Abstract
Objective:
To compare the audiological performance with the novel adhesive bone conduction hearing device (ADHEAR) to that with a passive bone conduction (BC) implant and to that with a bone conduction device (BCD) on a softband.
Study Design:
Prospective study in an acute setting, single-subject repeated measure in three situations: unaided, with conventional BCDs (passive implant or on softband), and with the ADHEAR.
Setting:
Tertiary referral center.
Patients:
Ten subjects with conductive hearing loss were evaluated with the ADHEAR. Five of these were users of a passive BC implant (Baha Attract with Baha4); five received a BCD (Baha4) on a softband for test purposes.
Intervention:
Use of non-invasive adhesive bone conduction system for the treatment of conductive hearing loss.
Main Outcome Measures:
Air and bone conduction thresholds, sound field thresholds, word recognition scores in quiet, and speech recognition thresholds in quiet and noise were assessed.
Results:
Users of the passive BC implant received comparable hearing benefit with the ADHEAR. The mean aided thresholds in sound field measurements and speech understanding in quiet and noise were similar, when subjects were evaluated either with the ADHEAR or the passive BC implant. The audiological outcomes for the non-implanted group were also comparable between the ADHEAR and the BCD on softband.
Conclusions:
Based on our initial data, the ADHEAR seems to be a suitable alternative for patients who need a hearing solution for conductive hearing loss but for medical reasons cannot or do not want to undergo surgery for a passive BC implant.
Keywords: Adhesive bone conduction device, Adhesive adapter, Bone conduction device, Nonsurgical
Current possibilities of compensating conductive hearing loss through bone conduction (BC) solutions include both nonsurgical and surgical interventions. The first group includes bone conduction devices (BCD) fixed on a softband, headband, or spectacle frame. The softband was a further development of the headband solution, which improved wearing comfort and placement stability, especially in children (1). As all these systems employ passive or skin-drive BC to improve hearing, the vibrations emitted by the BC transducer have to be relayed through the intact skin to the bone. However, due to dampening of energy by skin and soft tissue, especially in the high frequencies, the application of these devices has limitations. In the frequency range of 1 to 4 kHz this has been reported to be as much as 20 dB compared with percutaneous or direct-drive BC implants (1). In addition, a major drawback of these types of conventional nonsurgical BC solutions is that the transducer requires a relatively high static pressure of about 2 N on the skin to efficiently transmit vibrations to the cochlea, which may cause discomfort in long-term use (2,3).
Surgically implanted bone anchored hearing aids that are directly anchored in the temporal bone circumvent these problems (4), but their use is associated with an increased risk of dermatological complications due to the penetration of the skin surface necessitating constant wound care (5). Transcutaneous systems without skin penetration have been developed to reduce that risk. Again, as for the nonsurgical solutions the amount of transmitted energy in these systems is strongly related to the thickness of skin and subcutaneous tissue, and extended use may be associated with skin reactions such as paresthesia or numbness (6,7). In active BC implants the sound signal is transmitted between the external and the internal part by electromagnetic induction and vibrations are generated by an implanted transducer. Because of the size of the transducer, these implants have more specific requirements in terms of the temporal bone anatomy (4,8), which reduces the number of potential users. Still, there is no ideal nonsurgical solution for patients who cannot undergo anesthesia, need regular magnetic resonance imaging scans, or very young children with an insufficient mastoid size for an implantable solution (9).
In 2017 a novel, nonsurgical solution with a new auditory stimulation concept that uses adhesive retention of a BCD (Fig. 1) became available. The ADHEAR (MED-EL, Innsbruck, Austria) system consists of a bone conduction audio processor that is retained on the head with an adhesive adapter placed over the mastoid behind the auricle. The integrated transducer in the audio processor converts sound into mechanical vibrations that are transmitted via the adhesive adapter and relayed through the skin to the mastoid bone similar to other skin-drive solutions. Sound is transmitted via bone conduction to the inner ear, providing a hearing impression. The ADHEAR represents a nonsurgical adhesive bone conduction system for the treatment of conductive hearing loss and single-sided deafness that may be suitable for subjects of any age.
