Abstract

We report here the synthesis and thorough characterization of a new family of alkylbiguanides and alkylbiguanidium chlorides by 1H and 13C NMR and X-ray diffraction. Their critical micelle concentration was first determined by surface tension measurements. Hexylbiguanide was then studied as a surfactant in the micellar Suzuki–Miyaura cross-coupling reaction. The unexpected low reactivity of the system at high Pd/hexylbiguanide ratios was due to the change of the size and the shape of the aggregates, observed by transmission electron microscopy. The best catalytic activity was obtained for a 1:1 Pd/hexylbiguanide ratio for which the micellar conditions were conserved. Better results were obtained for several substrates, when compared to those previously obtained with metformin under the same reaction conditions. Higher yields and a better recyclability were obtained under micellar conditions with hexylbiguanide.
Introduction
Various biguanide derivatives (Figure 1) have been shown to possess multiple biological applications. Their antihyperglycemic properties have been extensively studied, and metformin hydrochloride became one of the most prescribed drugs for the treatment of type 2 diabetes patients.1 The antimalarial2 and antimicrobial3 properties of biguanides have also been reported, as a result of their membrane disruption activity.4 Recently, the capacity of biguanides to inhibit the proliferation of cancer cells has begun to be considered as a potential anticancer therapy, and even if their mechanism of action is still uncertain, the major limitation for their use is considered to be their inadequate ability to penetrate mitochondria in vivo.5 At physiological pH, biguanides are protonated and are usually found as hydrophilic chloride salts, also called biguanidium salts.6
Figure 1.
Representation of biguanide, biguanidium hydrochloride, and metformin hydrochloride.
Biguanides can be easily functionalized with different substituents at both ends (aryl, benzyl, alkyl, etc.) (Figure 1). The hydrophilic biguanide group can be incorporated in the design of amphiphilic compounds with surfactant properties. The self-assembly of surfactants allows the solubilization, transport, delivery, or extraction of hydrophobic compounds in the hydrophobic micellar environment7 and finds several applications as emulsifiers,8 as drug delivery systems,9 in absorption of lipids,10 and of course in micellar catalysis.11
Micellar catalysis is a strategy largely employed in the development of greener reactions in aqueous media, lowering the impact of chemistry on the environment.12 It allows water solubilization of insoluble reactants and also the decrease of the reaction temperature. Micellar catalysis usually requires the use of an innocent surfactant or a metallosurfactant. An innocent surfactant does not directly interact with the metal but only solubilizes a preformed catalyst and usually enhances its activity.12c A metallosurfactant includes a ligand moiety, where the metal is directly coordinated to the surfactant.13 The metallosurfactant acts as both a mass transfer agent and a ligand, resulting in a major atom economy of the process.
The self-aggregation properties of alkylguanidinium (Figure 2) were previously reported by Song et al.14 for alkyl chains containing 8–12 carbons, with critical micelle concentration (CMC) values varying from 5 to 75 mM. In 2011, Lin et al. used functionalized alkylguanidinium salts as ionic liquid solvents in the Suzuki–Miyaura coupling.15 They reported complete conversion after 2 h at 60 °C with 2 mol % Pd(OAc)2 and 5 equiv of dodecylguanidinium salt and proposed the formation of micelles to be responsible for the stabilization of the Pd nanoparticles, allowing the recycling up to five catalytic runs without significant loss of activity.
Figure 2.
Representation of guanidine and the guanidinium cation.
Regarding its chemical structure, a biguanide is composed of two guanidine moieties (Figure 2). Compared to a guanidine or a guanidinium salt, biguanide has a higher ability to bind metals because of the two “imine-like” functions and acts as a bidentate ligand. The coordination of different metals to biguanides has been first reported in 1961,16 but their use as ligands in metal-catalyzed cross-coupling reactions started only two decades ago. They have been incorporated in complex systems (mesoporous silica,17 carbon nanotubes,18 fullerene,19 and chitosan20) and used as ligands for several metal-catalyzed reactions in organic solvents or mixtures with water. In our seek to develop green catalytic reactions in neat water and use small ligands to increase the atom economy of the process, we recently reported the successful use of metformin hydrochloride as a ligand in the Suzuki–Miyaura coupling in pure water, at a very low palladium loading (0.0025 mol %).21 As the recycling of the metformin catalyst was unsuccessful as the catalytic species degraded in water after only four catalytic runs at 0.5 mol % Pd, we explored the possibility to using alkylbiguanides as surfactants under micellar conditions for the Suzuki–Miyaura reaction in water. Herein, we describe the synthesis, the characterization of alkylbiguanide surfactants, and their successful application under micellar conditions in the Suzuki–Miyaura reaction in water.
