Abstract
Highly efficient luminescent materials in solid states are promising candidates for the development of organic optoelectrical materials and devices and chemical and biological sensors. Aggregation-induced emission (AIE), a novel photophyscial phenomena coined in 2001 where the aggregate formation enhances the light emission, has drawn great attention because it provides a fantastic platform for the development of these useful luminescent materials. After 17 years of AIE research, diverse AIE luminogens with tunable color and high quantum yields have been explored, which finds diverse applications from optics and electronics to energy and bioscience. Most importantly, the concept of AIE has gradually changed people’s thinking way about the aggregation of luminogen and put forth a revolution of luminogen research both conceptually and technically. This perspective revisits our journey of AIE research, discusses our current understanding of the AIE mechanism, debates current challenges, and looks for the potential breakthroughs in this exciting research area.
1. Introduction
A scientific concept is an idea or model explaining some natural phenomena, which plays a critical role in the development of a new subject. During the process of finding evidence, the idea or model is discussed, reformed, proved and then can guide the further development of the subject and so is for the concept of aggregation-induced emission (AIE).
Before the birth of AIE, concentration quenching (CQ) is a general belief and a well-accepted concept for common organic luminophores, where the molecule quenches its own fluorescence at high concentrations.1 Problems arise when utilizing these luminophores, particular in solid states where the molecules like forming aggregates. As known from the CQ concept, people tend to believe that the aggregation formation is bad for luminophore light emission. Therefore, the concept of aggregation-caused quenching (ACQ) effect is gradually considered as a general belief.2 Then, various chemical and physical methodologies have been considered, blocking luminophore aggregation formation and decreasing the quenching effect when employing them in practical applications.3 However, the luminophores are commonly used as clusters, where they are treated as molecular assembly or nanoaggregates in microscopically or solid phase in macroscopically. From the single species to aggregates, there lays a huge gap conceptually and technologically.
Although the concept of CQ and ACQ is helpful in some areas and is commonly used to avoid the ACQ effect in molecular design, the derived methodologies have the intrinsic limitation in solving the ACQ problem and obtain limited success because they are against the natural process. The aggregate formation is the intrinsic behavior of luminophores in solid phase. The ACQ concept has reformed peoples’ idea that the aggregation is harmful for solid-state light emission. The long standing and established concept is gradually found to restrict people’s way of thinking and has to be reversed. Actually, few researchers have found some unique systems behaving “abnormally” whose emission at the aggregation state is stronger than that in solution.4 However, there is no continuous attention paid to these scattered findings. One reason is that people bury themselves in the ACQ concept deeply. The other reason is that the emerged abnormal phenomena lack reasonable explanations and working mechanisms. Therefore, a novel concept is highly demanded to reform people’s mind and guide the research, particularly focusing on the constructive effect of the aggregation formation.
For the past 17 years, our group started from occasional interesting findings and has established the concept of AIE, which provides a new platform for luminophore research.5 The constructive role of the aggregation for light emission is first paid continuous attention and enjoys throughout investigations. In this perspective article, we will briefly review what we have done in the past 17 years by revisiting the journey of AIE research and offer some perspectives on the future direction of the area. In detail, we want to emphasize the role of AIE that have guided important developments in some specific areas.
On the other hand, inspired by the investigations on light’s interaction with matter, lots of life-changing innovations have arisen.6 Luminescent materials are the cornerstone behind the area and attract extensive research efforts to explore new dyes with high efficiencies and multiple functionalities. For years, scientists have established quite a lot of concepts in the area based on the fundamental photophysical understanding, where luminophores are treated as single species such as existing in dilute solution or vapor.7 AIE changed the field revolutionally.
2. Journey of AIE Research
Highly efficient luminescent materials in solid states are promising candidates for the development of organic optoelectrical materials and devices and chemical and biological sensors.8 AIE, a novel photophyscial phenomena coined in 2001 where the aggregate formation enhances the light emission, has drawn great attention because it provides a fantastic platform for the development of these useful luminescent materials. Numerous AIE luminogens (AIEgens) with tunable color and high quantum yields have been reported and used in diverse applications from optics and electronics to energy and bioscience.7,9 This perspective article gives us the opportunity to revisit our research journey, discusses our current understanding of the AIE mechanism, debates current challenges, and looks for the potential breakthroughs in this exciting research theme. We present our journey in the following five aspects.
2.1. From ACQ to AIE
In the area of luminophore research, before the birth of AIE, ACQ is a well-known photophysical phenomenon commonly existing in most polyaromatic hydrocarbons, as mentioned by Birks in Photophysics of Aromatic Molecules.1 For example, as shown in Figure 1b, fluorescein is a typical ACQ dye.10 It is soluble in water but insoluble in common organic solvents. As molecularly dissolved in the good solvent, its water solution emits the bright green light (Figure 1a) upon UV irradiation. However, the emission is gradually quenched when the acetone is added, where the molecules form aggregates. Owing to the aggregate formation, the π–π coplanar interaction between the fluorescein molecules generates the excimer species that decay through nonradiative relaxation pathways (Figure 1b). On the contrary, AIE is an another photophysical phenomenon associated with luminogen aggregation, whose aggregates favor more intensive emission. A number of structurally similar systems have been found to exhibit the AIE effect.11 A typical AIEgen is shown in Figure 1d.12 Hexaphenylsilole (HPS) is nonemissive when it is molecularly dissolved in tetrahydrofuran (THF) but becomes highly emissive when forming aggregates (Figure 1c). HPS molecules have the twisted propeller-shaped structures (Figure 1d), which do not allow the π–π stacking.
Figure 1.
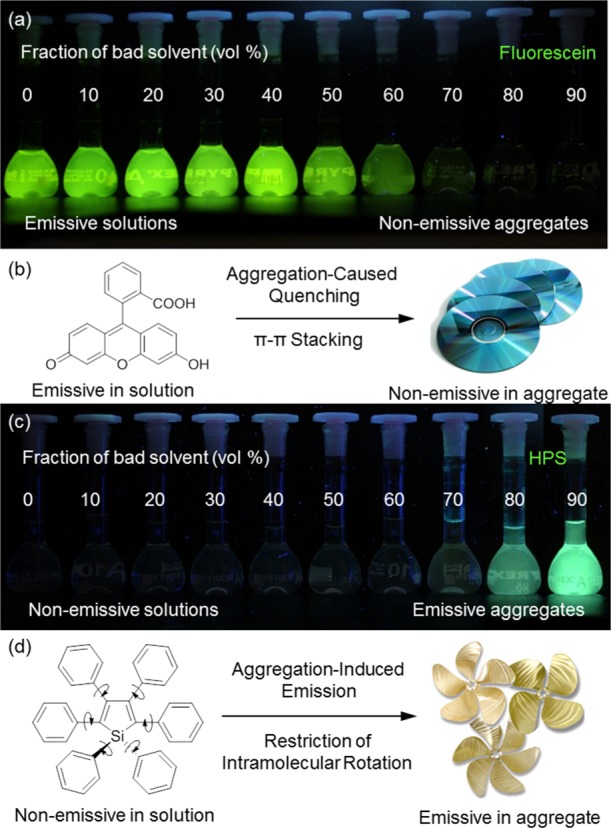
Fluorescent digital photographs of (a) fluorescein and (c) HPS solutions with different bad solvent fractions. (b) Fluorescein molecules are disk-shaped and become nonemissive when forming aggregates where strong π–π interaction quenches the emission. (d) HPS molecules are propeller-shaped and turn to highly emissive when forming aggregates where the intramolecular rotation is restricted.
Therefore, luminogen aggregate formation can play two different roles in the luminescence emission, ACQ and AIE. People applied a number of approaches against the ACQ effect when used them in the aggregation state; however, the aggregation cannot be totally blocked, because it is a natural process. In sharp contrast, the AIE effect makes it possible to utilize the aggregation process actively, rather than working against it passively.13 Most AIE-related studies utilize the idea that applies the aggregation to turn-on or boost the emission.
2.2. From Restriction of Intramolecular Rotation (RIR) to Restriction of Intramolecular Motion (RIM)
Why aggregation brightens emission of AIEgens? Efforts have been continuously devoted to decipher the AIE working principle. A number of possible working mechanisms, including conformational planarization, J-aggregate formation, E/Z isomerization, the restriction of twisted intramolecular charge transfer, and the excited-state intramolecular proton transfer, have been put forward, but none of them can be perfectly applicable to all the AIE systems.12 A correct decipherment of the working mechanisms of the AIE effect is of great importance to fundamental understanding, luminogen explorations, and advanced practical applications.
