Abstract

For uranium extraction from seawater, development of new stable and reusable sorbents with high affinity and good selectivity for U(VI) is required. Herein, a new phosphate-functionalized polyethylene (denoted PO4/PE) was synthesized via a simple Ar-jet plasma treatment of PE in concentrated H3PO4 and was employed in U(VI) extraction from seawater. The prepared PO4/PE shows superior performance in the extraction of trace U(VI) from seawater. The adsorption process followed the second-order kinetics model and the Langmuir model. The maximum adsorption capacity of PO4/PE for U(VI) reaches 173.8 mg/g at pH 8.2 and 298 K. PO4/PE can be effectively regenerated by 0.1 mol/L Na2CO3 and reused well even after eight cycles. Experimental results offer a new approach for U(VI) uptake from seawater.
Introduction
The widely used fossil fuels (petroleum and coal) are the main source of environmental pollution and greenhouse effect. The serious environmental impact and the limited reserves of fossil fuels have stimulated the search for alternative energy sources. Nuclear energy is widely considered as a good substitute for fossil fuels because of its unparalleled energy density (about 2.7 million times that of coal in theory) and low emission of CO2.1 The development of nuclear energy leads to a significant increase in the demands for uranium, which will be the basic material of nuclear plants in the future. However, the natural resource of uranium is limited and recycling of uranium from radioactive wastewater is difficult. The 4.5 billion tons of U(VI) in the ocean can be considered as a sustainable and environmentally friendly substitute of the uranium terrestrial supply to sustain the demands of the nuclear power industry in the future.2−4 The use of traditional adsorbents to recover U(VI) from seawater is very challenging because the concentration of U(VI) in seawater is very low (3.3 mg/L) and abundant competing ions (such as alkaline metals, alkaline earth metals, and heavy metals) coexist in seawater. Thereby, the development of adsorbents with a high affinity, excellent selective property, and simple preparation process is critical.5 From the initial inorganic adsorbents to the most studied polyamidoxime (PAO)-based adsorbents and to more recent metal–organic frameworks, great efforts have been devoted to developing efficient adsorbents for extracting U(VI) from seawater.
The properties of surface functional groups on sorbent surfaces determine its adsorption affinity and capacity. Among the various functional groups, phosphate groups (−PO4) have attracted increased attention and play an important role in actinide separation because of their excellent stability, strong irradiation resistance, and high complexation ability with actinide element ions (such as Pu(IV), Am(III), and U(VI)).1,6−11 Over the past years, various −PO4-functionalized adsorbents have been widely applied in U(VI) extraction from solution.1,8−19 However, −PO4-functionalized adsorbents are usually synthesized by chemical modification1,11−15 and polymerization techniques with appropriate organic reagents.8−10,16−19 To date, the development of a simple and efficient synthesis technique is important for the potential application of −PO4-functionalized adsorbents for U(VI) capture.
Herein, a new phosphate-functionalized polyethylene (PO4/PE) was synthesized by a simple Ar-jet plasma treatment of PE in H3PO4 and employed for U(VI) capture. PE was selected just because this is one of the most studied substrate materials in uranium extraction from seawater.
