Abstract

In this study, the in situ sol–gel method has been deployed to prepare the titanium dioxide/multiwalled carbon nanotubes (TiO2/MWCNTs) nanocomposite (NCs) powders with varying content of MWCNTs (0.01–1.0 wt %), to construct the dye-sensitized solar cells (DSSCs). First, binder-free NCs were deposited on a transparent-conducting F:SnO2 (FTO) glass substrate by a doctor-blade technique and then anchored with Ru(II)-based dyes to either N719 or ruthenium phthalocyanine (RuPc). The structural and optical properties and interconnectivity of the materials within the composite are investigated thoroughly by various spectral techniques (XRD, XPS, Raman, FT-IR, and UV–vis), electron microscopy (HRTEM), and BET analysis. The experimental results suggest that the ratio of MWCNTs and TiO2 in NCs, morphology, and their interconnectivity influenced their structural, optical, and photovoltaic properties significantly. Finally, the photovoltaic performances of the assembled DSSCs with different content of MWCNTs to TiO2 films anchored with two different dyes were tested under one sun irradiation (100 mW/cm2). The measured current–voltage (IV) curve and incident photon-to-current conversion efficiency (IPCE) spectra of TiO2/0.1 wt % MWCNTs (T@0.1 C) for N719 dye show three times more power conversion efficiency (η = 6.21%) which is opposed to an efficiency (η = 2.07%) of T@0.1 C for RuPc dye under the same operating conditions.
Introduction
Solar energy has become one of the fastest growing industries among all current energy industries. Among the various renewable energy sources, sunlight energy is the largest global energy source and reaches the earth’s surface at an average of 4.3 × 1020 J h–1,1 which is equal to the annual energy demand of today’s society. A few generations of solar cell research are already in place for the fabrication of solar cells that convert solar light to electrical power.2,3 However, for the last two decades, dye-sensitized solar cells (DSSCs) seem to be one of the most promising, due to their low cost, easy construction and also generate comparable power conversion efficiency to silicon solar cells.4 In brief, DSSCs consist of two electrodes, namely, working electrodes (dye-coated semiconductors) and counter electrodes (platinized ITO glass) having an organic-based redox electrolyte (I–/I3–) between these two electrodes. Most of the research has focused on either using a semiconductor TiO2 or various ruthenium(II) [Ru(II)] based dyes.5 However, the other critical parameters, such as electron density, the mobility of charge carriers, right alignment of energy levels (HOMO and LUMO) of the dye, or redox mediator with respect to valence band and conduction band energy level of the semiconductor, also play a pivotal role in the enhancement of photovoltaic performance of DSSCs.6 However, the power conversion efficiency (η) of these devices has reached up to 14.5%.7 This efficiency is less as compared to the S–Q limit (theoretical efficiency of 34% for a single p–n junction)8 of DSSCs or nanomaterial-based solar devices (theoretical efficiency up to 66%).9 Therefore, the devices with modified TiO2 (varied in optical and electronic) electrode, anchored with various Ru(II) sensitizers (either N719 or RuPc), would benefit from harvesting more photons from a broad range of the solar spectrum.
Various strategic synthetic routes have been employed, such as doping,10 supported with metal/metal oxides,11 or composites,12 to tune the optical, electrical, porosity, and structure-induced morphology of TiO2; however, importance has been placed on preparing the visible active composites of TiO2 nanoparticles (NPs) with highly conducting MWCNTs.13 TiO2-MWCNTs NCs research has focused more on design of the electrode for solar energy harvesting materials. The reported synthesis of TiO2/MWCNTs NCs requires high temperature, expensive chemicals, and materials, and moreover, their nonuniform morphology lowers the performance as working electrode materials. Earlier, Wang et al. synthesized TiO2/MWCNTs nanohybrids by a single-step laser pyrolysis technique via both in situ and ex situ ways. However, DSSC efficiencies comprised of these electrodes were limited to only 3.9% and 3.3%.14 Moreover, the pyrolysis techniques always require state-of-the-art advanced and expensive equipment which is not desirable for photovoltaic technology.15 Mahmood et al. reported the DSSC efficiency of 5.25% using 0.06 wt % MWCNTs along with commercially purchased TiO2 paste. These photoelectrodes were prepared by mixing MWCNTs in ethanol with the TiO2 paste using the sonochemical method.16 However, MWCNTs not uniformly decorated with TiO2 particles by this technique were confirmed by TEM analysis. The use of only bare MWCNTs always retards the electron transport between the semiconducting photoelectrode to the counter electrode, overall decreasing the power conversion efficiency of the cells. A high-temperature hydrothermal synthesis of TiO2/MWCNTs NCs was also reported and resulted in the device efficiency of up to 7.37%, which is higher than that of bare TiO2 NPs as well as Degussa P25 NPs.17 The 5 wt % MWCNTs content composites of TiO2/MWCNTs thin films prepared by an electrospinning technique reported a greater efficiency.18 The agglomeration is the key problem of this kind of TiO2/MWCNTs NCs. These NCs also absorb the incident photons actively which retard the electron–hole separation and lower the photovoltaic performance.19 Also, the electrospinning technique always produces the irregular and nonuniform morphology structure within the NCs which often results in low power energy conversion efficiencies.
Ru(II)-based sensitizers have played a significant role in the development of the solar cells as a chromophore. The efficiency of the DSSCs could be improved significantly if these dyes have absorption in the range of visible to NIR of the solar spectrum.20 Researchers have studied various chromophores including metal complexes and organic-based dyes. Among all dyes, Ru(II) polypyridine-based sensitizers, mainly black dye, N3, and N719, are the better chromophores for DSSCs due to their promising chemical and photochemical stability along with enhanced photovoltaic performance.21 Similarly, the RuPc dye is also used as an alternative dye for DSSCs due to its analogues to N719 dye’s structure, chemical and photochemical stability, and high molar extinction coefficient. Indeed, this intrinsic nature of RuPc dye promotes harvesting of more photons in the entire range of the visible to NIR solar spectrum. Also, the suitable electrochemical redox property of RuPc is utilized as a promising dye to anchor semiconducting metal oxides (TiO2) for other applications.22
In this work, we report the synthesis of TiO2/MWCNTs NCs with varying content of MWCNTs by in situ sol–gel method, and then these materials are well characterized by various spectroscopies (XRD, XPS, UV–vis, PL, FT-IR, and Raman), microscopy techniques (HRTEM), and BET measurements for knowing their structural, morphological, and optical properties. Thereafter, these materials are deposited on FTO glass substrates by a doctor blade technique to form the photoelectrodes. Finally, the photovoltaic performances of the assembled sandwiched devices made of a different composite of TiO2/MWCNTs are measured (IV and IPCE) under one sun condition (100 mW/cm2) and compared with two different dyes (N719 and RuPc).
Results and Discussion
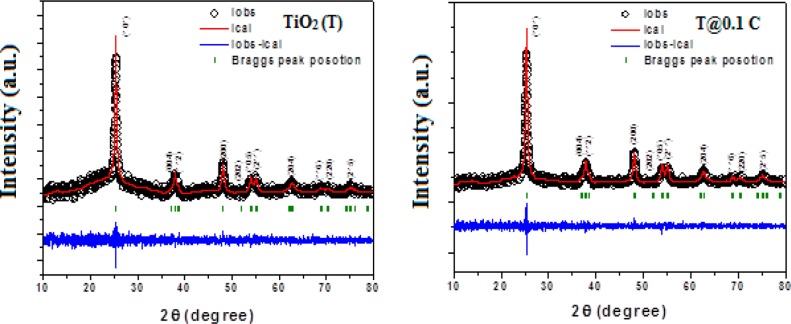
The thermal stability and existence of MWCNTs in the NC samples were studied by using thermogravimetric analysis (TGA) and illustrated in the Supporting Information (Figure S1, SI). The crystal structure of the pure phase of the materials (MWCNTs, bare TiO2 NPs, and TiO2/MWCNTs NCs) was characterized by using a Powder X-ray diffraction (XRD) technique. XRD pattern of MWCNTs (Figure 1(a)) shows a sharp peak at 26.17 corresponding to a (002) reflection, confirming the presence of elemental carbon (JCPDS No. 41-1487). XRD patterns of TiO2 and different TiO2/MWCNTs NCs are presented in Figure 1(b). The various peaks observed at ∼25.21 (101), ∼37.63 (004), ∼47.90 (200), ∼53.89 (105), ∼55.03 (211), ∼62.57 (204), ∼69.98 (220), and ∼74.93 (215) are the characteristic peaks of anatase TiO2 (JCPDS No. 21-1272). The sharp, intense peaks of the samples reveal the crystalline nature with crystallite size in the range between 15 and 19 nm. The structural cell parameters of the samples are calculated and presented (Table S1, SI). The expected peak position at 26.17 (002) reflection for MWCNTs did not appear in the TiO2/MWCNTs NCs because of either a tiny amount present in the composition or shielding by the most intense peak of anatase TiO2 appearing at ∼25.21 (101).23 As the content of MWCNTs increases in composite, a small change is observed in the peak positions with a definitive increase in the intensity of characteristic peaks of TiO2. The change in the peak positions of samples is reflected through the various cell parameters, crystallite size, and lattice strain,24 while the shifts in the intensity of the peaks are justified through the lattice sites of ions present in the TiO2 lattice (Table S1, SI).25
Figure 1.
