Abstract
Background:
Stiffness is a common reason for suboptimal clinical outcomes after primary total knee arthroplasty (pTKA). There is a lack of consensus regarding its definition, which is often conflated with its histopathologic subcategory—i.e., arthrofibrosis. There is value in refining the definition of acquired idiopathic stiffness in an effort to select for patients with arthrofibrosis. We conducted a systematic review and meta-analysis to establish a consensus definition of acquired idiopathic stiffness, determine its prevalence after pTKA, and identify potential risk factors for its development.
Methods:
MEDLINE, Embase, Cochrane Controlled Register of Trials (CENTRAL), and Scopus databases were searched from 2002 to 2017. Studies that included patients with stiffness after pTKA were screened with strict inclusion and exclusion criteria to isolate the subset of patients with acquired idiopathic stiffness unrelated to known extrinsic or surgical causes. Three authors independently assessed study eligibility and risk of bias and collected data. Outcomes of interest were then analyzed according to age, sex, and body mass index (BMI).
Results:
In the 35 included studies (48,873 pTKAs), the mean patient age was 66 years. In 63% of the studies, stiffness was defined as a range of motion of <90° or a flexion contracture of >5° at 6 to 12 weeks postoperatively. The prevalence of acquired idiopathic stiffness after pTKA was 4%, and this did not differ according to age (4%, I2 = 95%, among patients <65 years old and 5%, I2 = 96%, among those ≥65 years old; p = 0.238). The prevalence of acquired idiopathic stiffness was significantly lower in males (1%, I2 = 85%) than females (3%, I2 = 95%) (p < 0.0001) as well as in patients with a BMI of <30 kg/m2 (2%, I2 = 94%) compared with those with a BMI of ≥30 kg/m2 (5%, I2 = 97%) (p = 0.027).
Conclusions:
Contemporary literature supports the following definition for acquired idiopathic stiffness: a range of motion of <90° persisting for >12 weeks after pTKA in patients in the absence of complicating factors including preexisting stiffness. The mean prevalence of acquired idiopathic stiffness after pTKA was 4%; females and obese patients were at increased risk.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Stiffness is a common reason for failure of primary total knee arthroplasty (pTKA), contributing to up to 58% of reoperations or repeat interventions (such as manipulation under anesthesia) and >25% of 90-day hospital readmissions in some series1-3. Patients who develop this complication have poor functional outcomes and increased rates of knee pain, and their symptoms often are refractory to nonoperative and even operative management4,5. The incidence of TKA increased from 31.2 per 100,000 person-years from 1971 to 1976 to 220.9 per 100,000 person-years from 2005 to 20086. This trend, compounded by an increasing prevalence of obesity and a decreasing mean age of patients undergoing pTKA, will lead to an increased demand for revision TKAs7-9. It is therefore critically important to investigate and define one of the leading causes of pTKA failure.
Arthrofibrosis is characterized histopathologically by diffuse proliferation of scar tissue and results in a painful, restricted range of motion10. The process is hypothesized to originate from a pro-inflammatory insult that stimulates myofibroblast and mast cell proliferation, leading to pathologic amounts of type-I collagen deposition and subsequent joint contracture11. Diabetes, smoking, and the patient’s sex are also thought to contribute to its development. However, the precise mechanism has yet to be elucidated, and there is no definitive diagnostic test12. Furthermore, there is a paucity of data to support the implementation of a standardized clinical definition of arthrofibrosis10,13. In the absence of formal definitions, surgeons are forced to rely on clinical judgment and range-of-motion data to appropriately diagnose patients12. Therefore, definitions vary widely, from objective provider-obtained range-of-motion measurements to patient dissatisfaction with range of motion or pain10,13-16. Few authors have explicitly reported the exclusion of patients with periprosthetic joint infection, malpositioned or incorrectly sized components, ligamentous instability, patellar malalignment, an osseous block to motion, or complex regional pain syndrome from their arthrofibrotic cohort, despite the fact that these conditions may also lead to motion loss17-20. The terms “stiffness,” “flexion contracture,” and “arthrofibrosis” have often been incorrectly used interchangeably in the current literature to describe limitations of motion. This lack of consensus has led to a wide range of reported prevalences, from 0% to as high as 54%16,21,22.
In a recent consensus study, Kalson et al.10 developed comprehensive diagnostic and treatment guidelines for arthrofibrosis. Using 320 source studies, the authors highlighted the difficulty in studying arthrofibrosis in the absence of a widely accepted definition and generated multiple algorithms to allow clinicians to diagnose and classify these patients10. Additionally, a recent systematic review examined the treatment strategies for stiffness following pTKA23. This review included a host of etiologies and outcomes as well as common complications and reasons for revisions and reoperations. The patient cohorts from which these conclusions were drawn varied with respect to sample size, technique, diagnosis, and treatment. Furthermore, because no explicit numerical data analyses were performed, risk factors for the development of acquired idiopathic stiffness could not be determined.
We posit that a subset of patients with stiffness, specifically those for whom other identifiable causes have been ruled out, has acquired idiopathic stiffness. We hypothesize that this cohort most closely represents those who would receive the histopathologic diagnosis of arthrofibrosis. In light of the substantial patient cohort variability and absence of a concise standardized definition, we conducted a comprehensive systematic review and meta-analysis to (1) establish a range-of-motion threshold to define “acquired idiopathic stiffness” that can be used as a working clinical definition, (2) determine its prevalence after pTKA, and (3) identify potential risk factors for its development.
Materials and Methods
A protocol was designed for this systematic review and meta-analysis to define the population of interest, interventions of interest, and related outcomes. This study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology17.
Study Eligibility
All prospective or retrospective studies that recorded the prevalence of acquired idiopathic stiffness following TKA were included. Comparative studies with Level-I, II, or III evidence in which this disease was reported as an aseptic pTKA failure mechanism not attributable to another condition were also included. Exclusion criteria were (1) studies with non-routine inclusion criteria (e.g., only patients with organ transplants or connective-tissue disorders); (2) studies describing only patients with a preoperative flexion contracture or decreased range of motion preoperatively; (3) studies lacking a specific definition of stiffness, flexion contracture, or arthrofibrosis with numerical clinical data; and (4) studies of revision TKA without data describing overall prevalence of stiffness in the original patient cohort.
Literature Search
We searched databases, including MEDLINE, Embase, MEDLINE In-Process & Other Non-Indexed Citations, Cochrane Controlled Register of Trials (CENTRAL), Cochrane Database of Systematic Reviews, and Scopus, for studies published between January 2002 and October 2017 (see Appendix 1). We used MeSH and Emtree headings in several combinations and supplemented with free text to increase sensitivity. We manually searched the reference lists of relevant studies to identify any additional articles. The search strategy was designed and performed by an experienced librarian.
Study Selection
Three authors (M.E.T., A.K.L., and C.G.S.) independently identified all titles and abstracts using Covidence, an electronic screening form. Disagreements were resolved by consensus. Studies of patients who were <18 years of age and studies of stiffness in anatomical locations other than the knee were excluded. Editorials, reviews, symposia, basic-science papers, case reports, and case series were excluded as well.
