Abstract

D–A−π–A dyes differ from the traditional D−π–A framework having several merits in dye-sensitized solar cell (DSSC) applications. With regard to D−π–A dyes, D–A−π–A dyes red-shift absorption spectra and show particular photostability. Nevertheless, the effects of internal acceptor on the charge transfer (CT) probability are unclear. We employed density functional theory (DFT), time-dependent DFT (TD-DFT), and TD-DFT molecular dynamics (MD) simulations to investigate the effects of internal acceptor on the photophysical properties of D–A−π–A dyes on DSSCs. Our calculations show the absorption bands of D–A−π–A dyes with strong electron-withdrawing internal acceptors exhibiting significant characteristics of dual CT; the excited electron density is transferred to the internal and terminal acceptors simultaneously. Particularly, the internal acceptor traps a significant amount of electron density upon photoexcitation. The TD-DFT MD simulations at 300 K show that only a small amount of excited electron density is pushing and pulling between the internal acceptor and terminal acceptor moieties; the thermal energy is not high enough to drive the electron density from the internal acceptor to the terminal acceptor. Our study reveals the nature of CT bands of D–A−π–A dyes providing a theoretical basis for further rational engineering.
Introduction
The Sun has been considered as a promising sustainable energy source. Photovoltaic devices are regarded as optimal methods to directly convert solar energy into electricity. Various technologies,1−3 such as crystalline Si, semiconductors (e.g., GaAs-based cells), organic bulk heterojunction, and dye-sensitized solar cells (DSSCs), can be used. DSSCs have the advantage of being lightweight in comparison to inorganic semiconductor solar cells and have a higher conversion efficiency4 than polymer-based bulky heterojunction cells.5
In DSSC devices, the dye sensitizers play the first and crucial step in light harvesting. For higher light-harvesting efficiency, the absorption spectra of dye sensitizers should be optimally matched with the solar spectra and also have a larger extinction coefficient. In addition, the transition characters of absorption spectra of dye sensitizers influence and determine the efficiency of electron injection. Moreover, the stability of photoexcited dye sensitizers will determine the lifetime of DSSCs. To enhance the efficiency of DSSCs, researchers are dedicated to engineering configurations of dye sensitizers to optimize various entangled parameters as mentioned above.
DSSCs based on organic dyes have several advantages. Metal-free organic dyes are easily prepared and relatively less expensive. Moreover, the more interesting feature of metal-free organic dyes is that their absorption (wavelength range and extinction coefficients) and photoelectrical properties (related to frontier molecular orbital energy level) are adjustable through molecular engineering.6 Most metal-free organic sensitizers are typically designed to have a donor−π–acceptor (D−π–A) framework. D−π–A dyes with dipolar features are designed with the aim of performing efficient photoinduced charge transfer (CT). Previous studies have designed the dyes based on the D−π–A framework in terms of engineering the donors (e.g., triphenylamine (TPA), indoline, etc.)7,8 and π-spacer (e.g., benzene, thiophene, etc.). Probably due to its simple structure, the achievement of D−π–A DSSC is more limited. Nevertheless, variation of the D−π–A framework can lead to other frameworks of dyes. Recently, novel organic dyes with a D–A−π–A9 configuration made by introducing an internal acceptor into the traditional D−π–A structure have efficiencies up to 10%.10,11 D–A−π–A sensitizers employ an auxiliary electron-withdrawing group, known as internal acceptors A; commonly used internal acceptors are quinoxaline,12 diketopyrrolopyrrole,13 isoindigo,14 bithiazole,15 benzothiadiazole (BTD),16 benzotriazole (BT),17 2-methylbenzo[d]thiazole (DBT),18,19 and pyrido[3,4-b]pyrazine (PP).20 Previous comprehensive studies21 on the model of D–A−π–A showed that the internal electron-withdrawing acceptor can be regarded as an “electron trap” and exhibits some distinguishing characteristics: (i) Incorporation of an electron-withdrawing acceptor into the D−π–A configuration tunes the molecular energy gap; an acceptor owning a low-lying lowest unoccupied molecular orbital (LUMO) is expected to reduce the LUMO of the whole molecule red-shifting the absorption spectra when the highest occupied molecular orbital (HOMO) of the whole molecule remains the same. Wang10 and co-workers introduced a BTD group into one D−π–A dye, generating a new dye of D–A−π–A configuration; the new D–A−π–A dye (D2 in the original literature) performed a red-shifted absorption of 556 nm relative to that of the original D−π–A dye (λmax = 512 nm; D1 in the original literature). (ii) Furthermore, it may result in a new absorption band in the UV–vis region expanding the range of the light-harvesting response. In the work of Wang et al.,10 the D2 molecule has an extra absorption band at 466 nm and a prominent extinction coefficient of up to 30 500 M–1 cm–1, whereas the D1 molecule shows a blue-shifted band at 363 nm with a smaller extinction coefficient of 9300 M–1 cm–1. (iii) More importantly, it can significantly improve the photostability.16 (iv) The structural features of most internal acceptor units allow for facile structural modification. In addition, their N-containing heterocycles are considered to improve the Voc.22
The internal acceptor, no doubt having electron-withdrawing ability, is also expected to alter the electronic transition characteristics of the absorption band, which is crucial for electron injection for photo-to-current conversion. In particular, whether the internal acceptor promotes or retards the electron transfer (ET) is still unclear.21 Conceptually, it is anticipated that the internal acceptor can potentially trap a certain amount of photoexcited electron density at the initial stage. However, it is not known whether the D–A−π–A dye follows a two-step CT process driven by thermal energy. In a two-step CT process, the internal acceptor receives electron density from the donor upon photoexcitation. Thereafter, thermal energy drives the photoexcited electron density on the internal acceptor toward the terminal acceptor for electron injection. Moreover, introduction of an internal acceptor into a D−π–A dye is not assured to give a red-shifted spectra and a better performance of Jsc. In the work of Zhu et al.,23 the LS-1 molecule (in the original literature) with a D−π–A framework has a λmax of 483 nm and gives a Jsc value of 11.25 mA cm–2; retaining the same D and A moieties, the LS-2 with a D–A−π–A framework has a blue-shifted λmax of 442 nm and gives a lower Jsc value of 10.06 mA cm–2. Therefore, theoretical investigation of the effects of the internal acceptor in the D–A−π–A dyes on the photophysical properties, in particular the features of absorption spectra, as well as electron-transfer characters, is needed for rational engineering of novel D–A−π–A dyes for better DSSC applications.
To elucidate the effects of the internal acceptor underlying the complex D–A−π–A configurations, we employed density functional theory (DFT), time-dependent DFT (TD-DFT), and TD-DFT nonadiabatic (NA) molecular dynamics (MD) to investigate four D–A−π–A molecules and one D−π–A molecule, as displayed in Figure 1. The D−π–A molecule (Figure 1e) is used for parallel comparison. These five molecules were carefully selected from published literature containing rich experimental data. In particular, they share several common features, providing us with suitable systems to comparatively elucidate the effects of the internal acceptor on the photophysical properties from the complex interrelated experimental data. The five molecules studied have the same electron-donating group (triphenylamine, TPA), π-spacer (Th), and acceptor/anchor (cyanoacrylic acid, CAA). The only structural variation between the four D–A−π–A molecules is the internal acceptor located between the donor and the π-spacer. In addition, the internal acceptors have similar molecular structure but with different physical properties (e.g., HOMO–LUMO (H–L) gap). Moreover, our calculations in this study (vide infra) show that they own a planar A−π–A moiety and thus have similar molecular length. Nevertheless, they have distinct UV–vis spectra and photo-to-current conversion efficiency. Experimentally, the TPA-DBT-Th-CAA molecule (Figure 1c) has λmax at 423 nm, which is close to that of the D−π–A molecule (Figure 1e, λmax = 417 nm). Replacement of DBT by BTD gives the TPA-BTD-Th-CAA molecule having a significantly red-shifted spectrum of 497 nm. Our theoretical calculations reveal that the primary absorption of D–A−π–A molecules has electron density transferred to the internal acceptor and terminal acceptor moieties at the same time upon photoexcitation, which is significantly different from the commonly observed D-to-A transition in the D−π–A molecules. Moreover, we show that the thermal energy at 300 K has limited effect in further promoting the excited electron density on the internal acceptor to the terminal acceptor side.
