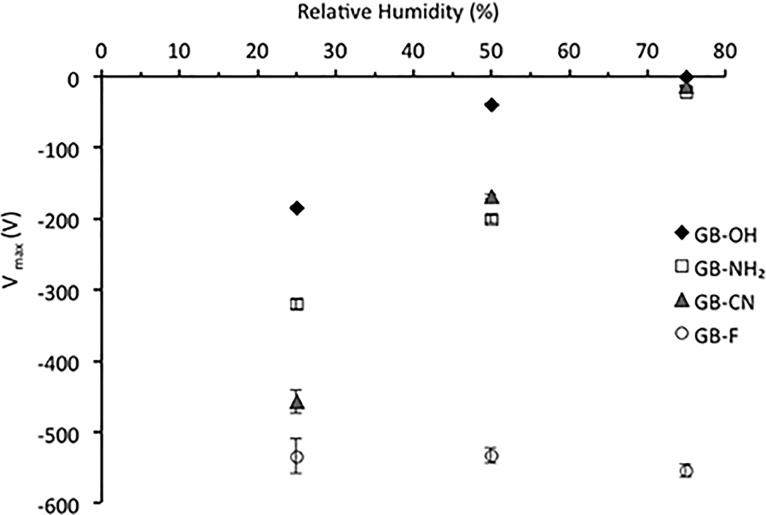
To assess the tribocharging behavior of each sample, the maximum electrostatic potential (Vmax) of each set of glass beads in contact with stainless steel was measured, as described in section 4.4.1. The method adopted here represents rather mild conditions for powder triboelectrification compared with more intensive industrial processes, such as conveying, that are characterized by much higher powder loadings and velocities. The maximum charge provides an indication of the ability of the powder to become electrostatically charged under applied tribocharging conditions. Figure 2 shows the maximum surface potential detected normalized with respect to the sample mass m (Vmax/m), for each sample.
Figure 2.
Maximum potential acquired by functionalized surfaces tribocharged by a stainless steel surface (25 °C, 20% RH; n = 6–10, mean ± SD).
As shown in Figure 2, each sample accumulated a distinct level of charge under the same conditions. The GB-OH sample acquired the overall surface potential of −1.9 ± 0.3 V/mg, followed by GB-NH2 and GB-CN, which acquired potentials equal to +2.1 ± 0.3 and −2.6 ± 0.5 V/mg, respectively. Out of the four surface functionalizations studied, the GB-F sample acquired the largest potential of −11.2 ± 2.8 V/mg. Therefore, the surfaces can be ranked in the order of increasing ability to accumulate charge, excluding polarity as follows
This order correlates very well with the order of hydrophobicity determined by the contact angle measurement and indicates that as the surface becomes more hydrophobic, it can accumulate a greater charge. A similar relationship between charge magnitude and hydrophobicity of two different silica coatings was reported by Jallo and Dave.23
The surfaces studied accumulate predominantly negative charge as a result of contact with stainless steel. Such behavior is typically expected when organic materials come in contact with metal surfaces, as the latter are believed to contain a large number of free electrons susceptible for donation. However, the two nitrogen functionalities, that is, amino and cyano, acquired positive and negative charges, respectively. The preference of the amino surface for positive charging cannot be explained on the basis of hydrophobicity.
