Abstract

Understanding the dynamic processes of CO2 capture in biosystems is important because of the great effect CO2 has on the carbon cycle, human health, the global climate, and living environments. After years of multidisciplinary studies, researchers have gained only basic mechanistic knowledge about how enzymes or protein-aggregates capture and deliver CO2, a process involving reversible bonding of CO2 with basic amino acid residues. However, vital mechanistic details of how the activated basic residues within these enzymes or protein-aggregates are initially formed, a crucial step for CO2 capture, are still lacking. Herein, we designed specific molecules, i.e., oxazolidines, which are able to reversibly change their alkalinity via ultrafast isomerizations. Serving as so-called transient bases, these oxazolidines mimic the activated/deactivated states of enzymes or protein-aggregates responsible for dynamic CO2 capture/release. A detailed mechanism for CO2 capture, which involves dynamic covalent bonding and multimolecular cooperative interactions among functional groups that occur with the help of a polyhydroxyl environment, is demonstrated by UV−vis and multiple NMR spectroscopies as well as theoretical calculations. Using suitable oxazolidine transient bases, applications for visual CO2 detection under different detection limit requirements were also developed. Insights for further understanding the process of dynamic CO2 capture in biosystems are also discussed. This oxazolidine-inspired biomimetic CO2 capture serves as a platform for the future development of additional biomimicking systems, as well as offers unique perspectives for other complicated life processes.
Introduction
Carbon dioxide (CO2), as an indispensable component of the atmosphere,1 plays important roles in the carbon cycle of the biosphere2 and has huge impacts on the global climate and human health.3,4 It is involved in many vital biological processes, such as photosynthesis,5 respiration,6 CO2 transport,7 and so on. Many detailed studies8−10 on CO2 are those that involve bioreactions and processes, especially with respect to how CO2 is fixed by enzymes and protein-aggregates found in cells. These investigations have revealed several key features that have led to a better understanding of intricate life processes. One particularly important process11,12 is the equilibrium between the noncarbamylated and carbamylated forms of the enzyme or protein, which is a key step for biotransformations involving CO2. A well-known example is the capture of CO2 in the atmosphere, which is then converted to a carbamate (RNHCOOH) by the activated Lys201ε-NH2 residue of ribulose-1,5-bisphosphate carboxylase (RuBisCO) during the Calvin cycle in plants10,13 (Scheme 1a). Another example of carbamylation involves hemoglobin (Hb), in which CO2 (proportion of 7%) is converted to carbamino-hemoglobin in blood by activated valyl-amine found in the β-chain of hemoglobin (or myohemoglobin) during respiration14 (Scheme 1b). Both RuBisCO and Hb require the formation of free-base amine groups in their related amino acid residues to be activated for CO2 capture. The resulting amines of these residues are alkaline enough to facilitate carboxylation with CO2. Nearby cations bound-up in the protein, such as Mg2+ or Fe2+, further stabilize the negative charge from the resulting carbamate anion (Scheme 1c). How the alkali residues are activated and deactivated for continuous and repetitive CO2 capture and release during these enzymatic processes, however, has not been understood completely yet. One convincing possible explanation for the reversible change in alkalinity is that the activation and deactivation of RuBisCO or Hb result in pH changes (from 7 to 9) of their local microenvironments.12,13 Nevertheless, many features or functions15−17 for macromolecules seem to be brought about by thermodynamic alteration of their molecular conformations (e.g., molecular vibration, rotation) or configurations (e.g., heterolytic cleavage of chemical bonds) for self-adaption or survival needs. Alkalinity changes among functional subunits of metabolic protein-aggregates (i.e., RuBisCO and Hb) may also be caused by thermodynamically adjusting their local structural configurations or conformations in response to an external stimulus (e.g., an increase in CO2 concentration), rather than a pH change in its environment. However, this bold conjecture is difficult to prove directly by current characterization techniques due to the complicated three-dimensional dynamic structures/conformations and behaviors for these protein-aggregates.
Scheme 1. Schematic Diagrams and Molecular Switches.
Schematic diagrams of CO2 capture (a) by the enzyme RuBisCO in plant cells and (b) by the protein hemoglobin in animal cells; schematic diagram showing (c) the common characteristic in forming free amines for capturing CO2 in the cases of RuBisCO and Hb.
(d) Molecular switches studied herein that can potentially form transient bases.
We became motivated by the idea that the essential principles of all of the molecular transformations should be universal, regardless of whether they are chemical or biochemical processes, that is, the underlying working principles deduced from simplified biomimetic reactions employing small organic molecules should be the same as those of large biomolecules (e.g., proteins and nucleic acids). This type of thinking might provide new trains of thought for the development of molecular bionics. Small molecules characterized by fleeting reversible alkalinity might display similar behaviors as those of biological systems nature uses for CO2 capture by means of a dynamic addition reaction. To verify this hypothesis, as well as to construct a simple platform for CO2 capture relying on dynamic covalent bonding, we went about designing some simple molecules that are able to reversibly change their alkalinity by way of ultrafast isomerization reactions. For the sake of discussion, herein we refer to molecules that possess this property of reversible alkalinity as “transient bases”. This momentary basicity is a result of the rapid, highly reversible, and repeated structural variations that occur at thermodynamic equilibrium, where one isomer possesses a particularly potent basic functionality. Numerous studies with chloroplasts and leaves have shown that a greater proportion of RuBisCO in the active form exists in response to a higher light intensity. Thus, we envision that photochromic molecules, such as spiropyrans,18 oxazines,19 and oxazolidines,20,21 which can undergo reversible isomerization between a ring-closed form (RCF) and a ring-open form (ROF) accompanied by obvious changes in color, might be ideal transient bases for developing simplified platforms for exploration of biomimetic CO2 capture (Scheme 1d). The reasons for this are threefold: (i) the quick dynamic equilibrium between RCF and ROF for these molecules happens at room temperature; (ii) the transiently formed zwitterionic ROFs contain anionic phenolate or ethanolate groups, which have undoubted similarity with the transiently formed alkali amines employed in those enzymetic reactions by nature; (iii) the process of CO2 capture and release can be conveniently visualized and monitored via color changes.