XRD patterns of (a) MWCNTs and (b) bare TiO2 NPs and TiO2/MWCNTs NCs with varying content of MWCNTs from 0.01 to 1.0 wt %.
The quantitative studies related to structural properties were further confirmed by the Rietveld refinement method using the Fullprof 2000 software package. Rietveld refined XRD patterns of the bare TiO2 NPs and T@0.1C NCs are shown in Figure 2(a,b), and the remaining patterns are also shown in the Supporting Information (Figures S3, S4, S5, and S6, SI).
Figure 2.
XRD Rietveld refined patterns of (a) bare TiO2 NPs and (b) T@0.1C NCs.
The lattice reflections such as (101), (004), (112), (200), (202), (105), (211), (204), (116), (220), and (215) in the Rietveld refined XRD patterns confirm the formation of the tetragonal anatase crystal structure. In the refinement, the oxygen positions (x, y, z) have been considered as free parameters, and fractional atomic positions have been taken as fixed. Other parameters such as lattice, temperature, occupancies, scale factors, and shape parameters have also been considered as free parameters. The quality of the Rietveld refinement quantified by the corresponding figures of merit, viz., Rwp, the goodness of fit (χ2), and pseudo-Voigt function, corrected the background of the pattern.
The atomic coordinates and occupancies of different atoms of the different samples are also presented (Table S2, SI). In the entire refined patterns, the value of “goodness of fit (χ2)” lies in the range of 1–1.5; this indicates well the extent of fitting. (The Rietveld refined factors such as χ2, Rwp, Rexp, RB, RF, etc. of all samples are summarized in Table 1.) The values of the various R factors are slightly higher, which may be due to the nanocrystalline nature of the samples and also could be assigned to the larger signal-to-noise ratio.26 Based on these Rietveld refinements, the average crystallite sizes (D) have been calculated using the Scherrer formula, which is observed from 17 to 24 nm. The increase in crystallite size, as well as little change related to lattice parameters of the samples, is observed with increasing composition of MWCNTs in the TiO2 host lattice.
Table 1. Rietveld Refinement Factors of Bare TiO2 and TiO2/MWCNTs NCs with Varying Composition of MWCNTs.
| samples |
||||||
|---|---|---|---|---|---|---|
| Rietveld refinement factors | TiO2 (T) | T@0.01 C | T@0.05 C | T@0.10 C | T@0.25 C | T@1.00 C |
| χ2 | 1.53 | 1.59 | 1.24 | 1.26 | 1.60 | 1.48 |
| RB (%) | 3.65 | 4.15 | 1.74 | 4.62 | 4.06 | 3.20 |
| RF (%) | 3.43 | 4.69 | 1.72 | 3.60 | 4.09 | 4.62 |
| Rwp | 29.8 | 34.4 | 26.12 | 34.2 | 33.5 | 22.1 |
| Rexp | 24.1 | 27.2 | 21.13 | 27.1 | 26.4 | 18.1 |
| D (nm) | 17 | 18 | 20 | 21 | 22 | 24 |
| a = b (Å) | 3.7880 | 3.7878 | 3.7874 | 3.7854 | 3.7851 | 3.7871 |
| c (Å) | 9.5025 | 9.5178 | 9.5191 | 9.5097 | 9.5096 | 9.5198 |
| V (Å3) | 136.35 | 136.55 | 136.54 | 136.21 | 136.20 | 136.51 |
| ρ (g/cm3) | 4.554 | 4.488 | 4.505 | 4.273 | 4.338 | 4.475 |
| O position (z) | 0.2004 | 0.2005 | 0.1993 | 0.2033 | 0.1993 | 0.2003 |
Raman spectra of the bare TiO2 NPs, MWCNTs only, and representative T@0.1 C NCs are shown in Figure 3(a). Raman spectrum of TiO2 NPs shows the characteristic peaks at 144.69, 398.4, 516.78, and 640.92 cm–1 corresponding to the Eg (1), B1g, A1g, and Eg (2) modes of vibrations, respectively.
Figure 3.
Raman spectra of bare TiO2 NPs, T@0.1C NCs, and MWCNTs (inset).
These peaks confirm the presence of the anatase phase only, which is in good agreement with the XRD results.27 Raman spectrum of MWCNTs is shown in the inset, which shows two bands, namely, the D band and G band. The D band is an indicative disorder in the graphitic structure at 1346.10 cm–1 due to the disorder induced by sp3 hybridization, whereas the G band (characteristic ordered graphitic structure) at 1585.01 cm–1 corresponds to ordered sp2 hybridization of MWCNTs. The intensity ratio (ID/IG) for the functionalized MWCNTs also reveals the presence of acidic functional moieties on the surface of MWCNTs with the conversion of the carbon atoms from sp2 to sp3 hybridization. Raman spectrum of functionalized MWCNTs with fitting results (Figure 3b) show graphitizable carbon activated by acidic functional moieties.28 Raman spectrum of a representative T@0.1 C NCs shows all the characteristic peaks of anatase TiO2 along with D (1350.96 cm–1) and G (1582.32 cm–1) bands of MWCNTs. It emphatically reveals the existence of MWCNTs composition in the NCs.29 The decrease in the peak intensity, with little shifting in the peak positions of all Raman bands for NCs, is shown in Figure 3 (c and d). It is usually due to the increase in the crystallite size with an increase in the MWCNTs content in the TiO2 host lattice.
Transmission electron microscopy (TEM), high-resolution TEM (HR-TEM) with seleced area electron diffraction (SAED) patterns of MWCNTs, bare TiO2 NPs and T@0.1 C NCs are shown in Figure 4 (a to i). TEM image (Figure 4(a)) of MWCNTs shows the cylindrical tubes having an average outer diameter in the range between 20 and 25 nm and a few micrometers in length. From HRTEM image (Figure 4(b)), the distance between the two successive inner layers of MWCNTs is around 0.33 nm, while the diameter between the two outermost shells is 23 nm. SAED pattern (Figure 4(c)) shows the bright ring patterns, which correctly match to the spacing of the (002), (100), and (006) reflections. Figure 4(d,e,f) shows TEM, HRTEM, and SAED patterns of the bare TiO2 NPs. A TEM image shows the spherical nanostructures having a mean particle diameter of 15–20 nm, while spacing for the (101) lattice fringes is 0.352 nm. SAED pattern indicates excellent crystallinity due to a clear ring structure with lattice points that directly match the anatase phase of TiO2. Figure 4(g,h,i) shows TEM, HRTEM, and SAED patterns of representative T@0.1 C NCs. TEM image shows the spherical nanostructures of the TiO2 NPs directly anchored on the surface of MWCNTs, and no bare MWCNTs is observed because of the high wall anchoring as well as density of TiO2 NPs.
Figure 4.
(a,d,g) TEM images, (b,e,h) HRTEM images, and (c,f,i) SAED patterns of MWCNTs, bare TiO2 NPs, and T@0.1 C NCs.
HRTEM micrograph shows that the clear fringes precisely match to the spacing of the (101) reflection of TiO2 NPs. However, uncleared fringes of MWCNTs are observed in the NCs because, in the in situ chemical route, a stronger chemical grafting occurs at the TiO2/MWCNT interface.30 The SAED pattern of the NCs shows good ring patterns with lattice points indicating crystallinity. The indexed ring designs closely match with the spacing of the various reflections of the anatase phase.
