Abstract
Background:
Anterior glenohumeral instability with >20% glenoid bone loss is a disorder that can be treated with the Latarjet stabilizing procedure; however, complications are common. The purposes of this study were to (1) evaluate the effect of an anatomic-specific titanium implant produced by 3-dimensional (3D) printing as a treatment option for recurrent shoulder instability with substantial glenoid bone loss and (2) compare the use of that implant with the Latarjet procedure.
Methods:
Ten fresh-frozen cadaveric shoulders (mean age at the time of death, 78 years) were tested in a biomechanical setup with the humerus in 30° of abduction and in neutral rotation. The shoulders were tested under 5 different conditions: (1) normal situation, (2) creation of an anterior glenoid defect, (3) implantation of an anatomic-specific titanium implant produced by 3D printing, and the Latarjet procedure (4) with and (5) without 10 N of load attached to the conjoined tendon. In each condition, the humerus was translated 10 mm anteriorly relative to the glenoid, and the maximum peak translational force that was necessary for this translation was measured.
Results:
After creation of the glenoid defect, the mean translational peak force decreased by 30% ± 6% compared with that for the normal shoulder. After restoration of the original glenoid anatomy, the translational force needed to dislocate the humeral head from the glenoid significantly increased compared with that in the defect condition—to 119% ± 16% of normal (p < 0.01) with the 3D-printed anatomic-specific implant and to 121% ± 48% of normal (p < 0.01) following the Latarjet procedure. No significant differences in mean translational force were found between the anatomic-specific implant and the Latarjet procedure (p = 0.72).
Conclusions:
The mean translational peak force needed to dislocate the humerus 10 mm anteriorly on the glenoid was higher after glenoid restoration with the 3D-printed anatomic-specific implant compared with when the glenoid had a 20% surface defect but also compared with when the glenoid was intact. No differences in mean translational peak force were found between the 3D-printed anatomic-specific glenoid implant and the Latarjet procedure, although there was less variability in the 3D-implant condition.
Clinical Relevance:
Novel 3D-printing technology could provide a reliable patient-specific alternative to solve problems related to traditional treatment methods for shoulder instability.
Anterior glenohumeral instability is a common disorder, typically affecting the young and active population, with an overall prevalence of 2%1,2. The shoulder joint is the most mobile joint in the human body; however, this mobility comes at the expense of stability1. A first dislocation often has a traumatic origin and is often followed by a disabling course as recurrent (sub)luxations occur in up to 94% of patients, especially younger ones3. Eventually, this can lead to chronic anterior shoulder instability, with presentations ranging from minor symptoms to frequent (sub)luxations1. Without adequate treatment, this condition often leads to more rapid degenerative arthropathy of the shoulder and major limitations in daily life2,4.
There are numerous surgical treatment options for the unstable shoulder joint, and they target different causes of a multifactorial problem. With all treatments, the aim is to lower the rate of recurrence of dislocations in combination with a low complication rate. The dynamic interactions of soft-tissues lesions and bone loss are an important factor in the choice of treatment5. The arthroscopic Bankart repair and the Latarjet procedure are the 2 most commonly used techniques2,6. Soft-tissue repairs such as the Bankart procedure often fail in the presence of substantial bone loss (>20% of the glenoid area), which is present in up to 67% of patients with recurrent shoulder instability7. In patients with severe glenoid loss, the Latarjet procedure seems to be the preferred treatment1,2,8. Currently, there are 2 commonly used and equivalent techniques for the Latarjet9 procedure: (1) the classic technique, with which the inferior surface of the coracoid is transferred to the anterior surface of the glenoid, and (2) the congruent-arc technique, with which the coracoid is rotated 90° and transferred with the medial side against the glenoid10.
The Latarjet procedure is known for its low rates of recurrent instability, even in high-intensity contact-sport athletes, but it can have severe complications in up to 30% of patients11-14. The recent literature contains claims of possible superiority of the Latarjet procedure relative to the Bankart repair14,15. However, although the split subscapularis tendon might provide dynamic stability by means of the sling effect by the coracobrachialis tendon, the bone block of the coracoid within the subscapularis tendon also prevents normal function of the subscapular muscles, which are major shoulder muscles. Another possible long-term problem with the Latarjet procedure is resorption of the coracoid bone block while it is fixed by 2 titanium screws16. Complications, donor site problems, and the nonanatomic nature of this procedure have spurred research on other graft sources, such as iliac crest autograft, allograft, and synthetics17.
In this study, as part of the PRosPERoS (PRinting PERsonalized orthopaedic implantS) project group, the first author (K.W.) designed a 3-dimensional (3D)-printed titanium implant that could circumvent these potential issues. The implant is placed extracapsularly, flush with the bone, to fill in the exact defect and with the joint capsule acting as the articulating surface.
