Abstract
Objective:
By discussing the design, findings, strengths, and weaknesses of available studies investigating the influence of angular insertion depth on speech perception, we intend to summarize the current status of evidence; and using evidence based conclusions, possibly contribute to the determination of the optimal cochlear implant (CI) electrode position.
Data Sources:
Our search strategy yielded 10,877 papers. PubMed, Ovid EMBASE, Web of Science, and the Cochrane Library were searched up to June 1, 2018. Both keywords and free-text terms, related to patient population, predictive factor, and outcome measurements were used. There were no restrictions in languages or year of publication.
Study Selection:
Seven articles were included in this systematic review. Articles eligible for inclusion: (a) investigated cochlear implantation of any CI system in adults with post-lingual onset of deafness and normal cochlear anatomy; (b) investigated the relationship between angular insertion depth and speech perception; (c) measured angular insertion depth on imaging; and (d) measured speech perception at, or beyond 1-year post-activation.
Data Extraction and Synthesis:
In included studies; quality was judged low-to-moderate and risk of bias, evaluated using a Quality-in-Prognostic-Studies-tool (QUIPS), was high. Included studies were too heterogeneous to perform meta-analyses, therefore, effect estimates of the individual studies are presented. Six out of seven included studies found no effect of angular insertion depth on speech perception.
Conclusion:
All included studies are characterized by methodological flaws, and therefore, evidence-based conclusions regarding the influence of angular insertion depth cannot be drawn to date.
Keywords: Angular insertion depth, Cochlear implants, Insertion depth, Speech perception, Systematic review
INTRODUCTION
Rationale
At present, a large variability and unpredictability in hearing performance is seen in individuals following cochlear implantation. In addition to different biological and audiological factors—e.g., age at implantation, residual hearing, and duration of hearing loss—position of the cochlear implant (CI) electrode array inside the cochlea is thought to contribute to variation in postoperative speech perception. The three seemingly most important electrode positional factors are; electrode scalar location, electrode-to-modiolus proximity, and electrode insertion depth.
The suggested influence of electrode positional factors is used by manufactures for design and marketing of their CI electrodes. However, controversy exists regarding whether the impact of various electrode position factors; in particular on electrode insertion depth. The range of CI electrode array lengths, that are currently in use by different manufactures, are: 15 to 31.5 mm. In theory, deep insertion of a CI electrode array into the apical region of the cochlea could enhance frequency alignment (1) and might give better experience of low-pitched sounds by stimulating the complete spiral ganglion covering deeper located areas (2). Yet, other theories suggest that deep electrode insertion: a) causes apical frequency pitch confusion (3), b) has a higher risk of trauma to cochlear structures possibly causing loss of residual hearing (4,5), and c) might reduce stimulation of the basal turn; due to potentially overly deep inserted electrodes (6).
Measurements of electrode insertion depth have been described in terms of linear distance in millimetres or insertion angle in degrees. In 2010, Verbist et al. (7) introduced an objective cochlear coordinate system to generate comparable measurements of cochlea dimensions and CI electrode positional measurements. An international panel of CI researchers and representatives of different manufacturers agreed that angular insertion depth, compared with measurement in millimetres, made allowance for variety in individual cochlear dimensions and intra-cochlear trajectories of the CI electrode. They recommended using a cylindrical coordinate system, which defined measurements of rotational insertion angle of a selected point along the trajectory of the CI electrode, such as different CI electrode contacts.
Concerning the influence of insertion depth, a variety of sometimes contradictory correlations are found in literature. In last decade, studies have reported findings of a positive (8,9), negative (6), or no demonstrated relationship (10–13) between insertion depth and speech perception with CI. There is, however, need for evidence-based conclusions on the influence of insertion depth.
OBJECTIVE
In this systematic review, we have systematically summarized available evidence on the influence of angular insertion depth on speech perception in CI patients. By discussing design, findings, strengths, and weaknesses of available studies, we intend to assess the status of current evidence for the influence of angular insertion depth on speech perception, which might contribute to determination of optimal CI electrode position.
METHODS
Protocol Registration
The review protocol can be accessed at the website of PROSPERO, the International Prospective Register of Systematic Reviews (www.crd.york.ac.uk/PROSPERO). The protocol was registered under the number CRD42018099186 on July 02, 2018.
ELIGIBILITY CRITERIA
Participants
Studies in adults with post-lingual onset of deafness, normal cochlear anatomy on preoperative imaging, and implanted with any type of CI system were considered eligible for inclusion in this systematic review.
Predictive Factor (PF)
Included studies had to investigate angular insertion depth measured on postoperative CT-scan, using the measurement method as advised by the Consensus Panel in Verbist et al. (7) in 2010, or one of the measurement methods on x-ray described as comparable. Studies measuring insertion depth in millimetres were excluded. Figure 1 shows an example of angular insertion depth measurement according the advised method of the Consensus Panel (7).
FIG. 1.
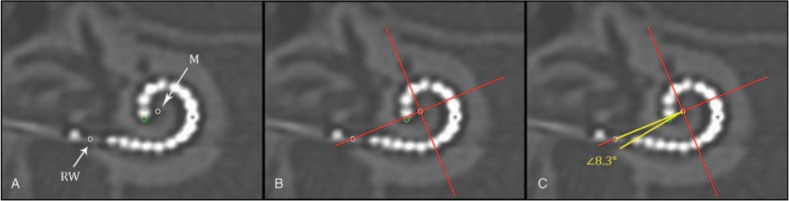
Method for angular insertion depth measurement on Computed Tomography (CT)-scan of an implanted electrode array with 16 electrode contacts. With in a three-dimensional cylindrical coordinate system all spatial information of the cochlea and an implant is measurable. By consensus this cochlear framework is defined by a plane of rotation through the basal turn of the cochlea and a z-axis through the modiolus. This can be applied on CT of the temporal bone by making a multiplanar reconstruction along the basal turn of the cochlea (A–C), and placing the z-axis through the center of the cochlea; the modiolus (M). A, An angular measurement of the insertion depth can then be made by indicating the center of the round window (RW) and the tip of the electrode array (dark grey circle). B, A 0 degree reference line between the modiolus (M) and the middle of the round window (RW), and a perpendicular line from the modiolus on the 0 degree reference line is drawn (cross). C, An angle is drawn (in white) from the modiolus over the 0 degree reference line, and through the most apical point the tip of the electrode array (dark grey circle). In this example the angular insertion depth of the most apical electrode contact is 368.3 degrees; the sum of four quadrants equal to 360 degrees plus the measured white angle equal to 8.3 degrees.