The optical properties of NCs powders were studied through UV–visible DRS spectra. Figure 5(a) includes UV–visible DRS spectra of bare TiO2 NPs and the representative TiO2/MWCNTs NCs. All samples show the absorption edge between 382 and 400 nm due to the excitation of electrons from the valence band to the conduction band of the TiO2 host material.29 With the increase in MWCNTs content in TiO2, not only the absorption capability but also the red shifting of the absorption edge of NCs are also observed. This optical absorption behavior reveals the strong interaction between the TiO2 and MWCNTs, which results in the enhancement of surface electric charge of the TiO2 NPs by MWCNTs.
Figure 5.
(a) UV–visible DRS spectra and (b) Kubelka–Munk function (αhυ)1/2 as a function of photon energy (hυ) of bare TiO2NPs and representative T@0.01 C, T@0.10 C, and T@1.00 C NCs.
It is also beneficial for the ease of charge transfer between TiO2 and MWCNTs and hence results in the enhancement of light absorption capability of TiO2 in the visible region, which are excellent aspects for light-harvesting ability of the photoanode material.31 To know the impact of varying content of MWCNTs on the optical properties of TiO2, the optical energy band gaps of all samples were determined. Optical energy band gaps of all the representative samples were calculated by using the Kubelka–Munk function (αhυ)1/2 (where α is the absorption coefficient) as a function of photon energy (hυ), which is shown in Figure 5(b). The optical energy band gap is recognized by plotting the intercept tangent to the x-axis in a graph, decreased from 3.2 to 2.85 eV with an increase in the content of MWCNTs in TiO2 (Table 2) (Figure S7, SI). The calculated optical band gap is firm evidence for the visible-light absorption by NCs as compared to bare TiO2 NPs.
Table 2. Optical Energy Band Gap, BET Surface Area, Amount of Dye Adsorbed (N719 and RuPc) on the Surface of the Bare TiO2, and Representative NCs Samples.
| amount
of dye adsorbed (mol/cm2) |
||||
|---|---|---|---|---|
| samples | optical band gap (eV) | surface area (m2/g) | N719 | RuPc |
| TiO2 (T) | 3.20 | 90.27 | 3.00 × 10–5 | 1.03 × 10–5 |
| T@0.1 C | 2.90 | 109.85 | 3.23 × 10–5 | 1.15 × 10–5 |
| T@1.0 C | 2.85 | 60.42 | 2.47 × 10–5 | -- |
To study the electronic behavior as well as separation of photogenerated charge carrier trapping with the fate of excitons in the semiconductor materials, the photoluminescence (PL) studies of the various materials were investigated. The PL spectra of bare TiO2 NPs and TiO2/MWCNTs NCs in the wavelength range between 450 and 650 nm are presented in Figure 6.
Figure 6.

Photoluminescence emission spectra of bare TiO2 NPs and TiO2/MWCNTs NCs with varying content of MWCNTs.
The emission peaks appeared at 486 and 527 nm, corresponding to the band–band emission and metal–nonmetal charge transfer transitions by excitation wavelength at 365 nm (Figure S8, SI).32 With the increase in the content of MWCNTs, the PL intensity of the respective emission peaks decreases. The decreasing behavior of PL is attributed to reductions in the radiative recombination of photoinduced electrons trapped at the surface of TiO2 with the content of MWCNTs, and hence NCs are best for the efficient charge separations. The detailed charge separation and energy level diagram of the photoelectrode are shown in the Supporting Information (Scheme S1, SI).
FT-IR spectra of MWCNTs, bare TiO2 NPs, and representative NCs are shown in Figure 7. FT-IR spectrum of MWCNTs shows the peaks at 3440, 2925, 2845, 1740, 1632, 1383, and 1110 cm–1 corresponding to the O–H stretching vibration, C–H stretching, C–OH stretching, C=O stretching, O–H deformation vibrations, and alkoxy C–O stretching vibrations, respectively.33,34
Figure 7.

FT-IR spectra of MWCNTs, bare TiO2 NPs, and representative T@0.1 C and T@0.25 C NCs.
FT-IR spectra of TiO2 NPs or NCs show the broad absorption band from 3000 to 3400 cm–1 assigned to the –OH stretching frequency vibration. The bands at the region 3000–3400 cm–1 broaden, with the content of MWCNTs, reflecting the increase in surface hydroxylation of NCs. A band in the range of 2925–2856 cm–1, analogous to the Ti–OH stretching vibration, is shifted to the lower frequency region at 2820–2730 cm–1 in the TiO2/MWCNTs NCs. The shifting is due to the −OH stretching frequency region of the Ti precursor overlapping with the other contributions like C=O, C–O, and O–C=O moieties of MWCNTs.23 Similarly, other peaks at 1374 cm–1 and 1590–1630 cm–1 in the NCs are also shifted to the longer frequency region, which reveals the interaction between the carboxylate groups of MWCNTs with the Ti precursors.30 The TiO2 NPs or NCs samples show the broad peak in the range between 550 and 900 cm–1, due to the various stretching vibrations such as Ti–O, O–Ti–O, Ti–O–C, and Ti–O–C=O.35
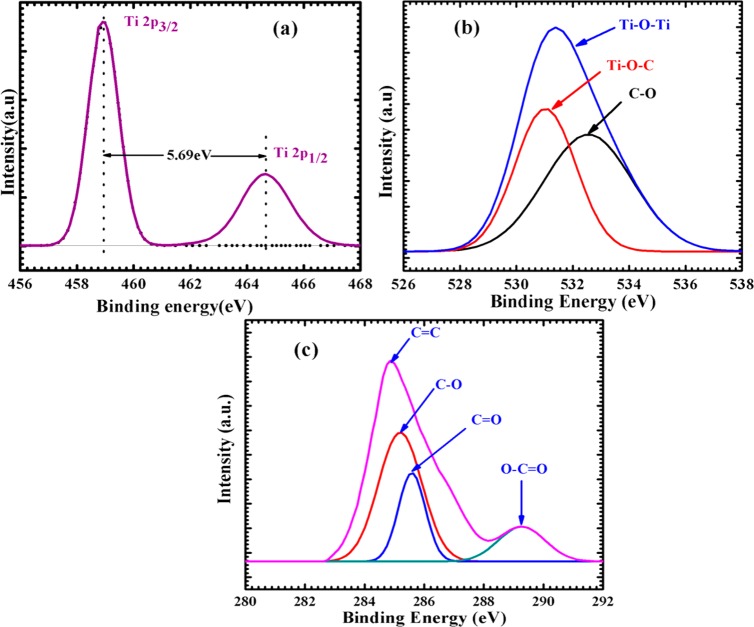
The chemical composition and the chemical states on the surface of the elements are studied by using EDAX, which is illustrated in the Supporting Information (Figure S2, SI) and XPS analysis shown in Figure 8. Figure 8(a) shows the high-resolution core level spectrum of the Ti ion, and it consists of two peaks at 458.95 and 464.66 eV corresponding to Ti 2P3/2 and Ti 2P1/2 states, respectively. The difference in binding energy between the two peaks (5.69 eV) corresponds to the Ti4+ state in the octahedral environment of the anatase TiO2.36Figure 8(b) shows the high-resolution core-level XPS spectrum of oxygen species. A major peak at 530.9 eV is due to the presence of lattice oxygen in the sample and is deconvoluted into two peaks at 531.4 and 532.6 eV and analogous to the carbonyl (−C=O) or carboxylic species from the TiO2/MWCNTs NC.29 The high-resolution C 1s core-level spectrum of the same sample is shown in Figure 8(c). A major peak at 284.8 eV is due to the sp2-hybridized carbon atoms, and it also deconvoluted into four peaks at 284.85, 285.26, 285.57, and 289.28 eV corresponding to the sp2-bonded carbon atoms of C=C, C–O, C=O, and ester groups (O–C=O), respectively. The existence of all these groups is beneficial for making the chemical bond formation between the TiO2 lattice and MWCNTs, and the absence of the other peak at 281 eV indicates that elemental carbon is not doped in the TiO2 lattice.37
Figure 8.
High-resolution XPS spectra of (a) Ti 2p, (b) C 1s, and (c) O 1s core-level spectra of T@0.1 C NCs powder.