Figure 1.
Chemical structures of studied dye sensitizers with the D–A−π–A framework: (a) TPA-BTD-Th-CAA, (b) TPA-BT-Th-CAA, (c) TPA-DBT-Th-CAA, (d) TPA-PP-Th-CAA, and (e) TPA-Ph-Th-CAA.
Results and Discussion
Studied Molecules
The sensitizers displayed in Figure 1 have similar molecular configuration having a triphenylamine (TPA) moiety as the electron-donating group, a thiophene (Th) unit as the π-spacer, and cyanoacrylic acid (CAA) as the terminal acceptor/anchor. These units were chosen mainly because they are frequently used in dye sensitizers:24−26 TPA belongs to the electron-rich aryl amine family; Th has excellent charge-transport properties; and the cyanoacrylic acid group consists of strong electron-withdrawing moieties of the cyano and carboxyl groups.1,27 The internal acceptors A have a low-lying LUMO and a smaller HOMO–LUMO gap. For convenience, these studied molecules were named by the sequence combination of the abbreviations of their donor, internal acceptor, π-spacer, and terminal acceptor/anchor. For example, the molecule TPA-BTD-Th-CAA (Figure 1a) uses one TPA as the donor, one benzothiadiazole (BTD) as the internal acceptor, one Th as the π-spacer, and CAA as the terminal acceptor/anchor. Therefore, our studied molecules have the molecular framework of TPA-A-Th-CAA except for TPA-Ph-Th-CAA (Figure 1e). TPA-Ph-Th-CAA is a reference D−π–A dye with a very similar structure to other TPA-A-Th-CAA molecules, but without the internal acceptor unit. It is used for parallel comparison.
Four acceptors were introduced into the abovementioned molecular framework as an internal acceptor and were investigated. TPA-BTD-Th-CAA (Figure 1a), developed by Tian, Wang, and co-workers,16 incorporates benzothiadiazole (BTD) as an internal acceptor to bridge the TPA (donor) and the Th (spacer). The BTD unit has been widely applied for engineering organic solar cells mainly due to its narrow band gap and strong electron-withdrawing ability.28−32 The introduced internal BTD acceptor is expected to function as an electron trap, which increases the efficiency of charge separation. TPA-BT-Th-CAA sensitizers (Figure 1b) have benzotriazole (BT) introduced into the sensitizer as an internal acceptor developed by Hua et al.17 In particular, BT is a close analogue of BTD; however, BT is electron-rich, leading to a higher LUMO energy level.33 TPA-DBT-Th-CAA (Figure 1c) was developed by Yu, Ma, and co-workers.18 2-methylbenzo[d]thiazole (DBT) has a nonplanar structure, which is expected to avoid the aggregation of the dyes and thus increases the lifetime of the excited state. TPA-PP-Th-CAA20 (Figure 1d) has a pyrido[3,4-b]pyrazine (PP) unit as the internal acceptor.20 PP is well known to have electron-withdrawing ability due to its unsaturated nitrogen atoms. It has shown promising photovoltaic properties in polymer structures for near-infrared light-emitting diodes.
Energy Level of Building Blocks
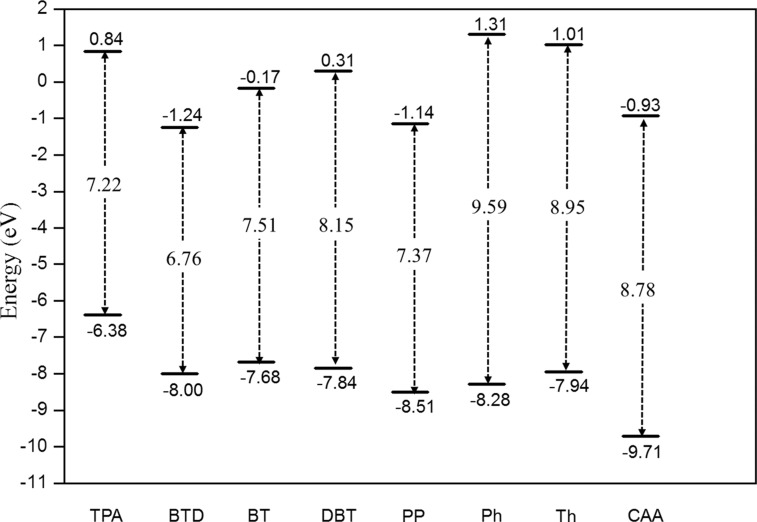
Figure 2 displays the HOMO and LUMO energy levels and HOMO–LUMO (H–L) energy gap of the “building blocks” of studied molecules, including TPA, Th, Ph, CAA, as well as four internal acceptors, BTD, BT, PP, and BTD, calculated at the CAM-B3LYP/6-31G(d,p) level based on their optimized structures by B3LYP/6-31G(d,p). The TPA moiety has the highest HOMO energy level, facilitating the donation of electrons. The CAA moiety has the lowest LUMO level and thus the largest driving force to accept electron density. The Ph and Th groups designed as π-spacers have relatively high-energy LUMO, which is less capable of trapping electrons, instead assisting the electron transportation and also extending the π-conjugation length.
Figure 2.
Energy diagram of the building blocks of studied molecules calculated at CAM-B3LYP/6-31G(d,p) level in tetrahydrofuran (THF). The bottom bars represent the HOMO energy; the top bars represent the LUMO energy. The HOMO–LUMO energy gaps are shown in the middle.
Four internal acceptors exploited in this study have low-lying LUMO levels and a smaller H–L energy gap. Nevertheless, they have distinct LUMO energy levels and an H–L energy gap, providing us suitable models to understand their effects on the photophysical properties of D–A−π–A organic sensitizers. Among them, BTD has the lowest-energy LUMO and a smaller H–L gap and DBT has the highest-energy LUMO and the largest H–L gap. The order of LUMO energy level of the four internal acceptors is DBT > BT > PP > BTD. The magnitude of the H–L gap of these four internal acceptors is correlated with their LUMO energy levels.
Ground-State Molecular Geometries
Figure 3 shows the lengths of C–C bonds connecting two neighboring building blocks and their associated dihedral angles. As the building blocks are aromatic rings, in which their structures are less sensitive to the chemical environments, instead, the dihedral angles and bond lengths between two neighboring aromatic rings can determine their photophysical properties. The bond lengths between two neighboring aromatic rings of studied molecules are between 1.34 Å (standard carbon–carbon double-bond length) and 1.54 Å (standard carbon–carbon single-bond length). This indicates that these bonds are partially conjugated within the molecule. We define four dihedral angles (depicted in Figure 3a), ϕ1 (between the Ph ring on TPA and internal acceptor ring), ϕ2 (between internal acceptor ring and Th ring), ϕ3 (between Th ring and C=C double bond), and ϕ4 (between CN and C=O groups), which may potentially change with different internal acceptors. As expected, the TPA moiety has a propeller-like nonplanar geometry. The Ph ring on TPA forms a certain twisted angle (ϕ1) with its neighboring ring; such a twisted angle may retard the electron back donation after photoexcitation. The ϕ2, ϕ3, and ϕ4 angles of TPA-BTD-Th-CAA, TPA-BT-Th-CAA, TPA-DBT-Th-CAA, and TPA-PP-Th-CAA are close to 0°, having a nearly planar A−π–A motif. Therefore, TPA-BTD-Th-CAA, TPA-BT-Th-CAA, TPA-DBT-Th-CAA, and TPA-PP-Th-CAA have similar molecular structures. The planar A−π–A motif can extend the π-conjugation length. On the other hand, ϕ2 of TPA-Ph-Th-CAA, due to the Th–Ph steric repulsion, is 20.7°, having a nonplanar Ph–Th motif, which reduces the effective π-conjugation length of the whole molecule.