The primary aim of this study was to investigate, in a cadaveric model, if use of an anatomic-specific glenoid implant in a severe glenoid defect could restore glenohumeral morphology and stability. The secondary aim was to compare the anatomic implant and the classic Latarjet procedure with regard to the translational forces needed to dislocate the humerus 10 mm anteriorly on the glenoid after the operation9. Our hypothesis was that the anatomic implant would increase these translational forces relative to those after the creation of the glenoid bone defect and that the forces would be comparable with those in a normal shoulder and those after the classic Latarjet procedure.
Materials and Methods
Thirteen fresh-frozen human shoulders were originally inspected for use in this study. Exclusion criteria were osseous defects (humeral and/or glenoid), rotator cuff tears, and moderate to severe osteoarthritis as demonstrated by direct inspection and computed tomography (CT). After exclusion, 10 shoulders (5 left and 5 right, and 5 from male donors and 5 from female donors) from 8 cadavers with a mean age at the time of death of 78 years (range, 71 to 86 years) were included.
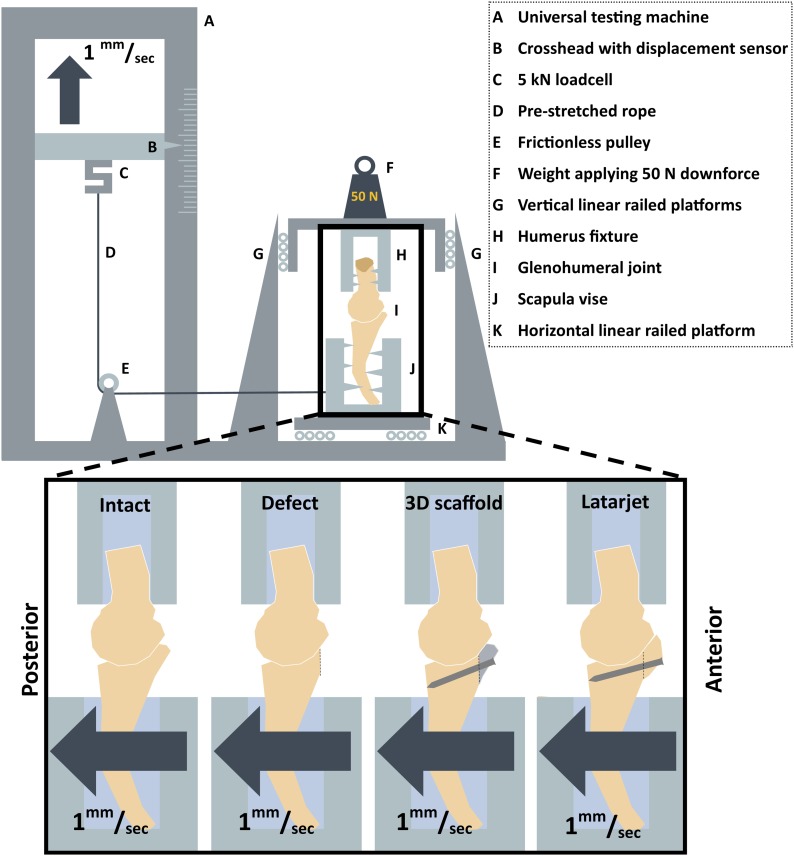
All specimens were disarticulated at the scapulothoracic joint and transected at the humeral shaft, about 15 cm distal to the greater tubercle. The shoulder girdle was dissected, with the deltoid muscle removed and the rotator cuff muscles, conjoined tendon, and joint capsule left intact. The scapula was rigidly fixed in a self-centering vice that was secured on 4 linear railed platforms (TRS15VN; TBI Motion Technology) placed parallel to the glenoid’s posterior-anterior axis and attached by prestretched rope (high-modulus polyethylene [HMPE]; Dyneema) to the crosshead of an LR5K universal testing machine equipped with an XLC 5kN load cell (Lloyd Instruments).
The proximal part of the humerus was rigidly fastened at its shaft with a custom-made fixture that allowed 30° of abduction and neutral rotation of the humerus in relation to the glenoid cavity. In this position, the osseous anatomy largely provides the stability, rather than the dynamic stabilizers and the capsuloligamentous structures18,19. The humeral fixture was attached to 4 vertically placed linear railed platforms and loaded with weights to allow a downward force of 50 N18-20 on the glenoid, ensuring that the humeral head found its original neutral anatomic position in the glenoid cavity. This neutral position was defined as the starting position for each test. The glenoid platform moved posteriorly to cause anterior translation of the humerus at a set rate of 1.0 mm/sec for a total of 10 mm measured by calipers on the horizontal rail21,22. The loads were recorded with NEXYGEN data acquisition software (Lloyd Instruments) (Fig. 1).