Outcome Measurement
There were no restrictions on type of speech perception test, setting of testing (quiet or in-noise) or loudness of stimulus. In the first year after implantation, speech perception is rising (10,14). Yet, after approximately 1 year, most CI recipients (about 90%) have reached a stable speech perception (10,13). Therefore, studies analyzing participants with speech perception measurements within first 12 months post-implantation were excluded.
Other Eligibility Criteria
Papers written in any language were eligible for inclusion. There were no restrictions in year of publication.
DATA SOURCES AND SEARCH STRATEGY
Assisted by a trained librarian, we systematically searched PubMed, Ovid EMBASE, Web of Science, and Cochrane Library up to June 01, 2018 for studies investigating influence of angular insertion depth on speech perception in adults with CI. Terms, and their synonyms, related to patient population, predictive factor, and outcome measurements were combined in the search strategy. Both keywords (MESH and Emtree) and free-text terms in title and abstract were used. Supplement I contains the full electronic search strategy in PubMed. Additionally, articles’ reference lists were scanned for any applicable studies.
Study Selection
Results of the search strategy were merged and duplicates were removed using EndNote reference management software (version X7, Thomas Reuters, New York City, NY). Two review authors (F.H. and S.dR.) individually screened titles and abstracts to identify relevant reports based on eligibility criteria outlined above. Full text versions of these potentially relevant studies were retrieved and independently assessed for eligibility by 2 review authors (F.H. and S.dR.). Any disagreements were resolved by discussion with the third reviewer (W.H.).
Data Extraction
Data were extracted using a predefined form that included: study design, participant details (total number of implantations, etiology of hearing loss, age at implantation, sex, history of hearing loss, and preoperative hearing ability), CI system, type of electrodes, type and details of surgical approach used, imaging details (type of imaging, timing of imaging, method used for measurement of angular insertion depth, and measurement of other electrode positional factors), speech perception measurement details (mean speech perception score, type of speech perception test, loudness of stimuli used, and timing of speech perception measurement), data on measured angular insertion depth(s) and speech perception outcome(s), correlation between angular insertion depth and outcome, and authors’ conclusions. Corresponding authors of included papers were contacted if relevant data were missing with the request to provide this information.
RISK OF BIAS ASSESSMENT
Risk of bias was independently assessed by two review authors (F.H. and S.dR.). Included studies were assessed using the Quality-in-Prognostic-Studies (QUIPS) tool (15). This tool contains six items judging risk of bias due to patient selection, attrition, measurement of prognostic factors, outcome measurement, confounding on statistical analysis, and confounding on reporting. Each of the six items in included studies was judged as low, moderate, or high risk. Confounding factors that were considered important because they possibly influence angular insertion depth, or relation of angular insertion depth on speech perception, were: age at implantation, history of hearing loss, preoperative speech perception score, preoperative residual hearing, electrode type(s), electrode scalar location, and electrode—to—modiolus proximity. Results of risk of bias assessment were graphically summarized using ReviewManager 5 (RevMan5) software (version 5.3.5, Cochrane Collaboration, London, England).
Data Synthesis
Details of included studies were structured, and an overview of effect sizes was created for the influence of angular insertion depth on speech perception. Ultimately, the studies included in our systematic review were too heterogeneous to perform the planned meta-analyses, as seen in Table 1. For this reason, effect estimates reported in individual studies are presented.
TABLE 1.
Characteristics of included studies
| Angular Insertion Depth Details | Outcome Measurements | Confounders | |||||||
| First Author Year | Number of Implants, Design, Surgical Approach | Electrodes Implanted | Mean (SD) | Range | Type of Test | Mean (SD) | Range | Collected | Included in Analysis |
| De Seta 2016 | 26 Prospective NRa | M1 | 643 (93) | 510–880 | Fournier word test In quiet @ 70 dB In noise @ SNR +5, SNR +10, SNR+15 | 64 (6) | NRa | Age at implantation, history of hearing loss, hearing aid use, cochlear diameter, cochlear height, electrode to modiolus distance @ 180 and 360 degrees | – |
| Hilly 2016 | 120 Retrospective C | AB1 | 382 (98) | 180–720 | HINT sentence test In quiet @ 60 dB | 76 (26) | NRa | Age at implantation, preoperative outcome score, preoperative residual hearing, number of intracochlear contacts | – |
| Holden 2013 | 114 Prospective C | AB1, AB2, AB3, C2, C3 | NRa | NRa | CNC word test In quiet @ 60 dB | 62 (21) | 3–89 | Age at implantation, educational level, cognitive measures, history of hearing loss, hearing aid use, lip-reading ability, preoperative outcome score, preoperative residual hearing, electrode type, scalar location, basal angular insertion depth, electrode to modiolus proximity | – |
| Marrinan 2004 | 28 Retrospective NRa | C2 | NRa | NRa | CNC word test In quiet @ 70 dB CUNY sentence test In quiet @ 70 dB In noise @ SNR +10 | NRa | NRa | Age at implantation, history of hearing loss, preoperative outcome score | – |
| O’Connell 2016 | 137 107 Retrospective RW 39% ERW 34% C 37% | M1, M2, M3, M4, C1, C3, AB1, AB4 | 420 (99) | 208–715 | CNC word test In quiet @ 60 dB AzBio sentence test In quiet @ 60 dB | 47 (23) 57 (28) | Age at implantation, category of electrode type, surgical approach, cochlear volume, Scalar location | Age at implantation, category of electrode type, surgical approach, cochlear volume, Scalar location | |
| Van der Beek 2005 | 15 Prospective C | AB2 | 439 (73) | 105–559 | CVC word test In quiet @ 65 and 75 dB | NRa | NRa | Age at implantation, Duration of deafness, Preoperative outcome score, Preoperative residual hearing, Electrode to modiolus proximity | - |
| Van der Marel 2015 | 162 Retrospective ERW | AB1, AB2 | 477 (70) | 303–678 | CVC word test In quiet average @ 65 + 75dB | Word score 54 (22) Phoneme score 74 (19) | 0–93 0–97 | Age at implantation, history of hearing loss, preoperative outcome score, electrode to modiolus proximity, linear insertion depth in millimeters | Duration of deafness Preoperative phoneme score Preoperative word score |
aNR, not reported in paper and missing information not able to retrieve after contact with author.