FIG. 1.

The ADHEAR system. Connecting the audio processor to the adhesive adapter.
The audio processor has dual microphones and signal processing technologies and is powered by a single P13 battery. The audio processor's push button allows users to switch between four predefined programs. Users can adjust the volume by using the wheel on the side of the audio processor. The signal processing technology employs an automatic classifier that controls the adaptive directional microphone system and feedback suppression. The adhesive adapter uses a non-toxic, non-allergenic medical adhesive tape to attach the adapter to the skin. The adhesive adapter is designed for single use and is water resistant, i.e., it can remain on the skin for 3 to 7 days. After attaching the adhesive adapter to the skin, the audio processor is connected to the adapter with a snap coupler without any pressure against the skin. The novel fixation mechanism using an adhesive adapter completely differs from other currently available non-invasive BC solutions, in which pressure has to be applied to the skin to efficiently transmit vibrations to the cochlea (2,3).
Based on the novel fixation method and the principles of bone conduction hearing, we hypothesized that the ADHEAR system achieves a similar audiological performance in subjects with conductive hearing loss as other passive transcutaneous solutions. In this pilot study, we particularly aimed to assess whether the ADHEAR system can achieve similar audiological performances in users of passive transcutaneous BC implants or not. In addition, we measured the audiological outcomes in comparison to that of a BCD on a softband in patients with conductive hearing loss who are not using any hearing solution.
MATERIALS AND METHODS
Study Subjects
The study was designed as a prospective single-subject repeated-measure study with each subject serving as his or her own control and was approved by the ethical commission of the Institute of Physiology and Pathology of Hearing in Warsaw. An acute test set-up was chosen, i.e., subjects were evaluated on 1 day without acclimatization to the test devices. Informed consent was obtained from all subjects before inclusion in the study. Ten subjects with conductive hearing loss (CHL) were enrolled. Subjects had to be 15 years of age or older and native speakers of the Polish language. Subjects were excluded from the study if they suffered from mixed hearing loss (i.e., a bone conduction PTA4 [four frequency pure tone average across frequencies 0.5, 1, 2, and 4 kHz] >25 dB HL), retrocochlear or central auditory disorders or skin, scalp, or surgical conditions that may preclude the attachment of the adhesive adapter.
Bone Conduction Hearing Devices
The 10 subjects were split into two groups which were both supplied with different bone conduction hearing solutions. Five subjects had previously been implanted with a passive transcutaneous bone conduction hearing aid (Baha Attract, CochlearTM, Melbourne, Australia) and had used their device for more than 12 months. These were evaluated with a Baha 4 test audio processor in combination with their passive BC implant (7) (referred to as pBC Implant group). The other five subjects have previously not been treated for their hearing loss with a hearing device and were provided with a test audio processor (Baha 4, CochlearTM, Melbourne, Australia) on a softband (10,11) (referred to as BCD Softband group). Both groups were also tested with the ADHEAR system as alternative BC device, i.e., all 10 subjects were evaluated in the unaided situation and aided with the ADHEAR.
Audiometric Testing
Subjects were evaluated acutely in one session split into two intervals. During the first part, the Baha 4 audio processor and ADHEAR settings were checked. Both devices were set up with default settings for conductive hearing loss. All subjects were using the same test audio processors. Air and bone conduction thresholds, thresholds for warble tones in sound field and speech understanding in quiet and noise were determined in the unaided condition. After a break, the patient's aided thresholds in sound field and speech understanding in quiet and noise with the different devices were evaluated. The order for measuring the patients first with the ADHEAR or the BC device was randomized. The pBC Implant group was tested with the Baha Attract device as well as the ADHEAR system, and the BCD Softband group was tested with the BCD on a softband and the ADHEAR system. The contralateral ear was always plugged and covered in all patients using foam earplugs (3M Earplugs 1100, attenuation expressed as single number rating [SNR] = 37 dB) and ear muffs (3M Peltor X5, SNR = 37 dB).