Data Extraction
The included studies were assessed by 3 reviewers (M.E.T., A.K.L., and C.G.S.) in accordance to the Newcastle-Ottawa Scale, which detects heterogeneity within groups and outcomes24. The studies were assessed for quality on the basis of the duration of follow-up, cohort selection, and outcome assessment. Data were extracted manually and then were modified as necessary to fit the outcomes of interest (Table I).
TABLE I.
Characteristics of Included Studies*
| Authors | Period of Study | Institution | Country | No. of Patients | No. of Knees | % with Arthrofibrosis | Definition of Arthrofibrosis/Stiffness/Flexion Contracture | Criteria for MUA |
| Abdel et al., 20171 | 2000-2013 | Mayo Clinic | U.S. | 5,098 | 2.1 | “Stiffness”: ROM <90° 6-12 wk postop. | “Stiffness” (ROM <90° 6-12 wk postop.) | |
| Anania et al., 20134 | Jan. 2006-Apr. 2011 | Hospital for Special Surgery | U.S. | 295 | 319 | 14.4 | “Flexion contracture” (loss of extension) ≥5° 6 wk postop. | |
| Barnes et al., 201343 | Sept. 2005-Apr. 2008 | Hip Knee Arkansas Foundation | U.S. | 755 | 755 | 4.1 | “Stiffness”: ROM <90° 6 wk postop. | |
| Bawa et al., 201328 | 1999-2007 | Case Medical Center | U.S. | 2,782 | 3,244 | 3.7 | Failure to reach 90° of flexion by 6 wk postop.; could be sooner if patient not on track to achieve this goal | |
| Bistolfi et al., 201352 | Jan. 1998-Sept. 2002 | Adelaide Hospital | Italy | 163 | 200 | 0 | “Flexion contracture” >10° | No MUA performed |
| Boldt et al., 200620 | 1988-1999 | Orthopedic University Hospital Balgrist | Switzerland | 3,058 | 1.6 | “Arthrofibrosis”: max. ROM <90°, flexion contracture >10° | ||
| Cates and Schmidt, 200929 | 2000-Jan. 2005 | Tennessee Orthopaedic Clinics | U.S. | 767 | 6 | Flexion <100° 4-8 wk postop. or <110° with functional restrictions later in recovery | ||
| Chalidis et al., 201144 | 1994-2000 | “G. Papanikolaou” Hospital | Greece | 345 | 393 | 1.5 | Flexion <90° <4 wk postop. | |
| Choi et al., 201545 | 2001-2011 | Massachusetts General Hospital | U.S. | 1,293 | 6.3 | Flexion <90° 4-6 wk postop. and/or flexion <90° and failure to gain ROM back 2-3 mo postop. | ||
| Curtin et al., 201419 | (1) Jan. 1998-May 2005/(2) June 2005-Dec. 2007 | VCU/MCV West Hospital | U.S. | 546 (1st study period/280 (2nd study period) | 4.2/2.1 | Flexion <90° 6 wk postop., or if flexion remained <90° up to 3 mo postop. | ||
| Dalury et al., 200330 | 3-yr period (published Oct. 2003) | University of Maryland | U.S. | 1,014 | 2.27 | “Stiffness”: flexion >80°-100° 3 mo postop.; severely stiff: flexion <80° 6 wk postop. and no MUA | Flexion <80° 6 wk postop. | |
| Dzaja et al., 201512 | May 2001-July 2012 | Western University | Canada | 6,043 | 1.2 | Flexion <90° unresponsive to PT | ||
| Everts et al., 200741 | Catharina Hospital | Netherlands | 85 (platelet gel and fibrin sealant/80 (no gel or sealant) | 10/2.3 | “Arthrofibrosis”: painful stiffness with scarring of soft tissue, flexion <80°, PF immobility, no heterotopic bone formation | |||
| Fosco et al., 201131 | Mar. 1997-Aug. 2009 | University of Bologna | Italy | 861 | 4.9 | “Stiff”: ROM <50° | ||
| Gandhi et al., 200632 | Sept. 1998-May 2002 | Henderson Hospital | Canada | 1,216 | 3.7 | “Stiffness”: flexion <90° 1 yr postop. | ||
| Geller et al., 201746 | Nov. 2005-Sept. 2015 | Columbia University | U.S. | 690 (sensor)/252 (no sensor) | 4.9/1.6 | ROM ≤90° 6-8 wk postop. | ||
| Harvie et al., 201347 | Royal Perth Hospital | Australia | 281 | 7.4 | Mean ROM 62° (range, 30°-75°); mean extension deficit 8° (range, 5°-30°) | |||
| Hommel and Wilke, 201742 | June 2011-Dec. 2013 | Krankenhaus Markisch Oderland | Germany | 257 | 257 | 0 | “Stiffness”: flexion <90° | |
| Husted et al., 201533 | Jan. 10, 2010-May 31, 2012 | Copenhagen University | Denmark | 3,145 | 2.2 | At surgeon’s discretion but all used flexion <90° and agreed to wait 6 wk (see various definitions in table on page 3/6) | ||
| Ipach et al., 201134 | Aug. 1, 2004-July 31, 2009 | Ortho University Tuebingen | Germany | 867 | 4.5 | If ROM >90° not achieved, patient kept in hospital for 14 days. | If ROM still not 90° and anatomical reasons were excluded, patient encouraged to undergo immediate MUA | |
| Kim et al., 200416 | 1997-2000 | Hospital of the University of Pennsylvania, Philadelphia, PA | U.S. | 981 | 1,000 | 1.3 | “Flexion contracture” >15° or flexion <75° | |
| Lavernia et al., 200835 | Orthopedic Institute, Mercy Hospital, Miami, FL | U.S. | 778 | 4.8 | Flexion <90° at 4 wk | |||
| McAllister and Stepanian, 200848 | Evergreen Orthopedic Center, Kirkland, WA | U.S. | 73 | 89 | 18.0 | If flexion <90° at 6 wk MUA considered; done by 3 mo if ROM had not improved; MUA done after 14% of traditional TKAs | ||
| McGinn et al., 201649 | Jan. 2013-Dec. 2014 | Rubin Institute for Advanced Orthopedics, Sinai Hospital, Baltimore, MD | U.S. | 127 | 8.7 | Need for MUA | Failure to reach 90° of flexion or 15° of extension by 6 wk postop. | |
| Mitsuyasu et al., 201153 | Jan. 2001-July 2006 | Kyushu University, Fukuoka | Japan | 85 (0°/<5°/<10° flexion contracture preop.) | 48 | 8/0/8.7 | Postop. flexion contracture in degrees (>5° was minimum) | |