However, amines are Brönsted bases, which means they can accept a proton; alternatively, they can be described as Lewis bases, which means they are electron donors. This theory supports the observed results well, where amino-functionalized particles give up electrons and become positively charged in contact with stainless steel surfaces. Even though the nitrogen in the cyano group also possesses a lone pair, the sp orbital hybridization provides it 50% s character and hence the electron density is held closer to the nucleus. Therefore, its basicity is significantly reduced.37 This can be further illustrated using pKa values of conjugated acids for common organic molecules, where the higher value of pKa indicates a stronger base and hence a better electron donor. Typical values of pKa for alkyl amines are 9–10, whereas the simple nitrile group has a pKa of −10.37
A similar behavior in polarity preference was previously observed for polymers, where positive surface charges were attributed to the dominating Lewis base character of the materials studied.38 This seems to be in agreement with the common triboelectric series that ranks amine-containing nylon above stainless steel, which means that it charges positively in contact with the stainless steel surface.39,40 The order of the triboelectric series can usually be predicted based on the work functions of conductive materials associated with the Fermi levels of the electrons. However, if insulators are involved in contact charging, their order in the triboelectric series tends to be determined based on the theoretically calculated apparent work function for an insulator and confirmed experimentally.9,40 In addition, it has been shown that the sites with a strong base character tend to accumulate H+ ions from the atmosphere in preference to OH– ions, which further supports the charge polarity observations made.24
2.2.1.2. Charge Accumulation by Corona Charging
Measurements were recorded using corona discharge, as outlined in section 4.4.2, to determine the maximum charge acquired by the samples. Corona discharge has been used extensively as a method for assessing the electrostatic properties of various materials ranging from thin films41,42 and fibers43 to polymers44,45 and powders,46 and the technique is believed to provide results comparable with tribocharging experiments.11
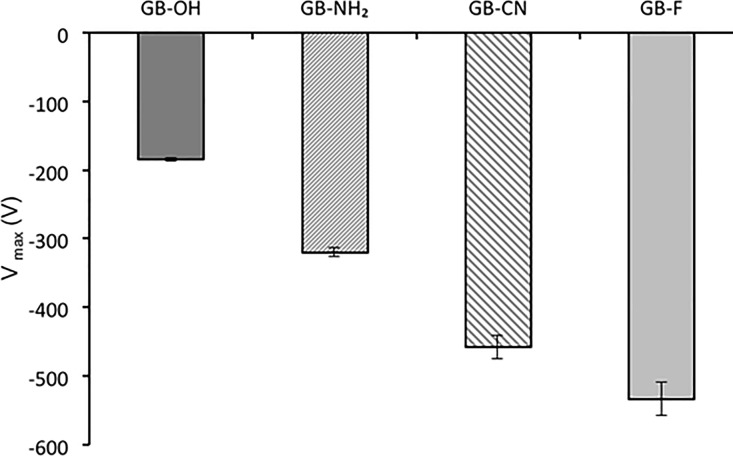
The results obtained when a negative corona discharge was applied to the modified glass bead samples are shown in Figure 3.
Figure 3.
Maximum potential acquired by functionalized surfaces charged by corona discharge (25 °C, 25% RH; n = 4, mean ± SD).
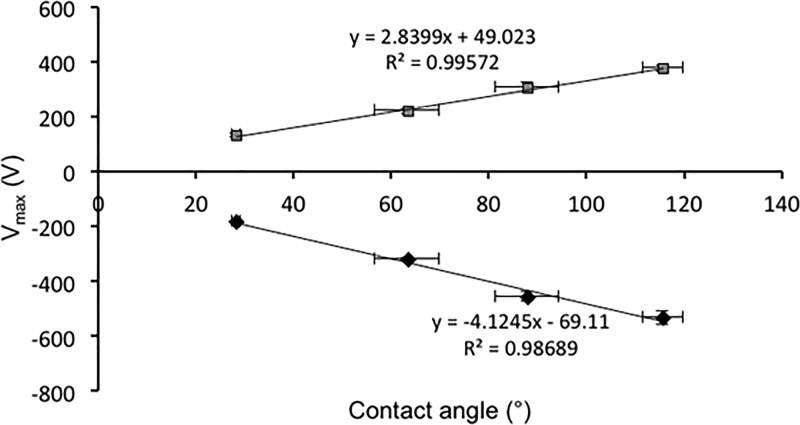
The four samples tested show a relatively strong charge accumulation and confirmed the same trend observed in the tribocharging measurements—the more hydrophobic the surface, the higher the acquired charge. A linear relationship between the maximum potential and surface hydrophobicity was obtained for both the negative and the positive corona discharge data (Figure 4).
Figure 4.
Maximum potential acquired by functionalized surfaces upon corona discharge as a function of surface contact angle (25 °C, 25% RH n = 4, mean ± SD).
In contrast to the data obtained using the tribocharging method (Figure 2), there is no clear preference of the amino-functionalized surface for the positive charge accumulated because of the positive corona deposition. The same linear trend is obtained across the samples irrespective of the corona polarity. This indicates that the ability of the surface studied to acquire charge could be primarily governed by surface hydrophobicity; however, this could be further altered by the nature of the contacting surface and its work function, with the latter being assessed using tribocharging methods only.
Furthermore, the results obtained from the tribocharging measurements (Figure 2) show that the fluorinated sample acquires an approximately six times greater charge compared with the other three samples. This difference could be because fluorine is the most electronegative element, with an electronegativity of 4.0 on the Pauling scale, which means that it has very high affinity toward free electrons, when they are available. A similar conclusion was made by Kwok et al., who studied charging properties of two fluorine-containing metered dose inhaler propellants. Their negative charge was attributed to the electronegativity of the fluorine atoms present.47
Such a strong preference for accumulation of the negative charge was not observed based on corona charging measurements under the same conditions. This implies that the strong affinity to electrons could be only a result of the interaction with the contacting surface. However, it is also important to highlight that under an applied corona discharge of 8 kV, the surfaces become saturated with charge, which would not be the case for the mild tribocharging conditions studied here.