Audiometric testing was conducted in an audiometric sound-attenuated room, using calibrated signals and equipment (ISO-389 Series, ISO-8253 Series, and ISO-60645 Series). The test ear of each patient was evaluated using standard air conduction (AC) audiometry at 0.5, 1, 2, 3, 4, 6, and 8 kHz and BC pure tone audiometry at 0.5, 1, 2, 3, and 4 kHz. Narrow-band noise was used for masking if applicable. Sound field (SF) thresholds were measured using warble tones with the frequencies of 0.5, 1, 2, 3, 4, 6, and 8 kHz with loudspeakers positioned 1 m in front of the patient. The contralateral side was plugged and covered. The PTA4 was calculated across the conversational frequencies of 0.5, 1, 2, and 4 kHz.
The speech recognition threshold (SRT) in quiet and the word recognition score (WRS) at 65 dB SPL (sound pressure level) in quiet were measured with speech coming from the front using the Pruszewicz monosyllabic Polish word test. The SRT in noise was determined using the Polish Sentence Matrix Test (12) with speech and stationary speech-shaped noise coming from the front. The noise level was fixed at 65 dB SPL and the signal level was changed adaptively. The SRT in noise is defined as the signal-to-noise ratio in dB SNR at which 50% intelligibility of the speech material is reached. Two training lists were performed before testing to acquaint the test person with the speech material and the test procedure.
Statistical Analyses
Nonparametric Wilcoxon signed-rank test was used to test for significant differences between the ADHEAR and the unaided condition in the audiological measurements. For smaller samples (n <10), i.e., differences compared with the BCD on the softband and the passive BC implant, statistical significance was not assessed due to the small sample size. In these cases only clinical significance was assessed. The Shapiro-Wilk test was used to determine if the data were normally distributed. Statistical significance was defined as p < 0.05. GraphPad Prism 6 (GraphPad Software, San Diego, CA) for Windows 2013, Version 6.02, was used for the analyses as well as the graphs.
RESULTS
Patients
Ten patients (five men and five women) with conductive hearing loss were included in the study. The main etiologies for the CHL were cholesteatoma, chronic otitis media, and congenital malformations of the outer or middle ear. Five were users of a passive transcutaneous bone conduction implant (pBC Implant group) and five had not been treated for their CHL before the study (BCD Softband group). The mean age in the pBC Implant group was 32.4 ± 21.0 years (mean ± standard deviation) (range, 16–65 yrs) and in the BCD Softband group 35.6 ± 10.6 years (range, 18–44 yrs). The pBC Implant group had a mean PTA4AC of 64 ± 3 dB HL and a mean PTA4BC of 12 ± 5 dB HL (Table 1). The BCD Softband group had a mean PTA4AC of 49 ± 13 dB HL and a mean PTA4BC of 11 ± 4 dB HL (Table 1). The pBC Implant group showed a mean air-bone gap of 53 ± 8 dB and the BCD Softband group of 38 ± 11 dB. Individual data including that of the contralateral ear can be found in the supplementary material (Supplementary Table 1).
TABLE 1.
Patients’ characteristics
| ID | Age (yr) | Tested Ear | Pathology (Tested Ear) | PTA4AC (dB HL) | PTA4BC (dB HL) | ABG (dB) |
| I1 | 38 | R | Congenital malformation of outer ear, reconstruction of right pinna | 65 | 10 | 55 |
| I2 | 22 | R | Tympanotomy, congenital malformation in middle ear | 66 | 13 | 54 |
| I3 | 65 | R | Chronic cholesteatoma, otitis media | 59 | 19 | 40 |
| I4 | 16 | R | Chronic otitis media, radical surgery | 64 | 4 | 60 |
| I5 | 17 | R | Congenital defect of the middle and external ear, microtia with atresia, reconstructive surgery of right pinna | 68 | 13 | 55 |
| S1 | 43 | L | Narrow external auditory canal, chronic cholesteatoma and otitis media, myringoplasty | 64 | 14 | 50 |
| S2 | 38 | R | Cholesteatoma, otitis media, tympanostomy | 39 | 4 | 35 |
| S3 | 18 | L | Chronic secretory middle ear obliterans, ventilation drainage | 39 | 9 | 30 |
| S4 | 34 | L | Congenital malformation external auditory canal | 61 | 13 | 49 |
| S5 | 42 | L | Chronic otitis media, radical surgery, myringoossiculoplasty | 41 | 14 | 28 |
| Overall mean ± SD | 33.3 ± 15.4 | – | – | 57 ± 12 | 11 ± 5 | 46 ± 12 |
I1 to I5: subjects with a passive BC implant (pBC Implant group); S1 to S5: subjects with a BCD on a softband (BCD Softband group). Individual data on the unaided air and bone conduction thresholds (PTA4AC, PTA4BC) and air-bone gap (ABG) of the tested ears are listed. The mean and standard deviation (SD) were calculated for all subjects (n = 10).