| Quah et al., 201236 | 2001-2006 | Royal Derby Hospital | U.K. | 1,626 | 9 | FFD (170 patients) >5° (124 patients, 91.2%) or >15° (12 patients, 8.8%) at 6-wk f/u | ||
| Ritter et al., 200754 | 1973-2002 | Center for Hip and Knee Surgery, Mooresville, IN | U.S. | 5,622 | 1.8 | “Flexion contracture” >10° | ||
| Rubinstein and DeHaan, 201018 | 1992-2007 | Portland Knee Clinic | U.S. | 800 | 4.6 | Flexion <90° 4-6 wk postop. and/or failure to gain motion over initial 2-3 mo | ||
| Sharma et al., 200850 | Jan. 2002-Dec. 2003 | Ranawat Orthopedic Center | U.S. | 251 (PCA)/ 248 (no PCA) | 286/292 | 2.4 | Patients with flexion <90°, flexion contracture >15°, or ROM <70°-80° given 2-3 wk of extensive supervised PT | No improvement after 2-3 wk of extensive supervised PT after ruling out malposition, PF overstuffing, patella baja, maltracking, infection |
| Smith et al., 201637 | 2012-2013 | Rothman Institute | U.S. | 372 | 3.8 | If flexion contracture >10°, extension orthosis applied 2-4×/day for 30-60 min. If this failed, patient offered study enrollment | ||
| Vanlommel et al., 201717 | 2004-2014 | University Hospitals Leuven | Belgium | 3,905 | 4,568 | 3.9 | Flexion <90° or lack of extension of >15° within 3 mo postop. | |
| Walton et al., 200540 | Jan. 1993-Dec. 2003 | Wakefield Orthopedic Clinic, Adelaide | Australia | 728 | 874 | 9.5 | Criteria for MUA defined as having arthrofibrosis according to Maloney | Flexion <80° at 6-8 wk f/u |
| White and Ranawat, 201651 | Jan. 2010-Nov. 2012 | Hospital for Special Surgery | U.S. | 42 | 38.1 | Flexion <90° or flexion contracture >10° at 6-wk or 3-mo f/u visit | Infection, implant malalignment, fracture, loosening contraindications | |
| Yercan et al., 200638 | 1987-2003 | Centre Livet-Hopital Croix Rousse, Caluire | France | 1,188 | 5.3 | Extension deficit >10° and/or flexion <95° 1st 6 wk postop. | Failure to achieve active knee flexion of 75° at end of 10 days and/or 95° of flexion within 3 mo postop. | |
| Yoo et al., 201539 | Mar. 2000-July 2004 | National Health Insurance Corporation Ilsan Hospital, Goyang | Korea | 329 | 1.2 | Patients with <80° of flexion or progressive stiffness despite flexion of >80° at discharge came back for visit at 1-2 wk | Flexion <80° at 1-2-wk visit |
MUA = manipulation under anesthesia, ROM = range of motion, PT = physical therapy, PF = patellofemoral, TKA = total knee arthroplasty, FFD = fixed flexion deformity, f/u = follow-up, and PCA = patient-controlled anesthesia.
Outcomes
The primary outcome of interest was the prevalence of acquired idiopathic stiffness following pTKA. We performed subanalyses according to the duration of follow-up (≤24 and >24 months), sex (female and male), age (<65 and ≥65 years old), and body mass index (BMI) (<30 and ≥30 kg/m2).
Statistical Analysis
The DerSimonian and Laird conservative random-effects model was used to pool log-transformed event prevalence and estimated 95% confidence intervals (CIs)25. Heterogeneity was quantified using the I2 statistic, which estimates the proportion of total variability between studies not due to chance alone26. Values of >50% were considered to be heterogeneous. Subgroup analyses of disease prevalence by duration of follow-up, sex, age, and BMI were also performed to further assess heterogeneity. P values of <0.05 were considered significant. All data were analyzed using STATA, version 14 (StataCorp)27.
Results
Eligible and Included Studies
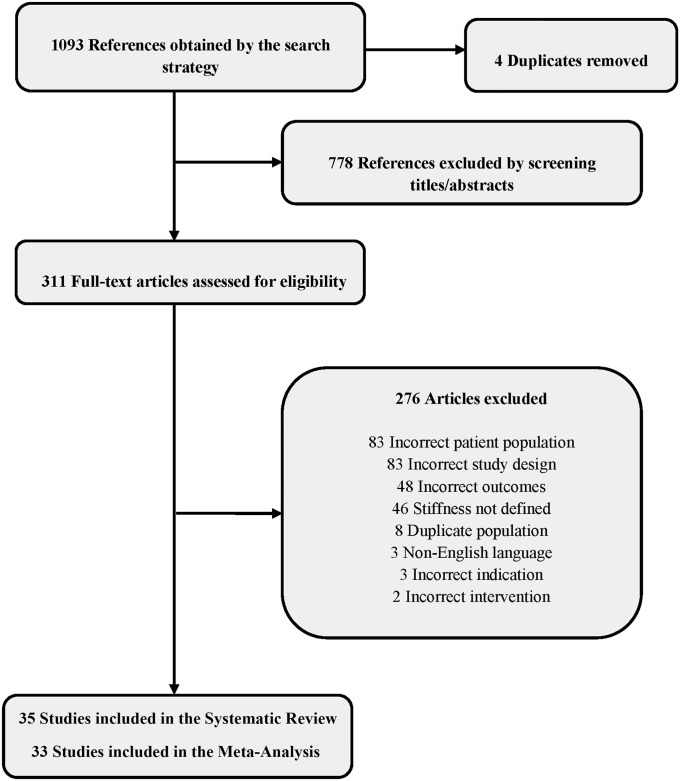
There were 1,089 potentially eligible articles; 778 were unrelated to the study question and thus excluded. The full text of 311 studies was reviewed, and 276 were excluded because of an incorrect or duplicate patient population, incorrect study design or outcomes, lack of criteria for stiffness, non-English language, and operative intervention not involving TKA. We further filtered these studies according to previously described inclusion and exclusion criteria as well as methodological quality. Ultimately, 35 studies (48,873 pTKAs) (Fig. 1) were selected for inclusion in the systematic review. Because 2 of them had no events, 33 were included in the meta-analysis. The mean patient age in the studies was 66 years.
Fig. 1.
Flowchart depicting PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) search strategy.
Study Characteristics
Eighteen (51%) of the studies4,12,16-18,20,28-39 (31,195 knees) contained sufficient data to allow analysis of age as a risk factor for developing acquired idiopathic stiffness following pTKA. This complication developed in 1,069 knees (3.4%) in patients with a mean age of 64.3 years (range among studies, 57 to 71 years). Patient sex was reported in 17 studies4,12,17-20,28,29,31,32,34-40 (49%) with a total of 16,720 patients (27,736 knees), 10,473 (63%) of whom were female and 6,247 (37%) of whom were male. Acquired idiopathic stiffness developed in 980 (6%) of these patients, 658 (67%) of whom were female and 322 (33%) of whom were male. BMI was reported in 10 studies (21,336 knees)4,12,17,20,28-30,32,35,39 and averaged 31 kg/m2 (range among studies, 18 to 54 kg/m2) for the patients who developed acquired idiopathic stiffness.