Fig. 1.
Top Schematic overview of the components of the custom-designed testing device. Bottom (enlarged area) The different testing conditions.
At the start of the study, a CT scan of the shoulder girdle was made. The images comprised the entire shoulder girdle and humerus with a slice thickness of 0.9 mm (250 mAs, 120 kV). The CT scans were transferred to commercially available image processing software (Mimics Medical 20.0; Materialise), which was used to segment a pre-defect 3D model of the osseous structures using standardized bone threshold values (≥226 Hounsfield units).
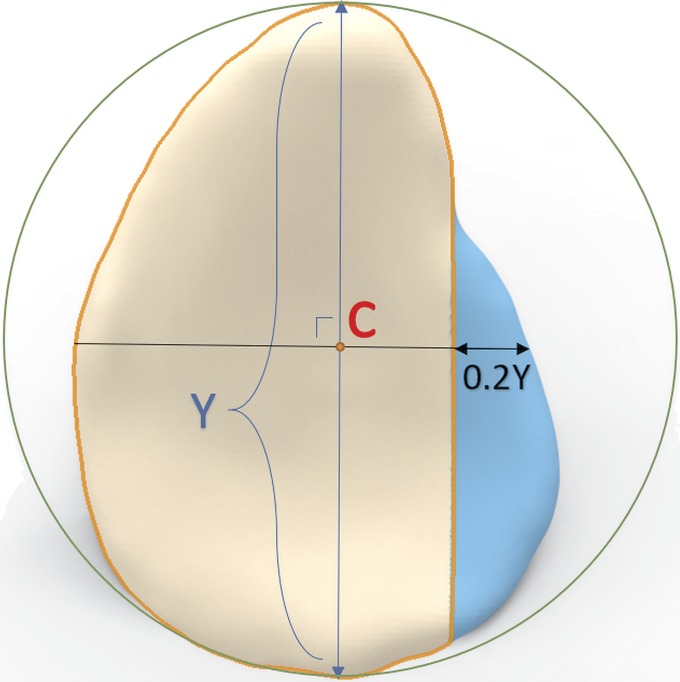
After imaging, an anterior critical defect of 20% of the glenoid length was created23 as described by Yamamoto et al.18,24. The anterior labrum was removed, and an osteotomy was made perpendicular to the joint surface using an anatomic-specific saw template (Fig. 2).
Fig. 2.
Schematic representation of the simulated glenoid defect as described by Yamamoto et al.18,24. A circle was drawn around the pear-shaped glenoid. The y axis was drawn through the superior and inferior points. The x axis was drawn perpendicular to the y axis, through the center (C) of the glenoid circle. The osseous defect was created at the anterior side of the glenoid with a total width equal to 20% of the glenoid length (0.2Y).
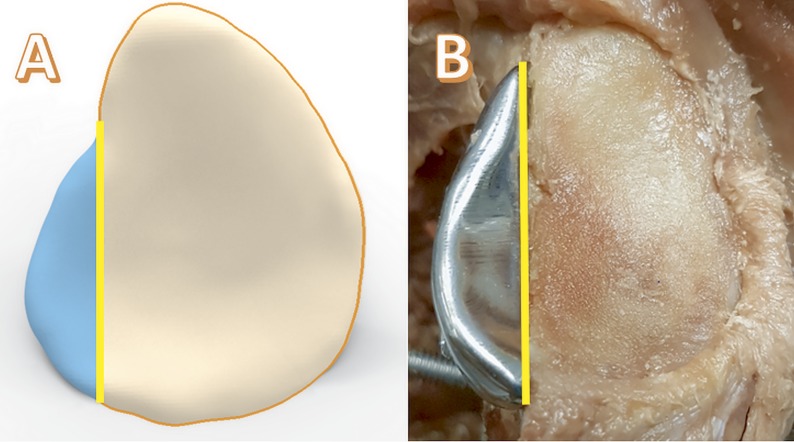
As part of the the PRosPERoS project, the defect-repairing titanium implants were designed by the first author (K.W.) using Geomagic Freeform Plus software (3D Systems). A simulation model of the glenoid defect was removed from the pre-defect model using CAD (computer-aided design) Boolean subtraction operatives, leaving the essential size of the implant. The created implant is therefore the size of the osteotomized glenoid rim and designed to be flush with the bone with the capsule as the overlying articulating surface. Additionally, 2 locking screws were added for angular stability, and their trajectories were digitally planned in the scaffold. The 3D printing was done with medical grade titanium (Ti-6Al-4V ELI [extra-low interstitial], grade 23) using an SLM (Selective Laser Melting) printer (ProX DMP 320; 3D Systems). Post-processing included polishing and screw wire tapping (Fig. 3).
