Abstract
Background:
Many individuals failing first-line antiretroviral therapy (ART) in sub-Saharan Africa never initiate second-line ART or do so after significant delay. For people on ART with a viral load more than 1000 copies/ml, the WHO recommends a second viral load measurement 3 months after the first viral load and enhanced adherence support. Switch to a second-line regimen is contingent upon a persistently elevated viral load more than 1000 copies/ml. Delayed second-line switch places patients at increased risk for opportunistic infections and mortality.
Methods:
To assess the potential benefits of a simplified second-line ART switch strategy, we use an individual-based model of HIV transmission, progression and the effect of ART which incorporates consideration of adherence and drug resistance, to compare predicted outcomes of two policies, defining first-line regimen failure for patients on efavirenz-based ART as either two consecutive viral load values more than 1000 copies/ml, with the second after an enhanced adherence intervention (implemented as per current WHO guidelines) or a single viral load value more than 1000 copies/ml. We simulated a range of setting-scenarios reflecting the breadth of the sub-Saharan African HIV epidemic, taking into account potential delays in defining failure and switch to second-line ART.
Findings:
The use of a single viral load more than 1000 copies/ml to define ART failure would lead to a higher proportion of persons with nonnucleoside reverse-transcriptase inhibitor resistance switched to second-line ART [65 vs. 48%; difference 17% (90% range 14–20%)], resulting in a median 18% reduction in the rate of AIDS-related death over setting scenarios (90% range 6–30%; from a median of 3.1 to 2.5 per 100 person-years) over 3 years. The simplified strategy also is predicted to reduce the rate of AIDS conditions by a median of 31% (90% range 8–49%) among people on first-line ART with a viral load more than 1000 copies/ml in the past 6 months. For a country of 10 million adults (and a median of 880 000 people with HIV), we estimate that this approach would lead to a median of 1322 (90% range 67–3513) AIDS deaths averted per year over 3 years. For South Africa this would represent around 10 215 deaths averted annually.
Interpretation:
As a step towards reducing unnecessary mortality associated with delayed second-line ART switch, defining failure of first-line efavirenz-based regimens as a single viral load more than 1000 copies/ml should be considered.
Keywords: AIDS, antiretroviral, antiretroviral therapy, efavirenz, HIV, modelling, second line, strategy, treatment failure, viral load strategy, virological failure
Introduction
In 2017, almost 22 million of 36.9 million people living with HIV globally have successfully initiated antiretroviral therapy (ART) [1]. For the individual and public health benefits of ART to be realized, antiretroviral programmes, previously focussed on ART initiation, must retain patients in care and achieve high rates of virological suppression. This requires optimizing management of those failing ART.
Viral load monitoring has been recommended by the WHO for the identification of treatment failure, to prompt enhanced adherence support and to allow for early identification of patients requiring a switch to second-line ART [2]. For patients with a viral load more than 1000 copies/ml, the WHO recommends a confirmatory viral load measurement 3 months after the first viral load and enhanced adherence support, with switch to second-line ART contingent upon a persistently elevated viral load. The main justification for this strategy is the preservation of costlier second-line ART for patients who may, after enhanced adherence support, resuppress virus without switch.
There are important limitations to this approach. First, existing research suggests that between 50 and 90% of patients experiencing virologic failure on first-line ART with a single viral load more than 1000 copies/ml have nonnucleoside reverse-transcriptase inhibitor (NNRTI) resistance [3,4,5,6,7,8,9,10,11,12]. Second, despite the increased availability of viral load monitoring, many programmes fail to switch failing patients promptly – with delays frequently exceeding 1 year – leading to avoidable morbidity and mortality, as well as elevating the risk for the development of additional drug resistance and transmission of drug-resistant virus [13,14,15,16,17]. Third, the evidence suggesting that enhanced adherence counselling leads to resuppression is limited [18,19]. Resuppression after a single viral load more than 1000 copies/ml has been reported to occur in 20–50% of individuals, but with suppression being particularly unlikely if drug resistance is present [20,21]. For those that do resuppress virus, the duration of resuppression is often limited, particularly if preceded by months of high-level viraemia, as is commonly the case [18,22,23,24,25]. Finally, although the CD4+ cell count at ART initiation has slowly increased in African contexts, patients who fail first-line ART continue to have advanced immunodeficiency at failure making rapid viral resuppression urgent [26]. For example, the median CD4+ cell count among a cohort of patients failing first-line ART in Johannesburg (recruited between 2008 and 2012) remained below 150 cells/μl [27].
The importance of this issue is likely to increase further in coming years. Data from several low–middle income countries suggest that the ART-experienced patients account for 10–30% of patients initiating or reinitiating first-line NNRTI-containing ART, a proportion that is likely to increase substantially following the rapid expansion in HIV treatment coverage [28,29]. Prior exposure to ART – regardless of viral load – in people restarting ART is associated with increased risk of virological failure [30]. Because of the higher risk of suboptimal treatment outcomes in this group, WHO's recent guidelines on HIV drug resistance indicated that consideration should be given to initiate non-NNRTI-based ART in patients restarting antiretrovirals [31]. In practice to date, however, this is rarely done.
From a public health perspective, it was hoped that replacing CD4+ cell count with viral load for the assessment of treatment failure would increase the proportion of patients failing ART promptly switched to second-line ART. However, these expected gains have generally not yet been achieved and the proportion of patients on second-line ART remains very low at an estimated 1–5% [32,33,34]. After 2 years on ART, programmes report failure rates of around 14%, highlighting the magnitude of unmet need for second line [35].
The viral load algorithm is one of many factors that may act to hamper appropriate use of second-line ART; others include access to medicines, incomplete coverage of viral load, inadequate communication of result and failure to appropriately action results received, as well as a lack of appreciation as to the high prevalence of failure among individuals with elevated viral loads, who may then be more likely to attribute viraemia to adherence problems. However, given the algorithm's importance and modifiability at the policy level, it warrants careful consideration from those seeking to address the large unmet need for second-line therapy.