Clinical Definitions
The definition(s) of “arthrofibrosis,” “stiffness,” and/or “flexion contracture” utilized as inclusion criteria in each study were assessed. Of the 35 studies included in our review, 2 used the clinical term “arthrofibrosis,”20,41 29 used “stiffness,”1,12,16-20,28-35,38-51 and 11 used “flexion contracture.”4,16,20,36-38,50-54 Five studies used a combination of the aforementioned clinical terminology as inclusion criteria16,20,38,41,51. For 12 studies12,18,19,28,29,33,35,44-48 that did not explicitly define inclusion criteria, but instead listed range-of-motion criteria for manipulation under anesthesia, definitive criteria for manipulation under anesthesia were used as surrogate definitions.
The numerical range-of-motion cutoffs used to differentiate among the above terms varied. Details and definitions can be found in Table I.
Outcomes
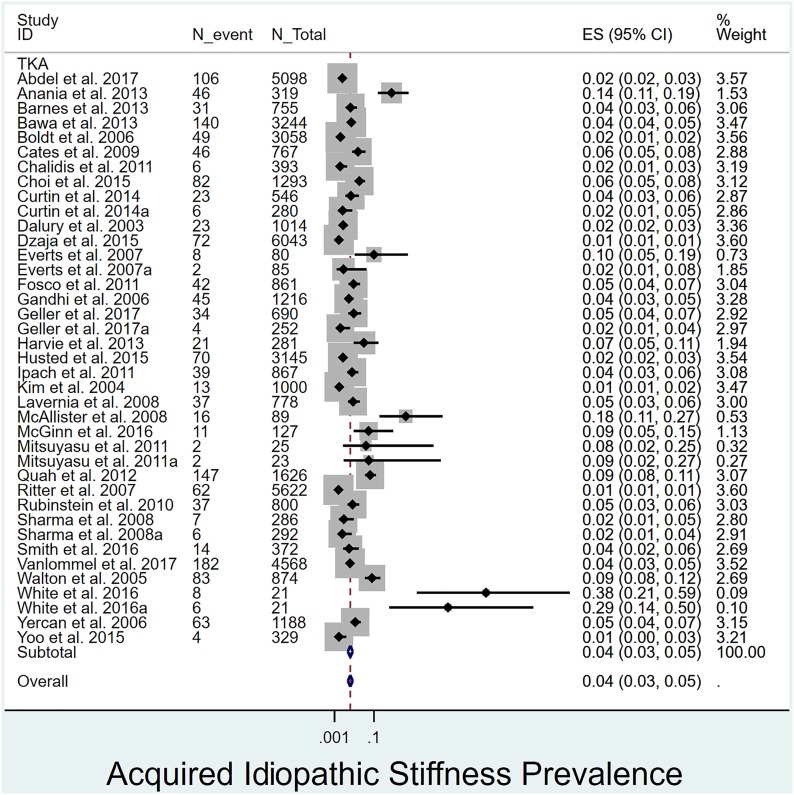
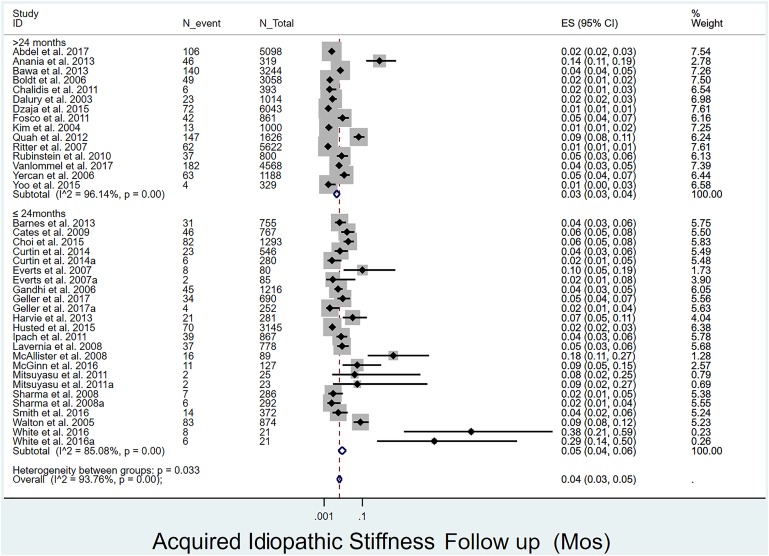
Overall prevalence of acquired idiopathic stiffness following pTKA: The overall prevalence of acquired idiopathic stiffness was 4% (Fig. 2). Its prevalence was significantly (p = 0.033) lower in studies with >24 months of follow-up1,4,12,16-18,20,28,30,31,36,38,39,44,54 (3%, I2 = 96%) than in those with ≤24 months of follow-up19,29,32-35,37,40,41,43,45-51,53 (4%, I2 = 85%) (Fig. 3).
Fig. 2.
Forest plot depicting the prevalence of acquired idiopathic stiffness among all included studies as well as the overall mean. ES (95% CI) = effect size (95% confidence interval). The values correspond to prevalences (expressed as decimal values rather than percentages).
Fig. 3.
Forest plot depicting the prevalence of acquired idiopathic stiffness according to duration of follow-up. ES (95% CI) = effect size (95% confidence interval). The values correspond to prevalences (expressed as decimal values rather than percentages).