RESULTS
Study Selection
A flow diagram of study selection is shown in Figure 2, derived from The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Group (16). After screening, based on title and abstract, full-texts of 63 studies were reviewed and 55 articles were excluded (1,3,6,9,11,12,14,17–64). Reasons for exclusion are listed in Figure 2. Eight papers (8,10,13,65–69) seemed eligible for inclusion.
FIG. 2.
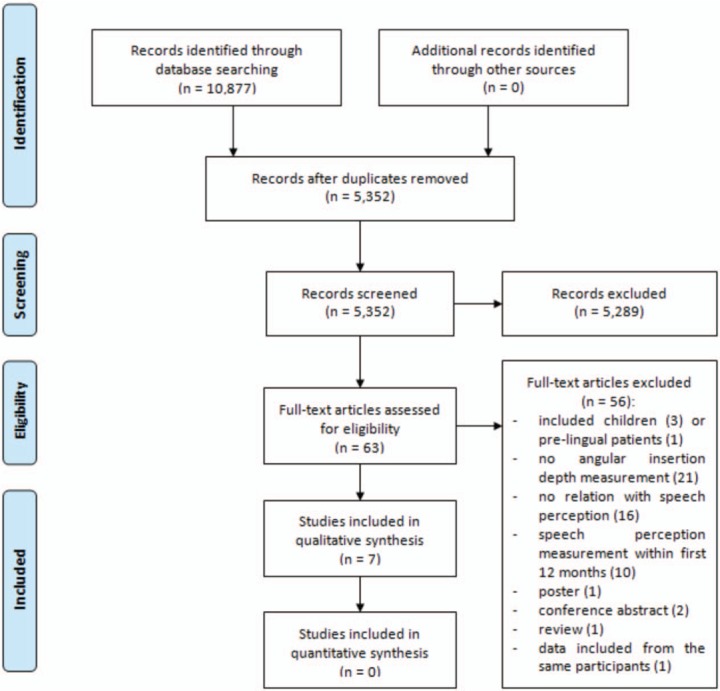
Flow diagram of the study selection.
After contact with corresponding authors, it was found the eligible study of van der Marel et al. (13) with 162 participants included a part of the 45 participants of the study in 2005 of van der Beek et al. (68), and included all 130 participants of the study of van der Beek et al. (69) in 2016. In this systematic review only unique, eligible participants (n = 15) of the study by van der Beek et al. (68) in 2005 were included. The study of van der Beek et al. (69) in 2016 was excluded. In total, seven papers (8,10,13,65–68) were included in this systematic review.
Study Characteristics
Table 1 shows characteristics of the seven included studies.
None of the included studies had a randomized design. Four studies (8,13,66,67) reviewed a retrospective acquired database and three (10,65,68) combined retrospective and prospectively collected data. Number of cochlear implantations in included studies varied between 15 and 220 implantation. Eleven different types of electrodes were implanted and 10 different speech perception tests were used. Measurement of and correction for confounding factors varied widely between studies.
Risk of Bias
QUIPS—Risk of Bias Assessment
Risk of bias assessment, using the QUIPS tool, is summarized in Figures 3 and 4. Three included studies (8,10,13) had noticeable lower risk on bias compared with the other four studies (65–68).
FIG. 3.
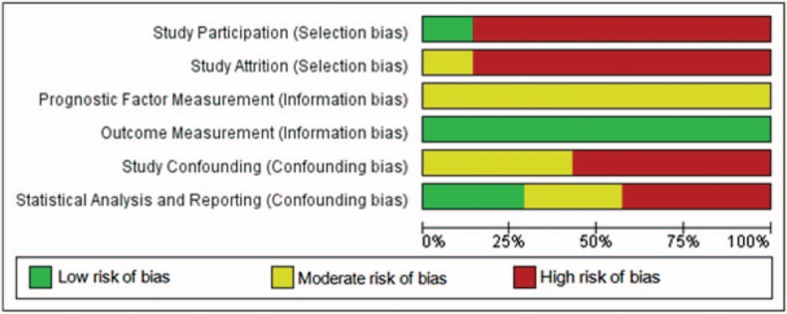
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
FIG. 4.