Herein, we have selected three types of related molecular switches as test molecular platforms and investigated the possible relationships between their structures and CO2-capture responses to screen for suitable transient bases that can serve as protein mimics. The visually reversible process of CO2 capture by transient bases in the form of oxazolidines was studied in detail. The mechanisms for CO2 capture, which involve dynamic reversible bonding as well as multimolecular cooperative interactions that occur with the help of a polyhydroxyl environment, are investigated and confirmed. Potential applications for the transient bases in visual CO2 detection under different detection limits were developed by fine tuning of the structures. Our study also offers a new feasible explanation for further understanding the process of dynamic CO2 capture in biosystems. Findings from this biomimetic exploration might hint at a simple way forward in developing biomimicking technologies and understanding complicated life processes, which will help stimulate innovations for vital industrial applications.
Results and Discussion
Designing and Screening Suitable Transient Bases
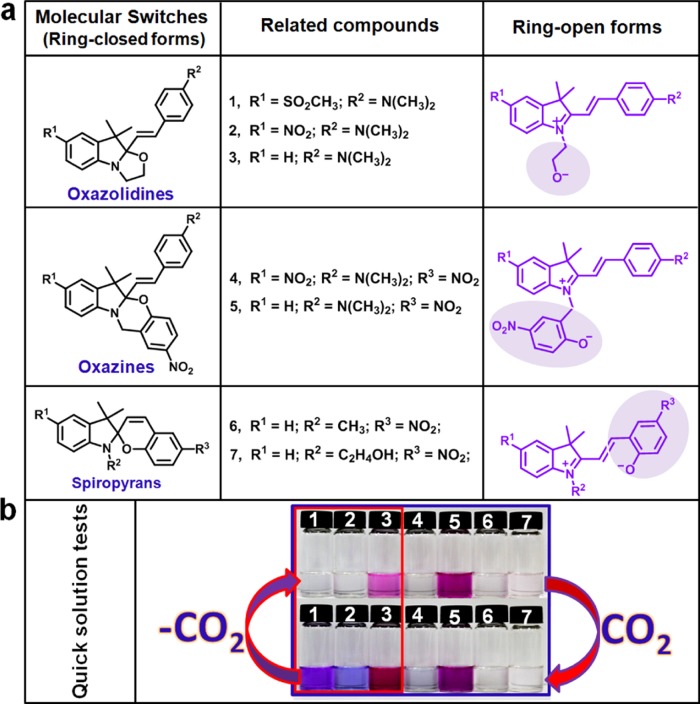
To test our assumptions about CO2 capture, we have selected and prepared three classes of seven structurally related molecules: oxazolidines (1–3), oxazines (4, 5), and spiropyrans (6, 7) (see Figure 1a). Ethanol (EtOH) was chosen as a solvent instead of water to mimic the polyhydric microenvironment within an enzyme, as well as to avoid confusion from possible acidochromism20 induced by carbonate or hydrochromism21 induced by water. The CO2-capture properties of these color switches were preliminarily evaluated by color changes with quick solution tests in anhydrous EtOH (see Figure 1b). Oxazines (4 and 5) are not optimal candidates due to the fact that they are either oversensitive (or nearly inactive) to stimulation by ethanol, and consequently, a considerable amount of their ROFs already exist (or hardly exist) in the solution. These properties render their responses to CO2 difficult to observe. The spiropyrans (6 and 7) were eliminated next for the reason that their ROFs can hardly be trapped by the introduction of CO2, even in aqueous solutions. Oxazolidines (1–3), by contrast, were selected as potential candidates because of their much better performances in response to CO2, which were accompanied by obvious color changes; when CO2 was purged from the solution by brief bubbling with the nitrogen or argon gas, the solutions of 1–3 in EtOH all returned to their initial states. These phenomena happen because the nucleophilicity of the zwitterionic ethanolates found in the ROFs of the oxazolidines is much stronger than that of the phenolates in the ROFs for the spiropyrans or oxazines. This inference is further supported by the order of their calculated alkalinities determined via B3LYP/6-31G(d,p) by Gaussian 1622 and the computational scheme suggested in the literature.23 These calculated alkalinities reflect their nucleophilicities to some extent, that is, oxazolidines (1–3) > oxazines (4, 5) > spiropyrans (6, 7) (Figure S1 and Table S1). These observations indicate that the CO2-capture process is greatly influenced by the nucleophilicity of the transient base.
Figure 1.
(a) Three classes of molecular switches 1–7 investigated in this study showed in their ring-closed forms (RCFs) and ring-open forms (ROFs). (b) Results of quick solution tests (photographs of solutions of 1–7 in ethanol after introduction or removal of CO2 under ambient conditions). The suitable compounds are boxed in red.