Results and Discussion
Characterization
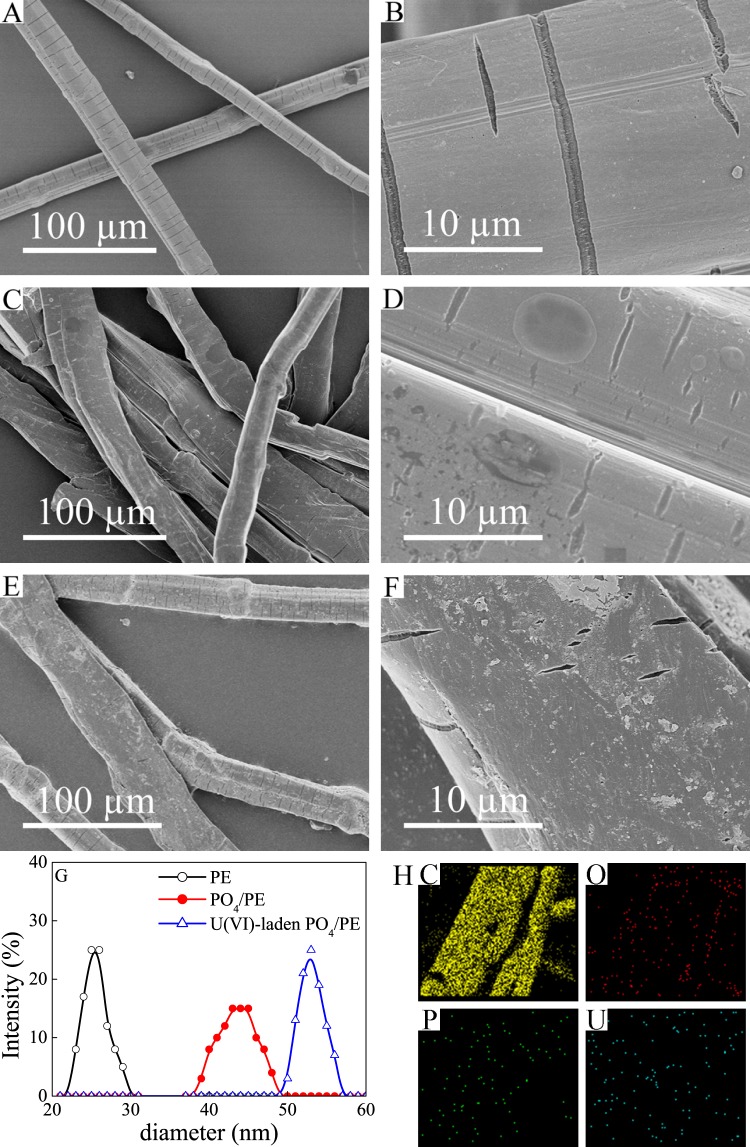
Scanning electron microscopy (SEM) photomicrographs of PE, PO4/PE, and PO4/PE after regenerating eight times are shown in Figure 1. The surface of PE (Figure 1A,B) is smooth with many microgrooves along the fiber, whereas that of PO4/PE (Figure 1C,D) is rough and covered with a thin layer. Meanwhile, no notable differences of PE surface morphology before and after immersion in H3PO4 are observed. Thereby, the thin layer is solid evidence for the successful induction of −PO4 on the surface of PE. Meanwhile, the sizes of PO4/PE become larger as compared to those of PE (Figure 1G), which can be ascribed to the penetration of water molecules into the PE polymeric structure and hydrolysis of the modified functional groups. After recycling eight times, PO4/PE (Figure 1E,F) still retained the stacking morphology as that of raw PO4/PE, indicating excellent stability and good reusability of PO4/PE. Elemental mapping images (Figure 1H) indicate that O, P, and U are homogeneously dispersed on the PO4/PE surface, which demonstrates the excellent adsorption capacity of PO4/PE for U(VI).
Figure 1.
SEM images of PE (A, B), PO4/PE (C, D), and PO4/PE after regenerating eight times (E, F). Particle size distributions of PE, PO4/PE, and U(VI)-laden PO4/PE (G) and elemental mappings of U(VI)-laden PO4/PE (H).