Fig. 3.
Fig. 3-A In silico simulation of the implant. The vertical line is the osteotomy or defect line. The implant is to the left of the osteotomy line, and the glenoid is to the right of the line. Fig. 3-B A specimen with an implanted scaffold. The shoulder capsule was removed for visualization purposes.
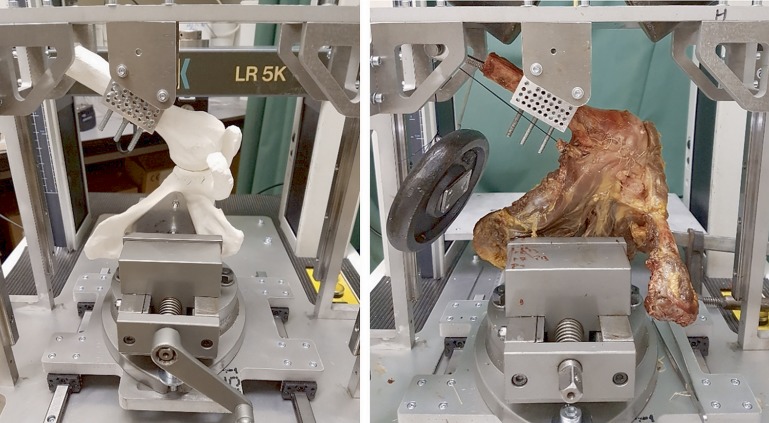
We tested 5 different conditions: (1) the “normal” situation, (2) after creation of an anterior glenoid bone defect, (3) after implantation of the 3D-printed titanium anatomic-specific implant (the “scaffold” condition), and after the classic Latarjet procedure (4) with and (5) without a 10-N load applied to the conjoined tendon by means of sutures to simulate the so-called sling effect19 (Fig. 4). The specimens were tested in the situation with either the 3D implant first (n = 5) or the Latarjet procedure first (n = 5), depending on the randomization. Every specimen was tested under all 5 conditions 5 times in 1 day. The specimens were sprayed with a 0.9% NaCl solution to prevent the quality of the soft tissue from deteriorating. A detailed description of the surgical technique is available in the Appendix (Supplementary Data 1).
Fig. 4.
Left A 3D-printed sample used for optimizing the setup. Right A specimen that underwent a test cycle under condition 4: the Latarjet procedure with 10 N of pull on the conjoined tendon.
After stability testing, another CT scan was performed for 5 shoulders with the 3D-printed implant in situ and 5 shoulders after the Latarjet for evaluation of the geometry of the defect repair. The images were uploaded into Mimics Medical to compare the glenoid width (the widest anteroposterior diameter measured parallel to the superior-inferior axis) and the cavity depth (measured as described by Willemot et al.25) among the intact, defect, and post-reconstruction conditions.
Data Analysis
A nonparametric Friedman test was performed to compare the mean peak translational forces needed to translate the humeral head 10 mm and the glenoid cavity width and depth among all of the different conditions. When a significant value was found, the related-samples Wilcoxon signed-rank test (SPSS, version 24; IBM) was used as a post hoc analysis for the distinct research questions. The sample size was calculated on the basis of prior data22,26-28. A mean effect size of 30% and a standard deviation of 25% were chosen. A minimum of 8 samples was needed to show a significant difference in translational force with a power of 0.8 and an alpha of 0.05. Ten samples were included in this study
Results
After dissection, all shoulder capsules and labra were found to be intact. During testing, no signs of damage to the specimens were observed. The mean superior-inferior glenoid diameter (and standard deviation [SD]) of the 10 specimens was 37.1 ± 3.9 mm as measured on CT scans. Therefore, 7.4 ± 2.1 mm—or 20% of the superior-inferior glenoid diameter—was the desired average width of the glenoid defect. The actual created mean width of the glenoid defect was 7.4 ± 1.9 mm, equivalent to 19.9% of the glenoid diameter, which was not significantly different from the desired width (p = 0.80).