Considering that inter/intramolecular dipole–dipole interactions are usually affected by their substituent groups, we anticipated that the CO2-capture rates and the associated color changes for the oxazolidines may be affected by different functional groups. According to our previous work,21 oxazolidines with electron-donating groups on the indole benzene ring prefer to exist in their ROFs even without CO2, whereas strong electron-donating groups on the styrene benzene ring increase color intensities. Thus, the three oxazolidines (1–3) in Figure 1a were designed with either electron-withdrawing or no substituents on their indole rings and N,N-dimethylamine on their styrene rings. Their X-ray crystal structures are shown in Figure 2a (for detailed information, see Table S2 and Figure S2). Experimental results reveal that 3, which has no substituent on its indole ring, shows a fast response rate (nearly instantaneous color change observed by eye upon addition of CO2), yet it appears light pink before the introduction of CO2. With electron-withdrawing groups (i.e., nitro or methylsulfoxide) on their indole rings, 2 and 1 exist as colorless RCFs in EtOH and turn to blue and purple after introduction of CO2, respectively. However, both sensitivity and response times with respect to CO2 capture for 1 and 2 are less than those for 3. The calculated alkalinity (or nucleophilicity) order for their ROFs is 3O > 1O > 2O (see Table S1). Notably, electron-withdrawing groups (i.e., nitro or methylsulfoxide) in conjugation with the indole ring can decrease the alkalinity of oxazolidine ROFs, even though they are not conjugated with their close-by ethanolates. This can be understood from Lewis acid/base theory, that is, acidity/basicity of an ionizable molecule is highly dependent on the relative acidic/basic intensities of the cation and anion of its ion pair. To further explain the substituent effect on their CO2-capture sensitivities, the free energies and activation barriers for their ring-opening and CO2-capturing reactions were calculated (Figure 2b). The results show that the ring-opening steps for forming the transient bases of 1–3 are spontaneous with negative changes in free energy (ΔGI), whereas CO2 capture by the transient base is the rate-controlling step with much higher free energy barriers (ΔGII) (see Figures 2b and S3–S6). The kinetics for CO2 captured by 1–3 were tested, which fit well with a first-order reaction mechanism at the early stage. The rate constants for 1, 2, and 3 were measured as 1.49 × 10–4, 9.52 × 10–5, and 2.16 × 10–3 s–1, respectively (Figures S7, S8 and Table S3). Taken together, both order of the free energy barriers for CO2 capture (ΔGII: 3 < 1 < 2) and rate constants (k: 3 > 2 > 1) are highly related to the order of the transient bases’ nucleophilicities (3O > 1O > 2O), and the relationship is shown in Figure 2c. These results further indicate that nucleophilicity is an important factor in determining the ability of a transient base to capture CO2.
Figure 2.

(a) Single-crystal X-ray structures of 1–3 in their ring-closed forms. (b) B3LYP/6-31g(d,p)-optimized structures for the transient bases of oxazolidine 1 taken as an example and the calculated free energies for the steps involved in CO2 capture. (c) Bar graphs showing the relationship between the alkalinity of 1O–3O, their free energy barriers (ΔGII) and rate constants (k) for capturing CO2.
Dynamic CO2 Capture with the Transient Bases Formed from the Oxazolidines
We take 1 as an example to discuss in detail dynamic CO2 capture by a transient base, which possesses a colorless ring-closed isomer 1C and colored ring-open isomer 1O′, a product formed by the addition of CO2 to the ring-open isomer 1O in anhydrous EtOH. The solution of 1 in anhydrous EtOH is initially colorless and has no detectable absorption in the visible region of the spectrum, an observation that indicates that 1C isomer is dominant before the addition of CO2. When CO2 was bubbled briefly into a solution of 1C, a purple color change was observed, an observation that indicates the formation of 1O′. This conclusion was supported by UV–vis spectroscopy, that is, a new absorption band appeared in the visible region around 568 nm, accompanied with a decrease in the absorption band at 298 nm (Figure 3a). When this purple solution was purged briefly with “inert” gases (air, nitrogen, or argon) lacking high concentrations of CO2, it returned to its initial colorless state, with the absorption bands returning to their original intensities. This process can be repeated many times without any detectable decomposition indicated by a stable isosbestic point at 400 nm (Figure 3b).
Figure 3.
(a) UV–vis spectra of 1 in ethanol (1 × 10–4 M) before and after purging with CO2; insets are the corresponding photographs of the solutions. (b) Five cycles of CO2 introduction to produce the ROF and CO2 removal (i.e., bubbling N2) to produce the RCF. (c) Molecular structures of 1C and 1O′ in alcohol solution without and after introduction of CO2, respectively; 1H NMR spectra in MeOD of 1C (spectrum i), mixture of 1C and 1O′ upon bubbling with CO2 (spectrum ii), and 1C again after purging with N2 to remove CO2 (spectrum iii). Peaks are assigned by using different color letters; peaks labeled with (×) are attributed to methanol (MeOH) and water.
Dynamic CO2 capture by reversible transient base 1 was further confirmed by 1H NMR spectroscopy (Figure 3c). 1 was observed to be nearly completely in its RCF in deuterium-substituted methanol (MeOD), even although the solution was slightly pink before the introduction of CO2 (Figure 3c,i). Upon introduction of CO2, the 1H NMR spectrum showed a new set of resonances, mostly shifted downfield, which arise from the protons of the colored, CO2-triggered structure 1O′ (Figure 3c,ii). The appearance of a new pair of resonances was observed at δ = 4.58 ppm for Hy and δ = 4.03 ppm for Hx instead of multiple peaks at δ = 3.79–3.51 ppm, as well as a single peak at δ = 1.88 ppm instead of two resonances at δ = 1.46 and 1.18 ppm for HMe, signals that are characteristic of ethane protons (i.e., −CH2–CH2–O–), and those of the two methyls on the indole of 1O′, respectively (Figure 3c,ii). The maximum ratio of 1O′ to 1C is 1:3, a value that is calculated by integrating the area of the characteristic peaks in the 1H NMR spectrum. When this purple solution was purged briefly with nitrogen, the 1H NMR signals shifted back to their initial positions (Figure 3c,iii). Analyzed in a similar manner as 1, the maximum ratios of 2O′ to 2C and 3O′ to 3C are 1:3.3 and 14.5:1, respectively, after bubbling with CO2 (Figures S9 and S10). This result is consistent with the afore-discovered sensitivity order of each oxazolidine’s CO2-capturing ability, that is, 3 > 1 > 2. All of these observations indicate clearly that CO2 can be reversibly captured and released by the transient bases formed from these oxazolidines.