Theoretically, an excited luminogen molecule can decay through the photophysical and/or photochemical pathways.14 The photophysical one includes nonradiative and radiative processes. The photochemical one includes a chemical reaction. Therefore, in solution, the excited AIEgens should decay mainly through nonradiative photophysical or photochemical processes. In aggregated states, they decay mainly through the radiative photophysical process. The collective effects give the unique AIE properties.15 We are working on the AIE mechanism through finding out the detailed decay processes that account for these photo-induced behaviors.
With persistent efforts, the RIR process has been first proposed as the mechanism for the AIE effect by our group.12,16 For demonstration, the four phenyl groups and the double bonds in TPE can rotate freely in dilute solutions in the excited states (Figure 2a), which dissipate the energy through the nonradiative decay channels. Hence, the TPE molecules in solution are nonemissive. These intramolecular rotations are then restricted in the aggregations owing to multiple intermolecular interactions, blocking nonradiative decay pathways, and enabling excited states to decay radiatively.17 The RIR mechanism has been successfully applied to explain different kinds of fluorescent and phosphorescent AIE systems. Recently, some newly emerging AIE systems that are absent from multiple rotors bring some ambiguous issues to the RIR mechanism. For luminogens with vigorously vibrative moieties such as THBA (Figure 2b), their AIE effects can be interpreted by the mechanism of RIV.18 Physical knowledge reveals that any intermolecular movement including rotation and vibration can dissipate energy. The active vibrational motions of the flexible parts in THBA play an important role in the radiationless dissipation of the excited states energy.19
Figure 2.
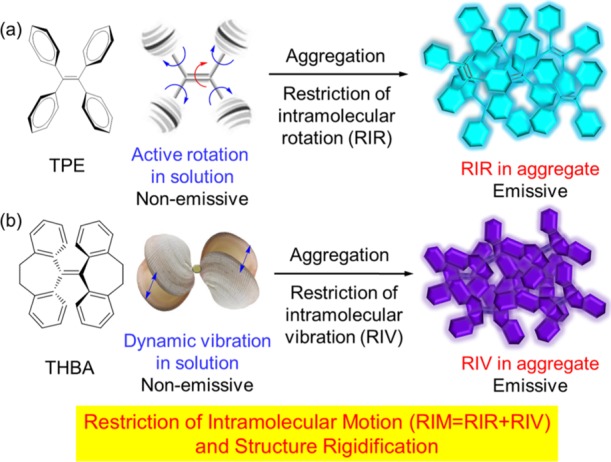
(a) Chemical structure of tetraphenylethene (TPE), governed the RIRs. (b) Chemical structure of 10,10′,11,11′-tetrahydro-5,5′-bidibenzo[a,d][7]-annulenylidene (THBA), a TPE derivative with the AIE effect, working under the restriction of intramolecular vibrations (RIVs).
In general, we integrated the RIR with the RIV to the RIM as a more comprehensive AIE mechanism (Figure 2).7a,18,19f The RIM mechanism with a broader content provides the simple, fundamental, and comprehensive AIE mechanism to work together for explaining and expanding the AIE family. Intrinsically, the intramolecular motion described here boosts the nonradiative decay rates, arising from the flexible isolated molecular structures. Upon aggregation, such intramolecular motion is restricted, contributing to the enhanced structural rigidification and dramatically decreased nonradiative decay rates.20 Thus, the radiative relaxation channels dominate. Generally, in an AIE system, the flexible structure weakens the molecular rigidity and promotes intramolecular motion to accelerate the nonradiative decay. The aggregation induces the structure rigidification and blocks the nonradiative decay channels, making intense fluorescence. Therefore, derived from RIM, we realized that the structural rigidification of a flexible luminogen is the intrinsic requirement for AIE systems.21
2.3. From Solid-State Fluorescence to Room-Temperature Phosphorescence
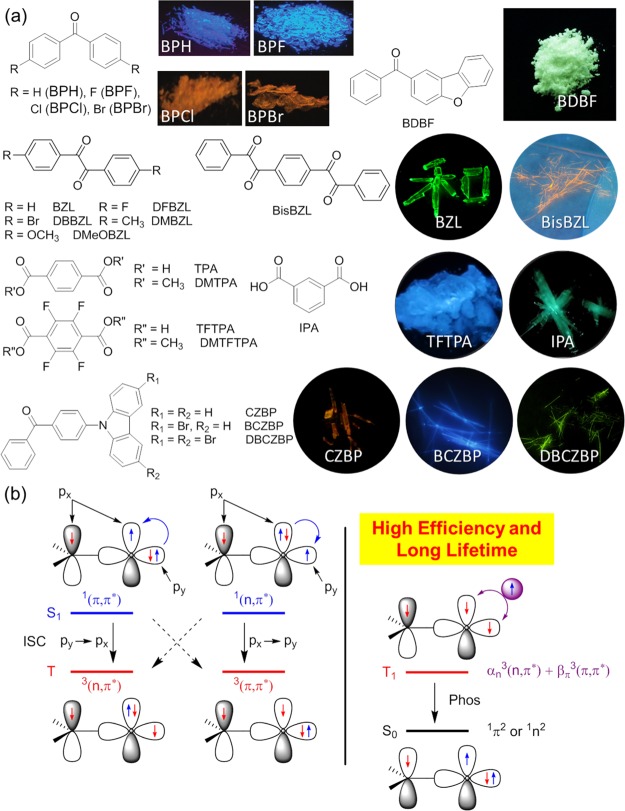
Compared to fluorescent luminogens, those with room-temperature phosphorescence (RTP) enjoy wide applications including the high-efficiency electroluminescence, bioimaging, photodynamic therapy, temperature monitoring, oxygen sensing, security inks, and so on, thanks to the long-lived triplet manifold.22 However, pure organic RTP phosphors are difficult to obtain, because the decay rate of phosphorescence is slow and triplet excitons could easily lose their energies through nonradiative decay pathways such as thermally vibrational, collisional processes and exposure to quenchers. To suppress these relaxation channels, several approaches have been developed.23 In 2010, our group discovered the crystallization-induced phosphorescence (CIP) phenomenon, where some pure organic luminogens such as benzophenone (BP) derivatives exhibit no emission in solution but become highly emissive in the crystals.24 Obviously, CIP is an extension of the AIE family. RIR by effective intermolecular interactions within the crystal lattice and isolation from oxygen and moisture are ascribed for the boosted phosphorescence. Moreover, CIP offers a new strategy for the fabrication of efficient pure-organic RTP luminogens through crystal engineering. Since then, more and more reports concerning on the RTP from pure-organic chromophores are demonstrated.25
As shown in Figure 3a, several BP luminogens, including BPH and its halogen derivatives (BPF, BPCl, BPBr), and BDBF are induced to emit RTP upon crystallization.24,26 These phosphors are nonemissive when they are dissolved in good solvents, because the intramolecular motions quench the triplet excitons through nonradiative decay pathways. In the crystalline state, intramolecular motions are restricted in the crystal lattice and multiple intermolecular interactions. Then, molecular conformation is rigidified, thus making them highly phosphorescent.
Figure 3.
(a) Chemical structures of BP, BZL, and CZBP and their corresponding derivatives; their photographs of the crystals taken after the stop of UV light irradiation. (b) Schematic representation of the El-Sayed’s rule for intersystem crossing and molecular orbital hybridization of the lowest triplet states for tuning the rate of phosphorescence decay. Generally, a phosphor with high efficiency and long lifetime requires a balanced hybridization of 3(n,π*) and 3(π,π*) orbitals.
As is well-known, carbonyl groups are effective spin–orbit coupling promoters for their enhanced intersystem crossing. Similar to BPH, BZL and its derivatives also exhibit the CIP feature with high efficiencies at crystalline states.27 Furthermore, we reported a unique phenomenon of crystallization-induced dual emission (fluorescence and phosphorescence) in pure-organic aromatic acids and esters.27 Specifically, long afterglow from terephthalic acid and isophthalic acid is observed (Figure 3a), which is rarely found for pure-organic luminogens.23c To further explore persistent RTP phosphors, we designed CZBP. Single crystals with dense crystal packing and multiple intermolecular interactions stabilize the triplet excitons from quenchers, block the vibrational dissipations, and yield persistent RTP.23e
To obtain efficient pure-organic RTP luminogens, several internal and external requirements should be fulfilled. First of all, intersystem crossing from the lowest excited singlet state (S1) to the triplet excited states (Tn) should be highly efficient. According to El-Sayed’s rule, intersystem crossing can be greatly promoted through efficient spin–orbit coupling by mixing the singlet and triplet states with different molecular orbital configurations (Figure 3b). In spite of efforts to suppress nonradiative decays by tuning the aggregation behaviors, we presented a rational molecular structure design principle to achieve efficient light emission and long lifetime. The hybrid (n,π*) and (π,π*) configurations of the excited states with the suitable proportion are the key factors in manipulating the RTP performance. For the proof-of-concept, aromatic subunits in arylphenones are modulated to change the energy level and the orbital configurations of the triplet excitons. Several long-lifetime and high-efficiency pure-organic phosphors were obtained with different colors at room temperature and in atmosphere, demonstrating the validity of our rational molecular structure design principle.26,28
2.4. From Traditional Luminophores to Nonconventional Luminogens
Traditional luminophores are generally constructed by aromatic groups and conjugated subunits, which function as chromophore centers. Recently, some nonconventional luminogens such as nonconjugated polymers and natural products which are free of aromatic building blocks have been reported.29 On one hand, these nonconventional luminogens are normally emissive in the aggregates, demonstrating their AIE characteristics. On the other hand, the emission working mechanism is quite difficult to decipher and still under dark. They are neither the typical AIEgen structure nor common conjugated building blocks.