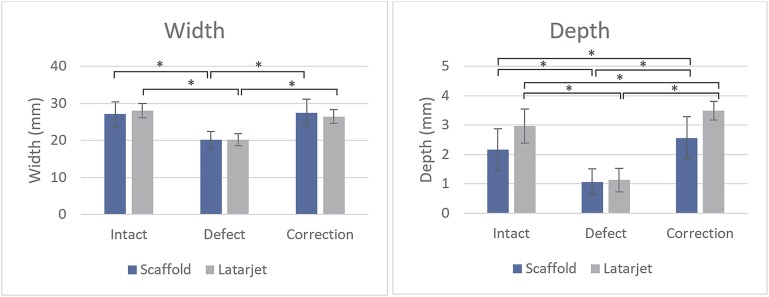
The glenoid width decreased significantly after creation of the bone defect in both the group that subsequently received the 3D-printed anatomic-specific scaffold (the “scaffold group”) (p < 0.05; n = 5) and the group that received the Latarjet procedure (p < 0.05; n = 5). The glenoid width increased to 100% and 96% of the normal width after restoration with the scaffold and Latarjet procedure, respectively. These widths did not differ significantly from the normal width in either the scaffold (p = 0.50) or Latarjet (p = 0.14) group (Fig. 5). The glenoid cavity depth decreased significantly after the creation of the bone defect in both the scaffold (p < 0.05) and the Latarjet (p < 0.05) group and increased to 118% of the normal depth after restoration with either procedure. This depth differed significantly from the normal depth in both the scaffold (p = 0.05) and the Latarjet (p < 0.05) group (Fig. 5).
Fig. 5.
The mean (and standard deviation [SD]) glenoid cavity width and depth (mm) in the normal, defect, and reconstructed (correction) conditions (scaffold or Latarjet procedure). *A significant difference (p ≤ 0.05).
Peak Translational Forces
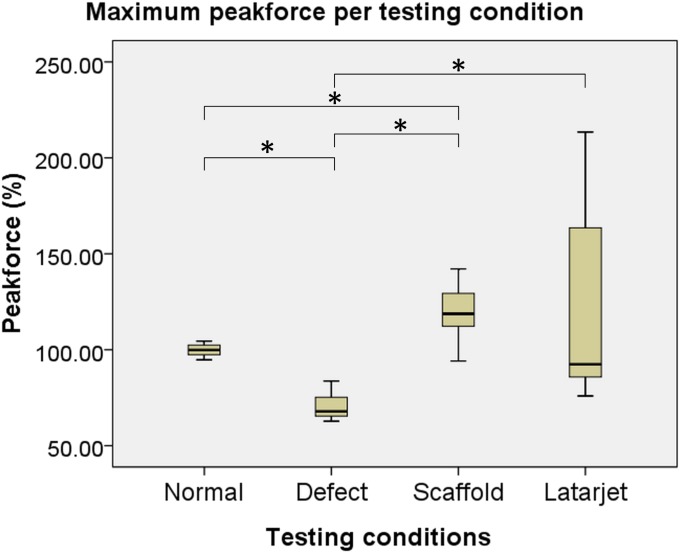
The mean maximum peak force needed to translate the humeral head 10 mm anteriorly in the intact specimens was 48.6 ± 15.8 N, which decreased significantly (by 30% ± 6%) to 33.8 ± 10.1 N after creation of the bone defect (p < 0.01). The mean force in the defect condition significantly increased after reconstruction—to 56.0 ± 16.4 N (p < 0.01) in the scaffold group and to 55.0 ± 16.2 N (p < 0.01) in the Latarjet group. Also, the mean translational peak force was significantly higher after reconstruction with the scaffold compared with that in the normal situation (p < 0.01). No significant difference was found when the reconstructions with the scaffold and the Latarjet procedure were compared (p = 0.72) (Table I). A box plot showing the peak forces, as percentages of the normal situation, under all of the different conditions is shown in Figure 6.
Fig. 6.
Maximum peak force (%) needed to translate the humeral head 10 mm anteriorly with respect to the glenoid in the normal, defect, scaffold, and Latarjet conditions. The humerus was in 30° of abduction and neutral rotation in all conditions. The normal healthy shoulder was used as the standard for the subsequent analyses. *A significant difference (p < 0.05). Horizontal line inside box = median, top and bottom of box = interquartile range, and top and bottom of whiskers = total range.
TABLE I.
Results of Related-Samples Wilcoxon Signed-Rank Test Comparing Various Testing Conditions*
| Z | P Value* | |
| Normal vs. defect | −2,803† | <0.01 |
| Scaffold vs. normal | −2,497‡ | <0.01 |
| Scaffold vs. defect | −2,803‡ | <0.01 |
| Latarjet vs. defect | −2,803‡ | <0.01 |
| Latarjet vs. scaffold | −0,357† | 0.72 |
| Latarjet vs. Latarjet without sling | −2,666† | <0.01 |
The level of significance was p < 0.05.
Based on positive ranks.
Based on negative ranks.
As an additional test, the translational forces were measured after the Latarjet procedure but without 10 N of load on the conjoined tendon. Under this condition, the force decreased by 21% ± 31%, compared with force in the Latarjet group with this load; this difference was significant (p < 0.01).