Detailed Mechanism of CO2 Capture with Oxazolidines
An in-depth understanding of the CO2-capture mechanism is essential to design new materials for selective detection or removal of CO2, as well as to assist in better understanding some of the complicated biological processes. There are three species in this reaction that need to be considered: CO2, oxazolidines, and the solvent. First, the media effects of different solvents on the capture of CO2 by oxazolidines are investigated. The effect of the surrounding medium provided by the solvent is analogous to the effects caused by the microenvironments found within enzymes or proteins. Various solvents were chosen to replace EtOH while the other conditions remained the same. Similar color changes also occurred in solutions of 1 in the protic solvents MeOH and propanol (PrOH) after the introduction of CO2, but not in aprotic solvents, such as ethyl acetate (EtOAc), dichloromethane (CH2Cl2), chloroform (CHCl3), dimethyl sulfoxide (DMSO), and acetonitrile (MeCN) (see Figure 4a). In addition, the ratio of 1O′ formed increases proportionally according to the hydrogen-bond-donating capability of the protic solvents (see Figure 4b). This result is due to the fact that hydrogen bonding between the proton of the solvent and 1O′ will greatly decrease free energy of 1O′, a hypothesis which is further supported by theoretical calculations (see Figures 4c, S11 and S12). It is to be noted that 1C and 1O coexist in most solvents (except in EtOAc) at room temperature via dynamic equilibrium even before the introduction of CO2 (see amplified graph in Figure 4a). These observations indicate that the formation of the transient base of 1O is greatly influenced by the nature of the polyhydric environment, a situation that is similar to water-manipulated microenvironments around enzymes, and these environments play important roles in CO2 capture by oxazolidines.
Figure 4.
UV–vis spectra of 1 (1 × 10–4 M) in (a) MeOH, EtOH, and various aprotic solvents (quartz cell: 10 mm, except for MeOH solution: 3.5 mm) and (b) protic solvents (quartz cell: 3.5 mm) before (solid) and after (dashed) bubbling with CO2; the inset shows the spectra of (a) partially magnified in the 450–650 nm region; PrOH/EtOH refers to a mixture of PrOH and EtOH with a volume ratio of 1:1 and EtOH/MeOH refers to a mixture of EtOH and MeOH with a volume ratio of 1:1. (c) Free energy curves (in kJ/mol) for transient base 1 (reactant) capturing CO2 to form 1O′ in different solvents in comparison to the gas-phase reaction. Inset is the B3LYP/6-31g(d,p)-optimized structure of the transition state for the reaction of 1O capturing CO2 stabilized by two solvent molecules of MeOH.
In situ 13C NMR experiments with the aid of two-dimensional correlation analyses were carried out in MeOD to further confirm the structural changes of the oxazolidines before and after the introduction of CO2 (see Figures S13–S16). Taking into consideration that the 13C signal strength of oxazolidines in their ROFs is related to their solubilities (1C: less than 2 mg/0.5 mL in MeOD; 3C: less than 5 mg/0.5 mL in MeOD) and the maximum ratios of ROF to RCF for each compound (1O′/1C = 1:3; 3O′/3C = 14.5:1), we chose 3 instead of 1 for 13C NMR investigation. When CO2 was purged into a saturated solution of 3, the solubility of 3 in MeOD was greatly increased (see Figure S17), an observation that is consistent with the reaction of CO2 with 3 to produce 3O′, whose solubility is larger than that of 3C. Compared to the 13C NMR spectra of 3C (see Figure 5a) and 3O(H) (formed by the addition of deuterium CF3COOH to a solution of 3C, Figure 5b), 3 in MeOD after the introduction of CO2 includes both 3C and 3O′, and 3O′ has nearly the same 13C signals as those of 3O (see Figure 5d). It is important to note that there are two additional peaks at δ = 161.5 and 126.3 ppm in comparison to the spectrum resulting from acid-triggered ring-opening by the addition of deuterium chloride, forming 3O(H) or 3O(H)+ (Figure S18). The peak at δ = 126.3 ppm arising from dissolved CO2 in MeOD is also observed after bubbling CO2 into pure MeOD (see Figure 5c). The peak at δ = 161.5 ppm is the C signal from the newly formed carbonate24,25 ester. However, this one-dimensional NMR experiment still cannot directly verify that the carbonate is a result of covalent bonding between the ethoxide of 3O and CO2. In situ 13C–DOSY NMR was carried out to provide additional evidence of the carbonate formed. By measuring any differences that arise in the diffusion coefficients that can be measured for each carbon signal, 13C–DOSY NMR26,27 is used to determine whether two or more molecules are covalently bonded together. Typically, molecules with different molecular weights are characterized by different diffusion coefficients. As shown in Figure 5e, the result clearly confirms that the carbonate is the product of transient base of 3O reacting with CO2, as the signal of this new carbonate has the same diffusion order as the signals for 3O′. The carbonate structure is further verified by the fact that a new peak at m/z = 379.2382 appears in the high-resolution mass spectrum for this colored solution (see Figure S19). Based on all of these data, we can conclude that the mechanism for capturing CO2, which is accompanied by a color change, is based on dynamic covalent bonds, i.e., reversible reactions between CO2 and the transient bases that result from the ROFs of the oxazolidines. The generated carbonate is stabilized by the dynamic poly H-bonds provided by protic solvents and disturbed by reducing the CO2 content simply via the introduction of inert gases.