Figure 3.
Two values close to a chemical bond represent the selected dihedral angles (deg) and their central bond length (Å) of the studied free molecules: (a) TPA-BTD-Th-CAA, (b) TPA-BT-Th-CAA, (c) TPA-DBT-Th-CAA, (d) TPA-PP-Th-CAA, and (e) TPA-Ph-Th-CAA.
UV–Vis Spectra of Dye in Solution
Table 1 lists the optical data of studied dyes in solution obtained from both experimental observations and TD-DFT calculations. Experimentally, the studied molecules have the λmax bands located at 417–500 nm. The λmax bands of TPA-BTD-Th-CAA, TPA-BT-Th-CAA, TPA-DBT-Th-CAA, TPA-PP-Th-CAA, and TPA-Ph-Th-CAA appear at 497, 454, 423, 500, and 417 nm, respectively. In particular, TPA-Ph-Th-CAA has the most blue-shifted band. These results reveal that replacement of the Ph by an internal acceptor, while maintaining the planarity of A-Th-CAA moiety, red-shifts the spectra. Furthermore, the magnitude of red shift in the spectra after introducing an internal acceptor moiety is correlated with the H–L gap and LUMO energy level of the internal acceptor. The calculated λmax bands of dyes are in good agreement with the experimental observations. The average absolute deviation between the calculated λmax values for these molecules in solution and those observed experimentally is 10.8 nm. The smallest deviation from the experimental value is only +2 nm (in TPA-BTD-Th-CAA), and the largest deviation from the experimental value is −25 nm (in TPA-PP-Th-CAA). Experimentally, TPA-BTD-Th-CAA (εmax = 13 000 M–1 cm–1) and TPA-PP-Th-CAA (εmax = 16 700 M–1 cm–1) have the smallest εmax values of the λmax bands. These results indicate that introduction of strong electron-withdrawing moieties, such as BTD and PP, reduces the absorption coefficients of the λmax bands. Similar results are also observed in other studies of D–A−π–A molecule. WS-2 (εmax = 16 700 M–1 cm–1) has a D–A−π–A framework using the BTD moiety, as internal acceptor has a smaller εmax value than its D−π–A analogue (LS-1 in the original literature; εmax = 21 000 M–1 cm–1).16 Computationally, TPA-BTD-Th-CAA gives the smallest oscillator strength, whereas the CAM-B3LYP/6-31G(d,p) method predicts that TPA-PP-Th-CAA has a similar oscillator strength to TPA-Ph-Th-CAA.
Table 1. Experimental and Calculated Absorptions of Studied Free Molecules.
| experiment |
calculation |
|||||
|---|---|---|---|---|---|---|
| dye | band | εmax (M–1 cm–1) | absorption (nm) | f | absorption (nm) | deviation from exp. |
| TPA-BTD-Th-CAA | λmax | 13 000 | 49716 a | 1.35 | 499d | +2 |
| #2 | 9500 | ∼39516 a | 0.12 | 378d | –17 | |
| TPA-BT-Th-CAA | λmax | 33 100 | 45417 b | 1.70 | 466b | +12 |
| #2 | 26 000 | ∼30017 b | 0.04 | 354b | +54 | |
| TPA-DBT-Th-CAA | λmax | 37 531 | 42318 c | 1.51 | 425c | +2 |
| #2 | 22 600 | ∼30018 c | 0.04 | 337c | +37 | |
| TPA-PP-Th-CAA | λmax | 16 700 | 50020 b | 1.57 | 475b | –25 |
| #2 | 28 000 | 42320 b | 0.08 | 370b | –53 | |
| TPA-Ph-Th-CAA | λmax | 23 000 | 41734 c | 1.56 | 410c | –6 |
| #2 | 15 000 | ∼31034 c | 0.10 | 330c | +20 | |
In CHCl3/CH3OH = 4/1.
In CH2Cl2.
In THF.
In dichloroethane (dielectric constant is close to the solvent used in experiment).
The D–A−π–A molecules exhibit second higher-energy absorption bands. Experimentally, the second bands for TPA-BTD-Th-CAA and TPA-PP-Th-CAA appear at 395 and 423 nm, respectively. More importantly, the second bands of TPA-BTD-Th-CAA and TPA-PP-Th-CAA appear in the visible (vis)-light region, which can potentially contribute to the photo-to-current conversion. Experimentally, the second absorption band of the TPA-Ph-Th-CAA molecule is located at ∼310 nm, which is poorly spectrally matched with solar spectra. Molecules with the introduction of larger H–L acceptors, such as TPA-BT-Th-CAA and TPA-DBT-Th-CAA, do not have red-shifted second absorption band. Except for TPA-PP-Th-CAA, other molecules have weaker second absorption band than the corresponding λmax bands. The calculated second absorption bands of dyes have larger deviations from the experimental observations than those of the λmax bands. The average absolute deviation between the calculated second absorption bands for these molecules in solution and those observed experimentally is 36.2 nm. These results indicate that the CAM-B3LYP method has better performance in reproducing the visible absorption than near UV absorption.
Table 2 lists the transition characters of the absorption bands. The major contribution of the electronic density transition of λmax bands is from HOMO to LUMO transition, and the minor contribution is from low-lying HOMO – 1 to LUMO transition. On the other hand, the major contribution of the electronic density transition of the second bands is from low-lying HOMO – 1 to LUMO transition, and the minor contribution is from HOMO to LUMO and from HOMO to higher-energy LUMO + 1 transitions. Table 3 lists the electron density difference map (EDDM) (where the excited electron density is coming from and going to) of absorption bands upon photoexcitation. The EDDM shows that the λmax and second bands of studied molecules have electron density mainly coming from the electron-donating TPA moiety; however, the character of excited electron density is distinctly different. The λmax bands of TPA-BTD-Th-CAA and TPA-PP-Th-CAA exhibit a dual charge-transfer character; the electron density is significantly transferred from the TPA moiety to both the internal acceptor and CAA moieties simultaneously upon photoexcitation. Upon excitation, TPA-BTD-Th-CAA has the electron density of the BTD and CAA moieties increased by 31 and 18%, respectively. For TPA-PP-Th-CAA, the electron density of the PP and CAA moieties is increased by 29 and 20%, respectively, upon excitation. Particularly, the internal acceptor moieties of TPA-BTD-Th-CAA and TPA-PP-Th-CAA receive more electron density than their corresponding CAA moieties. The short-range CT (to internal acceptor) has a higher probability than the long-range CT (to CAA). Similar EDDM results are also observed for the second absorption bands of TPA-BTD-Th-CAA and TPA-PP-Th-CAA; the second absorption bands of TPA-BTD-Th-CAA and TPA-PP-Th-CAA are dual CT bands. Interestingly, for the second absorption bands of TPA-BTD-Th-CAA and TPA-PP-Th-CAA, the electron density of Th moiety is nearly unchanged before and after excitation. On the other hand, the excited electron density of the λmax and second absorption bands of TPA-DBT-Th-CAA and TPA-Ph-Th-CAA is mainly transferred to the CAA moiety, and the electron density of the internal acceptor moiety is slightly decreased upon photoexcitation. These results indicate that the λmax and second absorption bands of TPA-DBT-Th-CAA and TPA-Ph-Th-CAA are single CT bands. For TPA-BT-Th-CAA, upon excitation on the λmax band, the excited electron density of the Th and CAA moieties is increased by 20 and 29%, respectively, and that of the BT is slightly increased by 8%. Similarly, upon excitation on the second absorption band of TPA-BT-Th-CAA, the excited electron density is mainly transferred to Th (increased by 10%) and CAA (increased by 23%) moieties.