The mechanical properties of PO4/PE is an important parameter for its real application in U(VI) extraction from seawater, which was studied at its break point (Figure 2A). The mechanical properties of substrates are usually significantly reduced in traditional synthesis processes (such as the γ-ray irradiation and electron-beam irradiation techniques). Xing et al.20 reported that the tensile strength of a PE fiber diminished significantly from 3.0 × 109 to 1.3 × 109 Pa after γ-irradiation for 50 kGy. In our case, the tensile strengths are 1.64 × 109 and 1.63 × 109 Pa for PE and PO4/PE, respectively. It means that the side effect of the traditional synthesis method is well-eliminated in this work. To evaluate the effect of the introduction of −PO4 by plasma treatment on the PE framework, PE and PO4/PE are characterized by powder X-ray diffraction (XRD) pattern (Figure 2B). The fairly similar XRD patterns of PE and PO4/PE further demonstrate no significant structure damages of PE after Ar-jet plasma treatment in H3PO4.
Figure 2.
Tensile strengths (A); XRD patterns (B); Raman spectra (C); and X-ray photoelectron spectroscopy (XPS) P 2p (D), XPS C 1s (E), and XPS O 1s spectra (F) of PE, PO4/PE, and U-laden PO4/PE.
The effect of the introduction of −PO4 by plasma treatment on the PE surface was also studied by the Raman spectroscopy technique. As shown in Figure 2C, PE presents “fingerprint” Raman peaks of C–C vibrations at ∼1061 (asymmetric vibrations) and ∼1128 cm–1 (symmetric vibrations) and of −CH2– vibrations at ∼1293 (twisting vibrations in all crystalline phases) and ∼1415 cm–1 (wagging vibrations in the orthorhombic crystalline phase).21 According to the relative intensity ratio of the peak at ∼1415 cm–1 to that at ∼1293 cm–1,22 the degree of orthorhombic crystallinity of PE was decreased after the introduction of −PO4 by plasma treatment. It suggests that parts of the C–C/C–H bonds were broken, which react with H3PO4 to form −PO4 groups on the PE surface. In the high-frequency region, the peak intensity ratio of −CH2– (∼2846 cm–1) to −CH3 (∼2880 cm–1) decreases after the Ar-jet plasma treatment in H3PO4, which indicates that the formation of −PO4 groups on the PE surface is mainly by breaking C–H bonds in −CH2–.
To study the existing form of −PO4 groups on the PO4/PE surface and its U(VI) adsorption mechanism, PE, PO4/PE, and U-laden PO4/PE were studied by the XPS technique. The P 2p spectra (Figure 2D) of PO4/PE and U-laden PO4/PE are deconvoluted into three components (Table 1): −PO4, polyphosphate (poly(−PO4) that contains P–O–P bonds), and metaphosphates (P–O–U(VI), in this work) at 133.2 ± 0.1, 133.9 ± 0.1, and 135.1 ± 0.1 eV, respectively.23−25 Besides −PO4, poly(−PO4) is also an important component on the PO4/PE surface. After U(VI) adsorption, U-laden PO4/PE also showed new peaks centered at ∼135.1 eV, indicating the effective binding of U(VI) on the surface of PO4/PE in the form of P–O–U(VI) bonds. The decreased XPS P 2p peak intensity of U(VI)-laden PO4/PE can be explained by the broadening of the XPS P 2p spectrum and the coverage of bound U(VI) on the surface of U(VI)-laden PO4/PE. Meanwhile, parts of poly(−PO4) would decompose into soluble −PO4 during the U(VI) adsorption process, which would dissolve in the solution. The C 1s spectral (Figure 2E) intensities significantly decreased after the Ar-jet plasma treatment in H3PO4 and deconvoluted into five components (Table 2) at 283.4 ± 0.1, 284.9 ± 0.1, 286.2 ± 0.1, 287.3 ± 0.1, and 288.8 ± 0.1 eV, which can be assigned to carbide carbon; C–C; and carbon atoms bound to one (C–OH and C–O–P), two (C=O), and three (−COOH) oxygen atoms, respectively.24 The carbide C and graphite carbon (C–C) are the main species of carbon on the PE and PO4/PE surfaces. The contents of carbide carbon and C–OH decreased after the Ar-jet plasma treatment in H3PO4, which indicates that the −PO4 groups on the PE surface are introduced mainly via the cleavage of carbide C and C–OH. The O 1s spectra of PE and PO4/PE (Figure 2F) can be deconvoluted into four components at 531.0 ± 0.1, 532.5 ± 0.1, 535.0 ± 0.1, and 537.5 ± 0.1 eV, which are related to the double-bonded oxygen (C=O), single-bonded oxygen (C–O and −O–P in −PO4), chemisorbed oxygen and water, and −OH, respectively (Table 3).24 The double- and single-bonded oxygen species as the main O components in PE and PO4/PE significantly decreased and increased, respectively. It confirms the successful introduction of −PO4 on the PE surface. The increased content of chemisorbed O and −OH in U-laden PO4/PE can be ascribed to the adsorbed U(VI), which confirms the high adsorption capability of PO4/PE for U(VI).