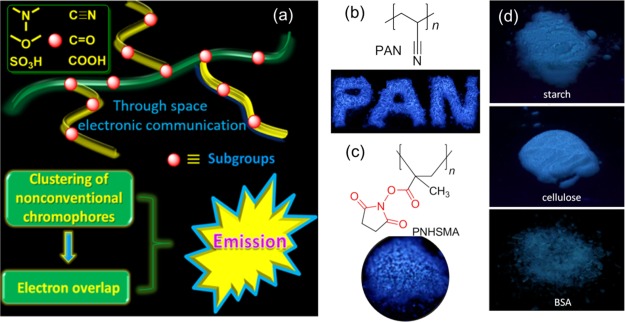
Individually, researchers attempted to figure out the effect emissive species to the luminescence. Typically, these polymers own several electron-rich subgroups, such as N, O, S, P with lone pair electrons and/or unsaturated CC, CO, and CN subgroups.29 (Figure 4a) These subgroups exist as common nonemissive organic groups, such as amide, imide, anhydride, ester units, and so on. However, when they are connected in macromolecules or clusters, they can become emissive upon aggregation, which has strong implications for the emission mechanism understanding. Figure 4a demonstrates the cross-linked and through-space interacted subgroups in the varying nonconventional luminogens clusters. In light of this, the clusteration-triggered emission (CTE) mechanism may well-explain the photophysical processes of these nonconventional systems,30 the clusteration of diverse subgroups with subsequent electron cloud overlapping, and molecular conformation rigidification.
Figure 4.
(a) Demonstration the clusters constructed by through-space conjugated subgroups. (b) Chemical structure of polyacrylonitrile (PAN) and fluorescent digital photograph of its blue solid powders. (c) Chemical structure of PNHSMA and fluorescent digital photographs of its blue solid powders. (d) Fluorescent digital photographs of starch, cellulose, and bovine serum albumin (BSA) solid powders.
As shown in Figure 4b, PAN is a cyanopolymer, which is virtually nonemissive in dilute dimethylformamide (DMF) solution. Its solid powders emit visible blue emission upon UV irradiation.30 The authors then designed and synthesized PNHSMA without any aromatic structures. PNHSMA is also virtually nonluminescent in dilute solutions but becomes highly emissive in concentrated solution and in the solid powders (Figure 4c).31 Furthermore, we reported intriguing bright blue emission from starch, cellulose, and BSA protein27 (Figure 4d). There are a number of isolated oxygen atoms in starch and cellulose, which cannot generate visible emission. However, their solid states can push these electron-rich atoms to a cluster, where the electron clouds are highly overlapped by the through-space interactions. Meanwhile, subsequent conjugations and rigidified conformations will also help the emission. As a result, these clustered subgroups can be excited and emit visible light. The CTE mechanism is put forth, trying to explain the interesting emission. Moreover, using this mechanism, we can also discover and design some other nonconventional luminogens.29a
2.5. From Through-Bond Conjugation to Through-Space Conjugation
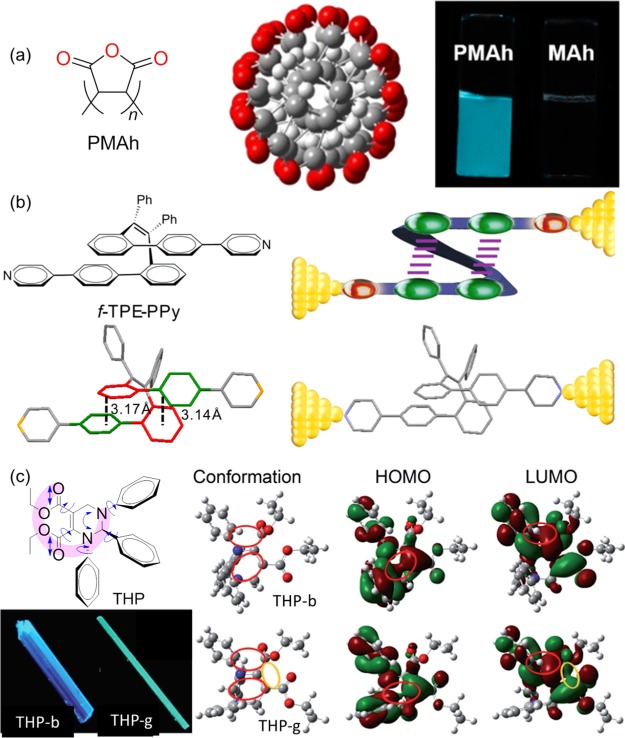
Derived from the nonconventional chromophores and CTE mechanism, we put forth the concept of through-space conjugation together with the through-bond conjugations to explain these unusual AIE systems. We found that the poly(maleic anhydride) (PMAh) oligomers emit bright blue light (Figure 5a).32 Theoretical calculations proved that the emission is related to the clusteration of the through-space electron interaction-locked anhydride subgroups. As shown in Figure 5b, the TPE derivative f-TPE-PPy with a folded conformation is designed and synthesized. It contains both through-bond and through-space electronic conjugations. The covalent bonds link the aromatic ring and alkene group, giving a large π-conjugation molecular structure. On the other part, the unique folding structure allows the interesting intramolecular through-space π–π interaction and generates an electronic delocalization conjugation.33 Lastly, tetrahydropyran (THP) is nonemissive in solution but highly emissive in crystalline state.34 Lots of oxygen and nitrogen atoms are electron-rich and packed tightly in the crystals to facilitate the through-space electronic interaction, revealing also from the theoretical calculations (pink crescent and cycles in Figure 5c).35
Figure 5.
(a) Chemical structure and calculated optimized conformation of PMAh and of DMF solution photograph taken when UV light irradiated. (b) Chemical and crystal structures of f-TPE-PPy and illustration of through-space conjugation represented by the circuit of f-TPE-PPy anchored onto gold electrodes. (c) Chemical structure of the racemic THP and its polymorphs under UV light and its molecular conformations, highest occupied molecular orbitals and lowest unoccupied molecular orbitals of polymorphs.
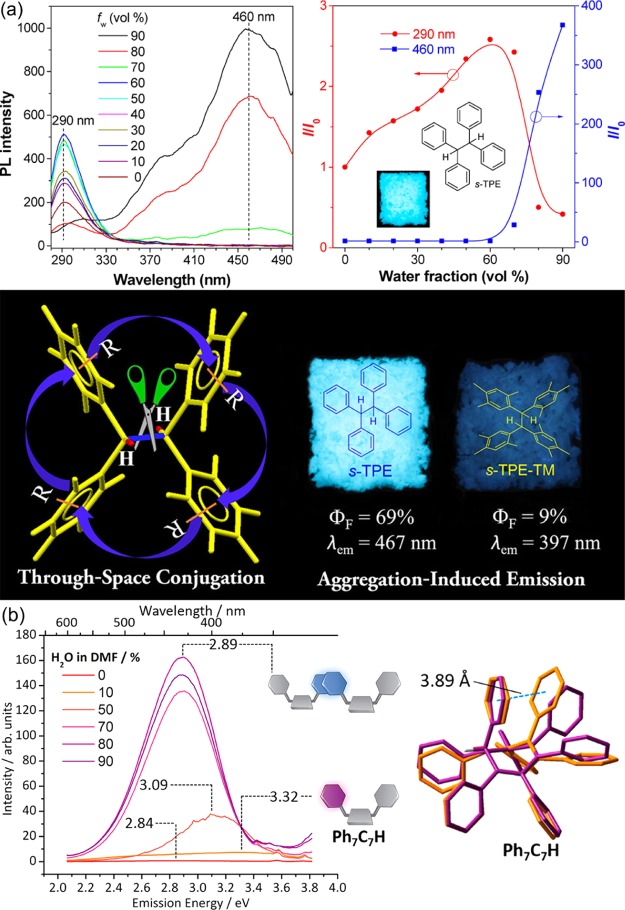
Recently, the concept of through-space conjugation was gradually accepted as a new mechanism of luminescent chromophores explaining some nonconjugated and/or nonpolar molecules emitting strong visible light. As shown in Figure 6a, for 1,1,2,2-tetraphenylethane (s-TPE), at a water content of 90%, a strong longwave emission peak can be seen, while the peak intensity at around 300 nm is also reduced. Under solid-state conditions, the fluorescence quantum yield can reach 70% while the emission wavelength reaches 470 nm. Such a long wavelength and high-efficiency fluorescence emission in the aggregation state cannot be explained by the traditional theory. The authors illustrated that in the excited state, the electron cloud of the two benzene rings with the same carbon will have a delocalization effect, meanwhile the band gap of the molecules will be drastically reduced, called through-space interaction.36Figure 6b shows that the luminescence intensity and wavelength of syn-heptaphenylcycloheptatriene (Ph7C7H) both increase with the increase of water content in DMF. In aggregates, the benzene rings from two molecules will be stacked in parallel and the electron cloud will be delocalized, that is, the through-space conjugation effect occurs.37 The through-space can illustrate the unusual AIE appearance reasonably, and it will lead to the electronic delocalization and reduce the band gap of the molecule, giving a red shift of the emission.