Table 2. Transition Characters and Absorption Bands of Studied Dyes at Protonated State in Solution.
| absorption/oscillator strength |
||||
|---|---|---|---|---|
| dye | state | fd | absorption (nm) | transitionse |
| TPA-BTD-Th-CAAa | λmax | 499 | 1.35 | H → L (72%) |
| H – 1 → L (21%) | ||||
| #2 | 378 | 0.12 | H – 1 → L (60%) | |
| H → L + 1 (16%) | ||||
| H → L (11%) | ||||
| TPA-BT-Th-CAAb | λmax | 466 | 1.70 | H → L (66%) |
| H – 1 → L (27%) | ||||
| #2 | 354 | 0.04 | H – 1 → L (56%) | |
| H → L (16%) | ||||
| H → L + 1 (14%) | ||||
| TPA-DBT-Th-CAAc | λmax | 425 | 1.51 | H – 1 → L (49%) |
| H → L (44%) | ||||
| #2 | 337 | 0.04 | H – 1 → L (39%) | |
| H → L (37%) | ||||
| H → L + 1 (10%) | ||||
| TPA-PP-Th-CAAb | λmax | 475 | 1.57 | H → L (72%) |
| H – 1 → L (19%) | ||||
| #2 | 370 | 0.08 | H – 1 → L (59%) | |
| H → L + 1 (18%) | ||||
| H → L (9%) | ||||
| TPA-Ph-Th-CAAc | λmax | 410 | 1.56 | H → L (48%) |
| H – 1 → L (44%) | ||||
| #2 | 330 | 0.10 | H – 1 → L (43%) | |
| H → L (32%) | ||||
| H → L + 1 (12%) | ||||
In dichloroethane.
In CH2Cl2.
In THF.
Oscillator strength.
H = HOMO; L = LUMO; H – 1 = HOMO – 1 and L + 1 = LUMO + 1.
Table 3. EDDMs of Studied Dyes in Solutione.
In dichloroethane.
In CH2Cl2.
In THF.
Oscillator strength.
Before (where the excited electron density is coming from) and after (where the excited electron density is going to) transition.
UV–Visible Spectra of Dye Adsorbed on TiO2
Table 4 lists the calculated transition characters and absorption bands of studied dyes adsorbed on TiO2 obtained from CAM-B3LYP/6-31G(d,p) calculations and their experimental absorption bands. The calculated λmax bands for TPA-BTD-Th-CAA, TPA-BT-Th-CAA, TPA-DBT-Th-CAA, TPA-PP-Th-CAA, and TPA-Ph-Th-CAA adsorbed on (TiO2)38 clusters in acetonitrile are 503, 479, 436, 486, and 417 nm, respectively. The root-mean-square deviation of calculated λmax values from the experimental values is 33 nm. TPA-BTD-Th-CAA is the most red-shifted molecule, and TPA-Ph-Th-CAA is the most blue-shifted molecule. Except for TPA-Ph-Th-CAA, the transition of the λmax band is mainly from HOMO to LUMO and partly from HOMO – 1 to LUMO. The λmax transition of TPA-Ph-Th-CAA is a mixed one, that is, from the two highest occupied orbitals to the two lowest unoccupied orbitals.
Table 4. Experimental Absorption, Calculated Absorption, and Transition Characters of the Bands of Studied Dyes Adsorbed on a (TiO2)38 Cluster in Acetonitrile.
| experiment | calculation |
||||
|---|---|---|---|---|---|
| dye | absorption (nm) | state | absorption (nm) | fa | transitionsb |
| TPA-BTD-Th-CAA | 48116 | λmax | 503 | 1.683 | H → L (69%) |
| H – 1 → L (23%) | |||||
| #2 | 382 | 0.198 | H – 1 → L (55%) | ||
| H → L (12%) | |||||
| TPA-BT-Th-CAA | 42617 | λmax | 479 | 2.115 | H → L (63%) |
| H – 1 → L (27%) | |||||
| #2 | 359 | 0.035 | H – 1 → L (53%) | ||
| H → L (17%) | |||||
| TPA-DBT-Th-CAA | λmax | 436 | 1.924 | H – 1 → L (47%) | |
| H → L (37%) | |||||
| #2 | 342 | 0.016 | H → L (38%) | ||
| H – 1 → L (33%) | |||||
| TPA-PP-Th-CAA | 47920 | λmax | 486 | 1.970 | H → L (70%) |
| H – 1 → L (20%) | |||||
| #2 | 376 | 0.138 | H – 1 → L (55%) | ||
| H → L (8%) | |||||
| TPA-Ph-Th-CAA | λmax | 417 | 1.970 | H – 1 → L (24%) | |
| H → L (21%) | |||||
| H – 1 → L + 1 (20%) | |||||
| H → L + 1 (18%) | |||||
| #2 | 335 | 0.057 | H – 1 → L (19%) | ||
| H → L (19%) | |||||
| H – 1 → L + 1 (16%) | |||||
| H → L + 1 (16%) | |||||
Oscillator strength.
H = HOMO; L = LUMO.
For the first band (λmax), the transition characters of four D–A−π–A molecules adsorbed on a (TiO2)38 cluster are mainly from the HOMO and HOMO – 1 to LUMO, which are similar to those in solution. On the other hand, the transition character of the TPA-Ph-Th-CAA dye mixes more higher-energy LUMO + 1 orbitals, whose electron densities are mainly localized on TiO2.
Table 5 lists the EDDM (before and after photoexcitation) for dyes adsorbed on a (TiO2)38 cluster. TPA-BTD-Th-CAA, after photoexcitation, has the electron density redistributed onto the BTD and CAA/(TiO2)38 moieties, increasing by the same amount of 25%. For TPA-PP-Th-CAA, the electron density on the PP and CAA/(TiO2)38 moieties is increased by 23 and 28%, respectively, after excitation. These results indicate that the λmax bands of TPA-BTD-Th-CAA and TPA-PP-Th-CAA are apparently dual-charge-transfer bands. These two dyes adsorbed on TiO2 have minor electron density delocalized onto TiO2 (<10%). In contrast, TPA-Ph-Th-CAA has most electron density delocalized onto TiO2 (61%), showing the λmax a strong single CT band. Upon adsorption on TiO2 and after photoexcitation, the electron density of DBT on TPA-DBT-Th-CAA is slightly decreased and moderate electron density is redistributed onto TiO2; λmax of TPA-DBT-Th-CAA is a single CT band. For TPA-BT-Th-CAA, the electron density on CAA/(TiO2)38 is significantly increased, a character of a single CT band. In general, upon adsorption onto TiO2 (a sink for electron acceptor), the capacity of the internal acceptor in receiving electron density is decreased relative to that in solution. For the blue-shifted band of a given dye, TiO2 receives more electron density than the λmax band. These results may be due to that the higher-energy transition of dyes (mainly LUMO) is better hybridized with the interfacial orbitals in the conduction band (CB) region of TiO2 with high density of states.
