Abstract
Background
Hepatitis B (HBV) or hepatitis C (HCV) virus co-infections in HIV are alarming during pregnancy due to the risk of vertical transmission and the eventual adverse effects on neonates. This study was conducted to ascertain the sero-prevalence of HIV/HBV and HIV/HCV co-infections, evaluate the effect of the co-infections on the immunological and virological characteristics and assess the association between some demographic and lifestyle characteristics and risk of HBV, HCV, HIV/HBV and HIV/HCV co-infections among pregnant women living in the Brong-Ahafo Region of Ghana.
Methods
This comparative cross-sectional study was conducted at the anti-retroviral therapy (ART) clinics of the St. Elizabeth Hospital and the Holy Family Hospital, Brong-Ahafo Region, Ghana. A total of 248 consecutive consenting pregnant Ghanaian women, 148 diagnosed with HIV [HIV (+)] and 100 who were HIV negative [HIV (-)], were recruited. Validated questionnaire was used to obtain demographic and lifestyle data. Venous blood samples were obtained and HCV status, HBV profile, CD4+ T cell count, and HIV-1 RNA load were determined.
Results
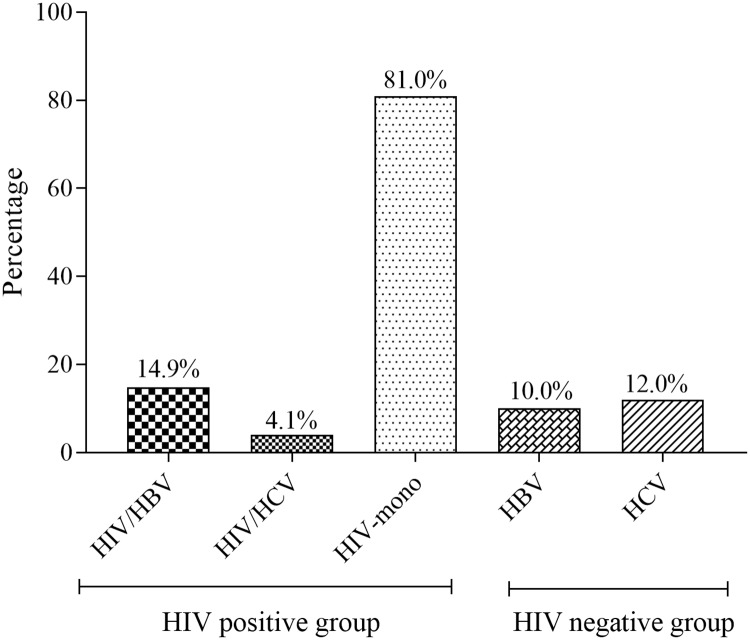
The sero-prevalence of HIV (+) /HBV, HIV (+) /HCV, HIV (-)/HBV, and HIV (-)/HCV infections were 22 (14.9%), 6 (4.1%), 10 (10.0%), and 12 (12.0%) respectively. HIV-1 viral load was not significantly different between HIV/HBV, HIV/HCV co-infection and HIV mono-infection. However, CD4+ T lymphocyte count (364 vs 512 vs 514 cells/μl; p = 0.0009) was significantly lower in HIV/HBV co-infection compared to HIV/HCV and HIV mono-infection respectively. There was no significant association between demographic and lifestyle characteristics and risk of HBV and HCV infections in HIV positive and negative subjects except for late diagnosis of HIV and history of sharing razors blades and pins, where increased odds of HIV (+) /HBV and HIV (-)/HBV infection were observed.
Conclusions
The prevalence of HIV (+)/HBV (14.9%), HIV (+)/HCV (4.1%), HIV (-)/HBV (10.0%), and HIV (-)/HCV (12.0%) are high among pregnant women in the Brong Ahafo Region of Ghana. HIV/HBV is associated with reduced CD4+ T lymphocyte count but not HIV-1 viral load. Early diagnosis of HIV and intensification of routine antenatal HBV and HCV are essential to abate the risk of maternal to child transmission.
Introduction
Human Immunodeficiency virus (HIV), Hepatitis B virus (HBV) and Hepatitis C virus (HCV) are the predominant cause of chronic viral infections globally [1]. The WHO Global Health Observatory reports that approximately 36.9 million people are infected with HIV worldwide, with more than half of these being women and 1.8 million being children [2]. Most of these HIV infected individuals are from low- and middle- income countries, and it is estimated that 66% of them are living in sub-Saharan Africa [3, 4]. Currently in Ghana, 310 000 people are living with HIV, of which 190 000 are women [4]. In the Brong Ahafo region of Ghana, an estimated number of adults living with HIV is 28,577, of which 20,455 are females [5].
HIV infected individuals are predisposed to other infections such as tuberculosis which can make the management of the disease arduous [3]. Reports show that, globally, approximately 325 million people are infected with HBV and HCV, and 1.34 million of these individuals expire annually [6]. The estimated number of individuals infected with HCV is 130–150 million [7] and about 2.3 million HIV infected individuals are co-infected with HCV; thus accounting for 6.2% of people living with HIV globally. In sub-Saharan Africa, the prevalence of HIV/HCV co-infection is estimated at 2.98–4.34% [8]. Additionally, the estimated prevalence of HBV surface antigen is 6.1% worldwide despite the introduction of universal hepatitis B vaccination and potent antiviral therapy [9]. HBV infection is endemic in sub-Saharan Africa and the prevalence of HIV/HBV co-infection in sub-Saharan Africa is between 6% and 25% [10]. In Ghana, the prevalence ranges between 8 and 15% [11], of which 6.4% and 15.6% are pregnant women and children respectively [12]. Co-infection of HBV and HCV in HIV infected persons is attributed to mutual routes of transmission [13].