Brunauer–Emmett–Teller (BET) analysis gives the specific surface area of the materials. Figure 9 shows the nitrogen (N2) adsorption–desorption isotherms of bare TiO2 NPs, representative T@0.1 C, and T@1.0 C NCs. The separation between the N2 adsorption–desorption curves indicates that samples exhibit type IV isotherms.38 T@0.1 C NCs as well as bare TiO2 show that the N2 adsorption rises abruptly due to the capillary condensation of N2 and leading to the formation of type H1 hysteresis loops. It also signifies the particles with spherical pore geometry and a high degree of pore size uniformity, while in the case of T@1.0 C NCs, the decrease in N2 adsorption is observed due to the reduction in surface area and also improper anchoring of TiO2 NPs on the surface of MWCNTs.39 The BET parameters of the samples are summarized in Table 2.
Figure 9.

N2 adsorption isotherms of bare TiO2 NPs and representative T@0.1 C and T@1.0 C NCs.
The specific surface area of bare TiO2 is found to be 90.27 m2/g, which is intermediate between the surface area of T@0.1 C (109.85 m2/g) and T@1.0 C (60.42 m2/g) samples. Overall, it reveals that the least addition of MWCNTs in the NCs increases the surface area to the TiO2 matrix due to the proper anchoring of TiO2 on the surface of the MWCNTs.40 In the design of the photoelectrode, the N719 dye is directly anchored on the surface of the NCs thin films through carboxylic groups of the N719 dye.41 However, due to the absence of carboxylic groups, RuPc is not directly anchored to the surface of the NCs thin films. Therefore, isonicotinic acid (INA) is used as a bridging ligand to connect RuPc with the surface of the NCs thin films, and hence the overall connectivity of RuPc with NCs through INA is similar to that of N719 dye. The bridging role of INA is confirmed by measuring the absorption spectra of TiO2/MWCNTs with and without INA, which is shown in the Supporting Information (Figure S9, SI). In comparison, the optical absorption of the TiO2/MWCNTs/INA/RuPc photoelectrode is higher than that without INA (TiO2/MWCNTs/RuPc). The optical behavior powerfully reveals that INA binds steadily to both dye and host materials, viz., the dye molecule through the pyridine ring and TiO2/MWCNTs through carboxylic acid moieties (Scheme 1).
Scheme 1. Schematic Representation of the Sandwich Structure of DSSCs Having either Dye (a) N719 or (b) RuPc Anchored Nanocrystalline TiO2/MWCNT Composites as a Working Electrode and Pt/ITO as the Counter Electrode along with the Redox Mediator.
After anchoring with dyes, the photoanodes further characterized by using UV–visible absorption spectroscopy. The UV–visible absorption spectrum of the TiO2-MWCNTs/N719 photoelectrode shows two characteristics absorption bands of N719 in the range 310–600 nm (Figure 10). The first band appeared at 370 nm due to the π–π* transition of the aromatic rings, and the second band is at 502 nm due to the internal charge transfer transition.42 Similarly, the TiO2/MWCNTs/INA/RuPc photoanode shows the characteristic Q-band of RuPc at 650 nm, where the maximum solar photons occur.43 In addition to the Q-band, it also absorbs the light to a small extent at the various regions of the electromagnetic spectrum. The bands in UV–visible patterns are characteristic absorptions of individual ingredients present in the photoelectrodes.
Figure 10.
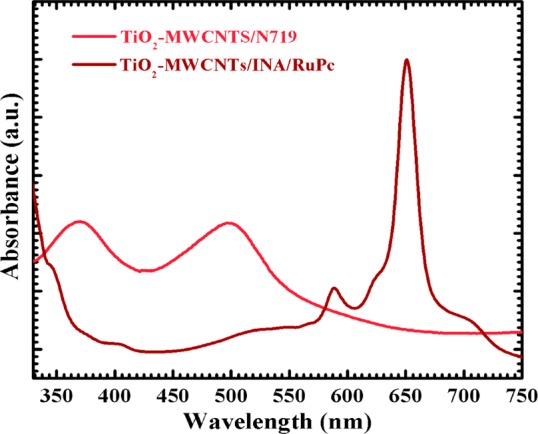
UV–visible absorption spectra of TiO2/MWCNTs/N719 and TiO2/MWCNTs/INA/RuPc based photoanodes.
After that, the amount of N719 and RuPc dyes was loaded on the surface of the NCs thin films measured by desorbing a 1.0 cm2 area of the dye adsorbed thin films into the 5 mL aqueous 1 mM KOH solution. It is evident from Figure 11(a) that the absorption spectra of N719 dye were detached from the TiO2 NPs and representative NCs thin films. It shows the two distinct absorption maxima at 370 and 500 nm, but the actual N719 dye shows maximum absorption peaks at 380 and 518 nm. The shifting to the 370 and 500 nm is due to the anchoring of the N719 dye molecules on the surface of the NCs thin films.42 RuPc shows the absorption maxima at 650 nm [Figure 11(b)] due to the characteristic Q-band. The quantification of dye adsorbed on the thin-film surface is calculated from Figure 11(a and b) and illustrated in Table 2. The observed result shows the T@0.1 C NCs photoelectrode having the maximum dye adsorption capability as compared to the bare TiO2 NPs and other NCs thin films. It is fascinating that the dye loading capacity of T@0.1 C NCs is higher as compared to the bare TiO2 NPs and other NCs based photoelectrodes. Hence, the high loading of dye offers the better harvesting of photons in the visible range of dyes and will increase the photocurrent density (Jsc) of the devices.
Figure 11.
UV–vis absorption spectra of solutions containing (a) N719 and (b) RuPc dyes detached from bare TiO2 and representative NCs thin films (all with 1.0 cm2 area) in 5 mL of aqueous solution of 1 mM KOH.
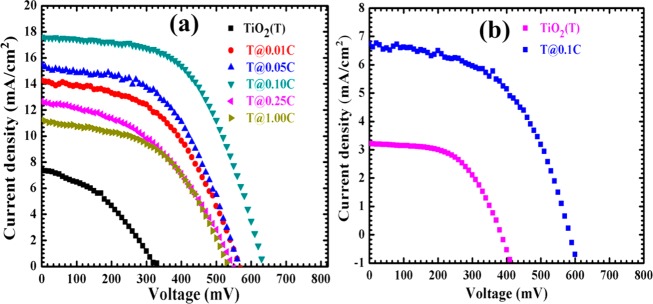
The sensitized photoanodes were used for sandwich-type DSSCs (Scheme S1, SI) and further tested for photovoltaic performance using a solar simulator under standard AM 1.5 one sun illumination (100 mW/cm2) with an active area of 0.25 cm2. In addition to the assessment of the sensitizer’s impact, the effect of MWCNTs on the photovoltaic properties of the TiO2 host lattice with the N719-based DSSC device showed different solar cell parameters. Figure 12(a,b) shows the current density–voltage characteristic curves of the samples with N719 and RuPc dyes, respectively.
Figure 12.
Photocurrent density vs voltage curves of (a) bare TiO2 and TiO2/MWCNTs NCs with varying composition of MWCNTs for N719 and (b) bare TiO2 and T@0.1C NCs for RuPc-based DSSC devices.
The different photovoltaic parameters such as photocurrent density (Jsc), open-circuit voltage (Voc), fill factor (FF), and light to electrical conversion efficiency (η) of the DSSCs are represented in Table 3.