Sound Field Audiometry
In the pBC Implant group, aided sound field thresholds improved from an average PTA4 of 58 ± 6 to 33 ± 6 dB HL with the passive BC implant and to 32 ± 9 dB HL with the ADHEAR (Fig. 2A). Aided thresholds were comparable using the ADHEAR and the passive BC implant. In the BCD Softband group, the mean PTA4 in sound field improved from unaided 47 ± 10 dB HL to a comparable level of 27 ± 6 dB HL with the softband and to 29 ± 6 dB HL with the ADHEAR (Fig. 2B). In both groups, the unaided sound field threshold improved significantly from a PTA4 of 53 ± 10 dB HL to an average of 30 ± 7 dB HL with the ADHEAR (n = 10, p = 0.002), resulting in a mean functional gain of 23 dB.
FIG. 2.
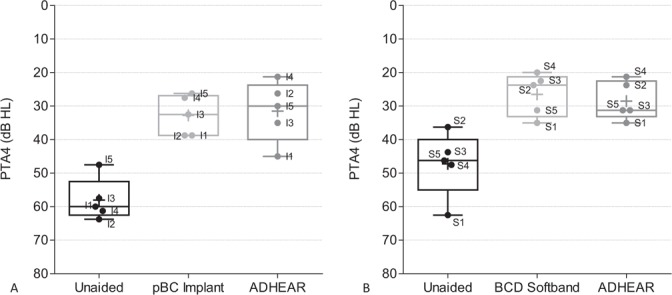
Sound field audiometry. PTA4 thresholds in sound field were determined in the unaided condition, with the ADHEAR and with the bone conduction device. Box plots indicate the median (centred horizontal line) with 25%- and 75%-quartiles (upper and lower horizontal lines) and minimum and maximum values (whisker). The cross indicates the mean. Individual data are labeled according to subject identifier. The pBC Implant group is displayed on panel A, the BCD softband group on panel B. pBC indicates passive bone conduction; BCD, bone conduction device; PTA4, four frequency pure tone average across frequencies 0.5, 1, 2, and 4 kHz.
Speech Understanding in Quiet
In the pBC Implant group, the mean aided WRS improved from unaided levels of 7% ± 16% to the same level with both the ADHEAR (66% ± 33%) and the passive BC implant (66% ± 24%) (Fig. 3A). In the BCD Softband group, aided scores with the Softband (83 ± 17%) were comparable to those with the ADHEAR (84% ± 13%) (Fig. 3B). In both groups, the WRS at 65 dB SPL increased significantly from a mean unaided score of 18% ± 31% to 75% ± 26% with the ADHEAR (n = 10, p = 0.002), resulting in an average improvement of 57%.
FIG. 3.