Against this background, it is necessary to explore potentially accelerating switch to second-line ART with simplification of the switch algorithm. We use an established model of HIV transmission, progression and the effect of ART which incorporates drug resistance to estimate the impact of simplifying the definition of first-line ART failure from two elevated viral loads to a single viral load more than 1000 copies/ml.
Methods
We used the HIV Synthesis Model, an individual-based simulation model of HIV transmission, progression and the effect of ART, considering specific drugs and resistance mutations, and which has previously been used to address policy questions in relation to HIV and ART programmes [36,37,38]. In brief, the model generates a population of adults who are each tracked over their lives in 3-month time periods for HIV testing, risk of condomless sex and risk of HIV acquisition. Those who acquire HIV are tracked with respect to viral load, CD4+ cell count, occurrence of WHO stage 3 and 4 conditions, clinic attendance and drop-out, current use of specific antiretroviral agents, presence of specific resistance mutations, adherence to ART and toxicities associated with ART.
We initially based the demographics and HIV/ART features of the population around those encountered in Malawi, but ran the model 500 times, each time sampling from a set of parameters to reflect the diversity of the epidemic across populations in sub-Saharan Africa, as illustrated in Table 1. Each of the model simulation runs that were executed reflects a different potential programmatic situation which we call a setting scenario.
Table 1.
Characteristics of setting scenarios in 2018, before consideration of a change in first-line failure definition.
| Characteristic | Median (90% range) across setting scenarios | Examples of observed data from settings in sub-Saharan Africa |
| HIV prevalence (age 15–49) | 8.8% (5.3–17.1%) | Lesotho (2014) 25%, Tanzania (2011) 5%, Uganda (2011) 9%, Zimbabwe (2015) 14% (2016) 14% [39,40,41,42] |
| HIV incidence (per 100 person-years; age 15–49) | 0.69 (0.31–1.40) | Malawi MPHIA 2016 (0.37%) [43], Zambia ZAMPHIA 2016 (0.66%) [44], Zimbabwe ZIMPHIA 2016 (0.45%) [45], (2.4%) [46], South Africa (0.39%) [47] |
| Among HIV-infected (% diagnosed) | 84% (73–92%) | Malawi MPHIA (73%) [43], Zambia ZAMPHIA (67%) [44], Zimbabwe ZIMPHIA (74%) [45], South Africa (75%) [47], Malawi [48] (77%) (see also [49], which suggests undisclosed diagnosed HIV) |
| Among those diagnosed with HIV (% on ART) | 87% (67–95%) | [50] |
| Among those on ART, % with an NNRTI resistance mutation (including minority variants) | 18% (12–31%) | No direct measures available to our knowledge |
| Among all people on ART, % with VL < 1000 copies/ml | 88% (78–93%) | South Africa (60–88% over districts), ZAMPHIA (89%) [44], MPHIA (91%) [43], ZIMPHIA (87%) [45], (91%) [48], (90%) [47] |
| Among those initiating ART, % with NNRTI resistance | 9% (1–28%) | Angola (14%), Botswana (8%), South Africa (14%) [12,13,14] Zimbabwe (11%) |
| Proportion of ART initiators with CD4+ cell count <350 at initiation of ART | 40% (30–52%) | |
| Among ART-experienced persons, percentage who have started second line | 4.8% (1.4–12.2%) | Malawi 1.5% (quarterly reports), 2.4% [34] |
| Overall rate of switch to second-line ART (per 100 person-years) | 1.9 (0.7–5.0) | 2.7 [34] |
| Among those receiving second-line ART, proportion with VL < 1000 copies/ml | 77% (65–83%) | 48%, 72% [51] South Africa [52] 77% [53], 86% [54], 85% [55] |
| Among those receiving second-line ART, proportion with a PI mutation | 3% (1–8%) | 6.5% [55], 7% [56] |
| Among those on ART, proportion with CD4+ cell count >500 cells/μl | 49% (38–55%) | |
| Death rate in persons on ART (per 100 person-years) | 2.2 (1.6–3.5) | |
| Death rate in persons on first-line ART (per 100 person-years) | 2.2 (1.5–3.5) | |
| Death rate in persons on second-line ART (per 100 person-years) | 3.3 (1.1–7.0) | |
| Death rate in persons who have stopped/interrupted ART (per 100 person-years) | 14.0 (6.2–23.1) | |
| AIDS death rate in persons with previous or current VL > 1000 while on ART (per 100 person-years) | 5.1 (2.2–9.6) | |
| Among persons on first-line ART with initial measured VL > 1000 copies/ml in past year, % with NNRTI resistance mutation | 76% (55–89%) | 84% (74–100%) [57]; 70% [58] |
| Of people defined as failing efavirenz-based first-line ART, % with NNRTI drug resistance | 98% (88–100%) | |
| Of people on first-line ART with initial VL > 1000 6 months ago, proportion with VL < 1000 | 30% (8–63%) | 22–50% [59] |
| Of people on ART who have first experienced VL > 1000 copies/ml 2 years ago, proportion on ART (first or second line) with VL < 1000 copies/ml | 23% (4–57%) | |
| Of people on first-line ART with initial VL > 1000 in past year rate of AIDS (per 100 person-years) | 6.1 (1.5–12.9) | |
| Of people on first-line ART with current VL > 1000, % with CD4+ cell count <200 cells/μl | 36% (27–45%) | |
| Of people on first-line ART with current VL > 1000, % classified as having fulfilled first-line failure criteria | 21% (6–43%) | |
| Of people who have been identified as having failed first-line ART in the past year, % who have been switched to second line | 25% (4–61%) | [33,34] |
| Of people switched to second line, proportion with drug resistance to at least 1 first-line drug | 100% (92–100%) | |
| Proportion of persons with drug resistance to efavirenz who have been switched to second-line ART | 21% (6–46%) | |
| Of persons on first-line ART with previous VL > 1000 (at least 6 months after start of ART), percentage with VL < 1000 copies/ml | 36% (22–54%) | |
| Number of AIDS deaths per year (in context of country of 10 million adults with median HIV prevalence 10%) | 21 500 (9000–44 000) | |
| Number of persons on second-line ART (assuming country of 10 million adults with a median HIV prevalence of 10%) | 31 000 (8000–105 000) |
ART, antiretroviral therapy; PI, protease inhibitor; VL, viral load.