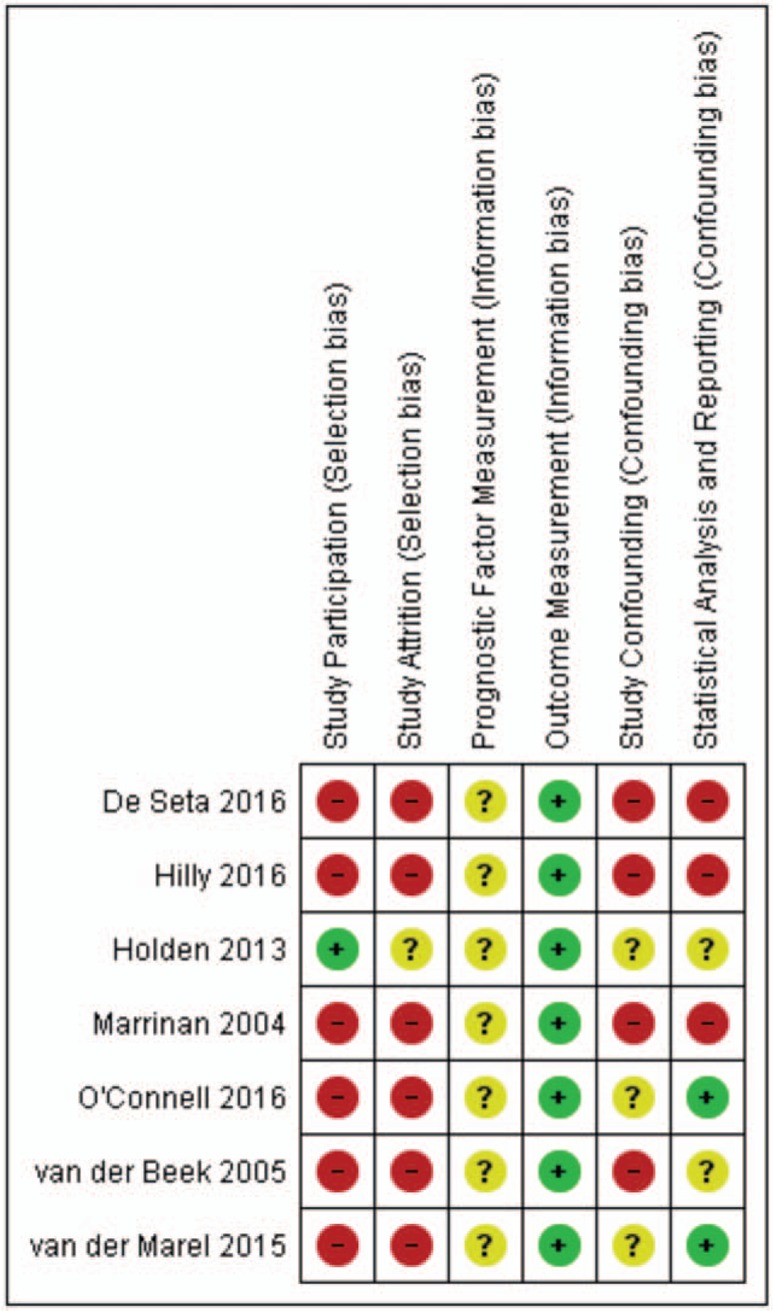
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Selection Bias
Overall risk on selection bias was high in most included studies. In six studies (8,10,13,65–67), eligible participants were excluded because of missing information, which mostly concerned imaging data. Additionally, two studies (8,10) included participants with different CI systems without stating criteria for allocation.
Information Bias
Overall risk on information bias was low in included studies. Even though two studies (66,67) used x-ray, and five studies (8,10,13,65,68) used CT-scan; all seven included studies measured angular insertion depth using one of the comparable methods advised by Verbist et al. (7). However, in all studies a time interval between imaging and speech perception measurement was present or at risk. When a time interval between imaging and outcome measurement is present, a non-negligible possibility exists of intra-cochlear changes to the electrode array position during this interval, e.g., extruding of electrode array outside the cochlea (70). In all studies, speech perception was measured at 12 months postoperatively. In four papers timing of imaging was provided: intraoperatively (67), first weeks postoperatively (13,68), or 5 years postoperatively (65). In three papers (8,10,66) timing of imaging was reported as “postoperative,” without reporting further details.
Confounding Bias
Overall risk on confounding bias was moderate to high in all included studies. Only one study (10) measured all confounding factors that we indicated as important. However, this study correlated angular insertion depth with speech perception without correction for these confounding factors. Four other studies (65–68) investigated the relationship between angular insertion depth and speech perception in univariate analysis, without reporting on possible confounding factors. Two studies (8,13) did include measured confounding factors in analysis, but measured confounders were incomplete.
The most important confounding factor, might be type(s) of electrode array implanted. In Table 1 types of electrodes implanted per study is shown. Table 2 shows an overview of characteristics and technical differences between different types of CI electrodes. Direct comparison of different electrode types can lead to bias in the following ways; firstly, different electrode designs might yield different results in speech perception in more ways than just angular insertion depth. For example, electrode types vary with respect to a) electrode-to-modiolus proximity, b) number of active contacts, c) spatial distance between active contacts along the array, d) length of the array in millimetres, or e) size of electrode contacts. Secondly, inserting a specific electrode too shallow or overly deep, compared with the design goals and prescription of manufacturers, might suggest a correlation between angular insertion depth and speech perception, while actually these studies might have found a difference in speech perception for surgical correctly placed electrode arrays when compared with surgically too shallow or overly-deep electrode placement. The surgical variation in depth of implantation should therefore be described in all studies on this topic. Degree of surgical insertion depth, or marker until were the electrode(s) in study participants were inserted, was not reported in five studies (8,65–68), therefore potential bias due to surgical depth of insertion in these studies was unclear. Two out of seven included studies (10,13) did address the possibility of shallow or deep inserted electrodes in more detail, as these studies measured angular insertion depth of the basal electrode contact and used this as a reference for degree of surgical insertion depth.
TABLE 2.
Characteristics of different electrodes implanted in included studies
| Brand | Name | Abbreviation Code in This Study | Type of Electrodea | Total Length in Millimetres | Active Length/Number of Active Electrodes | Spatial Distance Between Electrodes | Basal Diameter | Tip Diameter |
| Med-El | Standard | M1 | S | 31.5 | 26.4/12 | 2.4 | 1.2 | 0.5 |
| Flex 28 | M2 | S | 28 | 23.1/12 | 2.1 | 0.8 | 0.48–0.36 | |
| Flex 24 | M3 | S | 24 | 20.9/12 | 1.9 | 0.8 | 0.48–0.36 | |
| Medium | M4 | S | 24 | 20.9/12 | 1.9 | 0.8 | 0.38–0.36 | |
| Cochlear | Slim straight | C1 | S | 25 | 20/22 | 0.95 | 0.6 | 0.3 |
| Contour | C2 | MH | 18 | 15/22 | 0.71 | 0.8 | 0.5 | |
| Contour advanced | C3 | MH | 18 | 15/22 | 0.71 | 0.8 | 0.5 | |
| Advanced Bionics | HiFocus 1 | AB1 | S | 20 | 17/16 | 1.13 | 0.8 | 0.4–0.6 |
| HiFocus 1J | AB2 | S | 20 | 17/16 | 1.13 | 0.4–0.6 | ||
| Helix | AB3 | MH | 18.5 | 13.25/16 | 0.85 | 1.1 | 0.6 | |
| Mid-Scala | AB4 | MH | 18.5 | 15/16 | 1.0 | 0.7 | 0.5 |
aMH, modiolus hugging; precurved electrode; S, straight electrode.