Figure 6.
(a) PL spectra of s-TPE in THF/water mixture with different water fractions and plots of relative PL intensity (I/I0) at different emission wavelengths and through-space conjugation and AIE effect. (b) Steady-state photoluminescence spectra of Ph7C7H suspensions in H2O–DMF and DFT minimum energy geometries calculated for the S0 (purple) and S1 (orange) of Ph7C7H.
From small molecules to polymers, from traditional luminophores to the nonconventional luminogens, from organic to organometallic dyes, AIE has found its constructive roles in diversity luminescent systems. To make the situation simple and general, these AIEgens can be classified as the following: homogeneous AIE clustoluminogens and heterogeneous AIE clustoluminogens (Figure 7c). Illustrated by a string of small firecrackers, these small firecrackers can be electron-rich atoms, carbohydrates, amino acids, aromatic subgroups, and so on. They are clustered by covalent bonds, polymer chains, or even intermolecular interactions and emissive or nonemissive. After forming aggregates, they behave as whole luminogens where through-bond or through-space conjugation acts and RIM and/or CTE functions to make them luminescent. For instance, TPE can be considered as the homogeneous clustoluminogen, where phenyl groups and ethylene are clustered by the covalent bond, the through-bond conjugation makes the molecule as a whole luminophore. The luminescent polymers are also the homogeneous clustoluminogens. For example, the luminescent poly(9-vinylcarbazole), the emissive carbazoles are clustered together by covalent flexible polymer chain, and they emit both as individuals and groups.38 Even the nonconventional luminogen, the fluorescent dynamic glycopolymer is a homogeneous clustoluminogen. The fluorescence should result from the tightly packed structure of the polymer, where the hydrophobic core isolates and rigidly holds the aromatic chromophores (Figure 7a). The heterogeneous clusteroluminogens represent the hybrid systems, where organic counterparts and inorganic counterparts are clustered as a whole emissive species, no matter whether the counterparts are emissive or not.39Figure 7b shows an interesting AIE Au(I)–thiolate complex: strong luminescence emission with a quantum yield of ∼15%.40 The aggregates of Au(I)–thiolate complexes were prepared on in situ created Au(0) cores to generate Au(0)@Au(I)–thiolate core–shell nanoclusters. The strong luminescence was attributed to the AIE effect of Au(I)–thiolate complexes on the nanocluster surface and showed the size-dependent effect.
Figure 7.
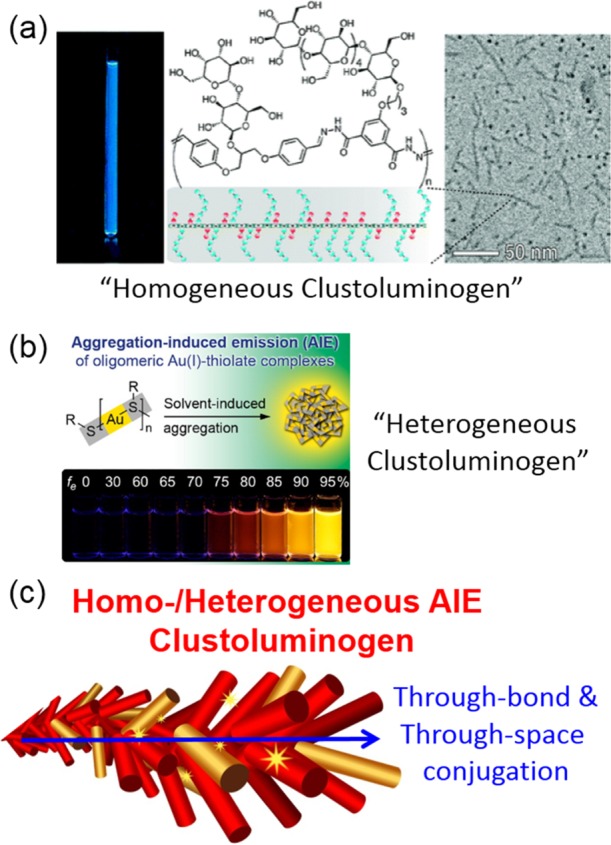
(a) Molecular structure of a dynamic glycopolymer as the fluorescent analogue of polysaccharides and its fluorescent assemblies characterized by TEM. (b) AIE effect of oligomeric Au(I)–thiolate complexes through solvent-induced aggregation and its luminescent photographs. (c) Illustration of homo-/heterogeneous clustoluminogens by “firecrackers” where through-bond and/or through-space conjugation within the subgroups provides effective luminescent chromophores.
From above examples, the homogeneous clustoluminogens involve pure-organic systems, they can be small molecules, polymers, supramolecular assemblies, and so on, whereas the heterogeneous clustoluminogens describe hybrid systems with organic and inorganic subgroups. There is no requirement of subgroups, even whether they are emissive or not. They are considered as a whole. The through-bond and through-space conjugation makes them behave as whole luminogens. Still governed by RIM and CTE, the luminescence can be lighted up. As a result, we greatly expand the scope of AIE and offer an attractive platform of future luminogen research.
3. Perspective and Remarks
Since the concept was coined in 2001 by our group, AIE has gradually changed people’s way of thinking about luminogen aggregation and put forth a revolution of the luminogen research both conceptually and technically. AIE has established a new platform in the area for novel luminogen exploration and for multidiscipline researchers to work on. Deciphering the nonradiative processes that lead to quenching luminogen emission in the solution and revealing the reasons that bring about radiative decay in the aggregate is cornerstone of the platform. On the platform, the AIE has grown up as a “big tree”, where the AIEgens has great structural diversity: they can be pure hydrocarbons, heteroatom-containing systems, organometallic molecules, synthetic and natural polymers, even nonconventional systems, and so on.
Among the AIE systems, luminogens without traditional chromophores are of particular interest and will find their bright future as they have huge structure tunability and multiple functionality. Their unorthodox luminescent processes are explained by the CTE mechanism and their emitting groups are attributed to the clusters of electron-rich atoms or subgroups by through-bond and through-space conjugation. In the clusters, the subgroups tied up with others to share their electrons and serve as effective clustoluminogens. Further studies should acquire in-depth insights into the detailed mechanisms. Also, RTP has been found in metal-free and even nonconventional AIEgen systems with the aid of the RIM mechanism. It can be envisioned that the RTP with high efficiency and long lifetime will be attained by taking advantage of the RIM process and our novel molecular design principle.
The study of AIE effect has far-reaching practical implications. AIE systems allow people to make use of the aggregate process actively, instead of fighting against it. The turn-on characteristic of the AIEgens makes them promising for real-time monitoring, optoelectronic devices, mechanochromism inks, and so on. The most thrilling fields of AIEgens are still in the life science and biomedical research, such as for in photoimaging of biological structures and bioprocesses. AIE-related research is booming, which has permeated a large number of research disciplines with a wide-spread influence. There are many opportunities as well as challenges on this platform. Grasping the opportunities and overcoming the challenges will deepen our understanding and create innovative light-based technologies.
Acknowledgments
The authors acknowledge the financial support from the Start-up Funding from HIT Shenzhen (HA45001033), the National Natural Science Foundation of China (51703042), the National Basic Research Program of China (973 Program; 2013CB834701 and 2013CB834702), the University Grants Committee of Hong Kong (AoE/P-03/08), the Research Grants Council of Hong Kong (16301614, 16305015, 16308016, and C2017-15G), and the Innovation and Technology Commission (ITC-CNERC14SC01). B.Z.T. thanks the support of the Guangdong Innovative Research Team Program (201101C0105067115).