Figure 5.
13C NMR spectra of 3 in (a) MeOD, (b) MeOD after the addition of CF3COOD, (c) MeOD after the introduction of CO2, (d) MeOD after bubbling with CO2, and (e) 13C–DOSY NMR spectrum of 3 in MeOD after the introduction of CO2.
Cooperative Interplay of Transient Bases for Dynamic Capturing of CO2
We asked whether this dynamic CO2 capture could occur through cooperative interactions between multiple transient bases and CO2. These types of cooperative interactions would be similar to the dynamic reaction centers of biological systems, such as enzymes and receptors. To test this possibility, we did further quantitative 13C NMR experiments on solutions of 3 in MeOD after the introduction of CO2. As shown in Figure 6a, we surprisingly found that oxazolidines capture CO2 at a constant ratio of two transient bases formed from the ROFs interacting with one molecule of CO2, regardless of whether the ratio of 3O′ to 3C is 14.5:1 (Figure 6a,i) or 9:1 (Figure 6a,ii). This observation indicates that the CO2-capture process by the transient bases of the oxazolidines is via dynamic multimolecular cooperative interactions (see Figure 6b). This dynamic multimolecular cooperative mechanism serves to improve the efficiency of CO2 capture and transport, a hypothesis that is supported by the fact that the relative proportion of dissolved molecules of CO2 in the solution also increased (from 4:1 to 7:1, ratio of dissolved CO2 to carbonated CO2) with the increase in 3O′. Subsequently, the CO2 involved in further downstream reactions in the same system will also be expected to be greatly improved.
Figure 6.
(a) Quantitative 13C NMR spectra of 3 (5.05 mg) in MeOD (0.5 mL) after inletting CO2 at different rates to obtain ratios of 3O′ to 3C of (i) 14.5:1 and (ii) 9:1, respectively. (b) Scheme of the cooperative interactions involved in the dynamic CO2 capture by oxazolidines.
Concentration Dependence of Dynamic CO2 Capture
From the perspective of reactions involving CO2 in nature, dynamic CO2-capture/release processes by the transient bases formed from functional groups within protein-aggregates are dependent on the amount of CO2, which can only passively diffuse in or out of the respiratory system of a given organism, i.e., CO2 is never “bubbled in”. These properties are also prerequisite for developing practical applications involving transient bases. With these considerations in mind, we first investigated the relationship between the amount of ROFs formed and the CO2 level. The UV–vis absorption spectra show that the colored 1O′ forms even when a gaseous mixture containing only 1% CO2 was bubbled into the solution (see Figure 7a). The absorption intensity of 1O′ at 568 nm gradually increases with CO2 content, accompanied by a gradually deepening of the color (see Figure 7b). It should be noted that purging each solution continuously with a large excess volume of the gaseous mixture did not lead to more 1O′. Nevertheless, the rate at which CO2 was bubbled in, which affects the rate at which intermolecular collisions occur, influences the proportion of 1O′ formed. As is illustrated in Figure 7c, it was found that the solutions of 1 in EtOH also changed color simply by blanketing the top of the liquid with a layer of CO2 above the solution instead of bubbling directly into it. Diffusion of the colored solution formed at the top to the bottom can be visually monitored. In addition, recovery of the purple solutions of 1O′ to their initial colorless states can be achieved by simply leaving the solution in open air. These results clearly suggest that CO2 capture/release by the transient bases of the oxazolidines can occur through passive diffusion of CO2 under ambient conditions, similar to some biological processes. Based on the distinct color-changing characteristics that occur upon capturing CO2, oxazolidines have great potential in various applications where visual CO2 detection is needed using only the naked eye without additional assay kits. We confirmed that the CO2 detection limits of 1, 2, and 3 are 2, 20, and 0.5%, respectively (see Figure 7d), and this system of oxazolidine/alcohol can resist interference from ordinary neutral air pollutants, especially carbon monoxide and methane (see Figure S20). Oxazolidines with different substituents may result in CO2-response limits and concomitant color changes that can be fined-tuned such that they can meet the needs of a diverse number of applications to be used for different occasions (see Supplementary Discussion 1 in the Supporting Information).
Figure 7.
(a) UV–vis spectra of the same solution of 1 (anhydrous ethanol solution, 1 × 10–4 M) upon purging with air (100 mL) with different percentages of CO2 (v/v). (b) A plot of absorbance at 568 nm against percentages of CO2 for (a). Inset: photo of solutions after the addition of increasing amounts of CO2. (c) Ethanol solution of 1 (1 × 10–4 M, 5 mL) in which the headspace is filled with 100% CO2 (7 mL). The pictures show the coloration of the colorless solution 1C and formation/diffusion process of the purple 1O′ with time (upper) and the obvious decoloration process of 1O′ inch by inch with time by simply leaving the solution (1 × 10–5 M, 8 mL) open to air (below). (d) Application potentials of 1–3 for CO2 visual detection. Color changes of ethanol solutions of oxazolidines before (left) and after (right) introduction CO2 up to the detection limit of each particular molecule. The middle shows the magnified photos.