Results and Discussion
Surfactants Synthesis
The alkylbiguanide dihydrochlorides were synthesized using the methodology developed by Suyama et al. in 1989.22 They were obtained in one step, with moderate to excellent yields, after only 90 min at 100 °C in 1,4-dioxane and purified as chloride salts by simple precipitation, by adding hydrochloric acid (Figure 3). It should be mentioned that the reaction was very sensitive to the concentration of reactants, that needed to be minimum 2 M to obtain high yields. Iron(II) chloride did not play a catalytic role in the reaction because lower amounts of iron resulted in lower yields or impure products. Moreover, a higher amount of iron (3 equiv as reported for some substrates by Suyama et al.22) resulted in no reaction at all, iron probably binding both dicyandiamide and amine, avoiding them to react. The reaction was also performed in greener solvents (ethyl acetate, water, acetone, methanol, and ethanol), but the reaction did not take place or the obtained product was impure.
Figure 3.
Synthesis of alkylbiguanide dihydrochlorides.
X-ray Diffraction
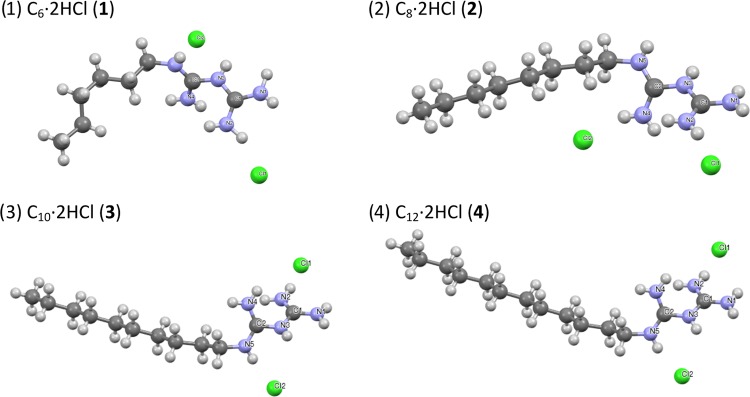
Single crystals of each alkylbiguanide were obtained by slow evaporation of methanol (1, 2, and 4) or by crystallization at low temperature in a mixture of methanol and acetone (3). They all appeared as overlapping plates (twinned crystals), which made their analysis difficult. Suitable crystals were selected and analyzed on a Bruker Venture MetalJet diffractometer (Figure 4).
Figure 4.
X-ray diffraction analysis of (1) hexylbiguanide dihydrochloride 1, (2) octylbiguanide dihydrochloride 2, (3) decylbiguanide dihydrochloride 3, and (4) dodecylbiguanide dihydrochloride 4.
Alkylbiguanides 1, 2, and 4 are monoclinic P21/c containing four molecules per primitive cell, and 3 is monoclinic C2/c with eight molecules per primitive cell. In the solid state, they all showed an organization similar to that of a lipid bilayer, where the hydrophobic alkyl chains were facing each other inside the primitive cell and the hydrophilic biguanidium moieties were facing the exterior of the primitive cell (Figure 5).
Figure 5.
Organization of the surfactant in the primitive cell for hexylbiguanide dihydrochloride (1), octylbiguanide dihydrochloride (2), decylbiguanide dihydrochloride (3), and dodecylbiguanide dihydrochloride (4).
The alkyl chains did not overlay in the same plane but organized themselves in two planes pointing toward opposite directions and forming an X (the pink and black molecules shown in Figure 5). The longer the alkyl chains were, the more organized they were in the solid state (Figures 5 and 6).
Figure 6.