Discussion
The force necessary to translate the humeral head 10 mm anteriorly in the glenoid significantly decreased, to 70% ± 7% of normal, after the creation of the glenoid defect. After restoration of the original glenoid anatomy with an anatomic-specific 3D-printed scaffold, the translational forces increased to 119% ± 16% of the forces in the intact glenohumeral joint. This was not significantly different from the increase after the Latarjet procedure (to 121% ± 48% of normal); however, this does not imply that the 2 procedures are the same (Fig. 6).
In 1947, Moseley described a metallic rim that could be fixed to the neck of the scapula29. This implant, which contained holes for suturing of the capsule to the bone on the joint side of the prosthesis, was placed in an extracapsular position29. More recently, Diederichs et al. presented an in silico method that compares the healthy contralateral glenoid with the affected glenoid to simulate the optimal reconstruction of a glenoid rim defect30. However, the current study is the first to use biomechanical testing of 3D-printed anatomic-specific titanium implants for reconstruction of severe glenoid defects in the human shoulder.
Since the 3D-printed glenoid scaffold should recreate the intact glenoid exactly it was expected that the mean translational peak force would be comparable between the 2 situations. However, several factors may have attributed to the greater forces measured after the scaffold reconstructions. First, although the bone cut used to create the glenoid defect was expected to be exactly parallel to the y axis and perpendicular to the glenoid surface, if the implant was not positioned perfectly perpendicular to the joint surface (i.e., if it was at a slight angle) the glenoid cavity could have become too wide and too deep. Second, capsular interposition and capsular suturing contribute to the translational force, as shown by Yamamoto et al.22. The capsule was envisioned to be as thick as the cartilage as the implant was placed and modeled to match the bone level. However, the thickness of the interpositioned capsule is difficult to predict as it is not visible on pre-defect CT. This might have affected the translational forces.
The secondary goal of this study was to compare the 3D-printed titanium implant with the classic Latarjet procedure, which is currently considered to be the standard for treating recurrent anterior glenohumeral instability when >20% of the glenoid bone has been lost2,8. However, the Latarjet procedure is not anatomically precise and has a high rate of complications, including malpositioning, problems with the screw trajectory, loss of the range of motion, and eventually the development of arthrosis13. A patient-specific implant can be a solution for some of these problems, as all aspects of the reconstruction can be planned with the aid of 3D-design software. However, this study was not performed to show inferiority or superiority of 1 procedure over the other; more research is needed for comparison of the 2 techniques.
Both clinical and biomechanical studies have demonstrated the working mechanism of the Latarjet procedure15,19,22,26,27. The downside of the 3D-printed scaffold method might be the absence of a dynamic muscle stabilizer, which is created during the Latarjet procedure using the conjoined tendon22,27,31. In our study, the conjoined tendon contributed approximately 21% of the force needed to translate the humerus. However, the variability in the restoration of glenohumeral stability by the Latarjet procedure was relatively large (Fig. 6), whereas the titanium implant was more predictable (had less variability) in the restoration of glenohumeral stability.
Some limitations must be considered when interpreting our findings. We performed a biomechanical cadaver study, thus eliminating large dynamic stabilizers (i.e., muscles), which may be 1 of the most important factors in shoulder instability. Also, the same specimens were used for both the Latarjet and the scaffold procedure, with the risk of tissue elongation during testing. However, no significant differences were found between the shoulders in which the scaffold was implanted after the primary Latarjet procedure and those in which the procedures were done in the reverse order. In addition, it would have been preferable for us to have created the defect before the implants were designed. However, we made a cutting template to accurately create the glenoid defects, which were nearly the same as the planned defects, with widths of 7.4 ± 1.9 mm and 7.3 ± 2.1 mm, respectively. By designing the implants before the creation of the defects, we were always able to perform all procedures within 24 hours after defrosting the specimen, thereby preventing degradation of the tissues as much as possible. Another limitation of the 3D-printed implant is that no soft-tissue lesions such as labral injuries were directly targeted.
In conclusion, the purpose of our study was to determine whether use of a 3D-printed anatomic-specific titanium implant in a severe glenoid defect would increase the mean peak force needed to translate the humerus 10 mm anteriorly to levels comparable with those in the healthy normal situation. We found that the mean translational peak force after restoration with the anatomic-specific implant was significantly higher than that in the normal situation. No significant difference in results was identified between the 3D-printed anatomic-specific implant and the classic Latarjet procedure. Restoration of glenohumeral stability with the 3D-printed anatomic-specific implant is not the same as the normal situation, although it is very consistent and is comparable with that following the Latarjet procedure.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJS/F299).
Footnotes
Investigation performed at the Department of Orthopaedics, University Medical Centre Utrecht, Utrecht, the Netherlands
Disclosure: This work was financially supported by the PRosPERoS (PRinting PERsonalized orthopaedic implantS) Project, funded by the Interreg VA Flanders—The Netherlands Program (PRosPERoS Project; CCI Grant 2014TC16RFCB046). The implants used in this project were produced by 3D Systems as part of the PRosPERoS research grant. The funding sources played no role in the preparation or performance of this study. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/F298).