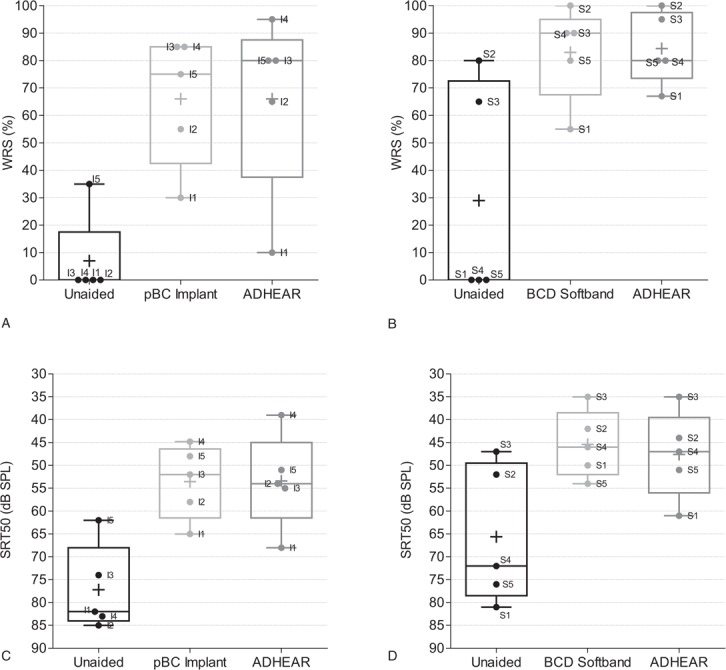
Speech understanding in quiet. WRS was determined at 65 dB SPL using the Pruszewicz monosyllabic Polish word test in the pBC Implant group (A) and BCD softband group (B). SRT in quiet was measured with the same test in the pBC Implant group (C) and BCD soft band group (D). Box plots indicate the median (centred horizontal line) with 25%- and 75%-quartiles (upper and lower horizontal lines) and minimum and maximum values (whisker). The cross indicates the mean. Individual data are labeled according to subject identifier. pBC indicates passive bone conduction; BCD, bone conduction device; SRT, speech recognition threshold; WRS, word recognition score.
No clinically relevant difference was seen between the devices and their capacity to improve speech understanding in quiet (Fig. 3C). In the BCD Softband group, a comparable improvement in SRT50%, with the BCD Softband and with the ADHEAR was seen (Fig. 3D). The SRT improved significantly with the ADHEAR in both groups (n = 10, p = 0.002), resulting in an average improvement of 20 dB.
Speech Understanding in Noise
Speech understanding in noise was assessed with the Polish Sentence Matrix Test at a fixed noise level of 65 dB SPL and adaptive speech level. In the pBC Implant group, speech understanding was restored from 2.7 ± 5.5 to –5.0 ± 1.0 dB SNR with the passive BC implant and –3.4 ± 2.4 dB SNR with the ADHEAR (Fig. 4A). In the BCD Softband group, the SRT in noise improved from 0.4 ± 2.5 dB SNR unaided to –4.4 ± 1.3 dB SNR with the Softband and –4.4 ± 2.0 dB SNR with the ADHEAR (Fig. 4B). With the ADHEAR the SRT in noise significantly improved from a mean of 1.5 ± 4.2 dB SNR unaided to –3.9 ± 2.1 dB SNR in the aided situation, when evaluating all subjects (n = 10, p = 0.002), resulting in an average improvement of 5.4 dB.
FIG. 4.
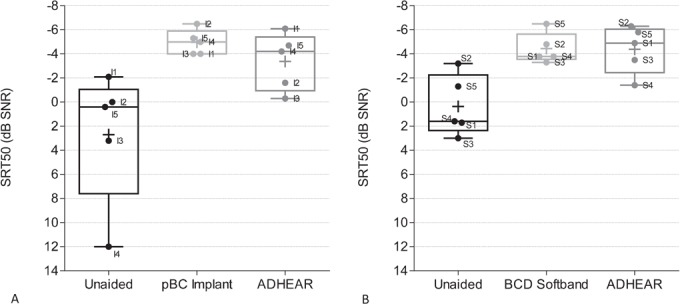
Speech understanding in noise. The SRT in noise was determined using the Polish Sentence Matrix Test, with speech and noise coming from the front at a fixed noise level of 65 dB SPL and adaptive speech level in the pBC Implant group (A) and in the BCD softband group. Box plots indicate the median (centered horizontal line) with 25%- and 75%-quartiles (upper and lower horizontal lines) and minimum and maximum values (whisker). The cross indicates the mean. Individual data are labeled according to subject identifier. pBC indicates passive bone conduction; BCD, bone conduction device; SRT, speech recognition threshold; WRS, word recognition score.