Biographies

Zikai He is currently an associate professor at the Harbin Institute of Technology (HIT), Shenzhen. He received his B.S. and Ph.D. degrees from the University of Science and Technology of China and the Chinese University of Hong Kong in 2009 and 2013, respectively. He then worked as a Postdoctoral Fellowship under the supervision of Prof. B.Z.T. at The Hong Kong University of Science and Technology (HKUST) from 2013 to 2016. He joined HIT in 2016. His research is mainly focused on the development of new functional organic small molecules and the exploration of their (opto)electronic applications.

Ben Zhong Tang received his B.S. and Ph.D. degrees from the South China University of Technology and Kyoto University, respectively. He conducted his postdoctoral research at the University of Toronto. He joined HKUST as an assistant professor in 1994 and was promoted to chair professor in 2008. He was elected to the Chinese Academy of Sciences and the Royal Society of Chemistry in 2009 and 2013, respectively. He is now serving as the Editor-in-Chief of Materials Chemistry Frontiers. His research interests include macromolecular chemistry, materials science, and biomedical theranostics. He is now spearheading the research on AIE. After 17 year journey of AIE research, homogeneous and heterogeneous AIE clustoluminogens were put forth here, representing the traditional and nonconventional AIEgens governed by CTE mechanisms. The through-bond and through-space conjugation within subgroups provides the effective luminescent chromophores. As a result, we greatly expand the scope of AIE and offer an attractive platform of future luminogen research.
The authors declare no competing financial interest.
References
- Photophysics of Aromatic Molecules; Birks J. B., Ed.; Wiley InterScience: London, 1970. [Google Scholar]
- a Thomas S. W.; Joly G. D.; Swager T. M. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev. 2007, 107, 1339–1386. 10.1021/cr0501339. [DOI] [PubMed] [Google Scholar]; b Qian G.; Zhong Z.; Luo M.; Yu D.; Zhang Z.; Wang Z. Y.; Ma D. Simple and Efficient Near-Infrared Organic Chromophores for Light-Emitting Diodes with Single Electroluminescent Emission above 1000 nm. Adv. Mater. 2009, 21, 111–116. 10.1002/adma.200801918. [DOI] [Google Scholar]
- a Fan C.; Wang S.; Hong J. W.; Bazan G. C.; Plaxco K. W.; Heeger A. J. Beyond superquenching: Hyper-efficient energy transfer from conjugated polymers to gold nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 6297–6301. 10.1073/pnas.1132025100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gaylord B. S.; Wang S.; Heeger A. J.; Bazan G. C. Water-Soluble Conjugated Oligomers: Effect of Chain Length and Aggregation on Photoluminescence-Quenching Efficiencies. J. Am. Chem. Soc. 2001, 123, 6417–6418. 10.1021/ja010373f. [DOI] [PubMed] [Google Scholar]; c Wang J.; Zhao Y.; Dou C.; Sun H.; Xu P.; Ye K.; Zhang J.; Jiang S.; Li F.; Wang Y. Alkyl and Dendron Substituted Quinacridones: Synthesis, Structures, and Luminescent Properties. J. Phys. Chem. B 2007, 111, 5082–5089. 10.1021/jp068646m. [DOI] [PubMed] [Google Scholar]; d Lim S.-F.; Friend R. H.; Rees I. D.; Li J.; Ma Y.; Robinson K.; Holmes A. B.; Hennebicq E.; Beljonne D.; Cacialli F. Suppression of Green Emission in a New Class of Blue-Emitting Polyfluorene Copolymers with Twisted Biphenyl Moieties. Adv. Funct. Mater. 2005, 15, 981–988. 10.1002/adfm.200400457. [DOI] [Google Scholar]
- Yang J.-S.; Swager T. M. Fluorescent Porous Polymer Films as TNT Chemosensors: Electronic and Structural Effects. J. Am. Chem. Soc. 1998, 120, 11864–11873. 10.1021/ja982293q. [DOI] [Google Scholar]
- a Mei J.; Leung N. L. C.; Kwok R. T. K.; Lam J. W. Y.; Tang B. Z. Aggregation-Induced Emission: Together We Shine, United We Soar!. Chem. Rev. 2015, 115, 11718–11940. 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]; b Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-induced emission: phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. 10.1039/b904665h. [DOI] [PubMed] [Google Scholar]; c Luo J.; Xie Z.; Lam J. W. Y.; Cheng L.; Tang B. Z.; Chen H.; Qiu C.; Kwok H. S.; Zhan X.; Liu Y.; Zhu D. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. 10.1039/b105159h. [DOI] [PubMed] [Google Scholar]; d Hong Y.; Lam J. W. Y.; Tang B. Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. 10.1039/c1cs15113d. [DOI] [PubMed] [Google Scholar]; e Hu R.; Leung N. L. C.; Tang B. Z. AIE macromolecules: syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. 10.1039/c4cs00044g. [DOI] [PubMed] [Google Scholar]; f Lou X.; Zhao Z.; Tang B. Z. Organic AIE Dots: Organic Dots Based on AIEgens for Two-Photon Fluorescence Bioimaging. Small 2016, 12, 6429. 10.1002/smll.201670247. [DOI] [PubMed] [Google Scholar]; g Kwok R. T. K.; Leung C. W. T.; Lam J. W. Y.; Tang B. Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. 10.1039/c4cs00325j. [DOI] [PubMed] [Google Scholar]; h Chen S.; Wang H.; Hong Y.; Tang B. Z. Fabrication of fluorescent nanoparticles based on AIE luminogens (AIE dots) and their applications in bioimaging. Mater. Horiz. 2016, 3, 283–293. 10.1039/c6mh00060f. [DOI] [Google Scholar]; i Dong Y. Q.; Lam J. W. Y.; Tang B. Z. Mechanochromic Luminescence of Aggregation-Induced Emission Luminogens. J. Phys. Chem. Lett. 2015, 6, 3429–3436. 10.1021/acs.jpclett.5b01090. [DOI] [PubMed] [Google Scholar]; j Roose J.; Tang B. Z.; Wong K. S. Circularly-Polarized Luminescence (CPL) from Chiral AIE Molecules and Macrostructures. Small 2016, 12, 6495–6512. 10.1002/smll.201601455. [DOI] [PubMed] [Google Scholar]
- a . Let there be light. Nat. Mater. 2015, 14 (), 453; 10.1038/nmat4287 [DOI] [PubMed] [Google Scholar]; b Colvin V. L.; Schlamp M. C.; Alivisatos A. P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. 10.1038/370354a0. [DOI] [Google Scholar]; c Tang C. W.; VanSlyke S. A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. 10.1063/1.98799. [DOI] [Google Scholar]
- a Mei J.; Hong Y.; Lam J. W. Y.; Qin A.; Tang Y.; Tang B. Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. 10.1002/adma.201401356. [DOI] [PubMed] [Google Scholar]; b Itoh T. Fluorescence and Phosphorescence from Higher Excited States of Organic Molecules. Chem. Rev. 2012, 112, 4541–4568. 10.1021/cr200166m. [DOI] [PubMed] [Google Scholar]
- a Bonacchi S.; Genovese D.; Juris R.; Montalti M.; Prodi L.; Rampazzo E.; Zaccheroni N. Luminescent Silica Nanoparticles: Extending the Frontiers of Brightness. Angew. Chem., Int. Ed. 2011, 50, 4056–4066. 10.1002/anie.201004996. [DOI] [PubMed] [Google Scholar]; b Schäferling M. The Art of Fluorescence Imaging with Chemical Sensors. Angew. Chem., Int. Ed. 2012, 51, 3532–3554. 10.1002/anie.201105459. [DOI] [PubMed] [Google Scholar]; c Cui Y.; Yue Y.; Qian G.; Chen B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. 10.1021/cr200101d. [DOI] [PubMed] [Google Scholar]; d Yang Y.; Zhao Q.; Feng W.; Li F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. 10.1021/cr2004103. [DOI] [PubMed] [Google Scholar]; e Maggini L.; Bonifazi D. Hierarchised luminescent organic architectures: design, synthesis, self-assembly, self-organisation and functions. Chem. Soc. Rev. 2012, 41, 211–241. 10.1039/c1cs15031f. [DOI] [PubMed] [Google Scholar]