Table 5. EDDM of Dyes on a (TiO2)38 Clusterb.
Oscillator strength.
Before (where the excited electron density is coming from) and after (where the excited electron density is going to) transition.
TPA-BTD-Th-CAA and TPA-PP-Th-CAA with strong internal acceptors have red-shifted absorption spectra and additional bands in the UV–vis region. Therefore, their absorption bands are better matched with solar spectra than other studied dyes, and in principle, they should have higher Jsc values. Nevertheless, their experimentally measured Jsc values (TPA-BTD-Th-CAA = 11.216 mA cm–2 and TPA-PP-Th-CAA = 7.120 mA cm–2) are smaller than or close to those of TPA-Ph-Th-CAA (13.8634 mA cm–2), TPA-BT-Th-CAA (11.8817 mA cm–2), and TPA-DBT-Th-CAA (10.0414 mA cm–2). One of the possible reasons for these results is that the strong internal acceptors, BTD and PP, trap the excited electron density and thus reduce the efficiency of charge transfer/injection to TiO2. This hypothesis is supported by our calculations that BTD and PP moieties trap significant amounts of excited electron density.
Excited-State Oxidation Potential and Photostability
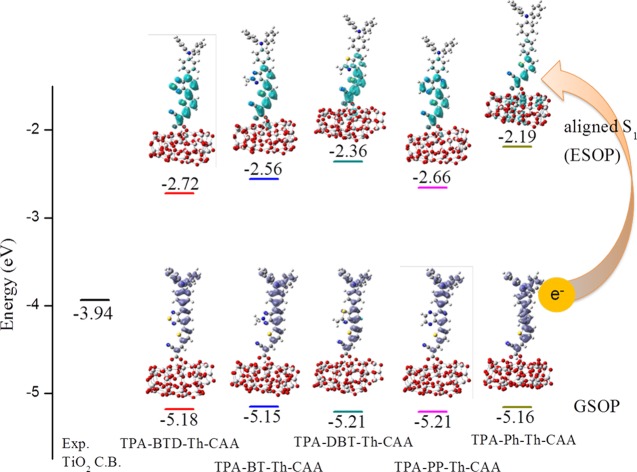
Figure 4 shows the calculated excited-state oxidation potential (ESOP) aligned by the method proposed by De Angelis and colleagues.35,36 The aligned excited-state energy also represents excited-state oxidation potential (ESOP). The exact ESOP is calculated by ESOP = (G – G+)ES, which is the free-energy difference between neutral and oxidized molecules in the excited state at the equilibrium geometry of neutral species. The ESOP can be approximated by ESOP ≅ GSOP – Eo–o, where GSOP is the ground-state oxidation potential (GSOP) calculated by (G – G+)GS and Eo–o is the energy difference between optimized excited and ground states. For large systems, such as dye adsorbed on TiO2, it is not feasible to optimize the excited-state geometry. The Eo–o is approximated by vertical excitation energy. We first calculated the ground-state oxidation potential (GSOP) by computing the energy difference of dyes adsorbed on a (TiO2)38 cluster in its neutral and cation states based on the B3LYP/6-31G(d,p) method. The ESOP is calculated by adding the absorption energy (Eabs) calculated at CAM-B3LYP/6-31G(d,p) level to the GSOP. In conjunction with the EDDM analysis above, it is seen that TPA-Ph-Th-CAA has a large driving force for electron injection as well as strong coupling with TiO2. On the other hand, the D–A−π–A dyes have a smaller driving force for electron injection to TiO2; moreover, the coupling of their excited state with the TiO2 is relatively weaker. In particular, introduction of a strong electron-withdrawing BTD group results in a small driving force as well as a weak coupling with TiO2.
Figure 4.
Energy alignment of dyes adsorbed on TiO2. The bottom bars represent the GSOP; the top bars represent the aligned ESOP. The experimental CB energy of TiO2 (−3.94 eV) is shown on the left.
The photostability of dyes will directly affect the duration time of DSSC devices. In principle, a photoexcited dye with lower energy is expected to be less active in undergoing photochemical reactions and vice versa. The calculated ESOP values (Figure 4) of TPA-BTD-Th-CAA, TPA-BT-Th-CAA, TPA-DBT-Th-CAA, TPA-PP-Th-CAA, and TPA-Ph-Th-CAA adsorbed on a (TiO2)38 cluster are −2.72, −2.56, −2.36, −2.66, and −2.19 eV, respectively. The four studied D–A−π–A dyes adsorbed on TiO2 have lower ESOP values than the D−π–A TPA-Ph-Th-CAA dye adsorbed on TiO2. These results indicate that the insertion of an internal acceptor into a D−π–A framework can potentially improve the photostability. In particular, TPA-BTD-Th-CAA has 0.53 eV lower ESOP than TPA-Ph-Th-CAA. Previous study has shown that the introduction of the electron-deficient BTD moiety can significantly improve the photostability of indoline-based organic sensitizers.16 Our calculations suggest that the D–A−π–A dye has lower ESOP and is potentially more photostable.
TD-DFT Nonadiabatic Molecular Dynamics Simulations
The fast photoinduced ET process from the donor to the acceptor was investigated by TD-DFT nonadiabatic (NA) molecular dynamics (MD) simulations on the femtosecond scale with atomistic details. These NAMD simulations address the CT mechanism of D–A−π–A molecules driven by thermal energy after photoexcitation. Thermal motions of atomic nucleus generate a nonuniform distribution of photoexcited states that are predominantly significant in driving the CT process. In the ground state, thermal motions of the atoms create the ensemble with slightly different molecular geometries corresponding to the inhomogeneous broadening of the absorption spectra that thus influences the energies of the donor and acceptor. On the other hand, the chemical bonds of photoexcited molecules are considered to be weaker than those in the ground state. Therefore, how thermal fluctuations of the atoms at the photoexcited state affect the energies of donor and acceptor and the CT character need to be investigated. Photoexcitation creates the initial charge separation. The EDDM analysis of the zero-temperature UV–vis spectra discussed above shows that the electron density of the photoexcited state is partially delocalized onto the acceptor moiety at the Franck–Condon state. In particular, the excited electron density of photoexcited TPA-BTD-Th-CAA is mainly delocalized onto the BTD and CAA moieties at the same time. One interesting question is whether the thermal energy promotes the second CT process from the internal to the terminal acceptor.
Figure 5 shows the EDDM (where the excited electron density is going to) of four building units of first singlet excited TPA-BTD-Th-CAA molecule as a function of simulation time. After photoexcitation, the excited electron has several possible pathways; it can delocalize more electron density to the acceptor or the electron density on the acceptor can move back to the internal acceptor and even to the donor. It is seen that the electron density on the TPA moiety remains in a steady state with low and stable electron population with a small variance of 0.0002. The electron population on the BTD unit has a large value and a relatively large fluctuation with a variance of 0.0017. On the CAA moiety, the electron population has a variance of 0.0006. No significant amount of electron density populated on CAA moiety is observed. These results indicate that only a small amount of electron density has been pushed and pulled between the internal BTD acceptor and the terminal CAA acceptor. The excited electron density redistribution is less sensitive to thermal motions.
Figure 5.
EDDM (where the electron density is going to) of four building units (TPA, BTD, Th, and CAA) of TPA-BTD-Th-CAA molecule at the S1 state as a function of simulation time.