Study Results
A summary of reported effect sizes is presented in Table 3.
TABLE 3.
Effect size(s) in included studies
| First Author, Year | Number of Implants | Type(s) of Analysis | Effect Size | Authors’ Conclusion |
| De Seta 2016 | 26 | Pearson's correlation | NR | No correlation |
| Hilly 2016 | 120 | Spearman's correlation | R = 0.16, p = 0.09 | No correlation |
| Holden 2013 | 114 | Spearman's correlation | NR | No correlation |
| Marrinan 2004 | 28 | Linear regression | NR | No correlation |
| O’Connell 2016 | 137 | Pearson's correlation | CNC: r = 0.23, p = 0.006 | Significant positive correlation between angular insertion depth and CNC word scores. |
| 107 | AzBio: NR | No correlation | ||
| 137 | Multivariate linear regression | CNC: coefficient 0.0006, 95% CI 0.0002–0.001, p = 0.009 | CNC word score increases 0.6% with every 10 degrees increase in angular insertion depth. | |
| Van der Beek 2005 | 45 | Pearson's correlation | R = 0.01, p > 0.8 | No correlation |
| Van der Marel 2015 | 162 | Multivariate partial correlation | R = 0.03, p = 0.69 | No correlation |
Speech Perception in Quiet
Speech perception in quiet is reported in all included studies. One out of seven studies (8) found a significant relationship between angular insertion depth and speech perception in quiet, and six studies (10,13,65–68) reported no correlation. Out of three studies (8,10,13) with noticeable lower risk of bias, Holden et al. (10) and van de Marel et al. (13), found no correlation between angular insertion depth and speech perception in quiet, while O’Connell et al. (8) did find a significant relationship. These studies are discussed in more detail in following paragraphs.
Holden et al. (10), found no correlation between angle of apical electrode insertion depth and speech perception in quiet. This study was the only included study measuring all confounding factors that were indicated as important in this systematic review. However, no multivariate analysis was performed. Interestingly, angular insertion depth of most basal electrode contact, and length of the electrode array measured in millimetres were found to significantly negatively correlate with speech perception outcome. Authors divided their participants in six outcome groups based on percentile ranking of participants CNC final score, and calculated mean values of independent variables of interest in these outcome groups. The effect size of linear relationship across the outcome groups mean angular insertion depth of most basal electrode contact was –2.42 (p ≤ 0.05) and of the groups mean array trajectory length was –2.10 (p ≤ 0.05). The negative relationship of these two variables suggest that electrodes of participants with deepest insertion in this study were probably inserted overly deep in comparison with design goals of included electrodes. In theory, this could decrease performance for two reasons (6). First, if such overly deep insertion occurs, the first part of the basal cochlea might be bypassed by the electrode, which otherwise would have been stimulated. Secondly, when the tip of the electrode array approaches the apex this might cause trauma which may reduce residual hearing. Furthermore, the tip might translocate to scala vestibuli, or the tip might fold inside scala tympani which might cause a decrease in apical stimulation (6). Authors concluded measurement of angular basal electrode contact, could be used to judge surgically too shallow or overly-deep surgical insertions (10).
Van de Marel et al. (13) found no correlation between angular insertion depth and postoperative CVC word scores, while correcting for age at implantation, duration of deafness, preoperative phoneme score, and preoperative word score (p = 0.89). In their analysis, van de Marel et al. did not correct for electrode scalar location and electrode-to-modiolus proximity. All participants were implanted with the same type of electrode (HiFocus I/IJ) and with the same surgical technique (extended round window approach). This homogeneity in implantation characteristics prevented bias of results caused by differences in CI systems and by differences in electrode designs which is a strength of this study. On the other hand, conclusions of this study only apply to this specific combination of electrode type and surgical technique.
O’Connell et al. (8) reported 0.6% increase of CNC word score for every 10 degrees increase in angular insertion depth (coefficient 0.0006, p = 0.03), while corrected for age at implantation, category of electrode type (lateral wall, perimodiolar, or mid-scalar electrode), surgical technique, cochlear volume, and scalar location. O’Connell et al. (8) did not measure possible confounding audiologic factors or electrode-to-modiolus proximity. Besides, the study of O’Connel et al. (8) included eight different electrode types, and despite grouping these electrodes into three categories, electrode types within these groups remained to differ significantly, as shown in Table 2. Thus, influence of array length and width, number of active electrodes, space between electrode contacts, and differences in fitting programs is unclear. Additionally, authors did not account for possible influence of shallow and/or deep inserted electrode arrays. Furthermore, O’Connell et al. (8) found no correlation between angular insertion depth and AzBio-sentence test scores in quiet.
Speech Perception in Noise
Speech perception in noise is reported in two studies (65,67). No correlation between angular insertion depth and CUNY-sentences and Fournier-word test in noise was found in these studies.
DISCUSSION
Summary of Evidence
This systematic review includes seven studies investigating the influence of angular insertion depth on speech perception, 1 year, or more after CI surgery in adults with post-lingual onset of deafness. Included studies demonstrate substantial heterogeneity in study design, electrodes implanted, speech perception test characteristics and confounding factors measured and accounted for in analysis. Risk of bias was judged high in all studies. Therefore, we did not perform a meta-analysis, but present effect size(s) reported in individual studies. Most studies found no relationship between angular insertion depth and speech perception test score in quiet. In all included studies correction for possible confounding factors was poor. None of the included studies found a relationship between angular insertion depth and speech perception in noise.