Reflections on CO2 Capture within Dynamic Protein-Aggregates
One significant goal in scientific research is to better understand and mimic the complex reactions or biological processes found in nature, an endeavor that directs us to develop new and ecofriendly technologies for human needs. Results from this biomimetic research as well as our previous related reports reveal the following inferences. (1) The long-conjectured mechanism, in which the alkaline amino residues important to some enzymes or protein-aggregates (e.g., RuBisCO or Hb) might be formed via thermodynamically controlled interatomic weak bond changes brought about by alteration of tertiary or quaternary conformations at the beginning of various biological reactions, is possible and reasonable. (2) Formation of transient bases is a prerequisite for CO2 capture by molecules. (3) The anticipated mechanism of reversible CO2 capture and release by the transient bases of oxazolidines is workable. These inferences also suggest that the previously conjectured mechanism on dynamic formation of carbamates from alkaline-free amines and CO2 is indeed feasible. (4) Dynamic alteration of molecular structure and conformation to generate transient bases for CO2 capture is highly dependent on their polyhydric microenvironments. More hydrophilic surroundings favor transient base formation. This also explains, at least to some degree, why life cannot exist without water and why the capability of photosynthesis is significantly reduced during times of drought. (5) Formation and decomposition of the carbamates or carbonates formed from the transient bases and CO2 are processes that occur at thermodynamic equilibrium, and are highly dependent on CO2 concentration. This result is consistent with previous findings that the limiting factor on photosynthesis in Britain during the summer is CO2 concentration. (6) Cooperative and competitive interactions between multiple transient bases seems to be a feasible and convincing route for continuous CO2 capture and transformation in biosystems. Within the reaction centers of proteins-aggregates, multiple amines from lysine residue side chains jostle one molecule of CO2 between each other, like juggling, forming temporary coordinate bonds therein. (7) Molecules with different rates of reversible conformational changes (such as spiropyrans versus oxazolidines, or oxazolidine 1 versus 2 or 3) have different sensitivities with respect to their responses to CO2 as a result of their specific stereochemical structures and nearby dipole effects. A similar phenomenon in the context of enzymes might provide another convincing explanation for why different enzymes have different rates of CO2 capture (e.g., carbonic anhydrase versus RuBisCO) rather than the generally accepted reason of oxygen competition. (8) Oxazolidines, which are both photochromic and hydrochromic, excel when it comes to CO2 capture, especially in comparison to the spiropyrans, which are only photoresponsive. Such a behavior might provide a clue as to why chloroplasts and leaves show a greater proportion of active RuBisCO in response to increased moisture and light intensity—light and water are vital for activating RuBisCO, even though CO2 fixation is a well-known dark reaction.
It is worth noting that even though there seems to be little structural similarity between oxazolidine derivatives and the bio-macromolecules RuBisCO or Hb, these two sets of molecules do have comparable behaviors and functions. The fundamental reason for these similar behaviors are likely the same, i.e., dynamic changes in molecular conformations (local stereostructure) and/or configuration is realized via thermo-driven coherent inter/intramolecular dipole–dipole interactions among multiple adjacent functional groups, whose interactions may also be under the influence of external field(s) and/or dynamically variable media.
Based on our results and numerous studies from other groups, we now speculate on the specific working principles of enzymes (i.e., RuBisCO), which capture CO2 in biological systems, in some detail. Because the three-dimensional conformations of these enzymes are maintained mostly by various weak interactions (e.g., hydrogen bonding, van der Waals forces, electrostatic interactions) between functional groups of adjacent polypeptide chains, their stereostructures around the active site can be easily adjusted by various sources of thermal energy, such as decomposition of adenosine triphosphate, hydration, enzymatic action (RuBisCO activase), rapid movement of electrons, etc., some of the processes that are crucial for cell survival. This thermal energy will surely stimulate the various stretching/contorting/rotating and change dipoles of atomic bonds nearby. When multiples of such atomic motions are striking the same subunit of the protein-aggregates, their synergetic forces will overcome the activation barrier for altering the conformation/configuration of the protein subunit, a process that generates the energetic transient base (e.g., an amino acid residue possessing an amine) and acid (e.g., amino acid residues with carboxyls or hydroxyls) by breaking their dipolar covalent bonds. The protein or enzyme then is activated, whereas also enlisting the help of the surrounding multihydroxyl environment in addition to metal cations (e.g., Mg2+, Fe2+).
The current experimental results further confirm that reversible covalent chemistry ongoing at thermodynamic equilibrium seems to be essential for the behavior of enzymes, or molecular-aggregates of proteins/nucleic acids. Having this understanding makes possible the further development of synthetic small molecules that mimic biological functions.
Conclusions
In summary, the design and synthesis of a kind of transient base utilizing a series of small molecular switches was achieved. These switches served as a molecular platform for mimicking some of the behavior of protein-aggregates and for further understanding the dynamic covalent CO2 capture in biochemical systems. The relationship between molecular structures, the ability at equilibrium to form transient bases, their associated nucleophilicities, and CO2-capture characteristics have been investigated systematically. The mechanisms of dynamic CO2 capture involve multimolecular cooperation and dynamic covalent interactions among the transient bases of the oxazolidines, which are aided by the polyhydroxyl microenvironment. Similar to some biosystems, CO2 capture by the transient bases of oxazolidines is a dynamic CO2-concentration-dependent process, regardless of whether CO2 is directly bubbled into the solution or blanketed on top. Due to different sensitivities and changes in color with respect to CO2 capture, these small molecules could be applied for the visual detection of CO2 at different detection limits. Furthermore, these small molecules provide insights into the mechanisms of CO2 capture within dynamic protein-aggregates, specifically as to how transient bases are formed within the protein-aggregates that are used for dynamic CO2 capture. The results herein suggest that the formation of transient bases is a spontaneous and thermodynamic process once the CO2 concentration becomes large enough.