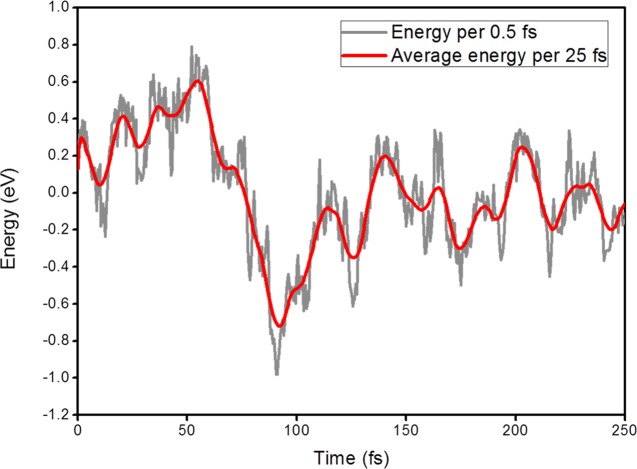
Figure 6 shows that the first excited-state energy of TPA-BTD-Th-CAA molecule evolves with time. The variance of energy is 0.12 eV, which is approximately equal to eight atoms that contribute to change in the electronic energies at 300 K. As shown in Figure 2, the BTD unit has a lower LUMO energy than the CAA moiety by 0.31 eV, which is larger than the variance of excited energy. One of the possible reasons that the excited electron density is rarely pushed to the CAA moiety by thermal energy is the thermal energy lower than the electronic energy difference between the BTD and CAA moieties. That is, the BTD unit traps a significant amount of excited electron density.
Figure 6.
First excited-state energy of TPA-BTD-Th-CAA as a function of simulation time. The thick line represents the average energy every 25 fs. The thin line shows the original energy every 0.5 fs.
Conclusions and Summary
In this study, we employed DFT, TD-DFT, and TD-DFT nonadiabatic MD simulations to investigate the photophysical properties of four D–A−π–A dyes and one D−π–A dye in solution and adsorbed on a (TiO2)38 cluster. Our main conclusions are summarized as follows:
-
1.
Introduction of a strong electron-withdrawing group into the D−π–A framework effectively induces red shifts in absorption due to the small band gap and low-lying LUMO of internal acceptor A.
-
2.
In addition, a strong electron-withdrawing group A, such as the BTD and PP moieties, generates a new band in the UV–visible region, which potentially contributes to the photo-to-current conversion.
-
3.
D–A−π–A dyes exhibit distinct transition characters of absorption. Strong electron-withdrawing groups, such as BTD and PP, induce dual CT bands. The excited electron density is transferred from the TPA donor to the internal and terminal acceptors simultaneously, whereas weaker electron-withdrawing group does not. The strong electron-withdrawing groups trap the electron density and hamper the electron density transferred to the terminal acceptor upon photoexcitation.
-
4.
TiO2 serving as the electron acceptor can assist in delocalizing the excited electron density from the internal acceptor to the CAA/TiO2 side upon photoexcitation.
-
5.
Thermal energy of 300 K, applied in TD-DFT nonadiabatic MD simulations, drives the excited electron density fluctuation between the internal and terminal acceptors, whereas it has limited effect in pushing significant electron density toward the terminal acceptor. It seems that the thermal energy is not populated high enough on proper vibrational modes to drive a significant amount of photoexcited electron density on internal acceptor to the terminal acceptor.
-
6.
Introduction of an internal acceptor with small band gap results in the D–A−π–A dyes a lower ESOP relative to the analogous D−π–A dyes, giving significant features of photostability. These results support experimental observations.
Our study provides new insights into the nature of the absorption bands of D–A−π–A dyes, giving pointers about how to improve the performance of newly designed D–A−π–A dyes.
Computational Methods
The density functional theory (DFT) implemented within Gaussian 09 program37 was exploited in our static ground-state calculations. The ground-state molecular geometries of the studied molecules displayed in Figure 1 were optimized by the Becke, three-parameter, Lee–Yang–Parr (B3LYP) functional38,39 with 6-31G(d,p) basis set.40 The solvation effect was implemented using conductor-like polarizable continuum model (C-PCM).41 Different solvents were used to match with experimental conditions. The long alkyl chain of the moiety of benzotriazole (BT) was replaced with a methyl group to reduce the computing resource because the long alkyl chain has less effect on the electronic structure of the backbone. The time-dependent DFT (TD-DFT) was exploited for UV–vis spectra calculations. To investigate the charge-transfer excitation properties, Coulomb-attenuating method (CAM)42 was applied (at CAM-B3LYP/6-31G(d,p) level) to calculate the UV–vis spectra of the studied molecules based on the optimized geometries by B3LYP/6-31G(d,p) methods.
To model the photophysical properties of dye sensitizers adsorbed on TiO2 thin films, a dye molecule adsorbed on a (TiO2)38 cluster was calculated. The geometries of the (TiO2)38 cluster were optimized at the B3LYP/6-31G(d,p) level. Deprotonated dyes are adsorbed on a (TiO2)38 supercluster43 with an anatase (101) surface in a bidentate mode;44 one proton is transferred to a nearby two-coordinated oxygen atom. The two oxygen atoms of the carboxylate are bound to the two neighboring five-coordinated Ti atoms on the (TiO2)38 cluster surface. The geometries of dye–(TiO2)38 complexes were optimized by the B3LYP/6-31G(d,p) method in the gas phase. All of the atoms of the dye–(TiO2)38 complexes are free for optimization. This model was used as a compromise between computational resources and the stated purpose of predictions. The UV–vis spectra of the optimized dye–(TiO2)38 complexes were performed at the CAM-B3LYP/6-31G(d,p) level using acetonitrile as solvent (C-PCM model) within the Gaussian 09 program. The acetonitrile was chosen to match with experimental conditions. The electron density difference maps (EDDMs), which indicate the electron density before and after excitation, were generated using GaussSum (version 3.0).
The TD-DFT nonadiabatic molecular dynamics (MD) simulations of free molecules were performed in terms of the Newton-X program45−48 (version 1.4.0-2) interfaced to Gaussian 09. The molecule we studied was first optimized using the Gaussian 09 program.37 Thereafter, we calculated vibrational frequencies for the studied molecule. The optimized structures and calculated frequencies were used to generate the initial conditions. A 1 ps adiabatic ground-state MD simulation (B3LYP/6-31G(d,p)) based on one of the initial conditions was performed to reach thermal equilibrium prior to the excited-state simulation (CAM-B3LYP/6-31G(d,p)). The simulation temperature was controlled at 300 K through Andersen thermostat.49,50 The thermostat was applied every 1 fs. The simulation time step was set to 0.5 fs. The final trajectory from the 1 ps adiabatic MD simulation at S0 was used as the initial condition for the following nonadiabatic MD simulations. We performed the nonadiabatic MD simulations at the first singlet excited state (S1) using time-derivative coupling method51 and fewest switching algorithm.52 The method to compute the global phase was set to overlap the h vectors. And hopping from one state to another surface was computed and is allowed at any time step. Butcher’s53 fifth-order method was used for the integration of time-dependent Schrödinger equation. Nonconsecutive states (e.g., S0 and S2) are all included in computing the nonadiabatic coupling. The output trajectories were saved every 5 fs. To maintain the stability of the system, the job was terminated when the energy difference between two neighboring steps or between the current step and the initial step was higher than 0.5 eV.
Acknowledgments
The authors acknowledge the Ministry of Science and Technology of Taiwan (Grant no.: MOST 106-2113-M-008-009) for financial support and the National Center for High-Performance Computing and the V’ger computer cluster at the National Central University of Taiwan for allowing access to computer time and facilities.