The ability of the surface to acquire charge was tested as the humidity increased to 50% RH and then finally to 75% RH. As shown in Figure 5, in contrast to other samples, the tendency for GB-F to accumulate a large negative charge is still strongly pronounced and does not seem to be affected by the substantial increase in the humidity. In all other samples, a significant charge reduction was observed as a result of moisture present in the environment.
Figure 5.
Maximum potential acquired by functionalized surfaces upon corona discharge as a function of humidity (n = 4, mean ± SD).
These observations imply that the charge initially accumulated by the surface could be strongly affected by the moisture present on the surface because of environmental conditions. Hydrophilic surfaces interact with the moisture easily, and as humidity increases, more water molecules are expected to deposit on the surfaces, which increases the overall conductivity. However, in the fluorine surface, because of its strong hydrophobic character, the levels of surface moisture might not be affected by the increased humidity, and therefore, the initial charging is not affected either.
The above observations highlight the importance of functional groups present on the surface of powders. These results could potentially be used to account for substantial differences in the charging behavior observed between active pharmaceutical ingredients (APIs) and excipients, where APIs tend to acquire considerably higher charges compared with excipients.5 Most of the common excipients, such as lactose monohydrate, mannitol, cellulose, or corn starch, possess hydroxyl groups on the surface and do not experience significant charge accumulation. By contrast, APIs may be more electrically resistive, which makes them more prone to acquiring charge. They may also possess a larger variety of elements, including halogens containing functionalities such as in fluticasone propionate, flurbiprofen, or aripiprazole;5,48 nitrogen atoms in theophylline, salmeterol xinafoate, and paracetamol; or sulfur in diltiazem, which may make them more susceptible to charge accumulation. This ability of acquiring large charges could be potentially magnified even further when coupled with typically small particle sizes of APIs, as these tend to accept greater charges compared with large particles.1
Overall, the surface chemistry may strongly affect the magnitude and polarity of charge acquired in contact with surfaces. In addition to the ability of powders to become electrostatically charged, it is important to determine how quickly the charge can be dissipated as this will affect the powder performance, with slow charge decay rates resulting in powder handling and processing issues.
2.2.2. Influence of Relative Humidity on Electrostatic Charge Decay Rate
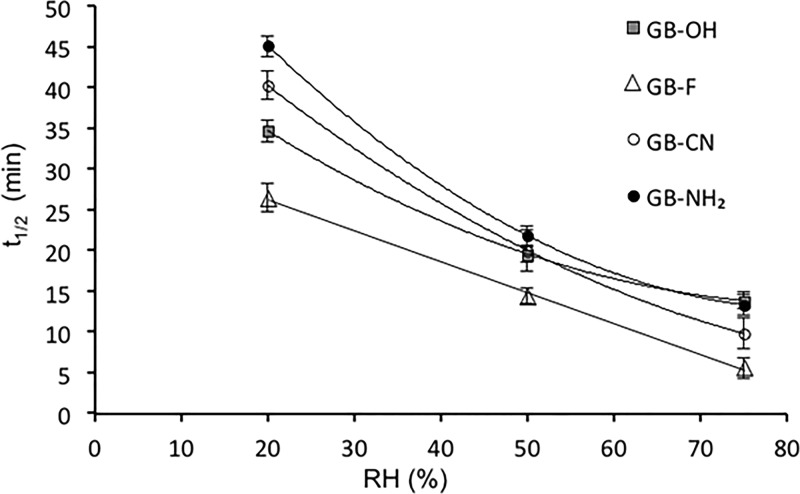
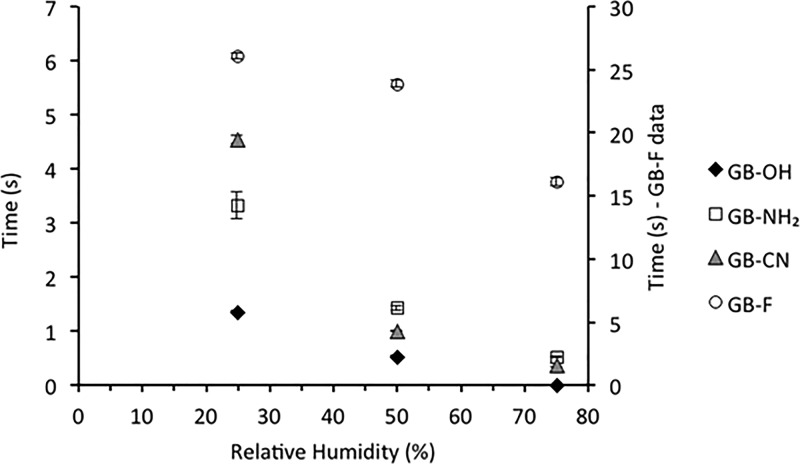
To determine the effect of surface chemistry on charge decay kinetics, charge decay curves were recorded for each sample at three different humidity levels using both tribocharging and corona charging methods. Figure 6 depicts the half-life time (t1/2) for all samples studied using the tribocharging method, whereas Figure 7 shows the time required (t1/e) for the charge to decrease to 36.7% of its initial level, for the same samples tested with a corona discharge. These data quantify how quickly surfaces lose charge when subjected to each respective charging method.