Notably, even though oxazolidine derivatives seem to have little structural similarity with the bio-macromolecules of RuBisCO or Hb, they do share comparable behaviors and functions. Considering that the essential physics and working principle for synthetic and biomolecules should be universal, we confidently infer that if a given chemical mechanism is possible for a small molecule, then the same working principle should also be able to exist in biosystems.
As far as we know, there are only a few successful examples of functional bionics in the form of small molecules. Typically, biomimicry demonstrations involve intricate macromolecules or supramolecular self-assembled systems.28,29 Thus, this work represents a new example of bionics that use small molecules to help better understand the complex dynamic CO2-capture and related processes in biosystems. In addition, it also provides a new way for designing functional small molecules for the capture or detection of CO2. An indubitable advantage of this small-molecule platform is the ease by which the dynamic processes can be understood visibly. Other techniques like single-crystal analysis of the more stable structural state and/or extremely difficult computer simulations require much more time and expertize. This means of visual detection will undoubtedly simplify and speed up the investigations and discoveries on biochemistry, molecular biology, cell biology, synthetic biology, epigenetics, biomimetic engineering and material science, etc. We welcome further verifications and investigations from global researchers on the aforesaid insights into the transient base formation within the CO2-capture enzymes.
Experimental Section
Materials and Methods
All of the solvents used were purified by literature methods. Chemicals and reagents of the highest grade commercial availability were used without further purification. CO2 and N2 gases used in this study were purged directly into the solutions inside either the NMR tube or the UV–vis cuvette before recording the spectra unless otherwise noted. Compounds 1–7 were synthesized according to our previous work.21
Characterizations
1H NMR (500, 600 MHz) and 13C NMR (126, 150 MHz) spectra were recorded on a Bruker AVANCE500 (or AVANCE 600) using tetramethylsilane (TMS) as the reference standard at room temperature. In 1H NMR spectra, chemical shifts (parts per million) were referenced to residual solvent protons (3.31 ppm in MeOD). In 13C NMR spectra, chemical shifts (parts per million) were referenced to the carbon signal of the deuterated solvent (49.0 ppm in MeOD). High resolution electrospray ionization mass spectrometry analysis was performed on an Agilent 1290-micrOTOF-Q II mass spectrometer. The UV–vis absorption spectra were measured from 230 to 800 nm at a scan rate of 5 nm/s using a Shimadzu UV-2550 PC double-beam spectrophotometer with a path length of 1 cm. The solutions for UV–vis measurements were prepared with a concentration of 1 × 10–4 M, unless otherwise specified. The kinetics was measured on an Analitik Jena Specord 210 plus UV–vis spectrophotometer.
Theoretical Calculations
The density functional theory (DFT) calculations were carried out with the Gaussian 1622 program at the B3LYP/6-31g(d,p) level. The UV–vis spectra of the transient bases of the oxazolidines were calculated at the time-dependent density functional theory (TD-DFT) level. The energy barriers were calculated in the gas phase, as well as by using a hybrid model (implicit model-polarizable continuum model and explicit cluster model with MeOH solvent molecules). As for the NMR calculations, geometry optimization was performed with the B3LYP method and 6-311+G(2d,p) basis set. The gauge-independent atomic orbital approximation30 in the Gaussian 16 package was also employed to calculate the NMR shifts. In the NMR shift calculations, TMS was used as the reference. Computed structures were illustrated using CYLVIEW drawings.31
Acknowledgments
L.S. thanks China NSF (No. 51603085), S.X.-A.Z. thanks China NSF (No. 21572079) and J.M. thanks China NSF (No. 21673111) for the financial support. We also thank Dr. Xue Zhang from Jilin Agricultural University for the help of art-drawing of Scheme 1 and Figure 6b.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b02028.
The authors declare no competing financial interest.