For each setting scenario, we assume a baseline date of October 2018 (2018.75). We compare predicted outcomes of two strategies, defining first-line regimen failure for people on efavirenz (EFV)-based ART by either two consecutive values more than 1000 copies/ml, at least 3 months apart, with the second after an enhanced adherence intervention (the current WHO recommended strategy) or a single value more than 1000 copies/ml (with the enhanced adherence intervention initiated at the time of the first viral load >1000 copies/ml under both scenarios). We refer to the latter as the simplified strategy.
We assume that from 2016 viral load monitoring, with differentiation of care based on whether a person is virologically suppressed, was introduced (i.e. reduced clinic visits for people with viral load <1000 copies/ml) [36]. We consider that while viral load testing is scheduled at 6 and 12 months immediately after ART initiation, and then annually, it is not always the case that a viral load measure is successfully carried out (the probability ranges from 0.2 to 0.85 before the baseline date of 1 October 2018). We assume that when a scheduled measure is not done it will be attempted 3 months later, with the same probability of success. Before baseline, the rate of switch to a second line regimen is determined for each setting scenario by randomly choosing a value from 0.05, 0.20 or 0.50 with equal probability. Within the model, we consider rates of interruption of ART with an associated risk of being lost to follow-up and subsequent probability of returning to care, a probability that is highest if a person becomes ill with a WHO stage 4 condition. The assumptions in the model around patterns of adherence and the effect of the enhanced adherence intervention lead to a median of 36% being resuppressed after the enhanced adherence intervention in the absence of a switch (Table 1).
To be able to identify effects of differences in definition of first-line failure we assumed (in our main analyses) that from the baseline date the rate of switch after detection of failure first-line failure (whatever the failure definition) is 0.85 per 3 months and the probability of viral load measures being performed is 0.85 for all setting scenarios. We explored lower probabilities in sensitivity analyses.
Results
Table 1 shows the range of characteristics of the setting scenarios in 2018, just before the consideration of the change in strategy for defining first-line EFV-based ART failure.
Over a 3-year follow-up period (Table 2), the strategy of using a single viral load more than 1000 to define failure of EFV-based first-line ART (simplified strategy), instead of two consecutive values (current strategy), is predicted to result in a 18% (90% range 6–30%) reduction of the AIDS death rate among people with previous or current viral load more than 1000 while on ART (Fig. 1). The simplified strategy is also predicted to reduce the rate of AIDS conditions by a median of 31% (90% range 8–49%) among people on first-line ART with a viral load more than 1000 copies/ml in the past 6 months. For a country of 10 million adults with 880 000 people with HIV, this is estimated to lead to a median of 1322 (90% range 67–3513) AIDS deaths averted per year over 3 years. For South Africa this would represent 10 215 deaths averted annually (refer to Supplementary Table 1). For a country of 10 million adults under the current strategy, we estimate 301 (90% range 33–1338) switches per year among those without resistance being present under the base case scenario, compared with 7285 (90% range 3538–14 653) such switches using the single viral load strategy (refer to Supplementary Table 2).
Table 2.
Comparison of effects of strategy of defining first-line failure of efavirenz-based regimens by a single viral load more than 1000 with strategy of two consecutive viral load more than 1000 copies/mla.
| Strategy for defining first-line failure of efavirenz-based regimen | |||
| Two consecutive VL > 1000 copies/ml (median 90% range over setting scenarios) | Single VL > 1000 copies/ml (median 90% range over setting scenarios) | Difference [(* or percentage reduction) between policies (mean 95% CI; median 90% range) over setting scenarios] | |
| Among ART-experienced persons, percentage who have started second line | 10.4% (5.6–19.1%) | 15.2% (9.5–26.5%) | +5.1% (+5.0, +5.2%) +4.8% (+3.4–+7.8%) |
| Of people on ART who have first experienced VL > 1000 copies/ml 2 years ago, proportion on ART (first or second line) with VL < 1000 copies/ml | 51% (33–68%) | 59% (36–80%) | +8% (+7–+8%) +8% (−3–+19%) |
| Of people on first-line ART with initial VL > 1000 in past year rate of AIDS (per 100 person-years) | 4.7 (2.4–8.4) | 3.2 (1.6–5.8) | 30% (28, 32%)* 31% (+8, +49%)* |
| AIDS death rate in people with previous or current VL > 1000 while on ARTb,c | 3.1 (1.7–6.8) | 2.5 (1.3–6.0) | 18% (18, 18%)* 18% (6, 30%)* |
| % Of people with drug resistance to efavirenz who have been switched to second-line ART | 48% (33–59%) | 65% (51–74%) | 17% (17, 17%) 17% (14, 20%) |
| Among those on ART (first or second line), % with VL < 1000 copies/ml | 92% (85–95%) | 94% (89–96%) | +2.9% (+2.8, +3.0%) +2.6% (+1.5, +4.8%) |
| Of people switched to second line, proportion with drug resistance to at least 1 first-line drug | 99% (95–100%) | 82% (68–91%) | –17% (–18, –16%) –17% (−28, –8%) |
| Of people defined as failing efavirenz-based first-line ART, % with NNRTI drug resistance | 97% (75–100%) | 72% (50–86%) | –23% (–24, –22%) –23% (–36, –12%) |
| Of persons on first-line ART with previous VL > 1000 (at least 6 months after start of ART), percentage with VL < 1000 copies/ml | 55% (41–65%) | 64% (50–72%) | 9% (9, 9%) 9% (6, 12%) |
ART, antiretroviral therapy; CI, confidence interval; NNRTI, nonnucleoside reverse-transcriptase inhibitor; VL, viral load.
aMean over 2018.75–2021.75 for each setting scenario, then summarized as mean and median over setting scenarios.
bMore than 6 months after (re)starting, and excluding people already started second line before baseline in 2018.75.
cAs shown in Fig. 1.