Figure 6.
Charge decay of functionalized surfaces at different humidity levels as a result of tribocharging by a stainless steel surface (n = 6, mean ± SD).
Figure 7.
Charge decay of functionalized surfaces at different humidity levels as a result of negative corona discharge (n = 4, mean ± SD).
In general, an increase in relative humidity resulted in a decrease in the charge relaxation times for all of the samples tested using both tribocharging and corona discharge methods. This general observation agrees with previously published data on the effect of humidity on charge decay kinetics.15
However, Figures 6 and 7 also show that the effect of humidity on charge decay rate is different for different surface chemistries. At lower humidity, a larger discrepancy between the decay times for each surface was observed. As the humidity level increased to 75% RH, these differences became smaller; that is, the charge decay times for different surfaces became more similar and therefore the surface chemistry itself became less pronounced. This trend is observed for three out of four surfaces studied, with the fluorine surface being an exception. The fluorine surface seems to be affected by the moisture to a much smaller extent compared with the other surface. However, considering that no effect of moisture was observed for the initial charge of GB-F (Figure 5), this suggests that charge relaxation properties could not only be dependent on the existing surface moisture but also be a function of the interaction with water and ions present in the surrounding environment. Surface charge relaxation via recombination with ions in air has been previously suggested as a possible route of charge decay.49 Furthermore, a tendency of hydrophobic surfaces toward the adsorption of OH– ions has been previously reported.18 This would imply that along with static charge dissipation, negative charge is being accumulated on the surface because of the moisture present effectively, which reduces the overall charge relaxation process.
An example discussed above proves that the total effect of humidity on the charge decay kinetics is surface-dependent. As the humidity increases from 25% RH to 75% RH, the time required for the charge to dissipate to 36.7% for the fluorine surface is improved by 1.6 times. Conversely, the cyano surface discharges 12.2 times faster under high-humidity conditions compared with the low-humidity conditions. This shows that although the general trend is valid for all of the surfaces, the overall effect is dependent on surface functionalization (Figure 7).
Finally, the results obtained using two methods (Figures 6 and 7) show that the discharge times vary significantly depending on the discharge route available. Much shorter times are observed (Figure 7) when a conductive pathway is provided; hence, utilizing conductive, grounded surfaces could be beneficial for improving the charge relaxation of highly chargeable powders. When a nonconductive pathway is provided, charge dissipates via air recombination only and hence the decay times observed (Figure 6) are significantly longer. This indicates that powders confined under insulating conditions, for example, in plastic containers, could remain charged for longer periods of time, potentially affecting their bulk structure, handling, and processability.
3. Conclusion
The present study demonstrates that surface chemistry may play a crucial role in determining the electrostatic properties of powders. Silanization reactions performed on glass beads resulted in chemically distinct surfaces with differing hydrophobicities without altering physical properties such as the size, surface area, and surface roughness of the particles. Consequently, a direct comparison of the impact of chemical functional groups on the electrostatic properties of surfaces was possible, with the polarity of the charge generated dependent on the electronic structure or electronegativity. An increase in surface hydrophobicity resulted in greater charge retention and, effectively, larger quantities of charge being accumulated during both triboelectrification and corona charging experiments with charge decay kinetics being strongly dependent on the surface chemistry and its interaction with the moisture present in the external environment. Finally, a comparison of data generated by the two methods demonstrated that corona charging experiments may provide a useful indication of the triboelectric charging behavior of a material in terms of the magnitude of charge build-up and charge decay kinetics, but not necessarily the polarity of the charge generated, which is also dependent on the difference in the effective work functions of the two interacting surfaces.