Supplementary Material
References
- Revelle R.; Suess H. E. Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades. Tellus 1957, 9, 18–27. 10.3402/tellusa.v9i1.9075. [DOI] [Google Scholar]
- Prentice I. C.; Farquhar G.; Fasham M. J. R.; Goulden M. L.; Heimann M.; Jaramillo V. J.; Kheshgi H. S.; LeQuéré C.; Scholes R. J.; Wallace D. W. R.. The Carbon Cycle and Atmospheric Carbon Dioxide. In Climate Change: the Scientific Basis; Cambridge University Press: Cambridge, 2001; pp 185–237. [Google Scholar]
- Cox P. M.; Betts R. A.; Jones C. D.; Spall S. A.; Totterdell I. J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. 10.1038/35041539. [DOI] [PubMed] [Google Scholar]
- Pierantozzi R.Encyclopedia of Chemical Technology, 4th ed.; Kroschwitz J. I., Ed.; Wiley: New York, 1991; pp 803–822. [Google Scholar]
- Lambers H.; Chapin F. S. III; Pons T. L.. Photosynthesis. In Plant Physiological Ecology; Springer: New York, 2008; pp 11–99. [Google Scholar]
- Valentini R.; Matteucci G.; Dolman A. J.; et al. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–865. 10.1038/35009084. [DOI] [PubMed] [Google Scholar]
- Roughton F. J. W. Recent work on carbon dioxide transport by the blood. Physiol. Rev. 1935, 15, 241–296. 10.1152/physrev.1935.15.2.241. [DOI] [Google Scholar]
- Bender M. M. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 1971, 10, 1239–1244. 10.1016/S0031-9422(00)84324-1. [DOI] [Google Scholar]
- Raven J. A.Carbon dioxide fixation. In Algal Physiology and Biochemistry; 1974, pp 434−455. [Google Scholar]
- Cleland W. W.; Andrews T. J.; Gutteridge S.; Hartman F. C.; Lorimer G. H. Mechanism of Rubisco: The carbamate as general base. Chem. Rev. 1998, 98, 549–562. 10.1021/cr970010r. [DOI] [PubMed] [Google Scholar]
- Gurd F. R. N.; Matthew J. B.; Wittebort R. J.; Morrow J. S.; Friend S. H.. The carbamate reaction with proteins: observation by 13C-NMR and evaluation of structural consequences. In Biophysics and Physiology of Carbon Dioxide; Springer: Berlin, Heidelberg, 1980; pp 89–101. [Google Scholar]
- Davis A. J.; Obrien P.; Nunn P. B. Studies of the stability of some amino acid carbamates in neutral aqueous solution. Bioorg. Chem. 1993, 21, 309–318. 10.1006/bioo.1993.1026. [DOI] [Google Scholar]
- Wang J.; Zhu S.; Xu C.. Biological Chemistry, 3rd ed.; Higher Education Press: Beijing, 2002; pp 219–220. [Google Scholar]
- Ferguson J. K. W. Carbamino compounds of CO2 with human haemoglobin and their role in the transport of CO2. J. Physiol. 1936, 88, 40–55. 10.1113/jphysiol.1936.sp003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Internal motions in proteins and gating kinetics of ionic channels. Biophys. J. 1988, 53, 877–884. 10.1016/S0006-3495(88)83168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W.; Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Conscious thought as simulation of behaviour and perception. Trends Cogn. Sci. 2002, 6, 242–247. 10.1016/S1364-6613(02)01913-7. [DOI] [PubMed] [Google Scholar]
- Zhang J. Z.; Schwartz B. J.; King J. C.; Harris C. B. Ultrafast studies of photochromic spiropyrans in solution. J. Am. Chem. Soc. 1992, 114, 10921–10927. 10.1021/ja00053a032. [DOI] [Google Scholar]
- Tomasulo M.; Sortino S.; Raymo F. M. A fast and stable photochromic switch based on the opening and closing of an oxazine ring. Org. Lett. 2005, 7, 1109–1112. 10.1021/ol050045a. [DOI] [PubMed] [Google Scholar]
- Mançois F.; Pozzo J. L.; Pan J.; Adamietz F.; Rodriguez V.; Ducasse L.; Castet F.; Plaquet A.; et al. and Champagne B. Two-way molecular switches with large nonlinear optical contrast. Chem. - Eur. J. 2009, 15, 2560–2571. 10.1002/chem.200801967. [DOI] [PubMed] [Google Scholar]
- Sheng L.; Li M.; Zhu S.; Li H.; Xi G.; Li Y. G.; Wang Y.; Li Q.; Liang S.; Zhong K.; Zhang S. X. A. Hydrochromic molecular switches for water-jet rewritable paper. Nat. Commun. 2014, 5, 3044 10.1038/ncomms4044. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.;. et al. Gaussian Inc 16, revision A. 03; Gaussian Inc.: Wallingford, CT, 2016.
- Hwang S.; Jang Y. H.; Wan C.; Chung D. S. Gas phase proton affinity, basicity, and pKa values for nitrogen containing heterocyclic aromatic compounds. Bull. Korean Chem. Soc. 2005, 26, 585–588. 10.5012/bkcs.2005.26.4.585. [DOI] [Google Scholar]
- Gassensmith J. J.; Furukawa H.; Smaldone R. A.; Forgan R. S.; Botros Y. Y.; Yaghi O. M.; Stoddart J. F. Strong and reversible binding of carbon dioxide in a green metal-organic framework. J. Am. Chem. Soc. 2011, 133, 15312–15315. 10.1021/ja206525x. [DOI] [PubMed] [Google Scholar]
- Kwak J. H.; Hu J. Z.; Hoyt D. W.; Sears J. A.; Wang C.; Rosso K. M.; Felmy A. R. Metal carbonation of forsterite in supercritical CO2 and H2O using solid state 29Si, 13C NMR spectroscopy. J. Phys. Chem. C 2010, 114, 4126–4134. 10.1021/jp1001308. [DOI] [Google Scholar]
- Botana A.; Howe P. W. A.; Caër V.; Morris G. A.; Nilsson M. High resolution 13C DOSY: the DEPTSE experiment. J. Magn. Reson. 2011, 211, 25–29. 10.1016/j.jmr.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Johnson C. S. Diffusion ordered nuclear magnetic resonance spectroscopy: principles and applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. 10.1016/S0079-6565(99)00003-5. [DOI] [Google Scholar]
- Sadownik J. W.; Mattia E.; Nowak P.; Otto S. Diversification of self-replicating molecules. Nat. Chem. 2016, 8, 264–269. 10.1038/nchem.2419. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Li H.; He Y.; Chen X.; Ma X.; Lee M. Collective helicity switching of a DNA-coat assembly. Nat. Nanotechnol. 2017, 12, 551–556. 10.1038/nnano.2017.42. [DOI] [PubMed] [Google Scholar]
- Wolinski K.; Hinton J. F.; Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. 10.1021/ja00179a005. [DOI] [Google Scholar]
- Legault C. Y.CYL view, 1.0 b; Université de Sherbrooke: Canada, 2009. http://www.cylview.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.