Fig. 1.
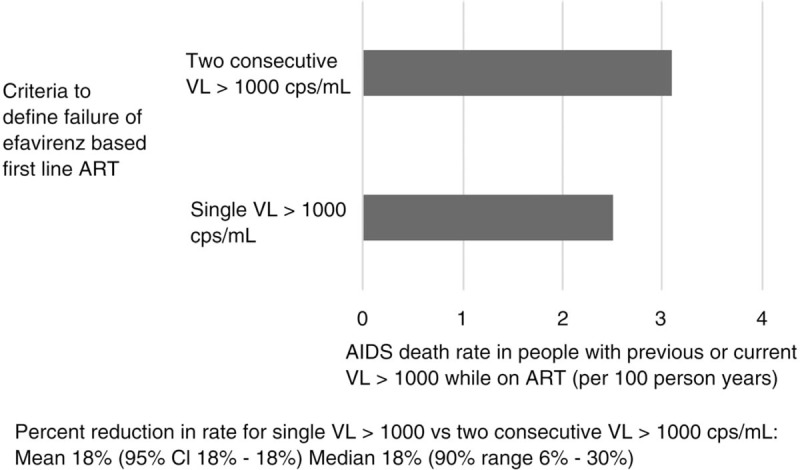
AIDS death rate (over 3 years; 2018.75–2021.75) in people with previous or current viral load more than 1000 while on antiretroviral therapy according to criteria to define failure of efavirenz-based first-line antiretroviral therapy (excluding people who had already switched to second-line antiretroviral therapy before baseline in 2018).
The effect of the simplified strategy was slightly less (from 18 to 17%) when we assumed that during the 3-year follow-up a substantially lower probability of viral load measures performed as planned (0.20 per 3 months instead of 0.85), and from 18 to 9% if the probability of switching to a second-line regimen after the failure criteria are met was substantially lower (0.20 per 3 months instead of 0.85) (Table 3). Variations in the extent to which people resuppress viral load (increasing to >40%) after the adherence intervention without a change in regimen has only a small impact on these results, as does the level of HIV incidence (Table 3).
Table 3.
AIDS death rate (2018.75–2021.75) in people with previous or current viral load more than 1000 copies/ml while on antiretroviral therapy according to strategy for defining first-line failure of efavirenz-based regimen: one-way sensitivity analysis.
| Strategy for defining first-line failure of efavirenz-based regimen | |||
| Two consecutive VL > 1000 copies/ml | Single VL > 1000 copies/ml | Percentage reduction between policies [mean (95% CI); median (90% range)] over setting scenarios | |
| Base casea | 3.1 (1.7–6.8) | 2.5 (1.3–6.0) | 18% (18, 18%)b 18% (6, 30%)b |
| Restricting to setting scenarios where: Of people on first-line ART with initial VL > 1000 6 months ago, % with VL < 1000 is >40% in 2018 | 3.2 (1.7–7.4) | 2.7 (1.3–6.3) | 17% (15, 19%) 17% (5, 29%) |
| Restricting to setting scenarios where HIV incidence in 2017 <0.5/100 person-years | 3.2 (1.7–6.3) | 2.5 (1.3–5.2) | 19% (17, 21%) 19% (5, 32%) |
| Probability of each scheduled viral load measure being done = 0.20 (0.85 in base case) | 3.9 (1.9–7.5) | 3.1 (1.5–6.7) | 17% (15, 19%) 16% (3, 31%) |
| Probability of switch to second-line (per 3 months) after first-line failure criteria fulfilled = 0.20 (0.85 in base case) | 4.0 (1.9–7.6) | 3.7 (1.5–7.0) | 9% (8, 10%) 9% (–1, 18%) |
ART, antiretroviral therapy; CI, confidence interval; VL, viral load.
a25% of those identified as having failed first-line ART in the past year switched to second line. Overall rate of switch to second-line ART 1.9/100 person-years (0.7–5.0).
b6 months after (re)starting, and excluding people who already started second line before baseline in 2018.75.
Under the simplified strategy, a higher proportion of people on first-line ART are predicted to be classified as having fulfilled first-line failure criteria [current strategy: 8% (90% range: 5–11%) vs. simplified strategy: 19% (90% range 14–26%)] and a higher proportion of people with drug resistance to EFV will have been switched to second-line ART [current strategy: 48% (90% range 33–59%) vs. simplified strategy 65% (90% range 51–74%)] (Table 2). Among those defined as failing EFV-based first-line ART, 99% (90% range 95–100%) vs. 82% (90% range 68–91%) are predicted to have drug resistance to at least one first-line drug for the current vs. the simplified viral load strategy respectively (Table 2).
Compared with the current approach, the simplified strategy is predicted to result in a higher proportion of individuals on ART being virologically suppressed, 92 vs. 94% (Table 2), a 2.9% increase in the ‘3rd 90’ of the 90–90–90 goals.
Discussion
We evaluated the predicted impact of dropping the requirement for a second viral load value of greater than 1000 copies/ml prior to switching to a second-line regimen by simulating the HIV epidemic in a range of setting-scenarios to reflect the diversity of the sub-Saharan epidemic. We found that such a change in strategy would be predicted to significantly reduce the rates of AIDS deaths and AIDS conditions among people with an elevated viral load on first-line ART. For a country of 10 million adults in the context of the range of HIV prevalences in our setting scenarios (a median 880 000 people living with HIV), the number of AIDS deaths averted per year in the 3 years from 2018 is a median of 1322 (90% uncertainty range 67–3513).