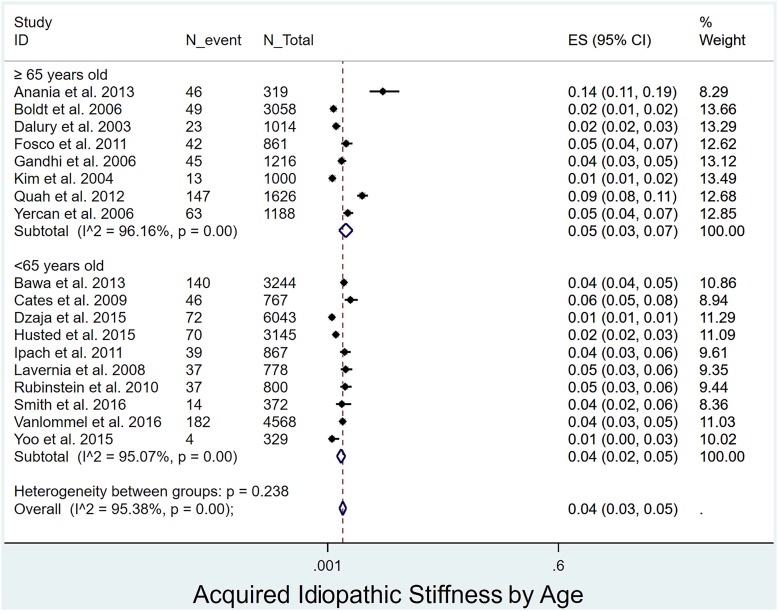
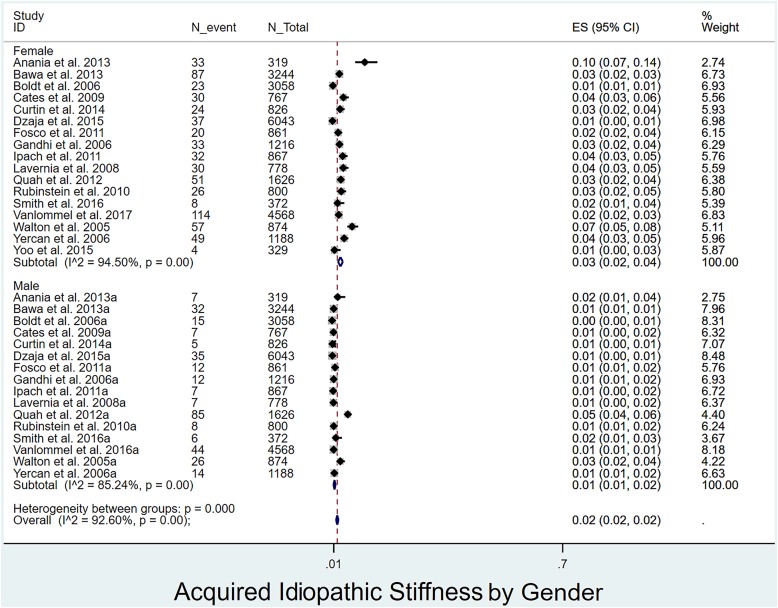
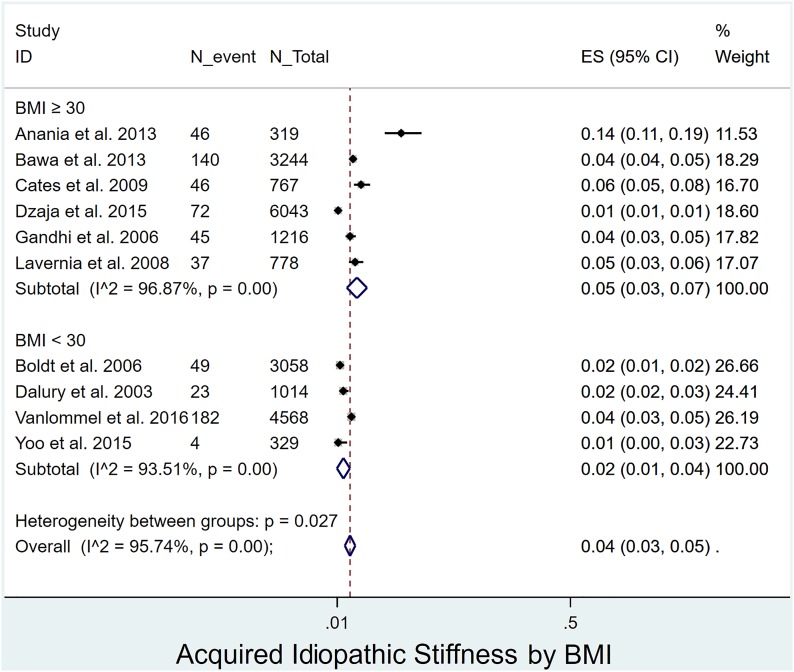
Prevalence of acquired idiopathic stiffness following pTKA by age, sex, and BMI: The prevalence of acquired idiopathic stiffness did not differ significantly (p = 0.238) between patients <65 years of age (4%, I2 = 95%)12,17,18,28,29,33-35,37,39 and those ≥65 years of age (5%, I2 = 96%)4,16,20,30-32,36,38 (Fig. 4). The prevalence was significantly lower (p < 0.001) in men4,12,17-20,28,29,31,32,34-38,40 (1%, I2 = 85%) than women (3%, I2 = 95%)4,12,17-20,28,29,31,32,34-40 (Fig. 5). Patients with a BMI of <30 kg/m2 (2%, I2 = 94%)17,20,30,39 had a significantly lower prevalence (p = 0.027) than those with a BMI of ≥30 kg/m2 (5%, I2 = 97%)4,12,28,29,32,35 (Fig. 6).
Fig. 4.
Forest plot depicting the prevalence of acquired idiopathic stiffness according to age. ES (95% CI) = effect size (95% confidence interval). The values correspond to prevalences (expressed as decimal values rather than percentages).
Fig. 5.
Forest plot depicting the prevalence of acquired idiopathic stiffness according to sex. ES (95% CI) = effect size (95% confidence interval). The values correspond to prevalences (expressed as decimal values rather than percentages).
Fig. 6.
Forest plot depicting the prevalence of acquired idiopathic stiffness according to BMI (kg/m2). ES (95% CI) = effect size (95% confidence interval). The values correspond to prevalences (expressed as decimal values rather than percentages).
Methodological quality and risk of bias assessment: All 35 studies included in this review were observational uncontrolled cohort studies and thus a high risk of bias was observed. A detailed assessment of methodological quality indicators is presented in Appendix 2. Overall, there was high statistical heterogeneity for all outcomes.
Diabetes mellitus and smoking status were examined; however, because of insufficient data reporting in the included studies, an analysis was not performed for these parameters.
Discussion
Despite being one of the most common reasons for failure of pTKA, the definition of acquired idiopathic stiffness is poorly understood and the entity probably is often misdiagnosed. The prevalence of this complication in the studies included in this review ranged from 1% to 38% (mean, 4%) even with strict inclusion criteria. Disease prevalence did not differ significantly according to age; however, female sex and a BMI of ≥30 kg/m2 were found to be risk factors.
In the majority (63%) of the studies, a range of motion of <90° or a flexion contracture of >5° was used to define postoperative acquired idiopathic stiffness1,12,17-20,28,32-35,38,42-46,48-51. Previous studies have demonstrated that 105° to 110° is the minimum knee flexion required to perform most activities of daily living (ADLs) in Western societies such as rising from a chair, walking, and ascending stairs55,56. A more recent kinematic analysis providing additional data on activities showed donning pants and getting in and out of a bathtub to require 78° and 123° to 143° of knee flexion, respectively57. Despite the results of these biomechanical studies, McClelland et al.58 recently demonstrated that patients use only 81° to 91° of their maximal passive knee flexion after TKA. Patients with a greater passive range of motion did have higher maximal flexion during ADLs but utilized only 68% to 77% of their maximal motion during more strenuous activities58. Several additional studies have shown that preoperative range of motion is the best predictor of final range of motion16,17,59, which justifies the extensive exclusion criteria that we utilized to select eligible studies. In order to accurately identify only those patients with a substantial postoperative loss of range of motion leading to limitations in ADLs, we propose a threshold range of motion of <90° persisting for >12 weeks after TKA to define acquired idiopathic stiffness. This should exclude patients with periprosthetic joint infection, a prosthetic or osseous block to motion (including malpositioned components), aseptic loosening of components, or preoperative stiffness. Use of this definition clinically, and in future studies, would allow improved understanding of the characteristics of at-risk patients and would facilitate treatment and prevention of this disease. We recognize that some of the above factors that are known to cause knee stiffness may create secondary tissue changes similar or identical to those seen in acquired idiopathic stiffness.