Individually, HIV, HCV and HBV cause chronic conditions. However, in co-infection states, they may lead to life-threatening and fatal conditions especially during pregnancy where there is a high risk of maternal complications and vertical transmission which is associated with foetal and neonatal hepatitis [14–16]. The clinical management of HIV/HBV and HIV/HCV co-infected individuals is challenging and evidence suggests that coinfection with HBV or HCV adversely affect the prognosis of HIV infection and results in complex interactions with antiretroviral therapy [17]. Despite the effect of HBV and HCV infections among pregnant women (e.g. cholestasis of pregnancy, gestational diabetes etc.) as well as their babies (e.g. preterm birth, low birth weight neonatal abstinence syndrome etc.) [18, 19], limited studies have addressed the issue of co-infection with HCV and/or HBV in HIV-positive pregnant women globally to date, with no study conducted in Ghana. It is against this background that this study was conducted to ascertain the sero-prevalence of HBV, HCV, HIV/HBV and HIV/HCV, to evaluate the effect of the co-infections on the immunological and virological characteristics in pregnant women and to identify the association between some demographic and lifestyle characteristics and risk of HBV and HCV infections in HIV positive and negative pregnant women in the Brong-Ahafo Region of Ghana.
Materials and methods
Study design/setting
This was a comparative cross-sectional study conducted at the antiretroviral therapy (ART) clinic of the St. Elizabeth Hospital, Hwidiem and the Holy Family Hospital, Techiman, all in the Brong-Ahafo Region of Ghana between May 2012 and 2013. Both hospitals are located in a semi-urban setting. The St. Elizabeth hospital is a Catholic health delivery facility which serves as the main district and referral hospital for both Asutifi North and South districts, offering antiretroviral therapy (ART), voluntary counseling and testing/ Prevention of Mother to Child Transmission (VCT/PMTCT) services. These services are provided in accordance with the WHO recommendations formulated for the 2013 guidelines on HIV testing and counselling, antiretroviral therapy (ART) and HIV service delivery [20]. The hospital also offers other healthcare services including curative, preventive, rehabilitative, diagnostic, and special programs to all its neighboring communities. The Holy Family Hospital is a 140 bed Missions hospital that serves the people of Techiman and the neighboring towns. The clients served by the Holy Family Hospital are predominantly farmers and traders.
Participants’ recruitment
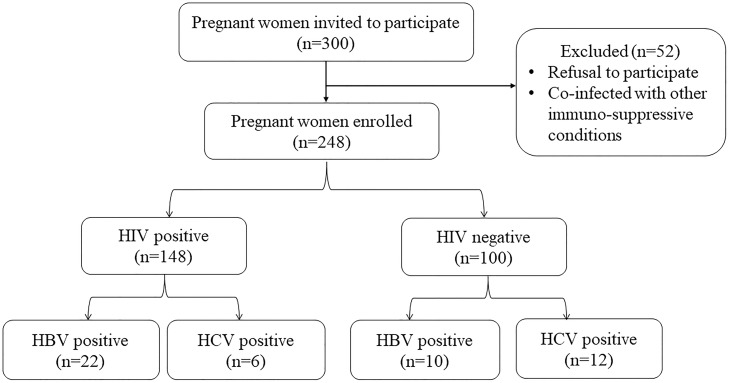
The sample size for the study was calculated using Fischer’s sampling formula (N = Z2 PQ/d2), where Z is the critical value of the normal distribution (1.96 at 95% CI); P is the estimated prevalence of HIV among pregnant women in Ghana (2.4%) [21]; d is the absolute precision or sampling error tolerated = 5%. From the above equation, the minimum sample size was 36 participants for each group. However, in the effort to enhance the statistical power, a total of 248 consecutive consenting pregnant Ghanaian women comprising 148 diagnosed with HIV and 100 who were HIV negative, living in the Brong-Ahafo Region were recruited during their routine clinic visit days. All HIV patients enrolled were on antiretroviral therapy (ART) at the time of study. Socio-demographic data were collected using an investigator-administered validated questionnaire in a language that they could easily comprehend. Additional information relevant to the study objectives were retrieved from the hospitals archive. Participants’ selection protocol is shown in Fig 1.
Fig 1. Flowchart of the protocol for the selection of subject.
Inclusion/ Exclusion criteria
Only pregnant women were included in this study. HIV positive pregnant women who have co-morbidities such as cancer, tuberculosis, and other immuno-suppressive conditions were excluded from this study.