We studied here the impact of the criteria for defining treatment failure in isolation. We have not assumed that the use of a single viral load measure definition is associated with any concomitant benefits in terms of propensity for viral load measures to be done as scheduled, or in the probability of a switch being made once the criteria are met. However, to understand the potential impact of this change, we have considered in our main analysis a relatively high rate of switch (0.85 per 3 months) in people with virological failure (regardless of the switch strategy). This means assuming that there are no other major constraints to prevent people from switching. In sensitivity analyses, we showed how the effect of changing the first-line EFV failure criteria was lower if we assume lower probabilities for viral load measurement being done and/or for switching once the failure criteria are met. This indicates that, to be most beneficial, a change in the failure criteria should be accompanied by an increase in the rate of switching once failure criteria are met. The likelihood of an increase in switching is not implausible given that the single switch strategy reduces the number of steps necessary to switch and the observed delays at every step of the current strategy.
Using this model we have previously found that use of a single value to define first-line failure, without a confirmatory value, is likely to be more effective (avert more disability adjusted life years) than use of a confirmatory test, but not to be cost effective in the context of low-income settings in sub-Saharan Africa due to the high cost of protease inhibitors [36]. Although the price of lopinavir/ritonavir and atazanavir/ritonavir have dropped from $243.00 and $243.00 in 2014 to $202.80 and $159.00 in 2017, respectively (procurement costs only) [60,61,62], this may not be sufficient to make the simplified switch strategy cost effective. However, as second-line ART becomes more affordable, the cost-effectiveness analysis may also eventually favour the single viral load strategy. Given that the strategy is more effective than the current strategy, were the costs of second line to approach those of EFV-based first line [as in the case of dolutegravir (DTG), for example] the single viral load switch strategy may be more favourable than the existing strategy also from an economic perspective. For implementers considering the case for a simplified switch to newer regimens it should be noted that the use of DTG, with an optimized NRTI backbone, is now recommended as a second-line option by WHO [63]. For patients failing existing EFV-based first line in settings in which DTG is being rolled out, these results provide support to programmes considering switching to DTG-based ART on the basis of one elevated viral load.
In keeping with the available literature [18,20,22,23,24,25], we capture in our model that very high levels (76%, refer to Table 1) of people with a single viral load value more than 1000 copies/ml have drug resistance to EFV and thus likely do require a change in regimen; underlining the limited scope for second-line preservation among failing patients.
In considering the role of the current strategy for switching to second-line ART it should be noted that there are many reasons why a patient may not be switched, including suboptimal uptake of viral load testing, slow turnaround of viral load test results and failure to make use of viral load test results, once obtained, in patient management decisions. Indeed only a small proportion of individuals with a single elevated viral load receive the second viral load, suggesting that patients with first-line regimen failure continue to be poorly served by existing algorithms [64].
Another problem with existing practices around first-line regimen failure are delays in the delivery of the enhanced adherence intervention. In our simplified scenario, the enhanced adherence intervention is still provided, but this occurs at the time of switch, removing the possibility that switch may be delayed by, or contingent upon completion of this intervention. The current use of the enhanced adherence intervention may be exacerbated by a conservative approach favouring the conservation of first-line ART, with some clinics having concerns as to the cost or availability of second-line ART [65], or wider concerns as to the options available for subsequent third line [66]. However, those with high viral loads are often more vulnerable patients including children, adolescents and those with more advanced disease [18], placing these individuals at increased risk of death. Hence, while the barriers to second-line switch are multiple, the requirement for a confirmatory viral load introduces an additional delay which in most routine settings is much longer than the intended 3 months, often with associated patient harm.
In terms of achieving sufficient adherence, while there is evidence to support counselling for adherence at the time of ART initiation [67], by the time a person previously suppressed on ART presents with a high viral load it is much less certain whether the intervention will be useful and whether any resuppression achieved will be durable [25,68]. For individuals who admit to having poor adherence it may still be reasonable to provide the opportunity to resuppress their viral load on first-line ART. In cases however where an individual states that they have adhered well, immediate switch is likely preferable, at which time an enhanced adherence intervention may still be commenced.
There are potential disadvantages of a simplified approach to second-line switch. Switching after a single viral load more than 1000 copies/ml will lead to an increase in the number of individuals without first-line ART resistance being switched unnecessarily. We estimate that this will be the case for around 18% of those switched (Table 2). For South Africa, we estimate that there would be 2326 individuals without antiretroviral resistance switched per year under the base case scenario, compared with 56 293 individuals without resistance switched were the simplified strategy employed (refer to Supplementary Table 2). The individual and programmatic disadvantages of unnecessary switch warrant consideration. Moving from once daily fixed-dose combination (FDC) first-line ART to protease inhibitor based second-line ART can entail an increased pill burden and additional toxicity. Ritonavir-boosted lopinavir containing regimens are associated with gastrointestinal toxicity and need to be taken twice daily, whereas atazanavir-based regimens exist in FDC and have a better toxicity profile. From a programmatic perspective, current second-line ART is more costly and used on a smaller scale than first-line ART, making it more susceptible to stockouts resulting from either forecasting or supply issues [69,70]. We have not assumed first and second-line ART to be affected differently by stockouts. Programmes may in the future replace protease inhibitor-based regimens with (DTG)-based regimens, including possibly FDC, but at this time this remains uncertain.