Author Present Address
† Department of Chemistry, National Taiwan University, No. 1, Section 4, Roosevelt Road, Taipei 10617, Taiwan (Y.-C.S.).
The authors declare no competing financial interest.
References
- Hagfeldt A.; Boschloo G.; Sun L.; Kloo L.; Pettersson H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. 10.1021/cr900356p. [DOI] [PubMed] [Google Scholar]
- Wöhrle D.; Meissner D. Organic Solar Cells. Adv. Mater. 1991, 3, 129–138. 10.1002/adma.19910030303. [DOI] [Google Scholar]
- Smestad G. P.Optoelectronics of Solar Cells; Spie Press, 2002; Vol. 115, p 118. [Google Scholar]
- Nazeeruddin M. K.; De Angelis F.; Fantacci S.; Selloni A.; Viscardi G.; Liska P.; Ito S.; Takeru B.; Grätzel M. Combined experimental and DFT–TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. 10.1021/ja052467l. [DOI] [PubMed] [Google Scholar]
- You J.; Dou L.; Yoshimura K.; Kato T.; Ohya K.; Moriarty T.; Emery K.; Chen C.-C.; Gao J.; Li G.; Yang Y. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446 10.1038/ncomms2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.-Y.; Taufany F.; Nachimuthu S.; Jiang J.-C.; Liaw D.-J. Design strategies of metal free-organic sensitizers for dye sensitized solar cells: Role of donor and acceptor monomers. Org. Electron. 2014, 15, 1205–1214. 10.1016/j.orgel.2014.03.022. [DOI] [Google Scholar]
- Xu W.; Peng B.; Chen J.; Liang M.; Cai F. New Triphenylamine-Based Dyes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2008, 112, 874–880. 10.1021/jp076992d. [DOI] [Google Scholar]
- Liu B.; Wu W.; Li X.; Li L.; Guo S.; Wei X.; Zhu W.; Liu Q. Molecular engineering and theoretical investigation of organic sensitizers based on indoline dyes for quasi-solid state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2011, 13, 8985–8992. 10.1039/c1cp20484j. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Zhu W.-H.; Zakeeruddin S. M.; Grätzel M. Insight into D–A−π–A Structured Sensitizers: A Promising Route to Highly Efficient and Stable Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 9307–9318. 10.1021/acsami.5b02475. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yang J.; Yu H.; Li F.; Fan L.; Sun W.; Liu Y.; Koh Z. Y.; Pan J.; Yim W.-L.; Yan L.; Wang Q. A benzothiazole–cyclopentadithiophene bridged D–A−π–A sensitizer with enhanced light absorption for high efficiency dye-sensitized solar cells. Chem. Commun. 2014, 50, 3965–3968. 10.1039/C4CC00577E. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Li X.; Hu Y.; Fan Y.; Yuan J.; Robertson N.; Hua J.; Marder S. R. Effect of an auxiliary acceptor on D–A−π–A sensitizers for highly efficient and stable dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 12865–12877. 10.1039/C6TA05588E. [DOI] [Google Scholar]
- Abe M.; Adam W.; Heidenfelder T.; Nau W. M.; Zhang X. Intramolecular and Intermolecular Reactivity of Localized Singlet Diradicals: The Exceedingly Long-Lived 2,2-Diethoxy-1,3-diphenylcyclopentane-1,3-diyl. J. Am. Chem. Soc. 2000, 122, 2019–2026. 10.1021/ja992507j. [DOI] [Google Scholar]
- Adam W.; Borden W. T.; Burda C.; Foster H.; Heidenfelder T.; Heubes M.; Hrovat D. A.; Kita F.; Lewis S. B.; Scheutzow D.; Wirz J. Transient Spectroscopy of a Derivative of 2,2-Difluoro-1,3-diphenylcyclopentane-1,3-diyl—A Persistent Localized Singlet 1,3-Diradical. J. Am. Chem. Soc. 1998, 120, 593–594. 10.1021/ja972977i. [DOI] [Google Scholar]
- Sauvage F.; Chen D.; Comte P.; Huang F.; Heiniger L.-P.; Cheng Y.-B.; Caruso R. A.; Graetzel M. Dye-sensitized solar cells employing a single film of mesoporous TiO2 beads achieve power conversion efficiencies over 10%. ACS Nano 2010, 4, 4420–4425. 10.1021/nn1010396. [DOI] [PubMed] [Google Scholar]
- He J.; Wu W.; Hua J.; Jiang Y.; Qu S.; Li J.; Long Y.; Tian H. Bithiazole-Bridged Dyes for Dye-Sensitized Solar Cells with High Open Circuit Voltage Performance. J. Mater. Chem. 2011, 21, 6054. 10.1039/c0jm03811c. [DOI] [Google Scholar]
- Zhu W.; Wu Y.; Wang S.; Li W.; Li X.; Chen J.; Wang Z.-s.; Tian H. Organic D–A−π–A Solar Cell Sensitizers with Improved Stability and Spectral Response. Adv. Funct. Mater. 2011, 21, 756–763. 10.1002/adfm.201001801. [DOI] [Google Scholar]
- Mao J.; Guo F.; Ying W.; Wu W.; Li J.; Hua J. Benzotriazole-bridged sensitizers containing a furan moiety for dye-sensitized solar cells with high open-circuit voltage performance. Chem. – Asian J. 2012, 7, 982–991. 10.1002/asia.201100967. [DOI] [PubMed] [Google Scholar]
- Ci Z.; Yu X.; Bao M.; Wang C.; Ma T. Influence of the benzo[d]thiazole-derived π-bridges on the optical and photovoltaic performance of D−π–A dyes. Dyes Pigm. 2013, 96, 619–625. 10.1016/j.dyepig.2012.11.004. [DOI] [Google Scholar]
- Pei K.; Wu Y.; Wu W.; Zhang Q.; Chen B.; Tian H.; Zhu W. Constructing Organic D–A−π–A-Featured Sensitizers with a Quinoxaline Unit for High-Efficiency Solar Cells: The Effect of an Auxiliary Acceptor on the Absorption and the Energy Level Alignment. Chem. – Eur. J. 2012, 18, 8190–8200. 10.1002/chem.201103542. [DOI] [PubMed] [Google Scholar]
- Ying W.; Yang J.; Wielopolski M.; Moehl T.; Moser J.-E.; Comte P.; Hua J. L.; Zakeeruddin S. M.; Tian H.; Grätzel M. New pyrido[3,4-b] pyrazine-based sensitizers for efficient and stable dye-sensitized solar cells. Chem. Sci. 2014, 5, 206–214. 10.1039/C3SC51844B. [DOI] [Google Scholar]
- Wu Y.; Zhu W. Organic sensitizers from D−π–A to D–A−π–A: effect of the internal electron-withdrawing units on molecular absorption, energy levels and photovoltaic performances. Chem. Soc. Rev. 2013, 42, 2039–2058. 10.1039/C2CS35346F. [DOI] [PubMed] [Google Scholar]
- Kusama H.; Orita H.; Sugihara H. TiO2 band shift by nitrogen-containing heterocycles in dye-sensitized solar cells: a periodic density functional theory study. Langmuir 2008, 24, 4411–4419. 10.1021/la703696f. [DOI] [PubMed] [Google Scholar]
- Li W.; Wu Y.; Zhang Q.; Tian H.; Zhu W. D–A−π–A featured sensitizers bearing phthalimide and benzotriazole as auxiliary acceptor: effect on absorption and charge recombination dynamics in dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2012, 4, 1822–1830. 10.1021/am3001049. [DOI] [PubMed] [Google Scholar]
- Sun L.; Hagfeldt A.; Hagberg D. P.; Yum J.-H.; Lee H.; De Angelis F.; Marinado T.; Karlsson K. M.; Humphry-Baker R.; Grätzel M.; Nazeeruddin M. K. Molecular Engineering of Organic Sensitizers for Dye-Sensitized Solar Cell Applications. J. Am. Chem. Soc. 2008, 130, 6259–6266. 10.1021/ja800066y. [DOI] [PubMed] [Google Scholar]