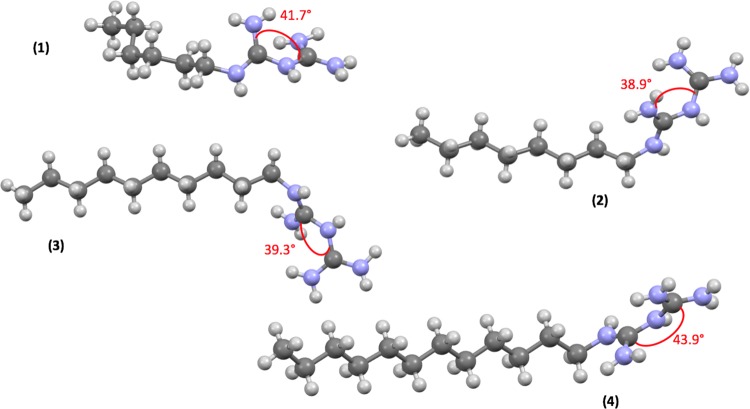
Torsion angles in the biguanidium cation.
Furthermore, we observed that the biguanide moiety was not planar, the torsion angles between the two guanidinium units being slightly different for the four salts, with values around 40°, as predicted by density functional theory calculations by Raczyńska et al.23 (Figure 6).
The four alkylbiguanidium dihydrochlorides had their double bonds positioned between C1 and N1 and C2 and N5. This is different from what was previously reported for metal-coordinated biguanides.24 As Raczyńska et al. previously reported, the protonation was favored on the imino groups, rather than the amino groups, because of the n−π conjugation.23 Finally, each molecule possesses two chlorides located in each plane of a guanidinium cation.
Surface Tension Measurement and Critical Micelle Concentration (CMC) Determination
The CMC of each alkylbiguanide was determined by measuring the evolution of the surface tension with its concentration. The surface tension was measured with a Wihelmy plate sensor on a dynamic contact angle meter and a tensiometer (DCAT11) in distilled water. The CMC was considered the concentration at which the surface tension reached a plateau (Supporting Information). The CMCs were determined at two temperatures (25 and 60 °C), in neutral and basic conditions (K2CO3 22 M), the basic conditions corresponding to the Suzuki–Miyaura reaction conditions (Table 1).
Table 1. Critical Micelle Concentration Values for Compounds 1–4 at 25 and 60 °C in Neutral and Basic Conditions (K2CO3 22 M) .
| compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| CMC at 25 °C (mM) | 2.30 | 2.10 | 1.80 | 0.22 |
| CMC at 60 °C (mM) | 2.25 | 1.50 | 0.72 | 0.16 |
| CMC in basic conditions at 25 °C (mM) | 0.40 | insoluble | insoluble | insoluble |
| CMC in basic conditions at 60 °C (mM) | 0.55 | insoluble | insoluble | insoluble |
The CMC values of the studied alkylbiguanides were influenced by the protonation state of the surfactant, the temperature, and the length of the alkyl chain. Compounds 1–4 were highly soluble in water. However, only 1 remained soluble in water in basic conditions, its CMC being 5–6 times lower than the one in neutral conditions. Ionic surfactants usually possess higher CMC values than those of their nonionic analogues because they are more water soluble.
At 60 °C, the CMC of biguanidium surfactants decreased. The CMC value of neutral surfactant 1 was slightly higher at 60 °C than the one at 25 °C. Both ionic and nonionic alkylbiguanide surfactants can form hydrogen bonds with water molecules, but water molecules stabilize better ionic surfactants because of their stronger interactions with the charged atoms. All of the protonated alkylbiguanidium salts showed lower CMCs at higher temperatures, probably due to the loss of some hydrogen bonds and a decrease of the hydration of the ions that resulted in an increase of hydrophobicity and favored the formation of micelles. The higher CMC obtained for the neutral hexylbiguanide at 60 °C can be due to the disruption of the network formed by the water molecules surrounding the smaller hexyl chain, disfavoring the formation of the micelles.25
Finally, the CMC values decreased, whereas the alkyl chain length increased. As a result of the increased hydrophobicity of the surfactant, the micelles started to form at 2.3 mM for the hexyl chain and decreased to 0.22 mM for the dodecyl chain in neutral conditions.
Suzuki–Miyaura Coupling Performed in Micellar Conditions with 1
We previously showed the efficiency of a 1:1 metformin/Pd(OAc)2 complex in the Suzuki–Miyaura cross-coupling reaction (Figure 7) in water. The catalytic species were preformed for 15 min at 100 °C, prior to the addition of the substrates. The coupling product was obtained in 95% yield after 15 min at 100 °C in pure water,21 and we were able to recycle the system up to four catalytic runs with no significant loss of efficiency, after which the catalytic species started to degrade.
Figure 7.