- a Zhao Z.; Lam J. W. Y.; Tang B. Z. Tetraphenylethene: a versatile AIE building block for the construction of efficient luminescent materials for organic light-emitting diodes. J. Mater. Chem. 2012, 22, 23726–23740. 10.1039/c2jm31949g. [DOI] [Google Scholar]; b Chi Z.; Zhang X.; Xu B.; Zhou X.; Ma C.; Zhang Y.; Liu S.; Xu J. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. 10.1039/c2cs35016e. [DOI] [PubMed] [Google Scholar]
- a Ding D.; Li K.; Liu B.; Tang B. Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. 10.1021/ar3003464. [DOI] [PubMed] [Google Scholar]; b Tolosa L.; Kostov Y.; Rao G.. Fluorescence-Based Sensors for Bioprocess Monitoring. In Fluorescence Sensors and Biosensors; Thompson R. B., Ed.; CRC Press, 2005; pp 333–351. [Google Scholar]
- Chen J.; Xu B.; Ouyang X.; Tang B. Z.; Cao Y. Aggregation-Induced Emission of cis,cis-1,2,3,4-Tetraphenylbutadiene from Restricted Intramolecular Rotation. J. Phys. Chem. A 2004, 108, 7522–7526. 10.1021/jp048475q. [DOI] [Google Scholar]
- Chen J.; Law C. C. W.; Lam J. W. Y.; Dong Y.; Lo S. M. F.; Williams I. D.; Zhu D.; Tang B. Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. 10.1021/cm021715z. [DOI] [Google Scholar]
- a Li Q.; Li Z. The Strong Light-Emission Materials in the Aggregated State: What Happens from a Single Molecule to the Collective Group. Adv. Sci. 2017, 4, 1600484. 10.1002/advs.201600484. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang C.; Li Z. Molecular conformation and packing: their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. 10.1039/c7qm00201g. [DOI] [Google Scholar]
- Modern Molecular Photochemistry of Oganic Molecules; Turro N. J., Scaiano J. C., Ramamurthy V., Eds.; University Science Books, 2010. [Google Scholar]
- a Peng Q.; Yi Y.; Shuai Z.; Shao J. Toward Quantitative Prediction of Molecular Fluorescence Quantum Efficiency: Role of Duschinsky Rotation. J. Am. Chem. Soc. 2007, 129, 9333–9339. 10.1021/ja067946e. [DOI] [PubMed] [Google Scholar]; b Zhang T.; Peng Q.; Quan C.; Nie H.; Niu Y.; Xie Y.; Zhao Z.; Tang B. Z.; Shuai Z. Using the isotope effect to probe an aggregation induced emission mechanism: theoretical prediction and experimental validation. Chem. Sci. 2016, 7, 5573–5580. 10.1039/c6sc00839a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ren Y.; Lam J. W. Y.; Dong Y.; Tang B. Z.; Wong K. S. Enhanced Emission Efficiency and Excited State Lifetime Due to Restricted Intramolecular Motion in Silole Aggregates. J. Phys. Chem. B 2005, 109, 1135–1140. 10.1021/jp046659z. [DOI] [PubMed] [Google Scholar]; b Li Z.; Dong Y.; Mi B.; Tang Y.; Häussler M.; Tong H.; Dong Y.; Lam J. W. Y.; Ren Y.; Sung H. H. Y.; Wong K. S.; Gao P.; Williams I. D.; Kwok H. S.; Tang B. Z. Structural Control of the Photoluminescence of Silole Regioisomers and Their Utility as Sensitive Regiodiscriminating Chemosensors and Efficient Electroluminescent Materials. J. Phys. Chem. B 2005, 109, 10061–10066. 10.1021/jp0503462. [DOI] [PubMed] [Google Scholar]; c Chen B.; Nie H.; Lu P.; Zhou J.; Qin A.; Qiu H.; Zhao Z.; Tang B. Z. Conjugation versus rotation: good conjugation weakens the aggregation-induced emission effect of siloles. Chem. Commun. 2014, 50, 4500–4503. 10.1039/c4cc00653d. [DOI] [PubMed] [Google Scholar]; d Fan X.; Sun J.; Wang F.; Chu Z.; Wang P.; Dong Y.; Hu R.; Tang B. Z.; Zou D. Photoluminescence and electroluminescence of hexaphenylsilole are enhanced by pressurization in the solid state. Chem. Commun. 2008, 2989–2991. 10.1039/b803539c. [DOI] [PubMed] [Google Scholar]
- a Zhang G.-F.; Chen Z.-Q.; Aldred M. P.; Hu Z.; Chen T.; Huang Z.; Meng X.; Zhu M.-Q. Direct validation of the restriction of intramolecular rotation hypothesis via the synthesis of novel ortho-methyl substituted tetraphenylethenes and their application in cell imaging. Chem. Commun. 2014, 50, 12058–12060. 10.1039/c4cc04241g. [DOI] [PubMed] [Google Scholar]; b Parrott E. P. J.; Tan N. Y.; Hu R.; Zeitler J. A.; Tang B. Z.; Pickwell-MacPherson E. Direct evidence to support the restriction of intramolecular rotation hypothesis for the mechanism of aggregation-induced emission: temperature resolved terahertz spectra of tetraphenylethene. Mater. Horiz. 2014, 1, 251–258. 10.1039/c3mh00078h. [DOI] [Google Scholar]; c Liang G.; Lam J. W. Y.; Qin W.; Li J.; Xie N.; Tang B. Z. Molecular luminogens based on restriction of intramolecular motions through host-guest inclusion for cell imaging. Chem. Commun. 2014, 50, 1725–1727. 10.1039/c3cc48625g. [DOI] [PubMed] [Google Scholar]
- Leung N. L. C.; Xie N.; Yuan W.; Liu Y.; Wu Q.; Peng Q.; Miao Q.; Lam J. W. Y.; Tang B. Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem.—Eur. J. 2014, 20, 15349–15353. 10.1002/chem.201403811. [DOI] [PubMed] [Google Scholar]
- a Yao L.; Zhang S.; Wang R.; Li W.; Shen F.; Yang B.; Ma Y. Highly Efficient Near-Infrared Organic Light-Emitting Diode Based on a Butterfly-Shaped Donor–Acceptor Chromophore with Strong Solid-State Fluorescence and a Large Proportion of Radiative Excitons. Angew. Chem., Int. Ed. 2014, 53, 2119–2123. 10.1002/anie.201308486. [DOI] [PubMed] [Google Scholar]; b Nishiuchi T.; Tanaka K.; Kuwatani Y.; Sung J.; Nishinaga T.; Kim D.; Iyoda M. Solvent-Induced Crystalline-State Emission and Multichromism of a Bent π-Surface System Composed of Dibenzocyclooctatetraene Units. Chem.—Eur. J. 2013, 19, 4110–4116. 10.1002/chem.201203952. [DOI] [PubMed] [Google Scholar]; c Yuan C.; Saito S.; Camacho C.; Kowalczyk T.; Irle S.; Yamaguchi S. Hybridization of a Flexible Cyclooctatetraene Core and Rigid Aceneimide Wings for Multiluminescent Flapping π Systems. Chem.—Eur. J. 2014, 20, 2193–2200. 10.1002/chem.201303955. [DOI] [PubMed] [Google Scholar]; d Sharma nee Kamaldeep K.; Kaur S.; Bhalla V.; Kumar M.; Gupta A. Pentacenequinone derivatives for preparation of gold nanoparticles: facile synthesis and catalytic application. J. Mater. Chem. A 2014, 2, 8369–8375. 10.1039/c4ta00397g. [DOI] [Google Scholar]; e Liu J.; Meng Q.; Zhang X.; Lu X.; He P.; Jiang L.; Dong H.; Hu W. Aggregation-induced emission enhancement based on 11,11,12,12,-tetracyano-9,10-anthraquinodimethane. Chem. Commun. 2013, 49, 1199–1201. 10.1039/c2cc38817k. [DOI] [PubMed] [Google Scholar]; f He Z.; Shan L.; Mei J.; Wang H.; Lam J. W. Y.; Sung H. H. Y.; Williams I. D.; Gu X.; Miao Q.; Tang B. Z. Aggregation-induced emission and aggregation-promoted photochromism of bis(diphenylmethylene)dihydroacenes. Chem. Sci. 2015, 6, 3538–3543. 10.1039/c5sc00900f. [DOI] [PMC free article] [PubMed] [Google Scholar]; g He Z.; Zhang L.; Mei J.; Zhang T.; Lam J. W. Y.; Shuai Z.; Dong Y. Q.; Tang B. Z. Polymorphism-Dependent and Switchable Emission of Butterfly-Like Bis(diarylmethylene)dihydroanthracenes. Chem. Mater. 2015, 27, 6601–6607. 10.1021/acs.chemmater.5b02280. [DOI] [Google Scholar]