Contemplation of Evidence in Light of Non-included Literature
The objective of present systematic review was to investigate the influence of angular insertion depth on speech perception performance. Since the increase in performance during rehabilitation is beyond the scope of this study, we only included studies describing analysis on stable speech perception scores. The exclusion criterion was therefore set at follow-up of less than 12 months. The decision to use the 12 months follow-up criterion is based on data of Holden et al. (10) who showed that most CI recipients reach stable speech perception performance after 1 year CI experience. Ten studies that were excluded from this review (6,9,11,14,44,53,56,59,61,64) investigated speech perception within the first year. Four out of 10 studies (40%), reported a significant positive correlation between angular insertion depth and speech perception, compared with one out of seven of the included studies (14%). Buchman et al. (14) randomly assigned 13 participants to receive either the standard electrode array (31.5 mm; mean angular insertion depth 657 degrees; SD 82 degrees), or the medium electrode array (24 mm; mean angular insertion depth 423 degrees; SD 23 degrees). A significant higher mean speech perception score, increasing over time in the first year was found in the standard electrode array group compared with the medium electrode array group. Comparing the percentage of studies showing a positive correlation of angular insertion depth with speech perception measured within the first year (four out of 10; 40%) to the studies included in the present systematic review investigating speech perception at or beyond the first year (one out of seven; 14%) suggests that deeper insertion might only make a difference in the period shortly after activation.
This hypothesis is supported by a recent study conducted by Buchner et al. (71), who compared three electrodes with different lengths (FLEX-series, Med-El Corp., Inssbruck, Austria). At 3-month post-activation, significant higher scores were found for FLEX28 electrode group for three measured speech perception tests when compared with FLEX20 group, and for two out of three tests when compared with FLEX24 group. However, these significant findings diminished at 6-month post-activation. It can be hypothesised that long electrodes used in this study give a better match to natural frequency placement after 3 months, but at 6 months brain plasticity copes with the mismatch for shorter electrodes, and early effect of electrode length diminishes. Further exploration of this theory goes beyond the scope of this review but should be addressed in future research.
Strengths and Limitations
This is the first systematic evaluation of evidence on the topic of influence of electrode insertion depth, measured in angular insertion depth, on speech perception performance beyond 1 year after CI surgery in adults with post-lingual onset of deafness. Considering the possibility to influence CI electrode position within the cochlea, potentially through surgical technique and more easily by electrode design, determination of influence of electrode position on performance is of high relevance to healthcare providers and patients. We conducted this systematic review with strict allegiance to our registered research protocol and followed PRISMA guidelines of reporting (16).
Several limitations are present in our systematic review. Most importantly, included individual studies were low to moderate quality, mainly due to risk of selection and confounding bias. Study designs were mostly retrospective, participants were excluded due to missing data, important confounding factors were not taken into account and reporting of data on angular insertion depth and outcome measurement was incomplete. Between study comparison was limited, due to 10 different outcome measurement tests being used, 11 different electrode types investigated, and large variation in number and definition of measured confounding factors.
Investigating influence of angular insertion depth, on speech perception in non-randomized, observational research is difficult because 1) differences in angular insertion depth are mostly due to differences in lengths in millimetres of used electrodes, and not due to surgical variation in insertion depth or anatomical variation in the cochlea of participants, and 2) comparing electrodes of different manufactures is automatically accompanied with other differences between electrodes then angular insertion depth, such as factors mentioned in this systematic review and shown in Table 2. These difficulties stress the need for randomized designs in future studies addressing insertion depth.
CLINICAL/FUTURE IMPLICATIONS
Identifying factors that may influence variability in CI outcome, which could be influenced by patient, surgeon, or manufactures, could potentially improve future speech discrimination capability after cochlear implantation. However, all studies investigating optimal insertion depth of CI electrode array are characterized by methodological flaws, and evidence-based conclusions regarding influence of angular insertion cannot be drawn to date. To fully assess influence of angular insertion depth on speech perception, a randomized trial with multiple identical electrodes of different lengths is preferred. Learning and developmental effects due to brain plasticity should be taken into account, and therefore it is recommended to measure speech perception outcomes beyond 12 months after implantation. Alternatively, prospective cohort studies addressing this topic should conduct analysis including important confounding audiologic, biographic, and electrode positional factors.
CONCLUSIONS
Although angular insertion depth is a much debated topic over the past decade in cochlear implantation research, the current body of evidence does not support firm conclusions on the effect of insertion depth on speech perception at 1 year or more after CI surgery.
Supplementary Material
Footnotes
Sources of support that require acknowledgment: For the preparation of this manuscript no sponsoring was obtained. Our institute; the department of Otorhinolaryngology–Head & Neck Surgery of the Radboudumc in the Netherlands, received an ongoing institutional grand from; Cochlear Ltd. (Sydney, Australia) and Advanced Bionics Corp. (California, United States of America), and an institutional grand in the past from Oticon Corp. (Smørum, Denmark) and Med-el Corp. (Innsbruck, Austria).
None of the authors have a personal conflict of interest to declare.