Safety
No adverse reactions towards the ADHEAR adhesive adapter occurred in the acute setting.
DISCUSSION
In our study the ADHEAR system significantly improved speech understanding in quiet and noise in patients suffering from conductive hearing loss caused by multiple etiologies. However, we did not only investigate if the ADHEAR system is capable to improve hearing in subjects with conductive hearing loss, in this pilot study we were primarily interested if the new system is capable to achieve a similar performance as an implantable device. The Baha Attract system served as comparator. For this passive transcutaneous implant a lower complication rate has been reported in comparison to percutaneous devices in a systematic review (7). However, there is still room for improvement: on the one side in terms of providing a more secure retention of the audio processor and on the other side in reducing pressure on the skin. Interestingly, results from this pilot study using an acute test set-up indicate that the ADHEAR system achieves a similar improvement in audiological outcomes as the passive BC implant that the tested subjects had already been using for more than 1 year.
However, a clear limitation of our pilot study is that the small sample size of only five subjects per group did not allow a thorough statistical analysis of the data. To address this problem, we assessed the statistical significance of our data using statistical models based on the test design. For standard audiometry with 5 dB steps as used in this study, a standard deviation of 10 dB was reported (13). Therefore, a change in threshold of more than 10 dB was defined as clinically significant. All subjects in the pBC Implant and BCD Softband group had clinically significantly better results with the Baha and ADHEAR compared with the unaided condition. Winkler and Holube (14) described the critical difference for the evaluation of significant differences between aided and unaided speech intelligibility in the Freiburger monosyllabic test. The critical difference for a monosyllabic test is calculated by modeling the speech test as a Bernoulli experiment with an underlying binomial distribution. The same applies for the Polish monosyllable test because both tests have monosyllables as test material and the WRS as outcome measure. The critical difference defines which WRS difference is significant, with an error rate of less than 5% depending on a baseline WRS, e.g., if a WRS of 50% is achieved in the unaided condition an aided score of at least 80% is significantly different (14). In our test set-up, five out of five subjects had significantly better WRS scores with the passive BC implant and four out of five subjects with the ADHEAR when evaluating the critical differences with the unaided condition as baseline score. For measurements with the SRT as the outcome, no clinical significance was evaluated because the critical difference could not be determined. The critical differences for SRTs depend on the slope of the discrimination function of each individual test subject (15) and reference data were not found for hearing-impaired subjects. In summary, our initial data show that the ADHEAR could be an excellent treatment option for persons with conductive hearing loss who – for medical reasons – cannot or do not want to undergo surgery for a BC implant.
A pressure of about 2 N is required to ensure an efficient transmission of bone-conducted sound (2,3). Verhagen et al. (11) reported that five out of eight users of the Baha Softband developed pressure points. In contrast, the ADHEAR requires no pressure for efficient transmission, which represents a clear benefit of this system compared to a softband. In our study we showed that the ADHEAR restores hearing to the same extent as a bone conduction device on a softband. The mean sound field PTA4, the WRS, and the SRT in quiet and noise were comparable with the ADHEAR and the softband device confirming initial reports (16,17). The results with the softband, with 27 dB HL aided soundfield threshold and 83% WRS at 65 dB SPL were slightly better than those previously published in this age group in subjects that suffered from atresia or chronic otitis media (18).
In conclusion, this study is the first to show that the audiological performance with the ADHEAR was comparable to that with a passive BC implant. Therefore, this system could be a valuable treatment option for patients who are not suitable for implant surgery due to age, difficult anatomies, or other reasons. We also demonstrated that the ADHEAR provides the same audiological benefits as a BCD on a softband without requiring pressure on the skin. Based on this, the ADHEAR represents a suitable alternative to hearing aids for patients with glue ears. However, further studies are needed to confirm these results in a larger cohort of patients and to investigate long-term patient satisfaction with the ADHEAR.
Supplementary Material
Acknowledgments
The authors appreciate the time and commitment given by the participants during this study.
Footnotes
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
ADHEAR test devices were provided by MED-EL. No other benefits were received. The authors declare they had no conflicts of interest in conducting this study.