We report a mean prevalence of acquired idiopathic stiffness of 4% after pTKA (Table I). The wide range of disease prevalence among the included studies (38% in 1 series51) can be attributed to variable and sometimes limited sample sizes. The severity of acquired idiopathic stiffness is a continuum; the authors of several studies have recognized that the time of follow-up as well as the extent of clinical intervention or rehabilitation play a large role in determining the reported prevalence of the disease. We found that the prevalence was significantly lower (p = 0.033) in studies with follow-up of >24 months1,4,12,16-18,20,28,30,31,36,38,39,44,54 (3%, I2 = 96%) than in those with ≤24 months of follow-up19,29,32-35,37,40,41,43,45-51,53 (4%, I2 = 85%). These data suggest that the disease process generates its most significant effects early; therefore, efforts to interrupt the process should be undertaken immediately postoperatively or in the perioperative period. Early clinical intervention such as intense physical therapy or manipulation under anesthesia has been advocated for patients who do not obtain at least 90° of flexion by the 4-week follow-up visit19,26,31,44. The decreased prevalence over time suggests that, in some patients, the stiff soft tissues eventually become more compliant, with subsequent improvements in range of motion.
Stratification by age did not demonstrate a significant difference in the prevalence of acquired idiopathic stiffness between patients ≥65 and those <65 years of age (p = 0.238). This corroborates the results reported by Kim et al.16, who did not identify age as a risk factor in 1,000 consecutive TKAs, 1.3% of which were followed by development of acquired idiopathic stiffness. In a review of 18,065 TKAs for which infection and stiffness were the most common reasons for failure, Pitta et al.60 found that increasing age was protective against TKA failure (hazard ratio [HR] = 0.61, p < 0.01). This is likely due to the decreased functional demands and stresses that older patients place on implants. Conversely, elevated BMI (≥30 kg/m2) significantly affected the prevalence of acquired idiopathic stiffness in our review (p = 0.027). While data on the prevalence of this disease in obese patients are limited, ample data have demonstrated that patients with a higher BMI have an increased risk of perioperative complications, decreased functional outcome scores, and a decreased range of motion61,62. Vazquez-Vela Johnson et al.63 found that, at 10 years, obese males ≤60 years of age had an implant survival rate of 35.7% compared with 99.4% in non-obese females who were >60 years old. The prevalence among females was 3-fold greater than that among males (p < 0.001) in our analysis, even though few series have demonstrated an association between female sex and the development of acquired idiopathic stiffness. This may be due to the fact that most studies are underpowered to detect sex-based differences. Pooling the data and subsequently performing analyses allows detection of these smaller differences.
Our review has a number of limitations. First, we were unable to stratify outcomes according to implant type, which may have contributed to the observed heterogeneity. To reduce and explore heterogeneity, we stratified analyses by the duration of follow-up, sex, age, and BMI. Second, publication bias is common in systematic reviews of observational studies. Third, it is not clear how the authors of each study managed the definition of postoperative stiffness as it relates to preoperative stiffness (for example, a 91° range of motion preoperatively compared with 88° postoperatively) and/or BMI.
We believe that the present study represents the most comprehensive assessment of acquired idiopathic stiffness following pTKA in the literature. It identified a mean prevalence of 4% after pTKA and showed female sex and a BMI ≥30 kg/m2 to be risk factors. It also provides a working definition for acquired idiopathic stiffness: a total range of motion of <90° persisting for >12 weeks after pTKA in patients with osteoarthritis in the absence of other complicating factors. This definition will allow more uniform diagnosis, treatment, and study of the disease process. Further research at the genetic, histological, and biochemical levels is necessary to determine the molecular etiology in patients with this clinical diagnosis who have the pathologic entity arthrofibrosis and to identify potential therapeutic targets.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/F317).
Footnotes
Investigation performed at the Mayo Clinic, Rochester, Minnesota
Disclosure: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR072597. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work and “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work (http://links.lww.com/JBJS/F316).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Abdel MP, Ledford CK, Kobic A, Taunton MJ, Hanssen AD. Contemporary failure aetiologies of the primary, posterior-stabilised total knee arthroplasty. Bone Joint J. 2017. May;99-B(5):647-52. [DOI] [PubMed] [Google Scholar]
- 2.Schroer WC, Berend KR, Lombardi AV, Barnes CL, Bolognesi MP, Berend ME, Ritter MA, Nunley RM. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013. September;28(8)(Suppl):116-9. Epub 2013 Aug 15. [DOI] [PubMed] [Google Scholar]
- 3.Schairer WW, Vail TP, Bozic KJ. What are the rates and causes of hospital readmission after total knee arthroplasty? Clin Orthop Relat Res. 2014. January;472(1):181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anania A, Abdel MP, Lee YY, Lyman S, González Della Valle A. The natural history of a newly developed flexion contracture following primary total knee arthroplasty. Int Orthop. 2013. October;37(10):1917-23. Epub 2013 Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrey ME, Abdel MP, Riester SM, Dudakovic A, van Wijnen AJ, Morrey BF, Sanchez-Sotelo J. Molecular landscape of arthrofibrosis: Microarray and bioinformatic analysis of the temporal expression of 380 genes during contracture genesis. Gene. 2017. April ;610:15-23. Epub 2017 Feb 3. [DOI] [PubMed] [Google Scholar]
- 6.Singh JA, Vessely MB, Harmsen WS, Schleck CD, Melton LJ, 3rd, Kurland RL, Berry DJ. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969-2008. Mayo Clin Proc. 2010. October;85(10):898-904. Epub 2010 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007. April;89(4):780-5. [DOI] [PubMed] [Google Scholar]