Previous results in the Suzuki–Miyaura coupling using metformin hydrochloride as a ligand.21
As our group also showed that the hydrophobic environment created by micelles can be used as a microreactor in the Suzuki–Miyaura reaction,13a we decided to use 1 as a metallosurfactant precursor in micellar conditions. Hexylbiguanide 1 was the only alkylbiguanide soluble in basic conditions and self-assembling in micelles.
The coupling reactions were performed over 24 h at 0.5 mol % Pd(OAc)2 and 5 mol % 1, at different temperatures (room temperature, 50, and 100 °C) and concentrations (0.5 and 5 mM) (Table 2). After 24 h, none of the reaction conditions allowed us to obtain satisfying results in terms of yield. We were expecting complete conversion at least for the reaction performed at 100 °C because this was obtained with the metformin ligand after only 15 min. The very low reactivity of hexylbiguanide could be explained by a possible segregation between the substrates and the catalyst. To explain these unexpected results, we studied the size and the shape of the aggregates formed by neutral 1 under the reaction conditions.
Table 2. Initial Reaction Conditions for the Micellar Suzuki–Miyaura Couplinga.
| entry | concentration of surfactant (mM) | temperature | yield (%)d |
|---|---|---|---|
| 1 | 0.5b | rt | <5 |
| 2 | 50 °C | <5 | |
| 3 | 100 °C | 16–25 | |
| 4 | 5c | rt | <5 |
| 5 | 50 °C | 10 | |
| 6 | 100 °C | 8–14 |
Reaction conditions: 0.5 mol % Pd(OAc)2 (1.12 mg), hexylbiguanide dihydrochloride 1 5 mol % (12.91 mg), 1 mmol (0.199 g) 4′-bromoacetophenone, 1 mmol (0.122 g) phenylboronic acid, 1.1 mmol (0.152 g) K2CO3, 24 h, in air.
In 100 mL distilled water.
In 10 mL distilled water.
Determined by 1H NMR.
The size and the shape of the micelles were determined by transmission electron microscopy (TEM) on a FEI Tecnai T12 (Eindhoven, The Netherlands) transmission electron microscope equipped with a LaB6 filament and operated at an acceleration voltage of 120 kV. Samples were prepared for TEM using a conventional negative staining procedure. A 3 μL drop of surfactant was adsorbed for 2 min onto a glow-discharged carbon-coated copper grid and stained for 1 min with freshly prepared 2% uranyl acetate in D2O. The TEM analysis of a 10 mM solution of 1 showed the formation of two populations of spherical micelles: one with 50 nm diameter and the other with 200 nm diameter (Figure 8a). After addition of Pd(OAc)2 to the micellar solution of 1 in the Suzuki–Miyaura coupling conditions, the formation of undefined aggregates was observed by TEM (Figure 8b). The micellar structure was probably lost due to the aggregation of several biguanides around a palladium center that resulted in the formation of nondefined domains and was responsible for the total lack of catalytic activity of the system.
Figure 8.
TEM analysis of (a) surfactant 1 at 10 mM in basic conditions (K2CO3 22 M) and (b) surfactant 1 at 10 mM in basic conditions (K2CO3 22 M)with 0.5 mol % Pd(OAc)2.
As the Pd/hexylbiguanide ratio (Table 3) seemed very important for the aggregation process, we decided to screen different ratios and study its influence on the catalytic activity of the system (Table 3). At a 1:2 Pd/hexylbiguanide ratio, higher yields were obtained, but the results were not reproducible (Table 3, entry 1). At a 1:1 ratio, complete conversion was obtained after 15 min with 0.5 mol % Pd(OAc)2 and 0.5 mol % 1 (1 mM), at 100 °C (Table 3, entry 2). The TEM analysis of the 1:1 Pd/hexylbiguanide ratio at 1 mM confirmed the formation of spherical micellar aggregates (Figure 9).
Table 3. Optimization of the Micellar Catalysis Conditions of the Suzuki–Miyaura Couplinga.
| entry | 1 (mol %) | Pd/1 ratio | reaction time (min) | yield (%) |
|---|---|---|---|---|
| 1 | 1 | 1:2 | 15 | 6–57 |
| 2 | 0.5 | 1:1 | 15 | 99 |
| 3 | 0.5 | 1:1 | 10 | 93 |
| 4 | 0.5 | 1:1 | 5 | 83 |
Reaction conditions: 0.5 mol % Pd(OAc)2 (1.12 mg), hexylbiguanide dihydrochloride 1, 1 mmol (0.199 g) 4′-bromoacetophenone, 1 mmol (0.122 g) phenylboronic acid, 1.1 mmol (0.152 g) K2CO3, in distilled water, at 100 °C, in air.