REFERENCES
- 1.Baskent D, Shannon RV. Interactions between cochlear implant electrode insertion depth and frequency-place mapping. J Acoust Soc Am 2005; 117 (3 pt 1):1405–1416. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner A, Rosen S, Norman C. The right information may matter more than frequency-place alignment: simulations of frequency-aligned and upward shifting cochlear implant processors for a shallow electrode array insertion. Ear Hear 2006; 27:139–152. [DOI] [PubMed] [Google Scholar]
- 3.Gani M, Valentini G, Sigrist A, et al. Implications of deep electrode insertion on cochlear implant fitting. J Assoc Res Otolaryngol 2007; 8:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suhling MC, Ketten DR, Salcher R, et al. The impact of electrode array length on hearing preservation in cochlear implantation. Otol Neurotol 2016; 37:1006–1015. [DOI] [PubMed] [Google Scholar]
- 5.Causon A, Verschuur C, Newman TA. A retrospective analysis of the contribution of reported factors in cochlear implantation on hearing preservation outcomes. Otol Neurotol 2015; 36:1137–1145. [DOI] [PubMed] [Google Scholar]
- 6.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol 2008; 29:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbist BM, Skinner MW, Cohen LT, et al. Consensus panel on a cochlear coordinate system applicable in histologic, physiologic, and radiologic studies of the human cochlea. Otol Neurotol 2010; 31:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connell BP, Cakir A, Hunter JB, et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol 2016; 37:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell BP, Hunter JB, Gifford RH, et al. Electrode location and audiologic performance after cochlear implantation: a comparative study between nucleus CI422 and CI512 electrode arrays. Otol Neurotol 2016; 37:1032–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013; 34:342–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kos MI, Boex C, Venail F, et al. Measurements of electrode position inside the cochlea for different cochlear implant systems. Acta Otolaryngol 2005; 125:474–480. [DOI] [PubMed] [Google Scholar]
- 12.Lee A, Jiang D, McLaren S, et al. Electric acoustic stimulation of the auditory system: experience and results of ten patients using MED-EL's M and Flex(EAS) electrodes. Clin Otolaryngol 2010; 35:190–197. [DOI] [PubMed] [Google Scholar]
- 13.van der Marel KS, Briaire JJ, Verbist BM, et al. The influence of cochlear implant electrode position on performance. Audiol Neurootol 2015; 20:202–211. [DOI] [PubMed] [Google Scholar]
- 14.Buchman CA, Dillon MT, King ER, et al. Influence of cochlear implant insertion depth on performance: a prospective randomized trial. Otol Neurotol 2014; 35:1773–1779. [DOI] [PubMed] [Google Scholar]
- 15.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158:280–286. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamczyk M, Bachor E, Bagus H, et al. [Cochlear implantation: relationship between speech development and insertion depth in children]. Laryngorhinootologie 2001; 80:123–126. [DOI] [PubMed] [Google Scholar]
- 18.Huang TC, Retzen SD, Marrinan MS, et al. Modiolar coiling, electrical thresholds, and speech perception after cochlear implantation using the nucleus contour advance electrode with the advance off stylet technique. Otol Neurotol 2006; 27:159–166. [DOI] [PubMed] [Google Scholar]
- 19.Nayak G, Panda NK, Banumathy N, et al. Deeper insertion of electrode array result in better rehabilitation outcomes - do we have evidence? Int J Pediatr Otorhinolaryngol 2016; 82:47–53. [DOI] [PubMed] [Google Scholar]
- 20.DeVries L, Scheperle R, Bierer JA. Assessing the electrode-neuron interface with the electrically evoked compound action potential, electrode position, and behavioral thresholds. J Assoc Res Otolaryngol 2016; 17:237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albu S, Babighian G. Predictive factors in cochlear implants. Acta Otorhinolaryngol Belg 1997; 51:11–16. [PubMed] [Google Scholar]
- 22.Aschendorff A, Kromeier J, Klenzner T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear 2007; 28 (2 suppl):75s–79s. [DOI] [PubMed] [Google Scholar]
- 23.Ball JB, Jr, Miller GW, Hepfner ST. Computed tomography of single-channel cochlear implants. AJNR Am J Neuroradiol 1986; 7:41–47. [PMC free article] [PubMed] [Google Scholar]
- 24.Basta D, Todt I, Ernst A. Audiological outcome of the pull-back technique in cochlear implantees. Laryngoscope 2010; 120:1391–1396. [DOI] [PubMed] [Google Scholar]
- 25.Blamey PJ, Pyman BC, Gordon M, et al. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol 1992; 101:342–348. [DOI] [PubMed] [Google Scholar]
- 26.Bredberg G, Lindstrom B. Insertion length of electrode array and its relation to speech communication performance and nonauditory side effects in multichannel-implanted patients. Ann Otol Rhinol Laryngol Suppl 1995; 166:256–258. [PubMed] [Google Scholar]
- 27.Chu KM, Au DK, Hui Y, et al. Short electrode insertion in cochlear implants: performance on speech perception. Cochlear Implants Int 2004; 5 suppl:126–128. [DOI] [PubMed] [Google Scholar]
- 28.Coombs A, Clamp PJ, Armstrong S, et al. The role of post-operative imaging in cochlear implant surgery: a review of 220 adult cases. Cochlear Implants Int 2014; 15:264–271. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald MB, Shapiro WH, McDonald PD, et al. The effect of perimodiolar placement on speech perception and frequency discrimination by cochlear implant users. Acta Otolaryngol 2007; 127:378–383. [DOI] [PubMed] [Google Scholar]
- 30.Hartrampf R, Dahm MC, Battmer RD, et al. Insertion depth of the nucleus electrode array and relative performance. Ann Otol Rhinol Laryngol Suppl 1995; 166:277–280. [PubMed] [Google Scholar]
- 31.Hiraumi H, Tsuji J, Kanemaru SI, et al. Cochlear implants in post-lingually deafened patients. Acta Otolaryngol 2007; 127 suppl:17–21. [DOI] [PubMed] [Google Scholar]
- 32.Johnston JD, Scoffings D, Chung M, et al. Computed tomography estimation of cochlear duct length can predict full insertion in cochlear implantation. Otol Neurotol 2016; 37:223–228. [DOI] [PubMed] [Google Scholar]
- 33.Kumakawa K, Takeda H, Ujita N. Determining the optimum insertion length of electrodes in the cochlear 22-channel implant: results of a clinical study. Adv Otorhinolaryngol 1997; 52:129–134. [DOI] [PubMed] [Google Scholar]
- 34.Long CJ, Holden TA, McClelland GH, et al. Examining the electro-neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol 2014; 15:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rader T, Baumann U, Stover T, et al. Management of cochlear implant electrode migration. Otol Neurotol 2016; 37:e341–e348. [DOI] [PubMed] [Google Scholar]