Sample collection and assay
Venous blood sample (5 ml) was obtained from each participant under aseptic conditions. Haemoglobin level, total white cell and platelet count was performed using the Sysmex KX N21 analyzer (Symex Corporation, Japan). Screening for HIV was performed using the First Response HIV-1/HIV-2 WB (PMC Medical India Pvt Ltd) and confirmed with OraQuick ADVANCE Rapid HIV-1/2 (OraSure Technologies, Inc.). In the case of discordant results, Enzyme-Linked Immunosorbent Assay (ELISA; Organon Teknika, Boxtel, Co., Ltd., Netherlands) was used to break the tie. All HIV positives included in this study were HIV-1. CD4+ T cell count and HIV-1 RNA loads were estimated using BD FacsCalibur analyzer (BD Biosciences, USA) and COBAS AmpliPrep/COBAS TaqMan Analyzer (lower limit of detection: <20 copies/ml) (Roche Diagnostics, USA) respectively. HBV profile and anti-HCV antibody testing were done using commercially available test kits (Guangzhou Wondfo Biotech Co. Ltd, Luogang District, China). The HBV profile kit determined HBV surface antigen (HBsAg), HBV surface antibody (HBsAb), HBV ‘e’ antigen (HBeAg), HBV ‘e’ antibody (HBeAb), and HBV core antibody (HBcAb) based on Immunochromatographic technique. The testing, reporting and interpretation of the results were performed following the manufacturer’s instructions. To ensure accuracy, two each of the HBV profile and anti-HCV kits were pre-tested with known positive and negative samples prior to testing of participants samples. Samples positive for HBV using the HBV profile kits were further confirmed for the presence HBsAg using the DRG ELISA kit (DRG International, Inc, New Jersey, USA) following the manufacturer’s specifications. Samples confirmed reactive by the DRG ELISA kit were considered positive for HBsAg.
Data analysis
All categorical data were presented as frequencies (percentages) and Chi square and Fishers exact test statistic were used to test for associations between the baseline characteristics and HIV infection where applicable. Continuous variables were presented as mean ± SD and significance of difference between groups were tested with independent sample t-test. One-way ANOVA, followed by Tukey post-hoc multiple comparison test was used to assess the significance of differences of HIV-1 viral load and CD4 count between HIV/HBV, HIV/HCV, and HIV mono-infection. Univariate logistic regression analysis was performed to determine the odds of the presence of serological markers in HIV-positive compared to the HIV negative participants. Multivariate logistic regression analysis, using the enter method for variables with p values < 0.25 after univariate analysis, was performed to identify possible risk factors for HBV and HCV infections in HIV positive and HIV negative pregnant women. All tests were two-sided and a p value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) software 25 (SPSS Inc., Chicago, IL, USA), and GraphPad Prism 7 version 7.04 (GraphPad Software, Inc., La Jolla, California USA).
Ethics
Ethical approval for this study was obtained from the Committee on Human Research Publication and Ethics (CHRPE) of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology and from the Research (CHRPE/RC/131/12) and Development Units of St. Elizabeth Hospital, Hwidiem and Holy Family hospital, Techiman. Written informed consent was obtained from all participants who opted to participate after the aims and objectives of the study was explained to them. Participation was voluntary, and respondents were assured that the information obtained was strictly for research and academic purposes only and were guaranteed the liberty to opt out from the study at their own convenience.
Results
Of the 248 participants recruited, 148 (59.7%) were HIV positive with mean age of 29.1 years whereas 100 (40.3%) were HIV negative with a mean age of 28.9 years. Overweight, reduced haemoglobin, and low WBC count were significantly associated with subjects with HIV compared to those without HIV. A higher proportion of the HIV-infected participants had history of sharing razors blades and pins compared to subjects without HIV (Table 1).
Table 1. Baseline characteristics of study population.
| Variables | HIV-Positive n = 148 (59.7%) |
HIV-Negative n = 100 (40.3%) |
P-value |
|---|---|---|---|
| Age (years) | 29.1 ± 6.1 | 28.9 ± 6.4 | 0.804* |
| 15–19 | 14 (46.7) | 16 (53.3) | 0.299† |
| 20–30 | 82 (61.2) | 52 (38.8) | |
| >30Years | 52 (61.9) | 32 (38.1) | |
| History of sharing razors blades and pins | <0.0001‡ | ||
| Ever | 60 (78.9) | 16 (21.1) | |
| Never | 88 (51.1) | 84 (48.9) | |
| History of blood transfusion | 0.408‡ | ||
| Ever | 28 (77.8) | 8 (22.2) | |
| Never | 120 (56.6) | 92 (43.4) | |
| Period of HIV diagnosis | N/A | ||
| Before pregnancy | 34 (23.0) | - | |
| During pregnancy | 114 (77.0) | - | |
| If during pregnancy, at what stage? | N/A | ||
| First trimester | 14 (12.3) | - | |
| Second trimester | 60 (52.6) | - | |
| Third trimester | 40 (35.1) | - | |
| Medication used | |||
| 3TC+AZT+EFV | 4 (2.7) | - | N/A |
| 3TC+TDF+EFV | 12 (8.1) | - | N/A |
| 3TC+AZT+NVP | 132 (89.2) | - | N/A |
| Marital status | 0.039‡ | ||
| Single | 44 (71.0) | 18 (29.0) | |
| Married | 104 (55.9) | 82 (44.1) | |
| Employment status | 0.007† | ||
| Formal | 14 (50.0) | 14 (50.0) | |
| Informal | 100 (56.2) | 78 (43.8) | |
| Unemployed | 34 (81.0) | 8 (19.0) | |
| BMI (kg/m2) | 26.90 ± 3.91 | 23.17 ± 3.55 | <0.0001* |
| Underweight | 16 (72.7) | 6 (27.3) | <0.0001† |
| Normal | 64 (45.7) | 76 (54.3) | |
| Overweight | 68 (79.1) | 18 (20.9) | |
| Haematology | |||
| Haemoglobin (g/dl) | 9.7 ± 1.81 | 10.75 ± 1.68 | <0.0001* |
| WBC (103/μL) | 5.60 ± 1.81 | 7.23 ± 2.13 | <0.0001* |
| Platelet count (103/μL) | 220.62 ± 86.73 | 209.38 ± 65.59 | 0.272* |
Continuous data is presented as Mean ± SD. Categorical data is presented as frequency (%).