Figure 9.
TEM analysis of 0.5 mol % surfactant 1 at 1 mM with 0.5 mol % Pd(OAc)2 in basic conditions (K2CO3 22 M).
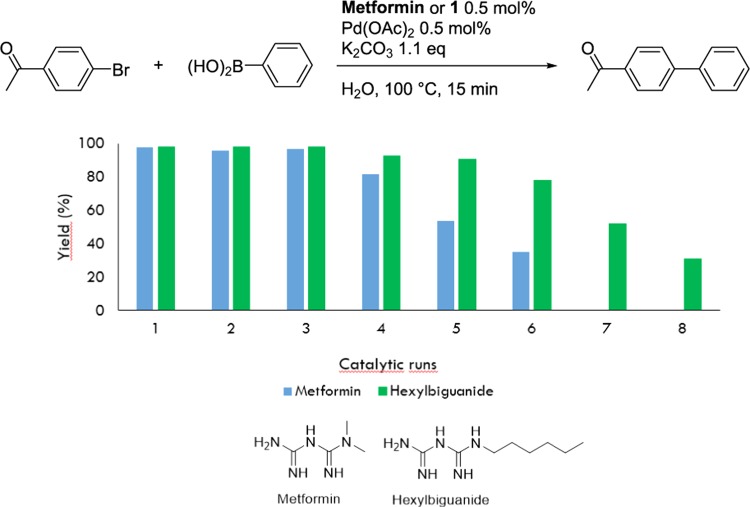
The substrate scope of the reaction performed with metformin as the catalyst showed limitations in the case of aryl iodides and electron-donating substituents on the aryl halides.21 We selected and tested these substrates in the micellar conditions with 0.5 mol % hexylbiguanide (1 mM) and 0.5 mol % Pd(OAc)2 during 15 min, at 100 °C (Table 4). Significantly higher yields were obtained for all of the substrates tested, especially for 4-iodoanisole and 4-iodoaniline (Table 4, entries 4 and 5) and 2-bromonaphtalene (Table 4, entry 6).
Table 4. Substrate Scope Comparison Using Metformin or Hexylbiguanide 1 as a Liganda.
Reaction conditions: 0.5 mol % Pd(OAc)2 (1.12 mg), 0.5 mol % metformin or 1, 1 mmol aryl halide, 1 mmol (0.122 g) phenylboronic acid, 1.1 mmol (0.152 g) K2CO3, in 5 mL distilled water, at 100 °C, in air.
At 1 mM.
The recyclability of the micellar system was studied under the same conditions and compared with that of the metformin catalyst (Figure 10). In the micellar system of hexylbiguanide 1, the palladium was kept active longer and the system was recycled up to six catalytic runs with no significant loss of activity. Therefore, the hydrophobic environment of the micelles not only allowed a better solubilization of the substrates and increased the reaction rates but also prevented palladium oxidation by water.
Figure 10.
Recyclability of the Suzuki–Miyaura reaction at 0.5 mol % Pd with metformin or hexylbiguanide 1 as a ligand.
Conclusions
We synthesized and fully characterized a new family of surfactants containing biguanide and biguanidium hydrophilic groups. All of the alkylbiguanidium chlorides in the solid state were double protonated and formed alternating layers of hydrophobic alkyl chains and hydrophilic biguanidium/chloride. Having determined the CMCs of each surfactant in neutral and basic conditions when it was possible, we performed the Suzuki–Miyaura reaction in micellar conditions in water, using 1 as the surfactant. We showed the importance of the Pd/hexylbiguanide ratio to obtain an efficient and recyclable catalytic system for the Suzuki–Miyaura reaction. Different aggregation states with and without Pd were observed by TEM, and we showed that a 1:1 Pd/hexylbiguanide ratio is necessary to maintain the micellar conditions and obtain a reproducible and efficient catalytic system.
Acknowledgments
We gratefully acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC), Green Chemistry Centre (CCVC), the Fonds Québécois de la Recherche sur la Nature et les Technologies (FRQ-NT), and the Université de Montréal for financial support. We also thank Le Centre Régional de RMN and Le Centre Régional de Spectrométrie de masse and X-ray diffraction facilities of Université de Montréal.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01962.