REFERENCES
- 1.Zarowski AJ, Verstraeten N, Somers T, Riff D, Offeciers EF. Headbands, testbands and softbands in preoperative testing and application of bone-anchored devices in adults and children. Adv Otorhinolaryngol 2011; 71:124–131. [DOI] [PubMed] [Google Scholar]
- 2.von Békésy G, Wever EG. Experiments in Hearing. New York: McGraw-Hill; 1960. [Google Scholar]
- 3.Reinfeldt S, Håkansson B, Taghavi H, Eeg-Olofsson M. New developments in bone-conduction hearing implants: a review. Med Devices Auckl NZ 2015; 8:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westerkull P. BAHA®: the direct bone conductor. Trends Amplif 2002; 6:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wazen JJ, Wycherly B, Daugherty J. Complications of bone-anchored hearing devices. Adv Otorhinolaryngol 2011; 71:63–72. [DOI] [PubMed] [Google Scholar]
- 6.Carr SD, Moraleda J, Procter V, Wright K, Ray J. Initial UK experience with a novel magnetic transcutaneous bone conduction device. Otol Neurotol 2015; 36:1399–1402. [DOI] [PubMed] [Google Scholar]
- 7.Dimitriadis PA, Farr MR, Allam A, Ray J. Three year experience with the cochlear BAHA attract implant: a systematic review of the literature. BMC Ear Nose Throat Disord 2016; 16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss BG, Bertlich M, Scheele R, et al. Systematic radiographic evaluation of three potential implantation sites for a semi-implantable bone conduction device in 52 patients after previous mastoid surgery. Eur Arch Otorhinolaryngol 2017; 274:3001–3009. [DOI] [PubMed] [Google Scholar]
- 9.Rahne T, Schilde S, Seiwerth I, Radetzki F, Stoevesandt D, Plontke SK. Mastoid dimensions in children and young adults: consequences for the geometry of transcutaneous bone-conduction implants. Otol Neurotol 2016; 37:57–61. [DOI] [PubMed] [Google Scholar]
- 10.Hol MK, Cremers CW, Coppens-Schellekens W, Snik AF. The BAHA Softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol 2005; 69:973–980. [DOI] [PubMed] [Google Scholar]
- 11.Verhagen CV, Hol MK, Coppens-Schellekens W, Snik AF, Cremers CW. The Baha Softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol 2008; 72:1455–1459. [DOI] [PubMed] [Google Scholar]
- 12.Ozimek E, Warzybok A, Kutzner D. Polish sentence matrix test for speech intelligibility measurement in noise. Int J Audiol 2010; 49:444–454. [DOI] [PubMed] [Google Scholar]
- 13.ANSI/ASA S3.21-2004 (R2009). AMERICAN NATIONAL STANDARD Methods for Manual Pure-Tone Threshold Audiometry. New York: Acoustical Society of America [ASA]. [Google Scholar]
- 14.Winkler A, Holube I. Test-retest reliability of the Freiburg monosyllabic speech test. HNO 2016; 64:564–571. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen ER, Juhl PM. Simulated critical differences for speech reception thresholds. J Speech Lang Hear Res 2017; 60:238–250. [DOI] [PubMed] [Google Scholar]
- 16.Mc Dermott A, Williams JS, Gill J, Child J. The ADHEAR HEARING System. A paediatric experience. Presented at the Osseo 2017 - 6th International Congress on Bone Conduction Hearing and Related Technologies, The Netherlands, Nijmegen, May 17–20, 2017. [Google Scholar]
- 17.Dahm V, Baumgartner WD, Liepins R, Arnoldner C, Riss D. First results with a new, pressure-free, adhesive bone conduction hearing aid. Otol Neurotol 2018; 39:748–754. [DOI] [PubMed] [Google Scholar]
- 18.Kara A, Iseri M, Durgut M, Topdag M, Ozturk M. Comparing audiological test results obtained from a sound processor attached to a Softband with direct and magnetic passive bone conduction hearing implant systems. Eur Arch Otorhinolaryngol 2016; 273:4193–4198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