4. Materials and Methods
4.1. Materials
Glass beads (150–210 μm) were purchased from Polysciences Europe GmbH, Eppelheim, and used as a model particulate system. Absolute ethanol (AnalaR NORMAPUR ACS) obtained from VWR West Sussex, UK, and deionized water (DI) were used for cleaning purposes. Concentrated 15.7 M nitric acid (Sigma-Aldrich, Dorset, UK); toluene (AnalaR NORMAPUR ACS) from VWR West Sussex, UK; trimethoxy(3,3,3-trifluoropropyl)silane (≥97.0%, Aldrich); 3-cyanopropyltriethoxysilane (≥98.0%, Aldrich); and 3-aminopropyltriethoxysilane (≥99.0%, Aldrich) were used as received for silanization reactions.
4.2. Surface Chemistry Modification
The glass beads were cleaned using DI water, ethanol, and again DI water followed by drying and then placing them in concentrated nitric acid at 65 °C for 5 h. The beads were then filtered, washed with DI water, and dried in a vacuum oven at 60 °C and 800 mbars for at least 4 h. The surface chemistry of the cleaned beads was then modified by placing the beads in toluene, stirring at room temperature for 10 min followed by the addition of a selected silane to yield 1% (v/v) silane solution in toluene. The reaction flask was heated to 100 °C under reflux and stirred overnight. After the time elapsed, the beads were filtered, rinsed first with toluene to remove any unreacted silane and then with ethanol. The beads were dried in a vacuum oven at 60 °C for a minimum of 4 h.
4.3. Contact Angle Measurements
Prepared glass beads were adhered to a glass microscope slide using a double-sided tape to form a glass bead monolayer. Static sessile drop measurements to determine the contact angle were taken with water as a probe liquid using an EasyDrop contact angle measuring instrument (Krüss GmbH, Hamburg, Germany). Five replicates were performed for each sample, under ambient conditions. The same procedure was used to measure the contact angle of the glass slide and the adhesive tape for reference.
4.4. Electrostatic Measurements
4.4.1. Tribocharging Measurements
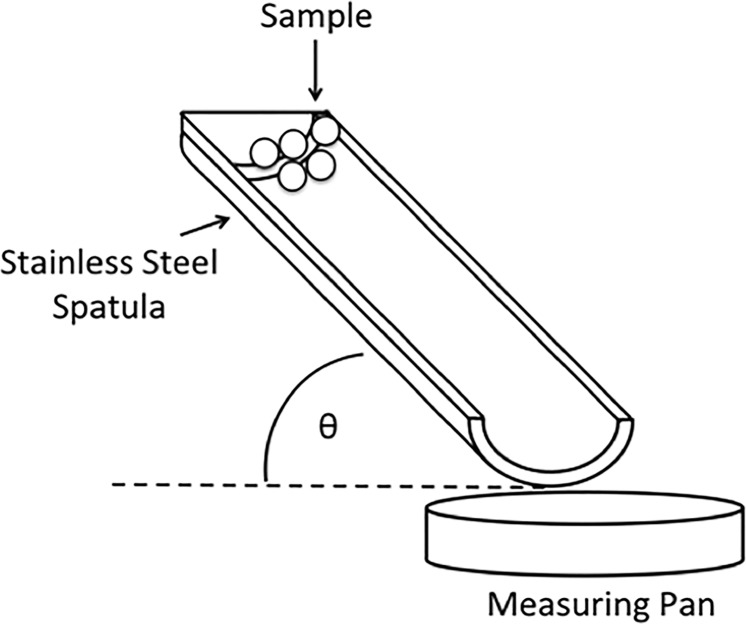
Triboelectrification of glass beads was measured using a capacitive probe method as reported previously.50 Briefly, glass beads were stored in a conductive-grounded container under controlled environmental conditions (25 °C, 30–35% RH) for a minimum of 18 h before measurement. A U-shaped stainless steel spatula was used to charge and load particles into the measuring pan, as illustrated in Figure 8. The spatula was tilted at an angle of approximately 45°, placed directly above the measuring pan, and between 10 and 30 mg of the sample was loaded at a time. Charge decay was measured at 25 °C and three different humidity levels: 20% RH, 50% RH, and 75% RH. The mass of sample used was recorded. At least six repeats were performed for each sample under each selected condition.
Figure 8.
Schematic of the tribocharging method used.
4.4.2. Corona Discharge Measurements
Glass beads were characterized for electrostatic properties using a charge decay time analyzer (JCI155 v6, Chilworth Technology Ltd. Southampton, UK). Samples were equilibrated under target conditions for a minimum of 18 h before measurement. Glass beads (2 g) were placed in the sample holder to form an even layer. Both a negative and a positive corona discharge of 8.0 kV were applied separately to the sample for a duration of 0.02 s. The data analysis was commenced at 0.07 s after the corona discharge, and the rate of charge decay was measured until 10% of the initial charge was reached. Four measurements were taken for each sample at 25 °C and three different humidity levels—25% RH, 50% RH, and 75% RH—in a temperature- and humidity-controlled cabinet (Safetech, Climatezone, Hampshire, UK).