- Liu B.; Zhu W.; Zhang Q.; Wu W.; Xu M.; Ning Z.; Xie Y.; Tian H. Conveniently synthesized isophorone dyes for high efficiency dye-sensitized solar cells: tuning photovoltaic performance by structural modification of donor group in donor−π–acceptor system. Chem. Commun. 2009, 1766–1768. 10.1039/b820964b. [DOI] [PubMed] [Google Scholar]
- Ning Z.; Zhang Q.; Wu W.; Pei H.; Liu B.; Tian H. Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J. Org. Chem. 2008, 73, 3791–3797. 10.1021/jo800159t. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Fischer M. K. R.; Bäuerle P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem., Int. Ed. 2009, 48, 2474–2499. 10.1002/anie.200804709. [DOI] [PubMed] [Google Scholar]
- Velusamy M.; Thomas K. R. J.; Lin J. T.; Hsu Y.-C.; Ho K.-C. Organic dyes incorporating low-band-gap chromophores for dye-sensitized solar cells. Org. Lett. 2005, 7, 1899–1902. 10.1021/ol050417f. [DOI] [PubMed] [Google Scholar]
- Hou J.; Chen H.-Y.; Zhang S.; Li G.; Yang Y. Synthesis, characterization, and photovoltaic properties of a low band gap polymer based on silole-containing polythiophenes and 2,1,3-benzothiadiazole. J. Am. Chem. Soc. 2008, 130, 16144–16145. 10.1021/ja806687u. [DOI] [PubMed] [Google Scholar]
- Li W.; Du C.; Li F.; Zhou Y.; Fahlman M.; Bo Z.; Zhang F. Benzothiadiazole-Based Linear and Star Molecules: Design, Synthesis, and Their Application in Bulk Heterojunction Organic Solar Cells. Chem. Mater. 2009, 21, 5327–5334. 10.1021/cm902611b. [DOI] [Google Scholar]
- Beaujuge P. M.; Pisula W.; Tsao H. N.; Ellinger S.; Müllen K.; Reynolds J. R. Tailoring structure–property relationships in dithienosilole–benzothiadiazole donor–acceptor copolymers. J. Am. Chem. Soc. 2009, 131, 7514–7515. 10.1021/ja900519k. [DOI] [PubMed] [Google Scholar]
- Tang Z.-M.; Lei T.; Jiang K.-J.; Song Y.-L.; Pei J. Benzothiadiazole containing D−π–A conjugated compounds for dye-sensitized solar cells: synthesis, properties, and photovoltaic performances. Chem. – Asian J. 2010, 5, 1911–1917. 10.1002/asia.201000158. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Peng B.; Liu B.; Pan C.; Li Y.; He Y.; Zhou K.; Zou Y. Copolymers from benzodithiophene and benzotriazole: synthesis and photovoltaic applications. Polym. Chem. 2010, 1, 1441. 10.1039/c0py00136h. [DOI] [Google Scholar]
- Chang Y. J.; Chow T. J. Dye-sensitized solar cell utilizing organic dyads containing triarylene conjugates. Tetrahedron 2009, 65, 4726–4734. 10.1016/j.tet.2009.04.024. [DOI] [Google Scholar]
- De Angelis F.; Fantacci S.; Mosconi E.; Nazeeruddin M. K.; Grätzel M. Absorption Spectra and Excited State Energy Levels of the N719 Dye on TiO2 in Dye-Sensitized Solar Cell Models. J. Phys. Chem. C 2011, 115, 8825–8831. 10.1021/jp111949a. [DOI] [Google Scholar]
- Pastore M.; Fantacci S.; De Angelis F. Modeling Excited States and Alignment of Energy Levels in Dye-Sensitized Solar Cells: Successes, Failures, and Challenges. J. Phys. Chem. C 2013, 117, 3685–3700. 10.1021/jp3095227. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Taroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam N. J.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas Ö; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09; Gaussian, Inc.: Wallingford, CT, 2009. [Google Scholar]
- Becke A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Lee C.; Yang W.; Parr R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B: Condens. Matter 1988, 37, 785 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]
- Petersson G. A.; Al-Laham M. A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. 10.1063/1.460447. [DOI] [Google Scholar]
- Cossi M.; Rega N.; Scalmani G.; Barone V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]
- Yanai T.; Tew D. P.; Handy N. C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. 10.1016/j.cplett.2004.06.011. [DOI] [Google Scholar]
- Yakhanthip T.; Jungsuttiwong S.; Namuangruk S.; Kungwan N.; Promarak V.; Sudyoadsuk T.; Kochpradist P. Theoretical investigation of novel carbazole-fluorene based D−π–A conjugated organic dyes as dye-sensitizer in dye-sensitized solar cells (DSCs). J. Comput. Chem. 2011, 32, 1568–1576. 10.1002/jcc.21735. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Cole J. M. Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. 10.1021/am507334m. [DOI] [PubMed] [Google Scholar]
- Barbatti M.; Granucci G.; Persico M.; Ruckenbauer M.; Vazdar M.; Eckert-Maksić M.; Lischka H.. NEWTON-X: A Package for Newtonian Dynamics Close to the Crossing Seam, 2011. www.newtonx.org.
- Barbatti M.; Ruckenbauer M.; Plasser F.; Pittner J.; Granucci G.; Persico M.; Lischka H. Newton-X: a surface-hopping program for nonadiabatic molecular dynamics. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 26–33. 10.1002/wcms.1158. [DOI] [Google Scholar]
- Barbatti M.; Granucci G.; Persico M.; Ruckenbauer M.; Vazdar M.; Eckert-Maksić M.; Lischka H. The on-the-fly surface-hopping program system Newton-X: Application to ab initio simulation of the nonadiabatic photodynamics of benchmark systems. J. Photochem. Photobiol., A 2007, 190, 228–240. 10.1016/j.jphotochem.2006.12.008. [DOI] [Google Scholar]
- Crespo-Otero R.; Barbatti M. Spectrum simulation and decomposition with nuclear ensemble: formal derivation and application to benzene, furan and 2-phenylfuran. Theor. Chem. Acc. 2012, 131, 1237 10.1007/s00214-012-1237-4. [DOI] [Google Scholar]
- Andersen H. C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. 10.1063/1.439486. [DOI] [Google Scholar]
- Tanaka H.; Nakanishi K.; Watanabe N. Constant temperature molecular dynamics calculation on Lennard-Jones fluid and its application to water. J. Chem. Phys. 1983, 78, 2626–2634. 10.1063/1.445020. [DOI] [Google Scholar]
- Pittner J.; Lischka H.; Barbatti M. Optimization of mixed quantum-classical dynamics: Time-derivative coupling terms and selected couplings. Chem. Phys. 2009, 356, 147–152. 10.1016/j.chemphys.2008.10.013. [DOI] [Google Scholar]
- Hammes-Schiffer S.; Tully J. C. Proton transfer in solution: Molecular dynamics with quantum transitions. J. Chem. Phys. 1994, 101, 4657–4667. 10.1063/1.467455. [DOI] [Google Scholar]
- Butcher J. C. A Modified Multistep Method for the Numerical Integration of Ordinary Differential Equations. J. ACM 1965, 12, 124–135. 10.1145/321250.321261. [DOI] [Google Scholar]