Table 3. Photovoltaic Parameters of Bare TiO2 NPs and TiO2/MWCNTs NCs with N719- and RuPc-Based DSSC Devices.
| solar
cell parameters |
|||||
|---|---|---|---|---|---|
| samples | MWCNT content (wt %) | JSC (mA cm–2) | VOC (V) | FF (%) | η (%) |
| For N719 Dye | |||||
| TiO2 (T) | 0.00 | 07.37 | 0.330 | 43.77 | 1.04 |
| T@0.01C | 0.01 | 14.27 | 0.560 | 49.48 | 3.95 |
| T@0.05 C | 0.05 | 15.50 | 0.560 | 51.72 | 4.49 |
| T@0.1 C | 0.10 | 17.60 | 0.630 | 56.08 | 6.21 |
| T@0.25 C | 0.25 | 12.64 | 0.540 | 44.61 | 3.04 |
| T@1.0 C | 1.00 | 11.24 | 0.530 | 50.21 | 2.99 |
| For RuPc Dye | |||||
| Bare TiO2 | 0.00 | 0.46 | 0.300 | 57.49 | 0.07 |
| T@0.1 C | 0.10 | 6.73 | 0.580 | 53.24 | 2.07 |
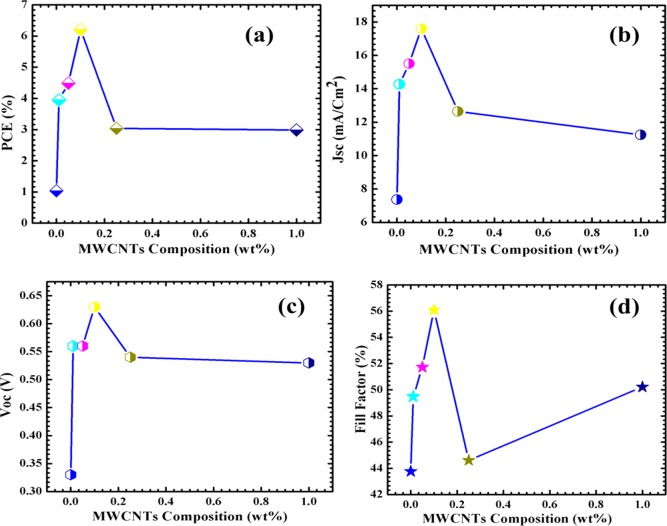
In the case of FTO/TiO2-MWCNTs/N719 DSSCs, the overall highest power conversion efficiency of 6.21% is noted for T@0.1 C NC-based DSSCs. This highest efficiency of T@0.1 C NC-based DSSCs is also reflected through its highest values of Jsc (17.60 mA/cm2), Voc (0.630 V), and FF (56.08%). However, the Jsc value of other NC-based DSSCs varied from 11.24 to 15.50 mA/cm2 (Figure 13(b)). Specifically, the Jsc value is increased up to T@0.1 C NC-based DSSCs, and after that, it decreased to 11.24 mA/cm2 for T@1.0 C NC-based DSSCs, which is shown in Table 3. It is also interesting that the Jsc value of NC-based DSSCs is higher than that of bare TiO2-based DSSCs. This change in Jsc value of NCs or bare TiO2-based DSSCs is collinear with the change in surface area of NCs as well as dye loading capacity of NC-based photoelectrodes (Table 2). In addition, the Jsc value of TiO2/MWCNTs/N719 DSSCs is higher than that of TiO2/MWCNTs/RuPc DSSCs; this is attributed to the reasons such as connectivity between the host material and dye and coverage of optical region. In the case of N719-based DSSCs, the direct connectivity between N719 and TiO2/MWCNTs is observed, while in RuPc-based DSSCs the INA acts as a bridging ligand between TiO2/MWCNTs and dye. Though absorption is higher for RuPc dye, the coverage region is small. Hence, the synergetic effect of these factors is responsible for an improved charge-carrier transport resulting in an increase in Jsc values of TiO2/MWCNTs/N719-based DSSCs.44
Figure 13.
Plot of MWCNTs content (wt %) in the TiO2 host lattice as a function of (a) η, (b) Jsc, (c) Voc, and (d) FF. [Conditions: substrate: FTO, dye: 0.3 mM N719 in 1:1 ratio of tertbutyl alcohol and acetonitrile, electrolyte: 1.0 M LiI + 0.06 M I2 in propylene carbonate, area: 0.25 cm2, counter: Pt-deposited ITO, light source: 300 W xenon lamp with AM 1.5G filter.]
A plot of Voc as a function of MWCNTs composition in TiO2 is shown in Figure 13(c). It revealed that no significant change is observed for Voc value in NC-based DSSCs except T@0.1 C NC-based DSSCs. The Voc value of NC-based DSSCs is seen around 0.550 V, which is still lower than that for bare TiO2-based DSSCs. The lower photovoltage is attributed to the nearly same absorption edge of the NC photoelectrodes, while in the case of FF value the change is similar to that of Jsc as well as the efficiency of DSSCs. Up to T@0.1 C NC-based DSSCs, the FF value increased to 56% from 43%, and after that, it decreased for higher content of MWCNTs in the TiO2 host lattice, which is shown in Figure 13(d). Moreover, the efficiency of all NCs-based DSSCs is in the range of 2.99–6.21%, which is three to six times more than that of bare TiO2-based DSSCs (1.04%) [Figure 12(a)]. From the photovoltaic analysis of TiO2/MWCNTs NCs with N719 dye sensitization, it is concluded that the highest solar to electrical conversion efficiency is observed for T@0.1 C NCs-based DSSCs, and hence, for further photovoltaic studies with RuPc dye, only the T@0.1 C NCs-based photoelectrode is used. In the case of RuPc, T@0.1 C NC-based DSSCs result in a power conversion efficiency of 2.07%. The other photovoltaic parameters of T@0.1 C NC-based DSSCs are Jsc (6.73 mA/cm2), Voc (0.580 V), and FF (53.24%). Overall, the power conversion efficiency of the TiO2-based DSSC device is low as compared to the TiO2/MWCNTs NC-based DSSC device. In DSSCs, the excited electrons (LUMO) from the dye are injected into the conduction band (LUMO) of TiO2 and finally transferred into the counter electrode through two ways, namely, electric field driven charge transport45 and a trap-limited diffusion process.46 However, in bare TiO2 the electron transport is negligible because the diffusion of electrons through the TiO2 network undergoes different interfaces, and these interfaces act as electron trap centers, and hence there is a possibile electron–hole pair recombination.47 However, in TiO2/MWCNTs NCs-based DSSC device the MWCNTs acts as a carrier transporter with proper channels, and hence it avoids the possibility of recombination; that is, it minimizes the charge transport resistance of the device. Once the excited electron from the dye is injected into the semiconductor, it has an efficient pathway to reach the counter electrode through MWCNTs, and hence it greatly enhances the photoresponse of the cell.48
In the end, with varying sensitizers, the photovoltaic performance of T@0.1 C NCs-based DSSCs is significantly higher. The power conversion efficiency of T@0.1 C NCs/N719 DSSCs is almost three times more than that of RuPc-based DSSCs, and hence the overall efficiency reaches 6.21% from either 1.04% of bare TiO2 with N719 or 0.07% of bare TiO2 with RuPc. It is interesting that although there is a resemblance in the connection of dyes with the surfaces of TiO2-MWCNTs the conversion efficiency of DSSCs with different dyes is different due to their optical absorption coverge. Overall, the energy conversion efficiency of T@0.1 C NCs/N719 DSSCs is higher than others (T@0.1 C NCs/RuPc, TiO2/N719, and TiO2/RuPc DSSCs). Along with the surface area of the host material, the higher efficiency is corelated to the well coverage of the electromagnetic spectrum by N719-based DSSCs, resulting in more absorption of light with the formation of efficient charge carriers, which is responsible for the significant enhancement of the conversion efficiency. The detailed absorption edge and absorption strength of the two different sensitizer-based DSSCs is discussed in the incident photon-to-charge carrier conversion efficiency (IPCE) measurement.
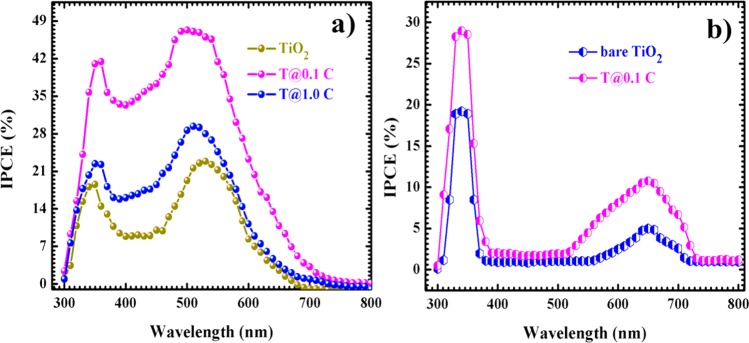
The photovoltaic performances of the N719 and RuPc-based DSSC devices are confirmed by using IPCE at different incident wavelengths and determined by using the following relation
 |
where Jsc is the short-circuit current density: λ is the wavelength of the incident light; and Iinc is the power of the incident light.49Figure 14(a, b) shows the IPCE spectra for the N719- and RuPc-based DSSCs, respectively. In the case of an N719 dye, the photocurrents for all TiO2-MWCNT NC-based DSSCs are generated in the range from 300 to 650 nm. The two distinct regions (viz., 300–400 nm and 475–650 nm) are observed for current generations.
Figure 14.