A limitation of this study is that we do not model cost effectiveness, largely as a result of current uncertainty over what will be the future first and second-line regimens in routine use. Affordability can be a leading consideration for national programmes, and this should be determined at the country level, noting that protease inhibitor-based second-line regimens costs range from approximately $160 to $200 per person-year [62,71]. To ensure that this health gain can be realized within resource constrained programmes it is imperative that the cost of existing second-line regimens decline further and that programmes identify alternative, less costly, more tolerable, second-line options. DTG is the backbone of one such regimen and WHO now recommends DTG-based ART as a second-line option [63]. It should also be noted that costs have declined since the time the current strategy was developed, at which time second-line ART cost up to 17 times more than first-line regimens. Protease inhibitor-based ART is now available at less than three times the cost first-line ART and DTG-based second line would be available from the same price (depending upon which NRTI were used) as current EFV-based first-line regimen [62,72]. These cost improvements make economic arguments against the wider use of second-line ART less relevant. Our results apply only to EFV containing first line and not for responding to DTG-based ART failure. Regimen-specific failure algorithms may be needed should the approach modelled here be implemented in a context where both drugs were in use.
WHO guidelines on the public health response to pretreatment HIV drug resistance published in 2017 recommend that when levels of NNRTI resistance among treatment initiators exceed 10% a change from a NNRTI-based first-line regimen to a non-NNRTI-based first-line regimen should be urgently considered [38]. Among people with a single viral load measurement documented viral load more than 1000 copies/ml while on treatment, the proportion of people with resistance to EFV/NVP ranges from 50 to 90% (WHO HIVDR report 2017), thus far exceeding the 10% threshold established for pretreatment drug resistance. Therefore, a rapid change in regimen after a single elevated viral load may be justifiable based on these guidelines.
To summarize, in our model 76% (90% range 55–89%) of individuals on ART with one elevated viral load have NNRTI resistance and do not go on to resuppress. Currently the majority of these patients are never initiated on second-line ART. We identify the current treatment failure algorithm as a contributor to this situation. The unmet need for effective therapy among failing individuals is highly consequential and we estimate that in a country the size of South Africa, the application of a single viral load switch strategy – assumed to address some but not all barriers to switch – could prevent an estimated 10 215 deaths per year (refer to Supplementary Table 2). In addition, such a change would improve progress towards the 3rd 90 of the UNAIDS goals by approximately 3%.
As the cost of second-line regimens decline, a change of strategy to define failure of EFV-based first-line ART after a single viral load value more than 1000 copies/ml should be considered, allowing faster switch to second line, boosting effort to achieve the 3rd 90 and reducing AIDS-related deaths.
Acknowledgements
Legion@UCL for computing the model results.
No specific funding was obtained for the analysis or writing of this article.
Given that this work involves modelling only, this was not required.
Authors’ contributions
A.S., G.V.C. and A.P. developed the research question. A.P. led the analysis. All authors contributed to the development of the article and its writing.
Conflicts of interest
The authors have declared that no competing interests exist.
Supplementary Material
References
- 1.UNAIDS. Fact sheet July 2018 – latest global and regional statistics on the status of the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2018. [Google Scholar]
- 2.World Health Organization. Department of HIV/AIDS – antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 3.Steegen K, Bronze M, Papathanasopoulos MA, van Zyl G, Goedhals D, Variava E, et al. HIV-1 antiretroviral drug resistance patterns in patients failing NNRTI-based treatment: results from a national survey in South Africa. J Antimicrob Chemother 2017; 72:210–219. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RK, Hill A, Sawyer AW, Cozzi-Lepri A, von Wyl V, Yerly S, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis 2009; 9:409–417. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization; United States Centers for Disease Control and Prevention & The Global Fund to Fight AIDS Tuberculosis and Malaria. HIV drug resistance report 2017. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 6.World Health Organization. The HIV drug resistance report - 2012. Geneva, Switzerland: World Health Organization. http://www.who.int/iris/handle/10665/75183. [Google Scholar]
- 7.Svärd J, Mugusi S, Mloka D, Neogi U, Meini G, Mugusi F, et al. Drug resistance testing through remote genotyping and predicted treatment options in human immunodeficiency virus type 1 infected Tanzanian subjects failing first or second line antiretroviral therapy. PLoS One 2017; 12:e0178942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigaloff KC, Kayiwa J, Musiime V, Calis JC, Kaudha E, Mukuye A, et al. Short communication: high rates of thymidine analogue mutations and dual-class resistance among HIV-infected Ugandan children failing first-line antiretroviral therapy. AIDS Res Hum Retroviruses 2013; 29:925–930. [DOI] [PubMed] [Google Scholar]
- 9.Tsai HC, Chen IT, Wu KS, Tseng YT, Sy CL, Chen JK, et al. High rate of HIV-1 drug resistance in treatment failure patients in Taiwan, 2009–2014. Infect Drug Resist 2017; 10:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etta EM, Mavhandu L, Manhaeve C, McGonigle K, Jackson P, Rekosh D, et al. High level of HIV-1 drug resistance mutations in patients with unsuppressed viral loads in rural northern South Africa. AIDS Res Ther 2017; 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.TenoRes Study Group. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis 2016; 16:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Global action plan on HIV drug resistance 2017–2021. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 13.Rohr JK, Ive P, Berhanu R, Shearer K, Maskew M, Long L, et al. Predictors of time to switch to second line ART after first line failure in Johannesburg South Africa. Top Antivir Med 2014; 22:280. [Google Scholar]