- a Tseng N.-W.; Liu J.; Ng J. C. Y.; Lam J. W. Y.; Sung H. H. Y.; Williams I. D.; Tang B. Z. Deciphering mechanism of aggregation-induced emission (AIE): Is E-Zisomerisation involved in an AIE process?. Chem. Sci. 2012, 3, 493–497. 10.1039/c1sc00690h. [DOI] [Google Scholar]; b Yang Z.; Qin W.; Leung N. L. C.; Arseneault M.; Lam J. W. Y.; Liang G.; Sung H. H. Y.; Williams I. D.; Tang B. Z. A mechanistic study of AIE processes of TPE luminogens: intramolecular rotation vs. configurational isomerization. J. Mater. Chem. C 2016, 4, 99–107. 10.1039/c5tc02924d. [DOI] [Google Scholar]
- He Z.; Zhao E.; Lam J. W. Y.; Tang B. Z.. New Mechanistic Insights into the AIE Phenomenon. Aggregation-Induced Emission: Materials and Applications Volume 1; American Chemical Society, 2016; Vol. 1226, pp 5–20. [Google Scholar]
- a Baldo M. A.; O’Brien D. F.; You Y.; Shoustikov A.; Sibley S.; Thompson M. E.; Forrest S. R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. 10.1038/25954. [DOI] [Google Scholar]; b Zhang G.; Palmer G. M.; Dewhirst M. W.; Fraser C. L. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat. Mater. 2009, 8, 747–751. 10.1038/nmat2509. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhao Q.; Huang C.; Li F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. 10.1039/c0cs00114g. [DOI] [PubMed] [Google Scholar]; d Kwon M. S.; Lee D.; Seo S.; Jung J.; Kim J. Tailoring intermolecular interactions for efficient room-temperature phosphorescence from purely organic materials in amorphous polymer matrices. Angew. Chem., Int. Ed. 2014, 53, 11177–11181. 10.1002/anie.201404490. [DOI] [PubMed] [Google Scholar]
- a Bergamini G.; Fermi A.; Botta C.; Giovanella U.; Di Motta S.; Negri F.; Peresutti R.; Gingras M.; Ceroni P. A persulfurated benzene molecule exhibits outstanding phosphorescence in rigid environments: from computational study to organic nanocrystals and OLED applications. J. Mater. Chem. C 2013, 1, 2717–2724. 10.1039/c3tc00878a. [DOI] [Google Scholar]; b Fermi A.; Bergamini G.; Peresutti R.; Marchi E.; Roy M.; Ceroni P.; Gingras M. Molecular asterisks with a persulfurated benzene core are among the strongest organic phosphorescent emitters in the solid state. Dyes Pigm. 2014, 110, 113–122. 10.1016/j.dyepig.2014.04.036. [DOI] [Google Scholar]; c Gong Y.; Zhao L.; Peng Q.; Fan D.; Yuan W. Z.; Zhang Y.; Tang B. Z. Crystallization-induced dual emission from metal- and heavy atom-free aromatic acids and esters. Chem. Sci. 2015, 6, 4438–4444. 10.1039/c5sc00253b. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li C.; Tang X.; Zhang L.; Li C.; Liu Z.; Bo Z.; Dong Y. Q.; Tian Y.-H.; Dong Y.; Tang B. Z. Reversible Luminescence Switching of an Organic Solid: Controllable On–Off Persistent Room Temperature Phosphorescence and Stimulated Multiple Fluorescence Conversion. Adv. Opt. Mater. 2015, 3, 1184–1190. 10.1002/adom.201500115. [DOI] [Google Scholar]; e Gong Y.; Chen G.; Peng Q.; Yuan W. Z.; Xie Y.; Li S.; Zhang Y.; Tang B. Z. Achieving Persistent Room Temperature Phosphorescence and Remarkable Mechanochromism from Pure Organic Luminogens. Adv. Mater. 2015, 27, 6195–6201. 10.1002/adma.201502442. [DOI] [PubMed] [Google Scholar]; f Yang Z.; Mao Z.; Zhang X.; Ou D.; Mu Y.; Zhang Y.; Zhao C.; Liu S.; Chi Z.; Xu J.; Wu Y.-C.; Lu P.-Y.; Lien A.; Bryce M. R. Intermolecular Electronic Coupling of Organic Units for Efficient Persistent Room-Temperature Phosphorescence. Angew. Chem., Int. Ed. 2016, 55, 2181–2185. 10.1002/anie.201509224. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Shimizu M.; Kimura A.; Sakaguchi H. Room-Temperature Phosphorescence of Crystalline 1,4-Bis(aroyl)-2,5-dibromobenzenes. Eur. J. Org. Chem. 2016, 467–473. 10.1002/ejoc.201501382. [DOI] [Google Scholar]; h Shimizu M.; Shigitani R.; Nakatani M.; Kuwabara K.; Miyake Y.; Tajima K.; Sakai H.; Hasobe T. Siloxy Group-Induced Highly Efficient Room Temperature Phosphorescence with Long Lifetime. J. Phys. Chem. C 2016, 120, 11631–11639. 10.1021/acs.jpcc.6b03276. [DOI] [Google Scholar]; i Ward J. S.; Nobuyasu R. S.; Batsanov A. S.; Data P.; Monkman A. P.; Dias F. B.; Bryce M. R. The interplay of thermally activated delayed fluorescence (TADF) and room temperature organic phosphorescence in sterically-constrained donor-acceptor charge-transfer molecules. Chem. Commun. 2016, 52, 2612–2615. 10.1039/c5cc09645f. [DOI] [PubMed] [Google Scholar]; j Zhang G.; Chen J.; Payne S. J.; Kooi S. E.; Demas J. N.; Fraser C. L. Multi-emissive difluoroboron dibenzoylmethane polylactide exhibiting intense fluorescence and oxygen-sensitive room-temperature phosphorescence. J. Am. Chem. Soc. 2007, 129, 8942–8943. 10.1021/ja0720255. [DOI] [PubMed] [Google Scholar]; k Bolton O.; Lee K.; Kim H.-J.; Lin K. Y.; Kim J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 2011, 3, 205–210. 10.1038/nchem.984. [DOI] [PubMed] [Google Scholar]; l Bolton O.; Lee D.; Jung J.; Kim J. Tuning the Photophysical Properties of Metal-Free Room Temperature Organic Phosphors via Compositional Variations in Bromobenzaldehyde/Dibromobenzene Mixed Crystals. Chem. Mater. 2014, 26, 6644–6649. 10.1021/cm503678r. [DOI] [Google Scholar]; m Hirata S.; Totani K.; Zhang J.; Yamashita T.; Kaji H.; Marder S. R.; Watanabe T.; Adachi C. Efficient Persistent Room Temperature Phosphorescence in Organic Amorphous Materials under Ambient Conditions. Adv. Funct. Mater. 2013, 23, 3386–3397. 10.1002/adfm.201203706. [DOI] [Google Scholar]; n Fermi A.; Bergamini G.; Roy M.; Gingras M.; Ceroni P. Turn-on phosphorescence by metal coordination to a multivalent terpyridine ligand: a new paradigm for luminescent sensors. J. Am. Chem. Soc. 2014, 136, 6395–6400. 10.1021/ja501458s. [DOI] [PubMed] [Google Scholar]; o Wang H.; Wang H.; Yang X.; Wang Q.; Yang Y. Ion-unquenchable and thermally “on-off” reversible room temperature phosphorescence of 3-bromoquinoline induced by supramolecular gels. Langmuir 2015, 31, 486–491. 10.1021/la5040323. [DOI] [PubMed] [Google Scholar]
- Yuan W. Z.; Shen X. Y.; Zhao H.; Lam J. W. Y.; Tang L.; Lu P.; Wang C.; Liu Y.; Wang Z.; Zheng Q.; Sun J. Z.; Ma Y.; Tang B. Z. Crystallization-Induced Phosphorescence of Pure Organic Luminogens at Room Temperature. J. Phys. Chem. C 2010, 114, 6090–6099. 10.1021/jp909388y. [DOI] [Google Scholar]