Synthetic procedures, characterization of the compounds, X-ray diffraction, and surface tension measurements (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Thorne D. P.; Lockwood T. D. Effects of insulin, biguanide antihyperglycaemic agents and beta-adrenergic agonists on pathways of myocardial proteolysis. Biochem. J. 1990, 266, 713–718. 10.1042/bj2660713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Helsby N. A.; Ward S. A.; Howells R. E.; Breckenridge A. M. In vitro metabolism of the biguanide antimalarials in human liver microsomes: evidence for a role of the mephenytoin hydroxylase (P450 MP) enzyme. Br. J. Clin. Pharmacol. 1990, 30, 287–291. 10.1111/j.1365-2125.1990.tb03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Patel D. S.; Ramesh M.; Bharatam P. V. CytochromeP450 isoenzyme specificity in the metabolism of anti-malarial biguanides: molecular docking and molecular dynamics analyses. Med. Chem. Res. 2012, 21, 4274–4289. 10.1007/s00044-011-9966-9. [DOI] [Google Scholar]
- James J. W.; Baker J. A.; Wiggins L. F. Synthesis of some heterocyclic derivatives of biguanide with antibacterial activity. J. Med. Chem. 1968, 11, 942–945. 10.1021/jm00311a006. [DOI] [PubMed] [Google Scholar]
- a Schaefer G.; Rieger E. Molecular basis for interaction of biguanides with the mitochondrial membrane. Eur. J. Biochem. 1974, 46, 613–623. [DOI] [PubMed] [Google Scholar]; b Schäfer G. Some new aspects on the interaction of hypoglycemia-producing biguanides with biological membranes. Biochem. Pharmacol. 1976, 25, 2015–2024. 10.1016/0006-2952(76)90424-X. [DOI] [PubMed] [Google Scholar]
- a Zhao Y.; Wang W.; Guo S.; Wang Y.; Miao L.; Xiong Y.; Huang L. PolyMetformin combines carrier and anticancer activities for in vivo siRNA delivery. Nat. Commun. 2016, 7, 11822 10.1038/ncomms11822. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cuyàs E.; Martin-Castillo B.; Bosch-Barrera J.; Menendez J. A. Metformin inhibits RANKL and sensitizes cancer stem cells to denosumab. Cell Cycle 2017, 16, 1022–1028. 10.1080/15384101.2017.1310353. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shafiei-Irannejad V.; Samadi N.; Salehi R.; Yousefi B.; Zarghami N. New insights into antidiabetic drugs: Possible applications in cancer treatment. Chem. Biol. Drug Des. 2017, 90, 1056–1066. 10.1111/cbdd.13013. [DOI] [PubMed] [Google Scholar]
- Langmaier J.; Pizl M.; Samec Z.; Zalis S. Extreme Basicity of Biguanide Drugs in Aqueous Solutions: Ion Transfer Voltammetry and DFT Calculations. J. Phys. Chem. A 2016, 120, 7344–50. 10.1021/acs.jpca.6b04786. [DOI] [PubMed] [Google Scholar]
- a Feng L.; Xie D.; Song B.; Zhang J.; Pei X.; Cui Z.. Aggregate evolution in aqueous solutions of a Gemini surfactant derived from dehydroabietic acid. Soft Matter 2018, 10.1039/C7SM02173A. [DOI] [PubMed] [Google Scholar]; b Mao J.; Zhang H.; Zhang W.; Fan J.; Zhang C.; Zhao J. Dissymmetric beauty: A novel design of heterogemini viscoelastic surfactant for the clean fracturing fluid. J. Ind. Eng. Chem. 2017, 10.1016/j.jiec.2017.10.048. [DOI] [Google Scholar]
- a Schulz E. N.; Ambrusi R. E.; Miraglia D. B.; Schulz E. P.; García S. G.; Rodriguez J. L.; Schulz P. C. Evaluation of oil-in-water emulsions with cationic-anionic surfactants mixtures for potential use in the oil industry. Colloids Surf., A 2016, 490, 145–154. 10.1016/j.colsurfa.2015.11.023. [DOI] [Google Scholar]; b Zaragoza-Contreras E. A.; Hernández-Escobar C. A.; Estrada-Monje A.; Kobayashi T. Synthesis of diphenylamine-co-aniline copolymers in emulsified systems using a reactive surfactant as the emulsifying agent and aniline monomer. Synth. Met. 2016, 214, 5–13. 10.1016/j.synthmet.2016.01.007. [DOI] [Google Scholar]