- 14.Narainsamy D, Mahomed S. Delays in switching patients onto second-line antiretroviral treatment at a public hospital in eThekwini, KwaZulu-Natal. South Afr J HIV Med 2017; 18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen ML, Tran L, Geng EH, Reynolds SJ, Kambugu A, Wood R, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS 2014; 28:2097–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadhani HO, Bartlett JA, Thielman NM, Pence BW, Kimani SM, Maro VP, et al. The effect of switching to second-line antiretroviral therapy on the risk of opportunistic infections among patients infected with human immunodeficiency virus in Northern Tanzania. Open Forum Infect Dis 2016; 3:ofw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy RA, Court R, Maartens G, Sunpath H. Second-line antiretroviral therapy in sub-Saharan Africa: it is time to mind the gaps. AIDS Res Hum Retroviruses 2017; 33:1181–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One 2015; 10:e0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCluskey SM, Boum Y, 2nd, Musinguzi N, Haberer JE, Martin JN, Hunt PW, et al. Brief report: appraising viral load thresholds and adherence support recommendations in the World Health Organization guidelines for detection and management of virologic failure. J Acquir Immune Defic Syndr 2017; 76:183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta RK, Goodall RL, Ranopa M, Kityo C, Munderi P, Lyagoba F, et al. High rate of HIV resuppression after viral failure on first-line antiretroviral therapy in the absence of switch to second-line therapy. Clin Infect Dis 2014; 58:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamers RL, Oyomopito R, Kityo C, Phanuphak P, Siwale M, Sungkanuparph S, et al. Cohort profile: the PharmAccess African (PASER-M) and the TREAT Asia (TASER-M) monitoring studies to evaluate resistance – HIV drug resistance in sub-Saharan Africa and the Asia-Pacific. Int J Epidemiol 2012; 41:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann CJ, Charalambous S, Grant AD, Morris L, Churchyard GJ, Chaisson RE. Durable HIV RNA resuppression after virologic failure while remaining on a first-line regimen: a cohort study. Trop Med Int Heal 2014; 19:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sörstedt E, Nilsson S, Blaxhult A, Gisslén M, Flamholc L, Sönnerborg A, Yilmaz A. Viral blips during suppressive antiretroviral treatment are associated with high baseline HIV-1 RNA levels. BMC Infect Dis 2016; 16:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greub G, Cozzi Lepri A, Ledergerber B, Staszewski S, Perrin L, Miller V, et al. Low-level HIV viral rebound and blips in patients receiving potent antiretroviral therapy. In 8th Conference on Retroviruses and Opportunistic Infections Abstract 522; 2001. [Google Scholar]
- 25.Hermans L, Tempelman H, Carmona S, Nijhuis M, Richman D, Grobbee D, et al. High rates of viral resuppression of first-line ART after initial virological failure. In Conference on Retroviruses and Opportunistic Infections (CROI), 1118; 2018. [Google Scholar]
- 26.Calmy A, Ford N, Meintjes G. The persistent challenge of advanced HIV disease and AIDS in the era of antiretroviral therapy. Clin Infect Dis 2018; 66:S103–SS105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossouw T, Nieuwoudt M, Manasa J, Malherbe G, Lessells RJ, Pillay S, et al. HIV drug resistance levels in adults failing first-line antiretroviral therapy in an urban and a rural setting in South Africa. HIV Med 2017; 18:104–114. [DOI] [PubMed] [Google Scholar]
- 28.Osler M, Hilderbrand K, Goemaere E, Ford N, Smith M, Meintjes G, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis 2018; 66:S118–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ousley J, Niyibizi AA, Wanjala S, Vandenbulcke A, Kirubi B, Omwoyo W, et al. High proportions of patients with advanced HIV are antiretroviral therapy experienced: hospitalization outcomes from 2 sub-Saharan African sites. Clin Infect Dis 2018; 66:S126–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta RK, Gregson J, Parkin N, Haile-Selassie H, Tanuri A, Andrade Forero L, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO guidelines on the public health response to pretreatment HIV drug resistance. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 32.Keiser O, Tweya H, Boulle A, Braitstein P, Schecter M, Brinkhof MW, et al. ART-LINC of IeDEA Study Group. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS 2009; 23:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas AD, Keiser O, Balestre E, Brown S, Bissagnene E, Chimbetete C, et al. Monitoring and switching of first-line antiretroviral therapy in adult treatment cohorts in sub-Saharan Africa: collaborative analysis. Lancet HIV 2015; 2:e271–e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madec Y, Leroy S, Rey-Cuille MA, Huber F, Calmy A. Persistent difficulties in switching to second-line ART in sub-saharan Africa – a systematic review and meta-analysis. PLoS One 2013; 8:e82724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS 2010; 24:563–572. [DOI] [PubMed] [Google Scholar]
- 36.Phillips A, Shroufi A, Vojnov L, Cohn J, Roberts T, Ellman T, et al. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015; 528:S68–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips AN, Venter F, Havlir D, Pozniak A, Kuritzkes D, Wensing A, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV 2019; 6:e116–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips AN, Cambiano V, Nakagawa F, Revill P, Jordan MR, Hallett TB, et al. Cost-effectiveness of public-health policy options in the presence of pretreatment NNRTI drug resistance in sub-Saharan Africa: a modelling study. Lancet HIV 2018; 5:e146–e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimbabwe National Statistics Agency. Zimbabwe Demographic and Health Survey 2015. Rockville, MD: The DHS Program, ICF International; 2015. [Google Scholar]
- 40.Demographic, Health Survey Program. Tanzania HIV/AIDS and malaria indicator survey. Calverton, MD: The DHS Program, ICF International; 2011. [Google Scholar]
- 41.Demographic, Health Survey Program. Uganda AIDS indicator survey. Calverton, MD: The DHS Program, ICF International; 2011. [Google Scholar]