IPCE spectra of bare TiO2 NPs and representative NCs for (a) N719 and (b) RuPc-based DSSCs.
The former region is found due to the characteristic absorption of TiO2 as well as the small extent of N719 dye, while the latter is due to the high absorption of the N719 dye.23 The maximum IPCE value of TiO2/MWCNTs NC-based DSSCs reached 47% for T@0.1 C NCs/N719-based DSSCs as compared to others, which is also in good agreement with the observed maximum Jsc of the TiO2/MWCNT NCs-based DSSCs. In comparison to bare TiO2-based DSSCs, the IPCE value for T@0.1 C NCs-based DSSCs is 2.5 times higher. Similar to I–V characterization, the loading of N719 dye and MWCNTs content in the TiO2 host lattice enhances the optical properties of the TiO2 host lattice, and it is better for the charge separation as well as the efficiency of the cells.28 In the end, IPCE response of T@0.1C NC with RuPc dye through INA was tested under the same operating conditions. It shows the two distinct absorption maxima, viz., between 300 to 400 nm and 600 to 700 nm, for the conversion of solar to electrical current. The first region is dominated due to the absorption of TiO2, and the second wide region is analogous to RuPc only, with minimum absorption to that of the N719 dye. Overall, the IPCE value of TiO2/MWCNTs NCs/N719 based DSSCs is almost three times that of the TiO2/MWCNTs NCs/INA/RuPc-based DSSCs. Because through carboxylic functional groups N719 is covalently connected to the surface of the TiO2/MWCNTs NCs, the connectivity of the different moieties results in the high absorption capacity of the cells. Due to the absence of carboxylic functional moieties, RuPc was anchored on host materials through INA, but it may also build up the resistivity of the cells and hence decrease the overall conversion efficiency of the RuPc-based DSSCs. The lower power conversion efficiency confirms that, along with MWCNTs content, the proper connectivity between the dye and MWCNTs plays a dominant role in capturing as much incident light as possible by its absorption strength with host semiconducting material and overlaps that possible absorption with the solar spectrum.
Conclusions
Thin films of TiO2/MWCNT NCs were successfully deposited on FTO-glass substrate using a binder-free doctor blade technique. The anchoring of TiO2 NPs to the surfaces of MWCNTs was confirmed by using HRTEM, FT-IR, Raman, and XPS analysis. With varying MWCNTs content in NCs, the structural parameters of the TiO2 host lattice were varied, which was also confirmed by Rietveld refinement studies (goodness of fit = ∼1.5). The optical absorption edge of TiO2 extended toward the red region of the electromagnetic spectrum with MWCNTs, and the optical energy band gap of samples turned from 3.2 to 2.85 eV. The TiO2/MWCNTs NCs are anchored with two different Ru(II)-based dyes, viz., N719 and RuPc, and these electrodes were used as photoanodes for efficient DSSCs. The different absorption and anchoring nature of sensitizers directly affected the solar energy power conversion efficiency of the devices. Among all devices, T@0.1C NCs with N719-based devices showed the highest Jsc, Voc, FF, and η (6.21%) as that of either other CNTs-based devices or the RuPc-based DSSCs device (η = 2.07%). The same materials were utilized toward the fabrication of solid-state DSSCs (using either p-type inorganic or p-type organic semiconductors as HTMs), and works are in progress in our lab.
Experimental Section
The commercial pristine MWCNTs were functionalized with the acid treatment method,50 and bare TiO2 NPs were synthesized by an earlier reported sol–gel method with slight modifications.51 The phthalocyanine (Pc) based Ru(II) complex (RuPc) was prepared by using a literature method with some modifications.52 The detailed experimental conditions of all these materials have been provided in the Supporting Information.
Synthesis of TiO2/MWCNTs Nanocomposites (NCs)
An in situ sol–gel method was used for synthesizing TiO2/MWCNT NCs with varying content of MWCNTs. The functionalized MWCNTs were dispersed in deionized water (DW) using an ultrasonicator bath, and these MWCNTs were added directly during the synthesis of the TiO2 NPs route after hydroxylation of titanium precursors. The blackish colored precipitate formed and was subsequently washed, dried, and annealed at 753 K for 2 h. The different contents of MWCNTs such as 0.01, 0.05, 0.1 0.25, and 1.0 wt % were added to the titanium precursor, and then these samples are designated as T@0.01 C, T@0.05 C, T@0.1 C, T@0.25 C, and T@1.0 C, respectively.
Fabrication of DSSCs with TiO2/MWCNTs Photoanodes
Binder-free NCs were deposited on FTO glass electrode using the doctor-blade technique. Primarily, FTO glass substrates were washed thoroughly with water (with detergent), acetone, and finally in ethanol using an ultrasonicator bath. The cleaned glass substrates were annealed at 373 K for 30 min. The NC powders were ultrasonically dispersed in both N,N-dimethylformamide (DMF) and acetonitrile (ACN) for 1 h and 30 min, respectively, and the upper organic layer was decanted. The remaining portion was stirred continuously to form slurry. This slurry was deposited on a cleaned FTO glass substrate using the doctor-blade method, and these films were sintered at 723 K for 2 h. The deposited thin films were sensitized using, namely, N719 and RuPc dyes separately. In sensitization protocol, thin films were immersed in 0.3 mM N719 dye solution (1:1 mixture of tert-butyl alcohol and ACN) for 18 h (room temp). The unbound dye was removed from the film after rinsing twice in a combination of tert-butyl alcohol and acetonitrile. The N719 dye-anchored photoelectrode was sandwiched between the platinum counter electrode (Pt/ITO) using 60 μm thick sealing Surlyn sheet. Finally, the electrolyte (1.0 M LiI + 0.06 M I2 in propylene carbonate) was impregnated and sealed (Scheme 1). The photovoltaic performance of the devices (with an active area of 0.25 cm2) was measured by the current–voltage (IV) and IPCE spectra. Similarly, the photovoltaic performance of the sandwich-type DSSCs with TiO2/MWCNTs (T@0.1 C) photoelectrode, sensitized with RuPc dye, was also measured.
Acknowledgments
Authors, SDD and AGD, thank to University Grant Commission and Department of Science and Technology, New Delhi, India, for a Raman postdoctoral fellowship at the Florida State University (UGC-No. F 5-88/2014 (IC) dated 9 Sep 2014), Rajiv Gandhi National Research Fellowship (UGC No. F./2014-15/RGNF-2014-15D-SC-MAH-61438 dated, April 2014) and fast track research scheme for young scientists (DST-No.SR/FT/CS-137/2010). Author SDD also acknowledges the late Sir Harold Kroto, Department of Chemistry and Biochemistry, Florida State University, Tallahassee, for giving him an opportunity to work with him under the Raman fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01316.