- a An Z.; Zheng C.; Tao Y.; Chen R.; Shi H.; Chen T.; Wang Z.; Li H.; Deng R.; Liu X.; Huang W. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. 10.1038/nmat4259. [DOI] [PubMed] [Google Scholar]; b Chen X.; Xu C.; Wang T.; Zhou C.; Du J.; Wang Z.; Xu H.; Xie T.; Bi G.; Jiang J.; Zhang X.; Demas J. N.; Trindle C. O.; Luo Y.; Zhang G. Versatile Room-Temperature-Phosphorescent Materials Prepared from N-Substituted Naphthalimides: Emission Enhancement and Chemical Conjugation. Angew. Chem., Int. Ed. 2016, 55, 9872–9876. 10.1002/anie.201601252. [DOI] [PubMed] [Google Scholar]; c Yang X.; Yan D. Strongly Enhanced Long-Lived Persistent Room Temperature Phosphorescence Based on the Formation of Metal–Organic Hybrids. Adv. Opt. Mater. 2016, 4, 897–905. 10.1002/adom.201500666. [DOI] [Google Scholar]; d Mieno H.; Kabe R.; Notsuka N.; Allendorf M. D.; Adachi C. Long-Lived Room-Temperature Phosphorescence of Coronene in Zeolitic Imidazolate Framework ZIF-8. Adv. Opt. Mater. 2016, 4, 1015–1021. 10.1002/adom.201600103. [DOI] [Google Scholar]
- Zhao W.; He Z.; Lam J. W. Y.; Peng Q.; Ma H.; Shuai Z.; Bai G.; Hao J.; Tang B. Z. Rational Molecular Design for Achieving Persistent and Efficient Pure Organic Room-Temperature Phosphorescence. Chem 2016, 1, 592–602. 10.1016/j.chempr.2016.08.010. [DOI] [Google Scholar]
- Gong Y.; Tan Y.; Li H.; Zhang Y.; Yuan W.; Zhang Y.; Sun J.; Tang B. Z. Crystallization-induced phosphorescence of benzils at room temperature. Sci. China: Chem. 2013, 56, 1183–1186. 10.1007/s11426-013-4930-9. [DOI] [Google Scholar]
- Wang S.; Yuan W. Z.; Zhang Y.. Pure Organic Luminogens with Room Temperature Phosphorescence. Aggregation-Induced Emission: Materials and Applications Volume 2; American Chemical Society, 2016; Vol. 1227, pp 1–26. [Google Scholar]
- a Yuan W. Z.; Zhang Y. Nonconventional macromolecular luminogens with aggregation-induced emission characteristics. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 560–574. 10.1002/pola.28420. [DOI] [Google Scholar]; b Ye R.; Liu Y.; Zhang H.; Su H.; Zhang Y.; Xu L.; Hu R.; Kwok R. T. K.; Wong K. S.; Lam J. W. Y.; Goddard W. A.; Tang B. Z. Non-conventional fluorescent biogenic and synthetic polymers without aromatic rings. Polym. Chem. 2017, 8, 1722–1727. 10.1039/c7py00154a. [DOI] [Google Scholar]; c Yan J.-J.; Wang Z.-K.; Lin X.-S.; Hong C.-Y.; Liang H.-J.; Pan C.-Y.; You Y.-Z. Polymerizing Nonfluorescent Monomers without Incorporating any Fluorescent Agent Produces Strong Fluorescent Polymers. Adv. Mater. 2012, 24, 5617–5624. 10.1002/adma.201202201. [DOI] [PubMed] [Google Scholar]; d Ruff Y.; Buhler E.; Candau S.-J.; Kesselman E.; Talmon Y.; Lehn J.-M. Glycodynamers: Dynamic Polymers Bearing Oligosaccharides Residues–Generation, Structure, Physicochemical, Component Exchange, and Lectin Binding Properties. J. Am. Chem. Soc. 2010, 132, 2573–2584. 10.1021/ja9082733. [DOI] [PubMed] [Google Scholar]; e Ruff Y.; Lehn J.-M. Glycodynamers: Fluorescent Dynamic Analogues of Polysaccharides. Angew. Chem., Int. Ed. 2008, 47, 3556–3559. 10.1002/anie.200703490. [DOI] [PubMed] [Google Scholar]; f Sun M.; Hong C.-Y.; Pan C.-Y. A Unique Aliphatic Tertiary Amine Chromophore: Fluorescence, Polymer Structure, and Application in Cell Imaging. J. Am. Chem. Soc. 2012, 134, 20581–20584. 10.1021/ja310236m. [DOI] [PubMed] [Google Scholar]; g Zhu S.; Zhang J.; Wang L.; Song Y.; Zhang G.; Wang H.; Yang B. A general route to make non-conjugated linear polymers luminescent. Chem. Commun. 2012, 48, 10889–10891. 10.1039/c2cc36080b. [DOI] [PubMed] [Google Scholar]; h Zhu S.; Song Y.; Shao J.; Zhao X.; Yang B. Non-Conjugated Polymer Dots with Crosslink-Enhanced Emission in the Absence of Fluorophore Units. Angew. Chem., Int. Ed. 2015, 54, 14626–14637. 10.1002/anie.201504951. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Cao B.; Zhu C.; Xu S.; Gong Y.; Yuan W. Z.; Zhang Y. Clustering-Triggered Emission of Nonconjugated Polyacrylonitrile. Small 2016, 12, 6586–6592. 10.1002/smll.201601545. [DOI] [PubMed] [Google Scholar]
- Bin X.; Luo W.; Yuan W.; Zhang Y. Clustering-Triggered Emission of Poly(N-hydroxysuccinimide Methacrylate). Acta Chim. Sin. 2016, 74, 935–941. 10.6023/a16080423. [DOI] [Google Scholar]
- Zhao E.; Lam J. W. Y.; Meng L.; Hong Y.; Deng H.; Bai G.; Huang X.; Hao J.; Tang B. Z. Poly[(maleic anhydride)-alt-(vinyl acetate)]: A Pure Oxygenic Nonconjugated Macromolecule with Strong Light Emission and Solvatochromic Effect. Macromolecules 2015, 48, 64–71. 10.1021/ma502160w. [DOI] [Google Scholar]
- a Chen L.; Wang Y.-H.; He B.; Nie H.; Hu R.; Huang F.; Qin A.; Zhou X.-S.; Zhao Z.; Tang B. Z. Multichannel Conductance of Folded Single-Molecule Wires Aided by Through-Space Conjugation. Angew. Chem., Int. Ed. 2015, 54, 4231–4235. 10.1002/anie.201411909. [DOI] [PubMed] [Google Scholar]; b Zhao Z.; Lam J. W. Y.; Chan C. Y. K.; Chen S.; Liu J.; Lu P.; Rodriguez M.; Maldonado J.-L.; Ramos-Ortiz G.; Sung H. H. Y.; Williams I. D.; Su H.; Wong K. S.; Ma Y.; Kwok H. S.; Qiu H.; Tang B. Z. Stereoselective Synthesis, Efficient Light Emission, and High Bipolar Charge Mobility of Chiasmatic Luminogens. Adv. Mater. 2011, 23, 5430–5435. 10.1002/adma.201102804. [DOI] [PubMed] [Google Scholar]
- Zhu Q.; Zhang Y.; Nie H.; Zhao Z.; Liu S.; Wong K. S.; Tang B. Z. Insight into the strong aggregation-induced emission of low-conjugated racemic C6-unsubstituted tetrahydropyrimidines through crystal-structure-property relationship of polymorphs. Chem. Sci. 2015, 6, 4690–4697. 10.1039/c5sc01226k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Zhao E.; Lam J. W. Y.; Tang B. Z. AIE luminogens: emission brightened by aggregation. Mater. Today 2015, 18, 365–377. 10.1016/j.mattod.2015.03.004. [DOI] [Google Scholar]
- Zhang H.; Zheng X.; Xie N.; He Z.; Liu J.; Leung N. L. C.; Niu Y.; Huang X.; Wong K. S.; Kwok R. T. K.; Sung H. H. Y.; Williams I. D.; Qin A.; Lam J. W. Y.; Tang B. Z. Why Do Simple Molecules with “Isolated” Phenyl Rings Emit Visible Light?. J. Am. Chem. Soc. 2017, 139, 16264–16272. 10.1021/jacs.7b08592. [DOI] [PubMed] [Google Scholar]
- Sturala J.; Etherington M. K.; Bismillah A. N.; Higginbotham H. F.; Trewby W.; Aguilar J. A.; Bromley E. H. C.; Avestro A.-J.; Monkman A. P.; McGonigal P. R. Excited-State Aromatic Interactions in the Aggregation-Induced Emission of Molecular Rotors. J. Am. Chem. Soc. 2017, 139, 17882–17889. 10.1021/jacs.7b08570. [DOI] [PubMed] [Google Scholar]
- Ye T.; Chen J.; Ma D. Electroluminescence of poly(N-vinylcarbazole) films: fluorescence, phosphorescence and electromers. Phys. Chem. Chem. Phys. 2010, 12, 15410–15413. 10.1039/c0cp00461h. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Chen B.; Susha A. S.; Wang W.; Reckmeier C. J.; Chen R.; Zhong H.; Rogach A. L. All-Copper Nanocluster Based Down-Conversion White Light-Emitting Devices. Adv. Sci. 2016, 3, 1600182. 10.1002/advs.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.; Yuan X.; Yu Y.; Zhang Q.; Leong D. T.; Lee J. Y.; Xie J. From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)–Thiolate Core–Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. 10.1021/ja306199p. [DOI] [PubMed] [Google Scholar]