References
- 1.Dodson CC, Cordasco FA. Anterior glenohumeral joint dislocations. Orthop Clin North Am. 2008. October;39(4):507-18, vii. [DOI] [PubMed] [Google Scholar]
- 2.Sofu H, Gürsu S, Koçkara N, Oner A, Issın A, Camurcu Y. Recurrent anterior shoulder instability: review of the literature and current concepts. World J Clin Cases. 2014. November 16;2(11):676-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroux T, Wasserstein D, Veillette C, Khoshbin A, Henry P, Chahal J, Austin P, Mahomed N, Ogilvie-Harris D. Epidemiology of primary anterior shoulder dislocation requiring closed reduction in Ontario, Canada. Am J Sports Med. 2014. February;42(2):442-50. Epub 2013 Nov 25. [DOI] [PubMed] [Google Scholar]
- 4.Hovelius L, Saeboe M. Neer Award 2008: arthropathy after primary anterior shoulder dislocation—223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009. May-Jun;18(3):339-47. Epub 2009 Feb 28. [DOI] [PubMed] [Google Scholar]
- 5.Momaya AM, Tokish JM. Applying the glenoid track concept in the management of patients with anterior shoulder instability. Curr Rev Musculoskelet Med. 2017. December;10(4):463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blonna D, Bellato E, Caranzano F, Assom M, Rossi R, Castoldi F. Arthroscopic Bankart repair versus open Bristow-Latarjet for shoulder instability: a matched-pair multicenter study focused on return to sport. Am J Sports Med. 2016. December;44(12):3198-205. Epub 2016 Aug 8. [DOI] [PubMed] [Google Scholar]
- 7.Pauzenberger L, Dyrna F, Obopilwe E, Heuberer PR, Arciero RA, Anderl W, Mazzocca AD. Biomechanical evaluation of glenoid reconstruction with an implant-free J-bone graft for anterior glenoid bone loss. Am J Sports Med. 2017. October;45(12):2849-57. Epub 2017 Aug 3. [DOI] [PubMed] [Google Scholar]
- 8.Ramhamadany E, Modi CS. Current concepts in the management of recurrent anterior gleno-humeral joint instability with bone loss. World J Orthop. 2016. June 18;7(6):343-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery SR, Katthagen JC, Mikula JD, Marchetti DC, Tahal DS, Dornan GJ, Dahl KD, Brady AW, Turnbull TL, Millett PJ. Anatomic and biomechanical comparison of the classic and congruent-arc techniques of the Latarjet procedure. Am J Sports Med. 2017. May;45(6):1252-60. Epub 2017 Feb 14. [DOI] [PubMed] [Google Scholar]
- 10.Burkhart SS, De Beer JF, Barth JRH, Cresswell T, Roberts C, Richards DP. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy. 2007. October;23(10):1033-41. [DOI] [PubMed] [Google Scholar]
- 11.Neyton L, Young A, Dawidziak B, Visona E, Hager JP, Fournier Y, Walch G. Surgical treatment of anterior instability in rugby union players: clinical and radiographic results of the Latarjet-Patte procedure with minimum 5-year follow-up. J Shoulder Elbow Surg. 2012. December;21(12):1721-7. Epub 2012 May 5. [DOI] [PubMed] [Google Scholar]
- 12.Latarjet M. [Treatment of recurrent dislocation of the shoulder]. Lyon Chir. 1954. Nov-Dec;49(8):994-7. French. [PubMed] [Google Scholar]
- 13.Griesser MJ, Harris JD, McCoy BW, Hussain WM, Jones MH, Bishop JY, Miniaci A. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013. February;22(2):286-92. [DOI] [PubMed] [Google Scholar]
- 14.Longo U, Loppini M, Rizzello G, Ciuffreda M, Maffulli N, Denaro V. Latarjet, Bristow, and Eden-Hybinette procedures for anterior shoulder dislocation: systematic review and quantitative synthesis of the literature. Arthroscopy. 2014;30(9):1184-211. [DOI] [PubMed] [Google Scholar]
- 15.An VVG, Sivakumar BS, Phan K, Trantalis J. A systematic review and meta-analysis of clinical and patient-reported outcomes following two procedures for recurrent traumatic anterior instability of the shoulder: Latarjet procedure vs. Bankart repair. J Shoulder Elbow Surg. 2016. May;25(5):853-63. Epub 2016 Jan 19. [DOI] [PubMed] [Google Scholar]