The thermal stability (TGA) and structural parameters of as-synthesized materials, position coordinates and atom occupancies of TiO2, XRD refined patterns of T@0.01 C, T@0.05 C, T@0.25 C, and T@1.00 C, MWCNTs composition (wt %) as a function of optical energy band gap, and photoluminescence excitation spectra (PDF)
Author Contributions
# S.D.D. and A.G.D. contributed equally
Author Contributions
The final version of the manuscript was approved by all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Lewis N. S.; Nocera D. G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 15729–15735. 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Wang F.; Yu J. C. Semiconductor/Biomolecular Composites for Solar Energy Applications. Energy Environ. Sci. 2011, 4, 100–113. 10.1039/C0EE00162G. [DOI] [Google Scholar]
- Dumortier M.; Tembhurne S.; Haussener S. Holistic Design Guidelines for Solar Hydrogen Production by Photo-Electrochemical Routes. Energy Environ. Sci. 2015, 8, 3614–3628. 10.1039/C5EE01821H. [DOI] [Google Scholar]
- Chen G.; Seo J.; Yang C.; Prasad P. N. Nanochemistry and Nanomaterials for Photovoltaics. Chem. Soc. Rev. 2013, 42, 8304–8338. 10.1039/c3cs60054h. [DOI] [PubMed] [Google Scholar]
- Grätzel M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. 10.1038/35104607. [DOI] [PubMed] [Google Scholar]
- Pastore M.; De Angelis F. Aggregation of Organic Dyes on TiO2 in Dye-Sensitized Solar Cells Models: An Ab Initio Investigation. ACS Nano 2009, 4, 556–562. 10.1021/nn901518s. [DOI] [PubMed] [Google Scholar]
- Kakiage K.; Aoyama Y.; Yano T.; Oya K.; Fujisawa J.-i.; Hanaya M. Highly-Efficient Dye-Sensitized Solar Cells with Collaborative Sensitization by Silyl-Anchor and Carboxy-Anchor Dyes. Chem. Commun. 2015, 51, 15894–15897. 10.1039/C5CC06759F. [DOI] [PubMed] [Google Scholar]
- Pazos-Outón L. M.; Lee J. M.; Futscher M. H.; Kirch A.; Tabachnyk M.; Friend R. H.; Ehrler B. A Silicon–Singlet Fission Tandem Solar Cell Exceeding 100% External Quantum Efficiency with High Spectral Stability. ACS Energy Lett. 2017, 2, 476. 10.1021/acsenergylett.6b00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima K.; Tamai Y.; Ohkita H.; Osaka I.; Takimiya K. High-Efficiency Polymer Solar Cells with Small Photon Energy Loss. Nat. Commun. 2015, 6, 10085. 10.1038/ncomms10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Peng W.; Chen Z.; Chen H.; Han L. Effect of Cerium Doping in the TiO2 Photoanode on the Electron Transport of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 19182–19190. 10.1021/jp3060735. [DOI] [Google Scholar]
- Rui Z.; Chen L.; Chen H.; Ji H. Strong Metal-Support Interaction in Pt/TiO2 Induced by Mild Hcho and Nabh4 Solution Reduction and Its Effect on Catalytic Toluene Combustion. Ind. Eng. Chem. Res. 2014, 53, 15879–15888. 10.1021/ie5029107. [DOI] [Google Scholar]
- Kim S.-B.; Park J.-Y.; Kim C.-S.; Okuyama K.; Lee S.-E.; Jang H.-D.; Kim T.-O. Effects of Graphene in Dye-Sensitized Solar Cells Based on Nitrogen-Doped TiO2 Composite. J. Phys. Chem. C 2015, 119, 16552–16559. 10.1021/acs.jpcc.5b02309. [DOI] [Google Scholar]
- Wang L.; Liu H.; Konik R. M.; Misewich J. A.; Wong S. S. Carbon Nanotube-Based Heterostructures for Solar Energy Applications. Chem. Soc. Rev. 2013, 42, 8134–8156. 10.1039/c3cs60088b. [DOI] [PubMed] [Google Scholar]
- Chen X.; Mao S. S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- Dai H.; Chen G.; Zhou C.; Fang Q.; Fei X. A Numerical Study of Ultraprecision Machining of Monocrystalline Silicon with Laser Nano-Structured Diamond Tools by Atomistic Simulation. Appl. Surf. Sci. 2017, 393, 405–416. 10.1016/j.apsusc.2016.10.014. [DOI] [Google Scholar]
- Nazeeruddin M. K.; Bessho T.; Cevey L.; Ito S.; Klein C.; De Angelis F.; Fantacci S.; Comte P.; Liska P.; Imai H. A High Molar Extinction Coefficient Charge Transfer Sensitizer and Its Application in Dye-Sensitized Solar Cell. J. Photochem. Photobiol., A 2007, 185, 331–337. 10.1016/j.jphotochem.2006.06.028. [DOI] [Google Scholar]
- Wang X.; Xi M.; Fong H.; Zhu Z. Flexible, Transferable, and Thermal-Durable Dye-Sensitized Solar Cell Photoanode Consisting of TiO2 Nanoparticles and Electrospun TiO2/SiO2 Nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 15925–15932. 10.1021/am503542g. [DOI] [PubMed] [Google Scholar]
- Dembele K. T.; Selopal G. S.; Soldano C.; Nechache R.; Rimada J. C.; Concina I.; Sberveglieri G.; Rosei F.; Vomiero A. Hybrid Carbon Nanotubes–TiO2 Photoanodes for High Efficiency Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 14510–14517. 10.1021/jp403553t. [DOI] [Google Scholar]
- Ahn J. Y.; Kim J. H.; Moon K. J.; Kim J. H.; Lee C. S.; Kim M. Y.; Kang J. W.; Kim S. H. Incorporation of Multiwalled Carbon Nanotubes into TiO2 Nanowires for Enhancing Photovoltaic Performance of Dye-Sensitized Solar Cells Via Highly Efficient Electron Transfer. Sol. Energy 2013, 92, 41–46. 10.1016/j.solener.2013.02.031. [DOI] [Google Scholar]
- O’Regan B. C.; López-Duarte I.; Martínez-Díaz M. V.; Forneli A.; Albero J.; Morandeira A.; Palomares E.; Torres T.; Durrant J. R. Catalysis of Recombination and Its Limitation on Open Circuit Voltage for Dye Sensitized Photovoltaic Cells Using Phthalocyanine Dyes. J. Am. Chem. Soc. 2008, 130, 2906–2907. 10.1021/ja078045o. [DOI] [PubMed] [Google Scholar]
- Ye M.; Wen X.; Wang M.; Iocozzia J.; Zhang N.; Lin C.; Lin Z. Recent Advances in Dye-Sensitized Solar Cells: From Photoanodes, Sensitizers and Electrolytes to Counter Electrodes. Mater. Today 2015, 18, 155–162. 10.1016/j.mattod.2014.09.001. [DOI] [Google Scholar]
- Martín-Gomis L.; Fernández-Lázaro F.; Sastre-Santos Á. Advances in Phthalocyanine-Sensitized Solar Cells (Pcsscs). J. Mater. Chem. A 2014, 2, 15672–15682. 10.1039/C4TA01894J. [DOI] [Google Scholar]
- Muduli S.; Lee W.; Dhas V.; Mujawar S.; Dubey M.; Vijayamohanan K.; Han S.-H.; Ogale S. Enhanced Conversion Efficiency in Dye-Sensitized Solar Cells Based on Hydrothermally Synthesized TiO2–MWCNT Nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 2030–2035. 10.1021/am900396m. [DOI] [PubMed] [Google Scholar]
- Dembele K.; Nechache R.; Nikolova L.; Vomiero A.; Santato C.; Licoccia S.; Rosei F. Effect of Multi-Walled Carbon Nanotubes on the Stability of Dye Sensitized Solar Cells. J. Power Sources 2013, 233, 93–97. 10.1016/j.jpowsour.2013.01.075. [DOI] [Google Scholar]
- Delekar S.; Yadav H.; Achary S.; Meena S.; Pawar S. Structural Refinement and Photocatalytic Activity of Fe-Doped Anatase TiO2 Nanoparticles. Appl. Surf. Sci. 2012, 263, 536–545. 10.1016/j.apsusc.2012.09.102. [DOI] [Google Scholar]
- Dinnebier R. E.Powder Diffraction: Theory and Practice; Royal Society of Chemistry, 2008. [Google Scholar]
- Pan D.; Jiao J.; Li Z.; Guo Y.; Feng C.; Liu Y.; Wang L.; Wu M. Efficient Separation of Electron–Hole Pairs in Graphene Quantum Dots by TiO2 Heterojunctions for Dye Degradation. ACS Sustainable Chem. Eng. 2015, 3, 2405–2413. 10.1021/acssuschemeng.5b00771. [DOI] [Google Scholar]