Chi square† and Fisher‡ exact test was performed to compare categorical variables.
*Independent t-test was performed to compare continuous variables.
Lamivudine (3TC), Zidovudine (AZT), Nevirapine (NVP), Tenofovir (TDF), Efavirenz (EFV). N/A: Not applicable, p < 0.05 was considered statistically significant (p values of significant variables are in bold print).
The sero-prevalence of HIV/HBV and HIV/HCV was 14.9% and 4.1% respectively. Among the HIV-negative pregnant women, the prevalence of HBV and HCV was 10.0% and 12.0% respectively. There was no triple infection (Fig 2).
Fig 2. The sero-prevalence of HBV and HCV among the study population.
Of the 22 HIV positive patients who were positive for HBsAg, 4 (18.2%) were positive for HBeAg. Additionally, HIV-infected participants were significantly less likely to sero-convert from HBeAg to HBeAb [OR = 0.21, 95% CI (0.10–0.43), p<0.0001]. On the other hand, HIV-positive participants were significantly less likely to be infected with HCV [OR = 0.31, 95% CI (0.11–0.86), p = 0.024] (Table 2).
Table 2. The prevalence of HBV and HCV serological markers stratified by the HIV status.
| Serological Marker | HIV-Positive n = 148 (59.7%) | HIV-Negative n = 100(40.3%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| HBsAg | 22 (68.8) | 10 (31.3) | 1.57 (0.71–3.48) | 0.265 |
| HBsAb (Anti-HBs) | 4 (50.0) | 4 (50.0) | 0.67 (0.16–2.73) | 0.573 |
| HBeAg | 4 (100.0) | 0 (0.0) | 6.26 (0.33–117.56) | 0.220 |
| HBeAb (Anti-Hbe) | 12 (28.6) | 30 (71.4) | 0.21 (0.10–0.43) | <0.0001 |
| HBcAb (Anti-HBc) | 44 (57.9) | 32 (42.1) | 0.90 (0.52–1.56) | 0.704 |
| Anti-HCV | 6 (33.3) | 12 (66.7) | 0.31 (0.11–0.86) | 0.024 |
Univariate logistic regression analysis was performed to determine the odds of the presence of serological markers in HIV-positive compared to the HIV negative participants. p < 0.05 was considered statistically significant (p values of significant variables are in bold print).
HIV-1 viral load was not significantly different between HIV/HBV, HIV/HCV co-infection and HIV mono-infection. However, CD4+ T lymphocyte count was significantly lower in HIV/HBV co-infection compared to HIV/HCV and HIV mono-infection respectively (Table 3).
Table 3. Virological and cellular markers of HIV among HIV positive participants.
| Parameters | HIV/HBV (a) | HIV/HCV (b) | HIV-mono (c) | p-value | Significant pairs |
|---|---|---|---|---|---|
| HIV-1 viral Load (log10 copies/ml) | 4.53 ± 3.70 | 2.72 ± 2.11 | 4.45 ± 3.72 | 0.523 | - |
| CD4 Count (cells/μl) | 364 ± 181 | 512 ± 123 | 514 ± 169 | 0.0009 | a&b; a&c |
One-way ANOVA followed by Tukey post hoc multiple comparison test was used to test for significance of difference between HIV/HBV, HIV/HCV co-infections and HIV mono-infection. p < 0.05 was considered statistically significant (p values of significant variables are in bold print).
Multivariate logistic regression analysis showed no statistically significant association between age, marital status, employment, history of sharing razors blades and pins, and history of blood transfusion with risk of HIV/HBV and HIV/HCV co-infection. However, late diagnosis of HIV (during pregnancy) was significantly associated with increased odds of HIV/HBV [aOR = 3.02 (0.16–14.92–306.5), p = 0.015] compared to HIV diagnosis before pregnancy. Similarly, there was no significant association between the risk factors and HBV and HCV infection in HIV negative pregnant women with the exception of having history of sharing razors blades and pins, where a significantly increased odd of HBV infection [aOR = 6.47, 95% CI (1.15–36.48), p = 0.030] was observed (Table 4).