Acknowledgments
The PhD studentship, supported by the Engineering and Physical Science Research Council and Pfizer for K.W.B., is gratefully acknowledged. K.W.B. gratefully acknowledges the useful discussions and comments from Dr. Daryl Williams. We thank Dr. Jin Wang Kwek for providing access to the capacitive probe sensor.
The authors declare no competing financial interest.
References
- Bailey A. G. Electrostatic phenomena during powder handling. Powder Technol. 1984, 37, 71–85. 10.1016/0032-5910(84)80007-8. [DOI] [Google Scholar]
- Bailey A. G. Charging of solids and powders. J. Electrost. 1993, 30, 167–180. 10.1016/0304-3886(93)90072-f. [DOI] [Google Scholar]
- Freund T. Tribo-electricity. Adv. Colloid Interface Sci. 1979, 11, 43–66. 10.1016/0001-8686(79)80003-2. [DOI] [Google Scholar]
- Hearn G. Static electricity: Concern in the pharmaceutical industry?. Pharm. Sci. Technol. Today 1998, 1, 286–287. 10.1016/s1461-5347(98)00078-9. [DOI] [Google Scholar]
- Šupuk E.; Zarrebini A.; Reddy J. P.; Hughes H.; Leane M. M.; Tobyn M. J.; Timmins P.; Ghadiri M. Tribo-electrification of active pharmaceutical ingredients and excipients. Powder Technol. 2012, 217, 427–434. 10.1016/j.powtec.2011.10.059. [DOI] [Google Scholar]
- Thomas A.; Saleh K.; Guigon P.; Czechowski C. Characterisation of electrostatic properties of powder coatings in relation with their industrial application. Powder Technol. 2009, 190, 230–235. 10.1016/j.powtec.2008.04.072. [DOI] [Google Scholar]
- Mizes H. A. Adhesion of small particles in electric fields. J. Adhes. Sci. Technol. 1994, 8, 937–951. 10.1163/156856194x00546. [DOI] [Google Scholar]
- Castle G. S. P. Industrial applications of electrostatics: The past, present and future. J. Electrost. 2001, 51–52, 1–7. 10.1016/S0304-3886(01)00068-7. [DOI] [Google Scholar]
- Matsusaka S.; Maruyama H.; Matsuyama T.; Ghadiri M. Triboelectric charging of powders: A review. Chem. Eng. Sci. 2010, 65, 5781–5807. 10.1016/j.ces.2010.07.005. [DOI] [Google Scholar]
- Matsusaka S.; Masuda H. Electrostatics of particles. Adv. Powder Technol. 2003, 14, 143–166. 10.1163/156855203763593958. [DOI] [Google Scholar]
- Chubb J. N. Comments on methods for charge decay measurement. J. Electrost. 2004, 62, 73–80. 10.1016/j.elstat.2004.04.004. [DOI] [Google Scholar]
- Peart J. Powder electrostatics: Theory, techniques and applications. KONA 2001, 19, 34–45. 10.14356/kona.2001009. [DOI] [Google Scholar]
- Ramirez-Dorronsoro J.-C.; Jacko R. B.; Kildsig D. O. Chargeability measurements of selected pharmaceutical dry powders to assess their electrostatic charge control capabilities. AAPS PharmSciTech 2006, 7, E133–E140. 10.1208/pt0704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elajnaf A.; Carter P.; Rowley G. Electrostatic characterisation of inhaled powders: effect of contact surface and relative humidity. Eur. J. Pharm. Sci. 2006, 29, 375–384. 10.1016/j.ejps.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Elajnaf A.; Carter P.; Rowley G. The effect of relative humidity on electrostatic charge decay of drugs and excipient used in dry powder inhaler formulation. Drug Dev. Ind. Pharm. 2007, 33, 967–974. 10.1080/03639040601134207. [DOI] [PubMed] [Google Scholar]
- Rezende C. A.; Gouveia R. F.; da Silva M. A.; Galembeck F. Detection of charge distributions in insulator surfaces. J. Phys.: Condens. Matter 2009, 21, 263002. 10.1088/0953-8984/21/26/263002. [DOI] [PubMed] [Google Scholar]
- Soares L. C.; Bertazzo S.; Burgo T. A. L.; Baldim V.; Galembeck F. A New Mechanism for the Electrostatic Charge Build-up and Dissipation in Dielectrics. J. Braz. Chem. Soc. 2008, 19, 277–286. 10.1590/S0103-50532008000200012. [DOI] [Google Scholar]