Table 1. Curve Fitting Results of XPS P 2p Spectra.
| peak | positiona (eV) | FWHMb (eV) | % | |
|---|---|---|---|---|
| PO4/PE | –PO4 | 133.20 | 1.59 | 11.0 |
| poly(−PO4) | 134.00 | 2.83 | 89.0 | |
| U-laden PO4/PE | –PO4 | 133.20 | 2.40 | 72.4 |
| poly(−PO4) | 133.85 | 0.47 | 7.03 | |
| P–O–U(VI) | 135.08 | 1.43 | 20.5 |
Binding energy.
Full width at half-maximum.
Table 2. Curve Fitting Results of XPS C 1s Spectra.
| peak | position (eV) | FWHM (eV) | % | |
|---|---|---|---|---|
| PE | carbide C | 283.30 | 1.91 | 40.5 |
| C–C | 284.95 | 1.47 | 56.2 | |
| C–O | 286.30 | 2.72 | 2.88 | |
| C=O | 287.36 | 1.00 | 0.00 | |
| COOH | 288.70 | 1.97 | 0.45 | |
| PO4/PE | carbide C | 283.30 | 2.08 | 34.2 |
| C–C | 284.92 | 1.67 | 64.6 | |
| C–O | 286.10 | 1.00 | 0.00 | |
| C=O | 287.40 | 1.21 | 0.81 | |
| COOH | 288.70 | 1.05 | 0.36 | |
| U-laden PO4/PE | carbide C | 283.30 | 1.03 | 0.74 |
| C–C | 284.82 | 1.36 | 83.5 | |
| C–O | 286.10 | 1.16 | 11.03 | |
| C=O | 287.38 | 1.58 | 4.22 | |
| COOH | 288.70 | 2.04 | 0.54 |
Table 3. Curve Fitting Results of XPS O 1s Spectra.
| peak | position (eV) | FWHM (eV) | % | |
|---|---|---|---|---|
| PE | C=O, P=O | 531.00 | 2.43 | 63.2 |
| C–O, –O–P | 532.50 | 1.84 | 32.5 | |
| chemisorbed O | 535.00 | 1.68 | 2.37 | |
| –OH | 537.50 | 2.50 | 1.99 | |
| PO4/PE | C=O, P=O | 531.00 | 2.47 | 29.4 |
| C–O, –O–P | 532.55 | 2.67 | 69.6 | |
| chemisorbed O | 535.08 | 1.00 | 0.65 | |
| –OH | 537.50 | 1.00 | 0.35 | |
| U-laden PO4/PE | C=O, P=O | 531.10 | 1.64 | 21.8 |
| C–O, –O–P | 532.40 | 2.44 | 56.8 | |