Table 4. Multivariate logistic regression analysis of risk factors for HBV and HCV co-infections in HIV positive and HIV negative pregnant women.
| Risk Factors | HIV positive | HIV negative | ||||||
|---|---|---|---|---|---|---|---|---|
| HBV | HCV | HBV | HCV | |||||
| aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age group (years) | ||||||||
| 15–19 | 1 | 1 | 1 | 1 | ||||
| 20–30 | 1.20 (0.24–6.58) | 0.794 | 0.81 (0.04–18.63) | 0.900 | 1.02 (0.08–8.93) | 0.870 | 3.85 (0.25–60.52) | 0.338 |
| >30 | 0.52 (0.07–3.85) | 0.525 | 23.16 (0.31–178.94) | 0.150 | 1.11 (0.12–10.34) | 0.930 | 11.23 (0.82–153.0) | 0.070 |
| Marital status | ||||||||
| Single | 1 | 1 | 1 | 1 | ||||
| Married | 0.49 (0.18–1.35) | 0.169 | 0.04 (0.02–0.45) | 0.310 | 0.35 (0.04–2.83) | 0.330 | 0.32 (0.06–1.66) | 0.173 |
| Employment status | ||||||||
| Unemployed | 1 | 1 | 1 | 1 | ||||
| Employed | 0.98 (0.30–3.16) | 0.971 | 0.13 (0.0–0.53) | 0.220 | 0.57 (0.05–6.73) | 0.650 | 0.44 (0.05–3.76) | 0.454 |
| History of sharing razors blades and pins | ||||||||
| Never | 1 | 1 | 1 | 1 | ||||
| Ever | 2.38 (0.83–6.78) | 0.105 | 9.56 (0.68–134.55) | 0.090 | 6.47 (1.15–36.48) | 0.030 | 3.57 (0.62–20.53) | 0.154 |
| History of blood transfusion | ||||||||
| Never | 1 | 1 | 1 | 1 | ||||
| Ever | 1.77 (0.50–6.36) | 0.379 | 5.98 (0.43–84.30) | 0.190 | 4.62 (0.55–39.19) | 0.160 | 1.09 (0.12–9.95) | 0.942 |
| Period of HIV diagnosis | - | - | - | - | ||||
| Before pregnancy | 1 | 1 | ||||||
| During pregnancy | 3.02 (0.61–14.92) | 0.015 | 0.04 (0.0–0.45) | 0.900 | ||||
| If during pregnancy, at what stage? | - | - | - | - | ||||
| First trimester | 1 | 1 | ||||||
| Second trimester | 7.47 (0.42–134.1) | 0.172 | 0.24 (0.01–12.59) | 0.480 | ||||
| Third trimester | 10.06 (0.54–187.5) | 0.122 | 2.38 (0.11–52.77) | 0.580 | ||||
Multivariate logistic regression analysis was performed to determine the odds of the sociodemographic, lifestyle and obstetric factors HBV and HCV infections in HIV positive and negative pregnant women. p < 0.05 was considered statistically significant (p values of significant variables are in bold print).
Discussion
HBV or HCV co-infection is a major health burden among HIV patients even in the era of Highly Active Antiretroviral therapy (HAART). These co-infections are even more alarming during pregnancy due to the risk of vertical transmission and the eventual adverse effects on the newborns [18, 19]. In Ghana however, no current study has addressed the issue of co-infection with HCV and/or HBV in HIV-infected pregnant women to date. Thus, the need for this study to provide update on this issue.
We found a high sero-prevalence of HBV (14.9%) and HCV (4.1%) infection among HIV positive pregnant women. This finding is similar to the finding of a study by Simpore et al. [15] among pregnant women in Burkina Faso. They reported a sero-prevalence of HIV/HBV and HIV/HCV of 11.6% and 4.8% respectively. On the contrary, our finding is higher than the findings of Rouet et al. [22] who reported sero-prevalence of HBV and HCV of 9.0% and 1.0% respectively among HIV-positive pregnant women in Côte d’Ivoire. This discrepancy could be due to the higher national HBV and HCV prevalence rates for women in Ghana (HBV: 12.3% and HCV: 3.2%) [23, 24] compared to Côte d’Ivoire (HBV: 9.9% and HCV: 2.2%) [24, 25]. Sagoe et al. [26] also reported the sero-prevalence of 13.0% and 3.6% for HBV and HCV respectively people living with HIV in Ghana; a finding similar to the findings of this present study. Additionally, the prevalence of HBV (10.0%) and HCV (12.0%) among HIV negative pregnant women in this study is similar to the finding of a study by Rouet et al. [22] among Ivorian women and Kumar et al. [27] among Egyptian women respectively. They reported HBV and HCV prevalence of 8.0% and 13% respectively in HIV negative women. Nonetheless, the higher sero-prevalence of HCV among HIV negative pregnant women compared to HBV is in dissonance with some findings of studies conducted locally [28, 29], and internationally [30]. This discrepancy may be due to fact the aforementioned studies utilized ELISA-based methods for the detection of HCV antibodies, which have higher specificity compared to the Immunochromatographic method employed in this study. Furthermore, the higher sero-prevalence of HIV/HBV compared to HIV/HCV in this study is also consistent with studies by Simpore et al. [15], Rouet et al. [22], and Sagoe et al. [26]. However, a study by Landes et al. [31] in Europe reported a prevalence of 4.9% and 12.3% for HBV and HCV co-infections among HIV-infected pregnant women respectively, showing a higher prevalence of HIV/HCV than HIV/HBV. The discrepancy with our finding may be due to differences in geographical location and lifestyle. Moreover, Europe has been reported to have a higher prevalence of HCV than HBV [32] compared to sub-Saharan Africa where the prevalence of HBV is usually higher than HCV.