- 42.The Demographic, Health Survey, Program. 2014 Lesotho demographic and health survey HIV factsheet. Rockville, MD: The DHS Program, ICF International; 2014. [Google Scholar]
- 43.Ministry of Health, Malawi. Malawi population-based HIV impact assessment (MPHIA) 2015-16: first report. Lilongwe, Malawi: Ministry of Health; November 2017. [Google Scholar]
- 44.Ministry of Health, Zambia. Zambia population-based HIV impact assessment (ZAMPHIA) 2016: first report. Zambia: Ministry of Health; December 2017. [Google Scholar]
- 45.Ministry of Health and Child Care (MOHCC), Zimbabwe. Zimbabwe population-based HIV impact assessment (ZIMPHIA) 2015-16: first report. Harare, Zimbabwe: MOHCC. July 2017. [Google Scholar]
- 46.ustman JE, Hoos D, Kalton G, Nyirenda R, Moyo C, Mugurungi O. Real progress in the HIV epidemic: PHIA findings from Zimbabwe, Malawi and Zambia. In CROI Abstract Number: 114LB; 2017. [Google Scholar]
- 47.Huerga H. Mbongolwane and eshowe HIV impact in population survey. Paris, France: Epicenter; 2014. [Google Scholar]
- 48.Maman D, Chilima B, Masiku C, Ayouba A, Masson S, Szumilin E, et al. Closer to 90-90-90. The cascade of care after 10 years of ART scale-up in rural Malawi: a population study. J Int AIDS Soc 2016; 19:20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim AA, Mukui I, Young PW, Mirjahangir J, Mwanyumba S, Wamicwe J, et al. Undisclosed HIV infection and antiretroviral therapy use in the Kenya AIDS indicator survey 2012. AIDS 2016; 30:2685–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.UNAIDS. Ending AIDS: progress towards the 90-90-90 targets. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2017. [Google Scholar]
- 51.Johnston V, Fielding K, Charalambous S, Mampho M, Churchyard G, Phillips A, Grant AD. Second-line antiretroviral therapy in a workplace and community-based treatment programme in South Africa: determinants of virological outcome. PLoS One 2012; 7:e36997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston V, Fielding KL, Charalambous S, Churchyard G, Phillips A, Grant AD. Outcomes following virological failure and predictors of switching to second-line antiretroviral therapy in a South African treatment program. J Acquir Immune Defic Syndr 2012; 61:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox MP, Cutsem GV, Giddy J, Maskew M, Keiser O, Prozesky H, et al. Rates and predictors of failure of first-line antiretroviral therapy and switch to second-line ART in South Africa. J Acquir Immune Defic Syndr 2012; 60:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Second-line antiretroviral treatment successfully resuppresses drug-resistant HIV-1 after first-line failure: prospective cohort in sub-Saharan Africa. J Infect Dis 2012; 205:1739–1744. [DOI] [PubMed] [Google Scholar]
- 55.Boender TS, Kityo CM, Boerma RS, Hamers RL, Ondoa P, Wellington M, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother 2016; 71:2918–2927. [DOI] [PubMed] [Google Scholar]
- 56.Wallis CL, Mellors JW, Venter WDF, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr 2010; 53:480–484. [DOI] [PubMed] [Google Scholar]
- 57.Gregson J, Kaleebu P, Marconi VC, van Vuuren C, Ndembi N, Hamers RL, et al. Occult HIV-1 drug resistance to thymidine analogues following failure of first-line tenofovir combined with a cytosine analogue and nevirapine or efavirenz in sub Saharan Africa: a retrospective multicentre cohort study. Lancet Infect Dis 2017; 17:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamers RL, Wallis CL, Kityo C, Siwale M, Mandaliya K, Conradie F, et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011; 11:750–759. [DOI] [PubMed] [Google Scholar]
- 59.Médecins Sans Frontières. Making viral load routine (Part 1: Programmatic strategies). Geneva, Switzerland: Médecins Sans Frontières; 2017. [Google Scholar]
- 60.Médecins Sans Frontières Access Campaign. Untangling the web of antiretroviral price reductions. 18th ed.Geneva, Switzerland: Médecins Sans Frontières; 2016. [Google Scholar]
- 61.Médecins Sans Frontières Access Campaign. HIV & opportunistic infection treatment: spotlight on access gaps. Geneva, Switzerland: Médecins Sans Frontières; 2016. [Google Scholar]
- 62.The Global Fund. Pooled procurement mechanism reference pricing: ARVs Q3 2018. 2018; Geneva, Switzerland: The Global Fund, Available at: https://www.theglobalfund.org/media/5813/ppm_arvreferencepricing_table_en.pdf. [Accessed 4 September 2018]. [Google Scholar]
- 63.World Health Organization. Update on antiretroviral regimens for treating and preventing HIV infection and update on early infant diagnosis of HIV. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 64.Gibb J, Chitsulo J, Chipungu C, Chivwara M, Schooley A, Hoffman RM. Supporting quality data systems: lessons learned from early implementation of routine viral load monitoring at a large clinic in Lilongwe, Malawi. J Clin Res HIV AIDS Prev 2017; 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mgosha PC. Barriers to switching patients to second-line antiretroviral treatment among clinicians in Tanzania. Minneapolis: MN: Walden University; 2017. [Google Scholar]
- 66.Srasuebkul P, Calmy A, Zhou J, Kumarasamy N, Law M, Lim PL. Impact of drug classes and treatment availability on the rate of antiretroviral treatment change in the TREAT Asia HIV Observational Database (TAHOD). AIDS Res Ther 2007; 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS 2014; 28:S187–S204. [DOI] [PubMed] [Google Scholar]
- 68.Bärnighausen T, Chaiyachati K, Chimbindi N, Peoples A, Haberer J, Newell M-L. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Lancet Infect Dis 2011; 11:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stop Stock Outs Consortium. Stop stock outs survey 2015. Johannesburg, South Africa: Stop Stock Outs Consortium; 2015. [Google Scholar]
- 70.Gils T, Bossard C, Verdonck K, Owiti P, Casteels I, Mashako M, et al. Stockouts of HIV commodities in public health facilities in Kinshasa: barriers to end HIV. PLoS One 2018; 13:e0191294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The Global Fund. Global fund procurement database. 2018; Geneva, Switzerland: The Global Fund, Available at: https://bip2-ext.theglobalfund.org/analytics/saw.dll?Dashboard. [Accessed 14 May 2018]. [Google Scholar]
- 72.UNAIDS. Price reduction of the dolutegravir-based antiretroviral therapy regimen. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