- 8.Singh JA, Lewallen DG. Time trends in the characteristics of patients undergoing primary total knee arthroplasty. Arthritis Care Res (Hoboken). 2014. June;66(6):897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Merchan EC. the influence of obesity on the outcome of TKR: can the impact of obesity be justified from the viewpoint of the overall health care system? HSS J. 2014. July;10(2):167-70. Epub 2014 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalson NS, Borthwick LA, Mann DA, Deehan DJ, Lewis P, Mann C, Mont MA, Morgan-Jones R, Oussedik S, Williams FM, Toms A, Argenson JN, Bellemans J, Bhave A, Furnes O, Gollwitzer H, Haddad FS, Hofmann S, Krenn V. International consensus on the definition and classification of fibrosis of the knee joint. Bone Joint J. 2016. November;98-B(11):1479-88. [DOI] [PubMed] [Google Scholar]
- 11.Abdel MP, Morrey ME, Barlow JD, Kreofsky CR, An KN, Steinmann SP, Morrey BF, Sanchez-Sotelo J. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J Orthop Res. 2012. May;30(5):713-9. Epub 2011 Nov 4. [DOI] [PubMed] [Google Scholar]
- 12.Dzaja I, Vasarhelyi EM, Lanting BA, Naudie DD, Howard JL, Somerville L, McCalden RW, MacDonald SJ. Knee manipulation under anaesthetic following total knee arthroplasty: a matched cohort design. Bone Joint J. 2015. December;97-B(12):1640-4. [DOI] [PubMed] [Google Scholar]
- 13.Ghani H, Maffulli N, Khanduja V. Management of stiffness following total knee arthroplasty: a systematic review. Knee. 2012. December;19(6):751-9. Epub 2012 Apr 24. [DOI] [PubMed] [Google Scholar]
- 14.Cheuy VA, Foran JRH, Paxton RJ, Bade MJ, Zeni JA, Stevens-Lapsley JE. Arthrofibrosis associated with total knee arthroplasty. J Arthroplasty. 2017. August;32(8):2604-11. Epub 2017 Feb 14. [DOI] [PubMed] [Google Scholar]
- 15.Moya-Angeler J, Bas MA, Cooper HJ, Hepinstall MS, Rodriguez JA, Scuderi GR. Revision arthroplasty for the management of stiffness after primary TKA. J Arthroplasty. 2017. June;32(6):1935-9. Epub 2017 Jan 19. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Nelson CL, Lotke PA. Stiffness after total knee arthroplasty. Prevalence of the complication and outcomes of revision. J Bone Joint Surg Am. 2004. July;86-A(7):1479-84. [PubMed] [Google Scholar]
- 17.Vanlommel L, Luyckx T, Vercruysse G, Bellemans J, Vandenneucker H. Predictors of outcome after manipulation under anaesthesia in patients with a stiff total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2017. November;25(11):3637-43. Epub 2016 Dec 29. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein RA, Jr, DeHaan A. The incidence and results of manipulation after primary total knee arthroplasty. Knee. 2010. January;17(1):29-32. Epub 2009 Aug 6. [DOI] [PubMed] [Google Scholar]
- 19.Curtin B, Yakkanti M, Malkani A. Postoperative pain and contracture following total knee arthroplasty comparing parapatellar and subvastus approaches. J Arthroplasty. 2014. January;29(1):33-6. Epub 2013 Apr 29. [DOI] [PubMed] [Google Scholar]
- 20.Boldt JG, Stiehl JB, Hodler J, Zanetti M, Munzinger U. Femoral component rotation and arthrofibrosis following mobile-bearing total knee arthroplasty. Int Orthop. 2006. October;30(5):420-5. Epub 2006 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scranton PE., Jr. Management of knee pain and stiffness after total knee arthroplasty. J Arthroplasty. 2001. June;16(4):428-35. [DOI] [PubMed] [Google Scholar]
- 22.Shoji H, Yoshino S, Komagamine M. Improved range of motion with the Y/S total knee arthroplasty system. Clin Orthop Relat Res. 1987. May;(218):150-63. [PubMed] [Google Scholar]
- 23.Cohen JS, Gu A, Lopez NS, Park MS, Fehring KA, Sculco PK. Efficacy of revision surgery for the treatment of stiffness after total knee arthroplasty: a systematic review. J Arthroplasty. 2018. September;33(9):3049-55. Epub 2018 Apr 30. [DOI] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010. September;25(9):603-5. Epub 2010 Jul 22. [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. September;7(3):177-88. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003. September 6;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed AT, Abdel-Rahman O, Morsy M, Mustafa K, Testini P, Aleem IS, Murad MH, Nassr A. Management of sacrococcygeal chordoma: a systematic review and meta-analysis of observational studies. Spine (Phila Pa 1976). 2018. October 1;43(19):E1157-69. [DOI] [PubMed] [Google Scholar]
- 28.Bawa HS, Wera GD, Kraay MJ, Marcus RE, Goldberg VM. Predictors of range of motion in patients undergoing manipulation after TKA. Clin Orthop Relat Res. 2013. January;471(1):258-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cates HE, Schmidt JM. Closed manipulation after total knee arthroplasty: outcome and affecting variables. Orthopedics. 2009. June;32(6):398. [DOI] [PubMed] [Google Scholar]
- 30.Dalury DF, Jiranek W, Pierson J, Pearson SE. The long-term outcome of total knee patients with moderate loss of motion. J Knee Surg. 2003. October;16(4):215-20. [PubMed] [Google Scholar]
- 31.Fosco M, Filanti M, Amendola L, Savarino LM, Tigani D. Total knee arthroplasty in stiff knee compared with flexible knees. Musculoskelet Surg. 2011. April;95(1):7-12. Epub 2011 Mar 9. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi R, de Beer J, Leone J, Petruccelli D, Winemaker M, Adili A. Predictive risk factors for stiff knees in total knee arthroplasty. J Arthroplasty. 2006. January;21(1):46-52. [DOI] [PubMed] [Google Scholar]
- 33.Husted H, Jørgensen CC, Gromov K, Troelsen A; Collaborative Group of the Lundbeck Foundation Center for Fast-Track Hip and Knee Replacement. Low manipulation prevalence following fast-track total knee arthroplasty. Acta Orthop. 2015. February;86(1):86-91. Epub 2014 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ipach I, Schäfer R, Lahrmann J, Kluba T. Stiffness after knee arthrotomy: evaluation of prevalence and results after manipulation under anaesthesia. Orthop Traumatol Surg Res. 2011. May;97(3):292-6. Epub 2011 Apr 11. [DOI] [PubMed] [Google Scholar]
- 35.Lavernia C, Cardona D, Rossi MD, Lee D. Multimodal pain management and arthrofibrosis. J Arthroplasty. 2008. September;23(6)(Suppl 1):74-9. [DOI] [PubMed] [Google Scholar]
- 36.Quah C, Swamy G, Lewis J, Kendrew J, Badhe N. Fixed flexion deformity following total knee arthroplasty. A prospective study of the natural history. Knee. 2012. October;19(5):519-21. Epub 2011 Oct 13. [DOI] [PubMed] [Google Scholar]
- 37.Smith EB, Shafi KA, Greis AC, Maltenfort MG, Chen AF. Decreased flexion contracture after total knee arthroplasty using Botulinum toxin A: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2016. October;24(10):3229-34. Epub 2016 Aug 11. [DOI] [PubMed] [Google Scholar]