- Burgo T. A. d. L.; Rezende C. A.; Bertazzo S.; Galembeck A.; Galembeck F. Electric potential decay on polyethylene: Role of atmospheric water on electric charge build-up and dissipation. J. Electrost. 2011, 69, 401–409. 10.1016/j.elstat.2011.05.005. [DOI] [Google Scholar]
- Murtomaa M.; Mäkilä E.; Salonen J. One-step method for measuring the effect of humidity on powder resistivity. J. Electrost. 2013, 71, 159–164. 10.1016/j.elstat.2013.01.010. [DOI] [Google Scholar]
- Ducati T. R. D.; Simões L. H.; Galembeck F. Charge partitioning at gas–solid interfaces: Humidity causes electricity buildup on metals. Langmuir 2010, 26, 13763–13766. 10.1021/la102494k. [DOI] [PubMed] [Google Scholar]
- Gouveia R. F.; Costa C. A. R.; Galembeck F. Water Vapor Adsorption Effect on Silica Surface Electrostatic Patterning. J. Phys. Chem. C 2008, 112, 17193–17199. 10.1021/jp803812p. [DOI] [Google Scholar]
- Gouveia R. F.; Galembeck F. Electrostatic Charging of Hydrophilic Particles Due to Water Adsorption. J. Am. Chem. Soc. 2009, 131, 11381–11386. 10.1021/ja900704f. [DOI] [PubMed] [Google Scholar]
- Jallo L. J.; Dave R. N. Explaining electrostatic charging and flow of surface-modified acetaminophen powders as a function of relative humidity through surface energetics. J. Pharm. Sci. 2015, 104, 2225–2232. 10.1002/jps.24479. [DOI] [PubMed] [Google Scholar]
- Gouveia R. F.; Bernardes J. S.; Ducati T. R.; Galembeck F. Acid–base site detection and mapping on solid surfaces by Kelvin force microscopy (KFM). Anal. Chem. 2012, 84, 10191–10198. 10.1021/ac3009753. [DOI] [PubMed] [Google Scholar]
- Karner S.; Littringer E. M.; Urbanetz N. A. Triboelectrics: The influence of particle surface roughness and shape on charge acquisition during aerosolization and the DPI performance. Powder Technol. 2014, 262, 22–29. 10.1016/j.powtec.2014.04.025. [DOI] [Google Scholar]
- Rowley G. Quantifying electrostatic interactions in pharmaceutical solid systems. Int. J. Pharm. 2001, 227, 47–55. 10.1016/s0378-5173(01)00784-0. [DOI] [PubMed] [Google Scholar]
- Ireland P. M. Triboelectrification of particulate flows on surfaces: Part I—Experiments. Powder Technol. 2010, 198, 189–198. 10.1016/j.powtec.2009.11.017. [DOI] [Google Scholar]
- Ireland P. M. Triboelectrification of particulate flows on surfaces: Part II—Mechanisms and models. Powder Technol. 2010, 198, 199–210. 10.1016/j.powtec.2009.11.008. [DOI] [Google Scholar]
- Williams M. W. Triboelectric charging of insulating polymers—Some new perspectives. AIP Adv. 2012, 2, 010701. 10.1063/1.3687233. [DOI] [Google Scholar]
- Wong J.; Kwok P. C. L.; Niemelä V.; Heng D.; Crapper J.; Chan H.-K. Bipolar electrostatic charge and mass distributions of powder aerosols—Effects of inhaler design and inhaler material. J. Aerosol Sci. 2016, 95, 104–117. 10.1016/j.jaerosci.2016.02.003. [DOI] [Google Scholar]
- Karner S.; Urbanetz N. A. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J. Aerosol Sci. 2011, 42, 428–445. 10.1016/j.jaerosci.2011.02.010. [DOI] [Google Scholar]
- Wong J.; Kwok P. C. L.; Chan H.-K. Electrostatics in pharmaceutical solids. Chem. Eng. Sci. 2015, 125, 225–237. 10.1016/j.ces.2014.05.037. [DOI] [Google Scholar]
- Adi H.; Kwok P. C. L.; Crapper J.; Young P. M.; Traini D.; Chan H.-K. Does electrostatic charge affect powder aerosolisation?. J. Pharm. Sci. 2010, 99, 2455–2461. 10.1002/jps.21996. [DOI] [PubMed] [Google Scholar]