- 16.Di Giacomo G, Costantini A, de Gasperis N, De Vita A, Lin BKH, Francone M, Rojas Beccaglia MA, Mastantuono M. Coracoid graft osteolysis after the Latarjet procedure for anteroinferior shoulder instability: a computed tomography scan study of twenty-six patients. J Shoulder Elbow Surg. 2011. September;20(6):989-95. Epub 2011 Mar 9. [DOI] [PubMed] [Google Scholar]
- 17.Willemot LB, Elhassan BT, Verborgt O. Bony reconstruction of the anterior glenoid rim. J Am Acad Orthop Surg. 2018. May 15;26(10):e207-18. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto N, Itoi E, Abe H, Kikuchi K, Seki N, Minagawa H, Tuoheti Y. Effect of an anterior glenoid defect on anterior shoulder stability: a cadaveric study. Am J Sports Med. 2009. May;37(5):949-54. Epub 2009 Mar 4. [DOI] [PubMed] [Google Scholar]
- 19.Wellmann M, Petersen W, Zantop T, Herbort M, Kobbe P, Raschke MJ, Hurschler C. Open shoulder repair of osseous glenoid defects: biomechanical effectiveness of the Latarjet procedure versus a contoured structural bone graft. Am J Sports Med. 2009. January;37(1):87-94. Epub 2008 Dec 4. [DOI] [PubMed] [Google Scholar]
- 20.Itoi E, Lee SB, Berglund LJ, Berge LL, An KN. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am. 2000. January;82(1):35-46. [DOI] [PubMed] [Google Scholar]
- 21.Itoi E, Yamamoto N, Kurokawa D, Sano H. Bone loss in anterior instability. Curr Rev Musculoskelet Med. 2013. March;6(1):88-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto N, Muraki T, An KN, Sperling JW, Cofield RH, Itoi E, Walch G, Steinmann SP. The stabilizing mechanism of the Latarjet procedure: a cadaveric study. J Bone Joint Surg Am. 2013. August 7;95(15):1390-7. [DOI] [PubMed] [Google Scholar]
- 23.Saito H, Itoi E, Sugaya H, Minagawa H, Yamamoto N, Tuoheti Y. Location of the glenoid defect in shoulders with recurrent anterior dislocation. Am J Sports Med. 2005. June;33(6):889-93. Epub 2005 Apr 12. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto N, Itoi E. Osseous defects seen in patients with anterior shoulder instability. Clin Orthop Surg. 2015;7(4):425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willemot LB, Akbari-Shandiz M, Sanchez-Sotelo J, Zhao K, Verborgt O. Restoration of articular geometry using current graft options for large glenoid bone defects in anterior shoulder instability. Arthroscopy. 2017. September;33(9):1661-9. Epub 2017 Jun 15. [DOI] [PubMed] [Google Scholar]
- 26.Patel RM, Walia P, Gottschalk L, Kuklis M, Jones MH, Fening SD, Miniaci A. The effects of Latarjet reconstruction on glenohumeral kinematics in the presence of combined bony defects: a cadaveric model. Am J Sports Med. 2016. July;44(7):1818-24. Epub 2016 Apr 15. [DOI] [PubMed] [Google Scholar]
- 27.Barrett Payne W, Kleiner MT, McGarry MH, Tibone JE, Lee TQ. Biomechanical comparison of the Latarjet procedure with and without a coracoid bone block. Knee Surg Sports Traumatol Arthrosc. 2016. February;24(2):513-20. Epub 2015 Dec 12. [DOI] [PubMed] [Google Scholar]
- 28.Kephart CJ, Abdulian MH, McGarry MH, Tibone JE, Lee TQ. Biomechanical analysis of the modified Bristow procedure for anterior shoulder instability: is the bone block necessary? J Shoulder Elbow Surg. 2014. December;23(12):1792-9. Epub 2014 Jun 9. [DOI] [PubMed] [Google Scholar]
- 29.Moseley HF. The use of a metallic glenoid rim in recurrent dislocation of the shoulder. Can Med Assoc J. 1947. March;56(3):320. [PMC free article] [PubMed] [Google Scholar]
- 30.Diederichs G, Seim H, Meyer H, Issever AS, Link TM, Schröder RJ, Scheibel M. CT-based patient-specific modeling of glenoid rim defects: a feasibility study. AJR Am J Roentgenol. 2008. November;191(5):1406-11. [DOI] [PubMed] [Google Scholar]
- 31.Giles JW, Boons HW, Elkinson I, Faber KJ, Ferreira LM, Johnson JA, Athwal GS. Does the dynamic sling effect of the Latarjet procedure improve shoulder stability? A biomechanical evaluation. J Shoulder Elbow Surg. 2013. June;22(6):821-7. Epub 2012 Sep 28. [DOI] [PubMed] [Google Scholar]