- 36.van Besouw RM, Forrester L, Crowe ND, et al. Simulating the effect of interaural mismatch in the insertion depth of bilateral cochlear implants on speech perception. J Acoust Soc Am 2013; 134:1348–1357. [DOI] [PubMed] [Google Scholar]
- 37.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014; 124 suppl:S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanna GB, Noble JH, McRackan TR, et al. Assessment of electrode placement and audiological outcomes in bilateral cochlear implantation. Otol Neurotol 2011; 32:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Li H, Galvin JJ, et al. Effects of insertion depth on spatial speech perception in noise for simulations of cochlear implants and single-sided deafness. Int J Audiol 2017; 56:S41–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuniga MG, Rivas A, Hedley-Williams A, et al. Tip fold-over in cochlear implantation: case series. Otol Neurotol 2017; 38:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyle PJ. The rational for a mid-scala electrode array. Eur Ann Otorhinolaryngol Head Neck Dis 2016; 133 suppl:S61–S62. [DOI] [PubMed] [Google Scholar]
- 42.Boex C, Baud L, Cosendai G, et al. Acoustic to electric pitch comparisons in cochlear implant subjects with residual hearing. J Assoc Res Otolaryngol 2006; 7:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyer E, Karkas A, Attye A, et al. Scalar localization by cone-beam computed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol 2015; 36:422–429. [DOI] [PubMed] [Google Scholar]
- 44.Chen JM, Farb R, Hanusaik L, et al. Depth and quality of electrode insertion: a radiologic and pitch scaling assessment of two cochlear implant systems. Am J Otol 1999; 20:192–197. [PubMed] [Google Scholar]
- 45.Deman PR, van Dijk B, Offeciers FE, et al. Pitch estimation of a deeply inserted cochlear implant electrode. Int J Audiol 2004; 43:363–368. [DOI] [PubMed] [Google Scholar]
- 46.Doshi J, Johnson P, Mawman D, et al. Straight versus modiolar hugging electrodes: does one perform better than the other? Otol Neurotol 2015; 36:223–227. [DOI] [PubMed] [Google Scholar]
- 47.Esquia Medina GN, Borel S, Nguyen Y, et al. Is electrode-modiolus distance a prognostic factor for hearing performances after cochlear implant surgery? Audiol Neurotol 2015; 18:406–413. [DOI] [PubMed] [Google Scholar]
- 48.Fama A, Carlson M, Driscoll C, et al. The use of micro-CT to evaluate cochlear implant electrode position and intracochlear damage. Laryngoscope 2010; 120:S205. [DOI] [PubMed] [Google Scholar]
- 49.Fischer N, Pinggera L, Weichbold V, et al. Radiologic and functional evaluation of electrode dislocation from the scala tympani to the scala vestibuli in patients with cochlear implants. AJNR Am J Neuroradiol 2015; 36:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grasmeder ML, Verschuur CA, Batty VB. Optimizing frequency-to-electrode allocation for individual cochlear implant users. J Acoust Soc Am 2014; 136:3313. [DOI] [PubMed] [Google Scholar]
- 51.Hassepass F, Aschendorff A, Bulla S, et al. Radiologic results and hearing preservation with a straight narrow electrode via round window versus cochleostomy approach at initial activation. Otol Neurotol 2015; 36:993–1000. [DOI] [PubMed] [Google Scholar]
- 52.Jolly CN, Gstottner W, Hochmair-Desoyer I, et al. Principles and outcome in perimodiolar positioning. Ann Otol Rhinol Laryngol Suppl 2000; 185:20–23. [DOI] [PubMed] [Google Scholar]
- 53.Lazard DS, Vincent C, Venail F, et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One 2012; 7:e48739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marsh MA, Xu J, Blamey PJ, et al. Radiologic evaluation of multichannel intracochlear implant insertion depth. Am J Otol 1993; 14:386–391. [PubMed] [Google Scholar]
- 55.Noble JH, Gifford RH, Hedley-Williams AJ, et al. Clinical evaluation of an image-guided cochlear implant programming strategy. Audiol Neurootol 2014; 19:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Connell BP, Hunter JB, Haynes DS, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope 2017; 127:2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puyalto De Pablo P, Fernandez JJS, Castilo NM, et al. Relationship between the insertion depth and the auditory results in patients with cochlear implants. Neuroradiology 2017; 59:186–187. [Google Scholar]
- 58.Roy AT, Penninger RT, Pearl MS, et al. Deeper cochlear implant electrode insertion angle improves detection of musical sound quality deterioration related to bass frequency removal. Otol Neurotol 2016; 37:146–151. [DOI] [PubMed] [Google Scholar]
- 59.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol Suppl 2007; 197:2–24. [PubMed] [Google Scholar]
- 60.Skinner MW, Ketten DR, Holden LK, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol 2002; 3:332–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Jagt MA, Briaire JJ, Verbist BM, et al. Comparison of the HiFocus mid-scala and HiFocus 1J electrode array: angular insertion depths and speech perception outcomes. Audiol Neurootol 2016; 21:316–325. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Noble J, Dawant B, et al. Cochlear implant outcomes with perimodiolar-positioned electrodes. Otolaryngol Head Neck Surg 2016; 155:105. [Google Scholar]
- 63.Wanna GB, Noble JH, Gifford RH, et al. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: preliminary results. Otol Neurotol 2015; 36:1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yukawa K, et al. Effects of insertion depth of cochlear implant electrodes upon speech perception. Audiol Neurootol 2004; 9:163–172. [DOI] [PubMed] [Google Scholar]
- 65.De Seta D, et al. The role of electrode placement in bilateral simultaneously cochlear-implanted adult patients. Otolaryngol Head Neck Surg 2016; 155:485–493. [DOI] [PubMed] [Google Scholar]
- 66.Hilly O, et al. Depth of cochlear implant array within the cochlea and performance outcome. Ann Otol Rhinol Laryngol 2016; 125:886–892. [DOI] [PubMed] [Google Scholar]
- 67.Marrinan MS, et al. Degree of modiolar coiling, electrical thresholds, and speech perception after cochlear implantation. Otol Neurotol 2004; 25:290–294. [DOI] [PubMed] [Google Scholar]
- 68.van der Beek FB, et al. Clinical evaluation of the Clarion CII HiFocus 1 with and without positioner. Ear Hear 2005; 26:577–592. [DOI] [PubMed] [Google Scholar]
- 69.van der Beek FB, et al. Intracochlear position of cochlear implants determined using ct scanning versus fitting levels: higher threshold levels at basal turn. Audiol Neurootol 2016; 21:54–67. [DOI] [PubMed] [Google Scholar]
- 70.van der Marel KS, et al. Electrode migration in cochlear implant patients: not an exception. Audiol Neurootol 2012; 17:275–281. [DOI] [PubMed] [Google Scholar]
- 71.Buchner A, et al. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One 2017; 12:e0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