- Ho R.; Hinder S. J.; Watts J. F.; Dilworth S. E.; Williams D. R.; Heng J. Y. Y. Determination of surface heterogeneity of d-mannitol by sessile drop contact angle and finite concentration inverse gas chromatography. Int. J. Pharm. 2010, 387, 79–86. 10.1016/j.ijpharm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Arkles B.; Kim Y. M.; Pan Y.. The role of polarity in the structure of silanes employed in surface modification. In Silanes and Other Coupling Agents, 1st ed.; Mittal K. L., Ed.; Koninklijke Brill NV: Leiden, 2009; Vol. 5, pp 51–64. [Google Scholar]
- Howarter J. A.; Youngblood J. P. Optimization of silica silanization by 3-aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. 10.1021/la061240g. [DOI] [PubMed] [Google Scholar]
- Troy D. B.; Beringer P.. Remington: The Science and Practice of Pharmacy, 21st ed.; Lippincott Williams & Wilkins: United States, 2006; pp 394–396. [Google Scholar]
- Németh E.; Albrecht V.; Schubert G.; Simon F. Polymer tribo-electric charging: Dependence on thermodynamic surface properties and relative humidity. J. Electrost. 2003, 58, 3–16. 10.1016/s0304-3886(02)00137-7. [DOI] [Google Scholar]
- Liu L.; Seyam A. M.; Oxenham W. Frictional Electrification on Polymeric Flat Surfaces. J. Eng. Fibers Fabr. 2013, 8, 126–136. [Google Scholar]
- Rowland M. G.Electrostatic Properties of Particles for Inhalation, Ph.D. Thesis; University of Bath, 2014. [Google Scholar]
- Rouagdia K.; Nemamcha M.; Herous L.; Dascalescu L.; Mellouki H. Surface potential decay of DC-corona-charged PET films on humid electrodes. J. Electrost. 2015, 78, 17–21. 10.1016/j.elstat.2015.09.004. [DOI] [Google Scholar]
- Xu Z.; Zhang L.; Chen G.. Measurement and analysis of electric potential decay in corona charged low-density polyethylene films. In International Conference on Solid Dielectrics; IEEE: Winchester, UK, 2007; pp 454–457. [Google Scholar]
- Chubb J. N. Corona charging of practical materials for charge decay measurements. J. Electrost. 1996, 37, 53–65. 10.1016/0304-3886(95)00059-3. [DOI] [Google Scholar]
- Herous L.; Remadnia M.; Kachi M.; Nemamcha N. Decay of electrical charges on polyethylene terephthalate surface. J. Eng. Sci. Technol. Rev. 2009, 2, 87–90. [Google Scholar]
- Kumara S.; Serdyuk Y.; Gubanski S. Surface charge decay on polymeric materials under different neutralization modes in air. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1779–1788. 10.1109/tdei.2011.6032850. [DOI] [Google Scholar]
- Carter P. A.; Rowley G.; Fletcher E. J.; Stylianopoulos V. Measurement of electrostatic charge decay in pharmaceutical powders and polymer materials used in Dry Powder Inhaler devices. Drug Dev. Ind. Pharm. 1998, 24, 1083–1088. 10.3109/03639049809089953. [DOI] [PubMed] [Google Scholar]
- Kwok P. C. L.; Noakes T.; Chan H.-K. Effect of moisture on the electrostatic charge properties of metered dose inhaler aerosols. J. Aerosol Sci. 2008, 39, 211–226. 10.1016/j.jaerosci.2007.11.004. [DOI] [Google Scholar]
- Hoe S.; Traini D.; Chan H.-K.; Young P. M. The influence of flow rate on the aerosol deposition profile and electrostatic charge of single and combination metered dose inhalers. Pharm. Res. 2009, 26, 2639–2646. 10.1007/s11095-009-9979-y. [DOI] [PubMed] [Google Scholar]
- Chen G.; Xu Z.; Zhang L. W. Measurement of the surface potential decay of corona-charged polymer films using the pulsed electroacoustic method. Meas. Sci. Technol. 2007, 18, 1453–1458. 10.1088/0957-0233/18/5/034. [DOI] [Google Scholar]
- Biegaj K.; Kwek J. W.; Lukas T.; Rowland M.; Heng J. Y. Y. Novel coupling of a capacitive probe with a dynamic vapor sorption (DVS) instrument for the electrostatic measurements of powders. Ind. Eng. Chem. Res. 2016, 55, 5585–5589. 10.1021/acs.iecr.6b00427. [DOI] [Google Scholar]