Taken together, the sero-prevalence of HBV was higher among pregnant women with HIV (14.9%) than pregnant women without HIV (10.0%); a finding which is consistent with the finding of a study by Simpore et al. [15] in Burkina Faso and Rouet et al. [22] in Côte d’Ivoire. The high prevalence of HBV in HIV is attributed to common routes of transmission [33, 34]. Furthermore, the high prevalence of HBV among pregnant women also suggests these pregnant women may serve as a possible potential pool of HBV to fuel hepatitis B endemicity in Ghana. Nonetheless, the low prevalence of HBeAg among HIV/HBV co-infected pregnant women and the fact that none of the HIV negative-HBV positive pregnant women in this study were positive for HBeAg suggest a low likelihood of perinatal transmission of HBV in Ghana, because perinatal transmission of HBV is largely effective in HBeAg positive mothers [35].
While some studies have reported a higher HIV viral load among HIV/HBV co-infected subjects’ relative to HIV/HCV and HIV mono-infection [33, 36], others report no significant association [37–39]. In this study, we observed no significant differences regarding HIV-1 viral load between pregnant women with HIV/HBV, HIV/HCV, and HIV mono-infection. However, it worthy of note that, despite being on ART, the viral loads of all the HIV positive subjects were high. This could possibly be linked to the high prevalence of non-adherence to ART among HIV patients in Ghana [40–42]. On the contrary, pregnant women with HIV/HBV presented with significantly lower CD4+ T-lymphocyte in comparison with HIV/HCV and HIV mono-infection respectively, which is in harmony with the finding of a study by Laurent et al. [38] and Omland et al. [39].
In assessing the risk factors for HIV/HBV and HIV/HCV, we found no significant association between age, marital status, employment, history of sharing razors blades and pins, and history of blood transfusion with risk of HBV and HCV infection among HIV positive pregnant women as consistent with a cross-sectional study by Zenebe et al. [43] in Ethiopia and Nimzing et al. [44] in Nigeria. However, late diagnosis of HIV (during pregnancy) posed greater risk of acquiring HIV/HBV compared to HIV diagnosis before pregnancy. This underscores the need for the development and implementation of a comprehensive policy for the screening of all females of reproductive age in the study area. Furthermore, there is the need to ensure early diagnosis of HIV during pregnancy since the risk of co-infection with HBV was also high when HIV was diagnosed in the second and third trimesters compared to the first trimester. On other hand, among HIV negative subjects, we observed that having history of sharing razors blades and pins, compared to those with no history, posed a significantly higher risk of HBV infection. This was expected because sharing sharps has been established as a risk factor for HBV infection [45]. This finding is partly in harmony with the finding of Ngaira et al. [46] among pregnant women attending antenatal clinic in Kenya and Obi et al. [47] among pregnant women in Nigeria.
Despite the non-significant associations, it is worth noting that, pregnant women aged 20–30 years old, those who have history of sharing razors blades and pins and blood transfusion had higher odds of HIV/HBV, HIV/HCV co-infections and HBV, HCV infections in HIV negative pregnant women. The higher risk of co-infection among women aged 20–30 years old could be attributed to the increased sexual activities among the youth. Furthermore, sharing razors blades and pins and blood transfusion has been reported to increase the risk of infections such as HIV, HBV and HCV. As such, public health education on safe sex, especially for young women, needs to be strengthened, and haemovigilance must be intensified to reduce and possibly eradicate transfusion-transmitted infections, particularly during pregnancy where there is increased demand for blood.
This study is nonetheless limited the fact that it was conducted in a semi-urban setting and thus the findings may not be generalizable to other areas especially rural areas. Furthermore, only a single brand of RDT was used to evaluate the prevalence of HCV and for the HBV profile. As such, the prevalence may not be the same when other commercially available test kits are employed. In addition, we did not perform anti-HBc IgM. Also, testing for HCV based on detection of antibodies rather than HCV RNA and the fact that we did not perform HBV DNA testing may have over-estimated the prevalence of HCV and HBV in this study. Additionally, the lower number of co-infected subjects obtained in this study limits some of the formal analysis. Thus, we recommend that further studies be conducted in the larger population.
Conclusion
The prevalence of HIV/HBV (14.9%), HIV/HCV (4.1%) co-infection, HBV (10.0%), and HCV (12.0%) are high among pregnant women in the Brong Ahafo Region of Ghana. HIV/HBV is associated with reduced CD4+ T lymphocyte count. Early diagnosis of HIV and intensification of routine antenatal HBV are essential to abate the risk of maternal to child transmission.
Supporting information
(XLSX)
Acknowledgments
The authors express their gratitude to all staff and patients of the ART clinic at the St. Elizabeth Hospital, Hwidiem and the Holy Family Hospital, Techiman in the Brong-Ahafo Region who actively participated in this study.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.WHO. Global hepatitis report 2017: World Health Organization; 2017. https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
- 2.WHO. Global Health Observatory (GHO) data 2018. http://www.who.int/gho/hiv/en/.
- 3.UNAIDS. AIDSinfo 2018. AIDSinfo.unaids.org.
- 4.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS DATA 2018: Global and regional data: UNAIDS; 2018. www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf.
- 5.Ghana Aids Commission. National and Sub-National HIV and AIDS Estimates and Projections: 2017 Report 2017. http://www.ghanaids.gov.gh/gac1/pubs/2017-2022_national_and_sub%20national_Estimates_Report.pdf.