- Yadav S. K.; Mahapatra S. S.; Cho J. W.; Lee J. Y. Functionalization of Multiwalled Carbon Nanotubes with Poly (Styrene-B-(Ethylene-Co-Butylene)-B-Styrene) by Click Coupling. J. Phys. Chem. C 2010, 114, 11395–11400. 10.1021/jp1028382. [DOI] [Google Scholar]
- Sadhu S.; Poddar P. Template-Free Fabrication of Highly-Oriented Single-Crystalline 1d-Rutile TiO2-MWCNT Composite for Enhanced Photoelectrochemical Activity. J. Phys. Chem. C 2014, 118, 19363–19373. 10.1021/jp5023983. [DOI] [Google Scholar]
- Zhang S.; Niu H.; Lan Y.; Cheng C.; Xu J.; Wang X. Synthesis of TiO2 Nanoparticles on Plasma-Treated Carbon Nanotubes and Its Application in Photoanodes of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2011, 115, 22025–22034. 10.1021/jp206267x. [DOI] [Google Scholar]
- Yan X.-b.; Tay B. K.; Yang Y. Dispersing and Functionalizing Multiwalled Carbon Nanotubes in TiO2 Sol. J. Phys. Chem. B 2006, 110, 25844–25849. 10.1021/jp065434g. [DOI] [PubMed] [Google Scholar]
- Wu X.; Chen X.; Wang J.; Liu J.; Fan Z.; Chen X.; Chen J. Functionalization of Multiwalled Carbon Nanotubes with Thermotropic Liquid-Crystalline Polymer and Thermal Properties of Composites. Ind. Eng. Chem. Res. 2010, 50, 891–897. 10.1021/ie1018029. [DOI] [Google Scholar]
- Liu Y.; Tang A.; Zhang Q.; Yin Y. Seed-Mediated Growth of Anatase TiO2 Nanocrystals with Core-Antenna Structures for Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2015, 137, 11327–11339. 10.1021/jacs.5b04676. [DOI] [PubMed] [Google Scholar]
- Koli V. B.; Dhodamani A. G.; Raut A. V.; Thorat N. D.; Pawar S. H.; Delekar S. D. Visible Light Photo-Induced Antibacterial Activity of TiO2-MWCNTs Nanocomposites with Varying the Contents of Mwcnts. J. Photochem. Photobiol., A 2016, 328, 50–58. 10.1016/j.jphotochem.2016.05.016. [DOI] [Google Scholar]
- Shevale V. B.; Dhodamani A. G.; Koli V. B.; Barkul R. P.; Jadhav J. P.; Delekar S. D. Efficient Degradation of Azorubin S Colourant in the Commercial Jam-Jelly Food Samples Using TiO2-CoFe2O4 Nanocomposites in Visible Light. Mater. Res. Bull. 2017, 89, 79–88. 10.1016/j.materresbull.2017.01.012. [DOI] [Google Scholar]
- Makowski M. J.; Galhenage R. P.; Langford J.; Hemminger J. C. Liquid-Jet X-Ray Photoelectron Spectra of TiO2 Nanoparticles in an Aqueous Electrolyte Solution. J. Phys. Chem. Lett. 2016, 7, 1732–1735. 10.1021/acs.jpclett.6b00445. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Guerra-Nuñez C.; Li M.; Michler J.; Park H. G.; Rossell M. D.; Erni R.; Utke I. High Conformity and Large Domain Monocrystalline Anatase on Multiwall Carbon Nanotube Core–Shell Nanostructure: Synthesis, Structure, and Interface. Chem. Mater. 2016, 28, 3488–3496. 10.1021/acs.chemmater.6b01209. [DOI] [Google Scholar]
- Agarwala S.; Kevin M.; Wong A.; Peh C.; Thavasi V.; Ho G. Mesophase Ordering of TiO2 Film with High Surface Area and Strong Light Harvesting for Dye-Sensitized Solar Cell. ACS Appl. Mater. Interfaces 2010, 2, 1844–1850. 10.1021/am100421e. [DOI] [PubMed] [Google Scholar]
- Kang C.; Jing L.; Guo T.; Cui H.; Zhou J.; Fu H. Mesoporous SiO2-Modified Nanocrystalline Tio2 with High Anatase Thermal Stability and Large Surface Area as Efficient Photocatalyst. J. Phys. Chem. C 2008, 113, 1006–1013. 10.1021/jp807552u. [DOI] [Google Scholar]
- Parveen N.; Ansari M. O.; Cho M. H. Route to High Surface Area, Mesoporosity of Polyaniline–Titanium Dioxide Nanocomposites Via One Pot Synthesis for Energy Storage Applications. Ind. Eng. Chem. Res. 2016, 55, 116–124. 10.1021/acs.iecr.5b02907. [DOI] [Google Scholar]
- Xie L.-Q.; Ding D.; Zhang M.; Chen S.; Qiu Z.; Yan J.-W.; Yang Z.-L.; Chen M.-S.; Mao B.-W.; Tian Z.-Q. Adsorption of Dye Molecules on Single Crystalline Semiconductor Surfaces: An Electrochemical Shell-Isolated Nanoparticle Enhanced Raman Spectroscopy Study. J. Phys. Chem. C 2016, 120, 22500–22507. 10.1021/acs.jpcc.6b07763. [DOI] [Google Scholar]
- De Angelis F.; Fantacci S.; Mosconi E.; Nazeeruddin M. K.; Grätzel M. Absorption Spectra and Excited State Energy Levels of the N719 Dye on TiO2 in Dye-Sensitized Solar Cell Models. J. Phys. Chem. C 2011, 115, 8825–8831. 10.1021/jp111949a. [DOI] [Google Scholar]
- Martin-Gomis L.; Fernandez-Lazaro F.; Sastre-Santos A. Advances in Phthalocyanine-Sensitized Solar Cells (Pcsscs). J. Mater. Chem. A 2014, 2, 15672–15682. 10.1039/C4TA01894J. [DOI] [Google Scholar]
- Yang M.; Ding B.; Lee S.; Lee J.-K. Carrier Transport in Dye-Sensitized Solar Cells Using Single Crystalline TiO2 Nanorods Grown by a Microwave-Assisted Hydrothermal Reaction. J. Phys. Chem. C 2011, 115, 14534–14541. 10.1021/jp2025126. [DOI] [Google Scholar]
- Yang W.; Vlachopoulos N.; Boschloo G. Impact of Local Electric Fields on Charge-Transfer Processes at the TiO2/Dye/Electrolyte Interface. ACS Ener. Lett. 2017, 2, 161–167. 10.1021/acsenergylett.6b00568. [DOI] [Google Scholar]
- Sajedi Alvar M.; Javadi M.; Abdi Y.; Arzi E. Enhancing the Electron Lifetime and Diffusion Coefficient in Dye-Sensitized Solar Cells by Patterning the Layer of TiO2 Nanoparticles. J. Appl. Phys. 2016, 119, 114302. 10.1063/1.4943772. [DOI] [Google Scholar]
- Wang J.; Lin Y.; Pinault M.; Filoramo A.; Fabert M.; Ratier B.; Bouclé J.; Herlin-Boime N. Single-Step Preparation of TiO2/MWCNT Nanohybrid Materials by Laser Pyrolysis and Application to Efficient Photovoltaic Energy Conversion. ACS Appl. Mater. Interfaces 2015, 7, 51–56. 10.1021/am507179c. [DOI] [PubMed] [Google Scholar]
- Bhande S. S.; Ambade R. B.; Shinde D. V.; Ambade S. B.; Patil S. A.; Naushad M.; Mane R. S.; Alothman Z.; Lee S.-H.; Han S.-H. Improved Photoelectrochemical Cell Performance of Tin Oxide with Functionalized Multiwalled Carbon Nanotubes–Cadmium Selenide Sensitizer. ACS Appl. Mater. Interfaces 2015, 7, 25094–25104. 10.1021/acsami.5b05385. [DOI] [PubMed] [Google Scholar]
- Liu J.; Kuo Y.-T.; Klabunde K. J.; Rochford C.; Wu J.; Li J. Novel Dye-Sensitized Solar Cell Architecture Using TiO2-Coated Vertically Aligned Carbon Nanofiber Arrays. ACS Appl. Mater. Interfaces 2009, 1, 1645–1649. 10.1021/am900316f. [DOI] [PubMed] [Google Scholar]
- Koli V.; Dhodamani A.; More K.; Acquah S. F.; Panda D. K.; Pawar S.; Delekar S. A Simple Strategy for the Anchoring of Anatase Titania on Multi-Walled Carbon Nanotubes for Solar Energy Harvesting. Sol. Energy 2017, 149, 188–194. 10.1016/j.solener.2017.03.036. [DOI] [Google Scholar]
- Koli V. B.; Dhodamani A. G.; Delekar S. D.; Pawar S. H. In Situ Sol-Gel Synthesis of Anatase TiO2-MWCNTs Nanocomposites and Their Photocatalytic Applications. J. Photochem. Photobiol., A 2017, 333, 40–48. 10.1016/j.jphotochem.2016.10.008. [DOI] [Google Scholar]
- Morandeira A.; López-Duarte I.; O’Regan B.; Martinez-Diaz M. V.; Forneli A.; Palomares E.; Torres T.; Durrant J. R. Ru (Ii)-Phthalocyanine Sensitized Solar Cells: The Influence of Co-Adsorbents Upon Interfacial Electron Transfer Kinetics. J. Mater. Chem. 2009, 19, 5016–5026. 10.1039/b904179f. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.