- 38.Yercan HS, Sugun TS, Bussiere C, Ait Si Selmi T, Davies A, Neyret P. Stiffness after total knee arthroplasty: prevalence, management and outcomes. Knee. 2006. March;13(2):111-7. Epub 2006 Feb 20. [DOI] [PubMed] [Google Scholar]
- 39.Yoo JH, Oh JC, Oh HC, Park SH. Manipulation under anesthesia for stiffness after total knee arthroplasty. Knee Surg Relat Res. 2015. December;27(4):233-9. Epub 2015 Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walton NP, Jahromi I, Dobson PJ, Angel KR, Lewis PL, Campbell DG. Arthrofibrosis following total knee replacement; does therapeutic warfarin make a difference? Knee. 2005. April;12(2):103-6. [DOI] [PubMed] [Google Scholar]
- 41.Everts PAM, Devilee RJJ, Oosterbos CJM, Mahoney CB, Schattenkerk ME, Knape JTA, van Zundert A. Autologous platelet gel and fibrin sealant enhance the efficacy of total knee arthroplasty: improved range of motion, decreased length of stay and a reduced incidence of arthrofibrosis. Knee Surg Sports Traumatol Arthrosc. 2007. July;15(7):888-94. Epub 2007 Feb 24. [DOI] [PubMed] [Google Scholar]
- 42.Hommel H, Wilke K. Good early results obtained with a guided-motion implant for total knee arthroplasty: a consecutive case series. Open Orthop J. 2017. February 24;11:51-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes CL, Lincoln D, Wilson B, Bushmaier M. Knee manipulation after total knee arthroplasty: comparison of two implant designs. J Surg Orthop Adv. 2013. Summer;22(2):157-9. [DOI] [PubMed] [Google Scholar]
- 44.Chalidis BE, Sachinis NP, Papadopoulos P, Petsatodis E, Christodoulou AG, Petsatodis G. Long-term results of posterior-cruciate-retaining Genesis I total knee arthroplasty. J Orthop Sci. 2011. November;16(6):726-31. Epub 2011 Sep 10. [DOI] [PubMed] [Google Scholar]
- 45.Choi HR, Siliski JM, Malchau H, Kwon YM. Effect of repeated manipulation on range of motion in patients with stiff total knee arthroplasty. Orthopedics. 2015. March;38(3):e157-62. [DOI] [PubMed] [Google Scholar]
- 46.Geller JA, Lakra A, Murtaugh T. The use of electronic sensor device to augment ligament balancing leads to a lower rate of arthrofibrosis after total knee arthroplasty. J Arthroplasty. 2017. May;32(5):1502-4. Epub 2016 Dec 24. [DOI] [PubMed] [Google Scholar]
- 47.Harvie P, Larkin J, Scaddan M, Longstaff LM, Sloan K, Beaver RJ. Stiffness after total knee arthroplasty: does component alignment differ in knees requiring manipulation? A retrospective cohort study of 281 patients. J Arthroplasty. 2013. January;28(1):14-9. [DOI] [PubMed] [Google Scholar]
- 48.McAllister CM, Stepanian JD. The impact of minimally invasive surgical techniques on early range of motion after primary total knee arthroplasty. J Arthroplasty. 2008. January;23(1):10-8. [DOI] [PubMed] [Google Scholar]
- 49.McGinn T, Chughtai M, Bhave A, Ali O, Mudaliar P, Khlopas A, Harwin SF, Mont MA. Innovative multi-modal physical therapy reduces incidence of manipulation under anesthesia (MUA) in non-obese primary total knee arthroplasty. Surg Technol Int. 2016. October 26;29:328-33. [PubMed] [Google Scholar]
- 50.Sharma V, Maheshwari AV, Tsailas PG, Ranawat AS, Ranawat CS. The results of knee manipulation for stiffness after total knee arthroplasty with or without an intra-articular steroid injection. Indian J Orthop. 2008. July;42(3):314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White PB, Ranawat AS. Patient-specific total knees demonstrate a higher manipulation rate compared to “off-the-shelf implants”. J Arthroplasty. 2016. January;31(1):107-11. Epub 2015 Aug 1. [DOI] [PubMed] [Google Scholar]
- 52.Bistolfi A, Massazza G, Lee GC, Deledda D, Berchialla P, Crova M. Comparison of fixed and mobile-bearing total knee arthroplasty at a mean follow-up of 116 months. J Bone Joint Surg Am. 2013. June 19;95(12):e83. [DOI] [PubMed] [Google Scholar]
- 53.Mitsuyasu H, Matsuda S, Miura H, Okazaki K, Fukagawa S, Iwamoto Y. Flexion contracture persists if the contracture is more than 15° at 3 months after total knee arthroplasty. J Arthroplasty. 2011. June;26(4):639-43. Epub 2010 Jun 11. [DOI] [PubMed] [Google Scholar]
- 54.Ritter MA, Lutgring JD, Davis KE, Berend ME, Pierson JL, Meneghini RM. The role of flexion contracture on outcomes in primary total knee arthroplasty. J Arthroplasty. 2007. December;22(8):1092-6. [DOI] [PubMed] [Google Scholar]
- 55.Thomsen MG, Husted H, Otte KS, Holm G, Troelsen A. Do patients care about higher flexion in total knee arthroplasty? A randomized, controlled, double-blinded trial. BMC Musculoskelet Disord. 2013. April 8;14:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurosaka M, Yoshiya S, Mizuno K, Yamamoto T. Maximizing flexion after total knee arthroplasty: the need and the pitfalls. J Arthroplasty. 2002. June;17(4)(Suppl 1):59-62. [DOI] [PubMed] [Google Scholar]
- 57.Hyodo K, Masuda T, Aizawa J, Jinno T, Morita S. Hip, knee, and ankle kinematics during activities of daily living: a cross-sectional study. Braz J Phys Ther. 2017. May-Jun;21(3):159-66. Epub 2017 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McClelland JA, Feller JA, Menz HB, Webster KE. Patients with total knee arthroplasty do not use all of their available range of knee flexion during functional activities. Clin Biomech (Bristol, Avon). 2017. March;43:74-8. Epub 2017 Feb 7. [DOI] [PubMed] [Google Scholar]
- 59.Aderinto J, Brenkel IJ, Chan P. Natural history of fixed flexion deformity following total knee replacement: a prospective five-year study. J Bone Joint Surg Br. 2005. July;87(7):934-6. [DOI] [PubMed] [Google Scholar]
- 60.Pitta M, Esposito CI, Li Z, Lee YY, Wright TM, Padgett DE. Failure after modern total knee arthroplasty: a prospective study of 18,065 knees. J Arthroplasty. 2018. February;33(2):407-14. Epub 2017 Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perry KI, MacDonald SJ. The obese patient: a problem of larger consequence. Bone Joint J. 2016. January;98-B(1)(Suppl A):3-5. [DOI] [PubMed] [Google Scholar]
- 62.Collins JE, Donnell-Fink LA, Yang HY, Usiskin IM, Lape EC, Wright J, Katz JN, Losina E. Effect of obesity on pain and functional recovery following total knee arthroplasty. J Bone Joint Surg Am. 2017. November 1;99(21):1812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazquez-Vela Johnson G, Worland RL, Keenan J, Norambuena N. Patient demographics as a predictor of the ten-year survival rate in primary total knee replacement. J Bone Joint Surg Br. 2003. January;85(1):52-6. [DOI] [PubMed] [Google Scholar]