- Kaur P.; Garg T.; Rath G.; Murthy R. S.; Goyal A. K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 727–38. 10.3109/10717544.2014.935530. [DOI] [PubMed] [Google Scholar]
- Bochenek W. J.; Slowinska R.; Kapuscinska B.; Mrukowicz M. Inhibition of lipid absorption by non-ionic hydrophobic surfactant Pluronic L-81, and its benzoyl esters. J. Pharm. Pharmacol. 1984, 36, 834–836. 10.1111/j.2042-7158.1984.tb04887.x. [DOI] [PubMed] [Google Scholar]
- a Mishra M.; Muthuprasanna P.; Surya Prabha K.; Sobhita Rani P.; Satish Babu A.; Sarath Chandiran I.; Arunachalam G.; Shalini S. Basics and Potential Applications of Surfactants - A Review. Int. J. PharmTech Res. 2009, 1, 1354–1365. [Google Scholar]; b Brals J.; Smith J. D.; Ibrahim F.; Gallou F.; Handa S. Micelle-Enabled Palladium Catalysis for Convenient sp2-sp3 Coupling of Nitroalkanes with Aryl Bromides in Water Under Mild Conditions. ACS Catal. 2017, 7, 7245–7250. 10.1021/acscatal.7b02663. [DOI] [Google Scholar]; c Klumphu P.; Desfeux C.; Zhang Y.; Handa S.; Gallou F.; Lipshutz B. H. Micellar catalysis-enabled sustainable ppm Au-catalyzed reactions in water at room temperature. Chem. Sci. 2017, 8, 6354–6358. 10.1039/C7SC02405C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lipshutz B. H.; Abela A. R.; Bošković Ž. V.; Nishikata T.; Duplais C.; Krasovskiy A. “Greening Up” Cross-Coupling Chemistry. Top. Catal. 2010, 53, 985–990. 10.1007/s11244-010-9537-1. [DOI] [Google Scholar]; b Lipshutz B. H.; Ghorai S.; Leong W. W.; Taft B. R.; Krogstad D. V. Manipulating micellar environments for enhancing transition metal-catalyzed cross-couplings in water at room temperature. J. Org. Chem. 2011, 76, 5061–73. 10.1021/jo200746y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lipshutz B. H.; Isley N. A.; Fennewald J. C.; Slack E. D. On the way towards greener transition-metal-catalyzed processes as quantified by E factors. Angew. Chem., Int. Ed. 2013, 52, 10952–8. 10.1002/anie.201302020. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Xu D.; Wang H.; Pan Z.; Zhang T. The kinetics and effect of a new gemini surfactant on the efficiency of micellar catalysis for the hydrolysis reaction of 4-nitrophenyl acetate. J. Mol. Liq. 2018, 250, 223–228. 10.1016/j.molliq.2017.12.008. [DOI] [Google Scholar]; e Donner A.; Hagedorn K.; Mattes L.; Drechsler M.; Polarz S. Hybrid Surfactants with N-Heterocyclic Carbene Heads as a Multifunctional Platform for Interfacial Catalysis. Chemistry 2017, 23, 18129–18133. 10.1002/chem.201703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kairouz V.; Schmitzer A. R. Imidazolium-functionalized β-cyclodextrin as a highly recyclable multifunctional ligand in water. Green Chem. 2014, 16, 3117–3124. 10.1039/C4GC00365A. [DOI] [Google Scholar]; b Ghosh K. K.; Gupta B.; Bhattacharya S. Metallosurfactant Aggregates as Catalysts for the Hydrolytic Cleavage of Carboxylate and Phosphate Esters. Curr. Organocatal. 2016, 3, 6–23. 10.2174/2213337202666150713174927. [DOI] [Google Scholar]; c Liu Y.; Ma X.; Xie J.; Liu P.; Dai B.; He R. Metallomicelles of palladium(II) complexes as efficient catalysts for the Suzuki-Miyaura reaction in neat water. Appl. Organomet. Chem. 2013, 27, 494–498. 10.1002/aoc.3021. [DOI] [Google Scholar]