- 6.WHO. World Hepatitis Day 2018: Test. Treat. Hepatitis: World Health Organization; 2018. https://www.who.int/campaigns/world-hepatitis-day/2018.
- 7.WHO. Hepatitis C: World Health Organization; 2016. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c.
- 8.Rao VB, Johari N, Cros P, Messina J, Ford N, Cooke G. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2014;15(7):819–24. [DOI] [PubMed] [Google Scholar]
- 9.Spearman C, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. The lancet gastroenterology & hepatology. 2017. 2(12):900–9. [DOI] [PubMed] [Google Scholar]
- 10.Stockdale AJ, Geretti A. Chronic hepatitis B infection in sub-Saharan Africa: a grave challenge and a great hope. Journal of Virology. 2015;109:421–2. [DOI] [PubMed] [Google Scholar]
- 11.Mutocheluh M, Owusu M, Kwofie TB, Akadigo T, Appau E, Narkwa P. Risk factors associated with hepatitis B exposure and the reliability of five rapid kits commonly used for screening blood donors in Ghana. BMC Research Notes. 2014;7:873 10.1186/1756-0500-7-873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankson A, Wiredu E, Gyasi R, Adjei A, Tettey Y. Sero-Prevalence of Hepatitis B and C Viruses in Cirrhosis of the Liver in Accra, Ghana. Ghana Med J. 2005;39(4):132–7. [Google Scholar]
- 13.Easterbrook P, Johnson C, Figueroa C, Baggaley R. HIV and hepatitis testing: global progress, challenges, and future directions. AIDS Rev. 2016;18(1):3–14. [PubMed] [Google Scholar]
- 14.Elsheikh R, Daak A, Elsheikh M, Karsany M, Adam I. Hepatitis B virus and hepatitis C virus in pregnant Sudanese women. Virology Journal. 2007;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpore J, Savadogo A, Ilboudo D, Nadambega M, Esposito M, Yara J, et al. Toxoplasma gondii, HCV, and HBV seroprevalence and co-infection among HIV-positive and-negative pregnant women in Burkina Faso. Journal of medical virology. 2006;78(6):730–3. 10.1002/jmv.20615 [DOI] [PubMed] [Google Scholar]
- 16.UNAIDS. A progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive: UNAIDS; 2012. www.unaids.org.
- 17.Nyirenda M, Beadsworth M, Stephany P, Hart C, Hart I, Munthali C, et al. Prevalence of infection with hepatitis B and C virus and coinfection with HIV in medical inpatients in Malawi. Journal of Infection. 2008;57(1):72–7. 10.1016/j.jinf.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Dunkelberg J, Berkley E, Thiel K, Leslie K. Hepatitis B and C in pregnancy: a review and recommendations for care. Journal of perinatology. 2014;34(12):882 10.1038/jp.2014.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safir A, Levy A, Sikuler E, Sheiner E. Maternal hepatitis B virus or hepatitis C virus carrier status as an independent risk factor for adverse perinatal outcome. Liver international. 2010;30(5):765–70. 10.1111/j.1478-3231.2010.02218.x [DOI] [PubMed] [Google Scholar]
- 20.WHO. HIV/AIDS: Summary of new recommendations, Consolidated ARV guidelines, June 2013: World Health Organization; 2013 [23/05/2019]. https://www.who.int/hiv/pub/guidelines/arv2013/intro/summarynewrecommendations.pdf?ua=1.
- 21.Ghana Aids Commission. Summary of the 2016 HIV sentinel survey report. 2016.
- 22.Rouet F, Chaix M, Inwoley A, Msellati P, Viho I, Combe P, et al. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Côte d’Ivoire: The ANRS 1236 study. Journal of medical virology. 2004;74:30–40. [DOI] [PubMed] [Google Scholar]
- 23.Ofori-Asenso R, Agyeman AA. Hepatitis B in Ghana: a systematic review & meta-analysis of prevalence studies (1995–2015). BMC infectious diseases. 2016;16(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonderup MW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L, et al. Hepatitis C in sub-Saharan Africa: the current status and recommendations for achieving elimination by 2030. The Lancet Gastroenterology & Hepatology. 2017;2(12):910–9. [DOI] [PubMed] [Google Scholar]
- 25.Magoni M, Ekra K, Aka L, Sita K, Kanga K. Effectiveness of hepatitis-B vaccination in Ivory Coast: the case of the Grand Bassam health district. Annals of Tropical Medicine & Parasitology. 2009;103(6):519–27. [DOI] [PubMed] [Google Scholar]
- 26.Sagoe W, Agyei A, Ziga F, Lartey M, Adiku T, Seshi M, et al. Prevalence and Impact of Hepatitis B and C Virus Co-Infections in Antiretroviral Treatment Naive Patients With HIV Infection at a Major Treatment Center in Ghana. Journal of Medical Virology. 2012;10(September 2011):6–10. [DOI] [PubMed] [Google Scholar]
- 27.Kumar RM, Frossad PM, Hughes PF. Seroprevalence and mother-to-infant transmission of hepatitis C in asymptomatic Egyptian women. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1997;75(2):177–82. [DOI] [PubMed] [Google Scholar]
- 28.Lassey A, Damale N, Bekoe V, Klufio C. Hepatitis C virus seroprevalence among mothers delivering at the Korle-Bu Teaching Hospital, Ghana. East African medical journal. 2004;81(4):198–201. [DOI] [PubMed] [Google Scholar]
- 29.Apea-Kubi K, Yamaguchi S, Sakyi B, Ofori-Adjei D. HTLV-1 and other viral sexually transmitted infections in antenatal and gynaecological patients in Ghana. West African journal of medicine. 2006;25(1):17–21. [DOI] [PubMed] [Google Scholar]
- 30.Aniszewska M, Kowalik-Mikołajewska B, Pokorska-Lis M, Kalinowska M, Cianciara J, Marczyrńska M. Seroprevalence of anti-HCV in pregnant women. Risk factors of HCV infection. Przeglad epidemiologiczny. 2009;63(2):293–8. [PubMed] [Google Scholar]
- 31.Landes M, Newell M, Barlow P, Fiore S, Malyuta R, Martinelli P, et al. Hepatitis B or hepatitis C coinfection in HIV-infected pregnant women in Europe. HIV medicine. 2008;9(7):526–34. 10.1111/j.1468-1293.2008.00599.x [DOI] [PubMed] [Google Scholar]
- 32.European Centre for Disease Prevention and Control. Systematic review on hepatitis B and C prevalence in the EU/EEA. Stockholm: ECDC, 2016. [Google Scholar]
- 33.Konopnicki D, Mocroft A, De Wit S, Antunes F, Ledergerber B, Katlama C, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. Aids. 2005;19(6):593–601. [DOI] [PubMed] [Google Scholar]
- 34.Nikolopoulo G, Paraskevis D, Hatzitheodorou E, Moschidis Z, Sypsa V, Zavitsanos X, et al. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clinical Infectious Diseases. 2009. 48(12):1763–71. 10.1086/599110 [DOI] [PubMed] [Google Scholar]
- 35.Kfutwah A, Tejiokem M, Njouom R. A low proportion of HBeAg among HBsAg-positive pregnant women with known HIV status could suggest low perinatal transmission of HBV in Cameroon. Virology journal. 2012;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Idoko J, Meloni S, Muazu M, Nimzing L, Badung B, Hawkins C, et al. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clinical infectious diseases. 2009;49(8):1268–73. 10.1086/605675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Firhaber C, Reyneke A, Schulz D, Malope B, Macphail P, Sanne E, et al. The prevalence of hepatitis B co infection in a South African (SA) urban government HIV clinic. South African Medical Journal. 2008;98(7):541–4. [PMC free article] [PubMed] [Google Scholar]
- 38.Laurent C, Bourgeois A, Mpoudi-Ngolé E, Kouanfack C, Ciaffi L, Nkoue N, et al. High rates of active hepatitis B and C co-infections in HIV-1 infected Cameroonian adults initiating antiretroviral therapy. HIV medicine. 2010;11(1):85–9. 10.1111/j.1468-1293.2009.00742.x [DOI] [PubMed] [Google Scholar]
- 39.Omland L, Weis N, Skinhøj P, Laursen AL, Christensen PB, Nielsen HI, et al. Impact of hepatitis B virus co-infection on response to highly active antiretroviral treatment and outcome in HIV-infected individuals: a nationwide cohort study. Hiv Medicine. 2008;9(5):300–6. 10.1111/j.1468-1293.2008.00564.x [DOI] [PubMed] [Google Scholar]
- 40.Obirikorang C, Selleh PK, Abledu JK, Fofie CO. Predictors of adherence to antiretroviral therapy among HIV/AIDS patients in the upper west region of Ghana. Isrn Aids. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prah J, Hayfron-Benjamin A, Abdulai M, Lasim O, Nartey Y. Factors Affecting Adherence to Antiretroviral Therapy among HIV/AIDS Patients in Cape Coast Metropolis. Ghana J HIV AIDS. 2018;4(1). [Google Scholar]
- 42.Okotah A, Korbuvi J. Adherence to Antiretroviral Therapy (Art) Among Adult Hiv Positive Patients in Volta Regional Hospital, Ghana. Value in Health. 2014;17(7):A329. [DOI] [PubMed] [Google Scholar]
- 43.Zenebe Y, Mulu W, Yimer M, Abera B. Sero-prevalence and risk factors of hepatitis B virus and human immunodeficiency virus infection among pregnant women in Bahir Dar city, Northwest Ethiopia: a cross sectional study. BMC infectious diseases. 2014;14(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nimzing L, Busari B, Ladep N. Seroprevalence of Hepatitis C Virus in HIV/AIDS Patients in Jos, Nigeria. Bangladesh Liver Journal. 2009;6(1):28–33. [Google Scholar]
- 45.WHO. Hepatitis B: World Health Organization; 2018. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- 46.Ngaira JAM, Kimotho J, Mirigi I, Osman S. Prevalence, awareness and risk factors associated with Hepatitis B infection among pregnant women attending the antenatal clinic at Mbagathi District Hospital in Nairobi, Kenya. The Pan African Medical Journal. 2016;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obi S, Onah H, Ezugwu F. Risk factors for hepatitis B infection during pregnancy in a Nigerian obstetric population. Journal of obstetrics and gynaecology. 2006;26(8):770–2. 10.1080/01443610600963986 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.