- a Song Y.; Li Q.; Li Y. Self-aggregation and antimicrobial activity of alkylguanidium salts. Colloids Surf., A 2012, 393, 11–16. 10.1016/j.colsurfa.2011.10.015. [DOI] [Google Scholar]; b Song Y.; Li Q.; Li Y.; Zhi L. Surface and aggregation properties of heterogemini surfactants containing quaternary ammonium and guanidine moiety. Colloids Surf., A 2013, 417, 236–242. 10.1016/j.colsurfa.2012.11.004. [DOI] [Google Scholar]
- Li S.; Lin L.; Li Y.; Zhang S. Enhancing Activity of Suzuki Reactions in Water by Using Guanidinium Ionic Liquid Stabilized Palladium Micelle Catalyst. Synlett 2011, 2011, 1779–1783. 10.1055/s-0030-1260810. [DOI] [Google Scholar]
- Ray P. Complex Compounds of Biguanides and Guanylureas with Metallic Elements. Chem. Rev. 1961, 61, 313–359. 10.1021/cr60212a001. [DOI] [Google Scholar]
- Veisi H.; Kordestani D.; Faraji A. R. Palladium nanoparticles supported on an organosuperbase denderon-modified mesoporous SBA-15 as a heterogeneous catalyst in Heck coupling reaction. J. Porous Mater. 2013, 21, 141–148. 10.1007/s10934-013-9758-3. [DOI] [Google Scholar]
- Veisi H.; Khazaei A.; Safaei M.; Kordestani D. Synthesis of biguanide-functionalized single-walled carbon nanotubes (SWCNTs) hybrid materials to immobilized palladium as new recyclable heterogeneous nanocatalyst for Suzuki-Miyaura coupling reaction. J. Mol. Catal. A: Chem. 2014, 382, 106–113. 10.1016/j.molcata.2013.10.028. [DOI] [Google Scholar]
- Veisi H.; Masti R.; Kordestani D.; Safaei M.; Sahin O. Functionalization of fullerene (C60) with metformine to immobilized palladium as a novel heterogeneous and reusable nanocatalyst in the Suzuki-Miyaura coupling reaction at room temperature. J. Mol. Catal. A: Chem. 2014, 385, 61–67. 10.1016/j.molcata.2014.01.007. [DOI] [Google Scholar]
- Veisi H.; Ghadermazi M.; Naderi A. Biguanidine-functionalized chitosan to immobilize palladium nanoparticles as a novel, efficient and recyclable heterogeneous nanocatalyst for Suzuki-Miyaura coupling reactions. Appl. Organomet. Chem. 2016, 30, 341–345. 10.1002/aoc.3437. [DOI] [Google Scholar]
- Fortun S.; Beauclair P.; Schmitzer A. R. Metformin as a versatile ligand for recyclable palladium-catalyzed cross-coupling reactions in neat water. RSC Adv. 2017, 7, 21036–21044. 10.1039/C7RA01197K. [DOI] [Google Scholar]
- Suyama T.; Soga T.; Miyauchi K. A Method for the Preparation of Substituted Biguanides. Nippon Kagaku Kaishi 1989, 5, 884–887. 10.1246/nikkashi.1989.884. [DOI] [Google Scholar]
- Raczyńska E. D.; Gal J. F.; Maria P. C.; Michalec P.; Zalewski M. Exceptionally High Proton and Lithium Cation Gas-Phase Basicity of the Anti-Diabetic Drug Metformin. J. Phys. Chem. A 2017, 121, 8706–8718. 10.1021/acs.jpca.7b09338. [DOI] [PubMed] [Google Scholar]
- a Jalový Z.; Padělková Z.; Jirásko R.; Matyáš R.; Holčapek M.; Němec O.; Novotná M.; Mišková L. Syntheses, crystal structures and properties of copper(II) complexes of 1-amidinoisourea and biguanide nitrates. Polyhedron 2012, 44, 88–100. 10.1016/j.poly.2012.06.029. [DOI] [Google Scholar]; b Das B. C.; Dey I.; Biswas G.; Banerjee R.; Iitaka Y.; Banerjee A. Crystal and molecular structure of a new biguanide metal complex, [Cu(AMNH)2]Cl2. J. Crystallogr. Spectrosc. Res. 1993, 23, 509–512. 10.1007/BF01182528. [DOI] [Google Scholar]
- Chen L.-J.; Lin S.-Y.; Huang C.-C.; Chen E.-M. Temperature dependence of critical micelle concentration of polyoxyethylenated non-ionic surfactants. Colloids Surf., A 1998, 135, 175–181. 10.1016/S0927-7757(97)00238-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.