| chemisorbed O | 535.00 | 2.53 | 7.86 | |
| –OH | 537.40 | 2.71 | 13.5 |
Adsorption
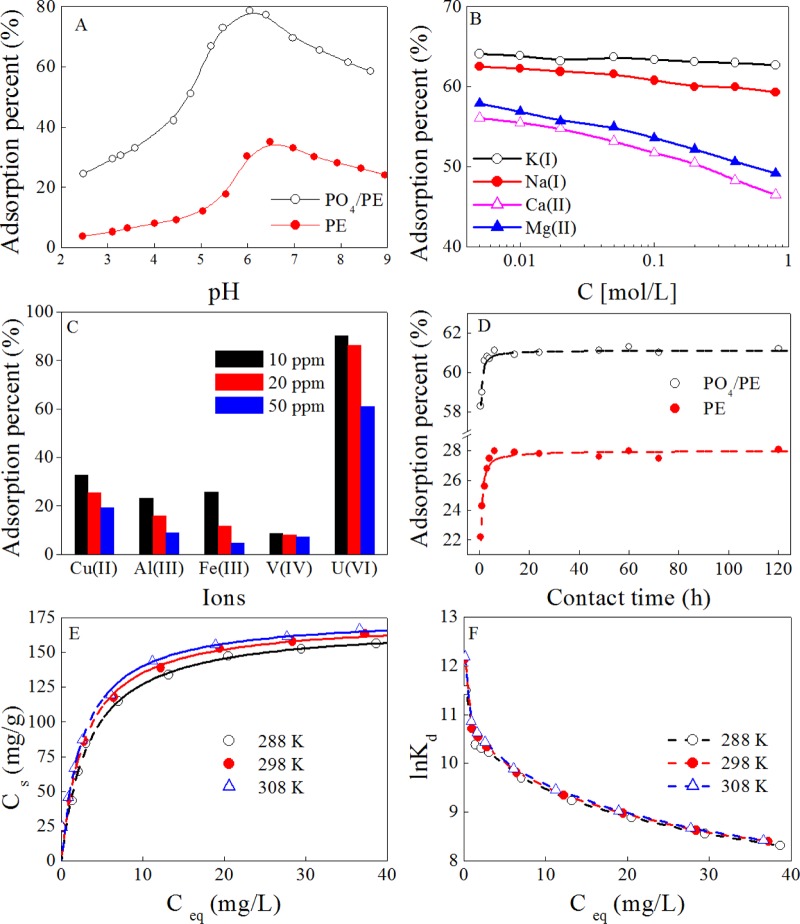
The prepared PO4/PE shows excellent performance in selective adsorption of U(VI) from seawater. As shown in Figure 3A, the introduced −PO4 groups increase the enrichment of U(VI) on PO4/PE under experimental conditions. Moreover, the adsorption of U(VI) on PE and on H3PO4-dispersed PE (data are not shown) is similar under the experimental uncertainties. Thereby, the enhanced U(VI) adsorption can be attributed to the strong complexation of U(VI) and −PO4 groups26 on the PE surface. Meanwhile, the adsorption U(VI) on PE and on PO4/PE increases with increasing pH and then slowly decreases with a further increase in pH. It reveals that U(VI) adsorption on the PO4/PE surface is fairly pH-dependent. The charges of the U(VI) species in the solution are affected by solution pH. Meanwhile, the available −PO4 groups gradually deprotonate with increasing solution pH.27
Figure 3.
Effect of pH (A), ionic strength (B), selective adsorption (C), and contact time (D) on and adsorption isotherms for (E) the adsorption of U(VI) from solution onto PE and PO4/PE, and the related Ce and ln Kd (F). m/V = 0.20 g/L. (A) T = 25 ± 1 °C, I = 0.1 mol/L NaCl, contact time: 24 h, and C[U(VI)]initial = 50.0 mg/L. (B) T = 25 ± 1 °C, pH = 8.2 ± 0.1, contact time: 24 h, and C[U(VI)]initial = 50.0 mg/L. (C) T = 25 ± 1 °C, pH = 8.2 ± 0.1, I = 0.1 mol/L NaCl, and contact time: 24 h. In (D), T = 25 ± 1 °C, pH = 8.2 ± 0.1, I = 0.1 mol/L NaCl, and C[U(VI)]initial = 50.0 mg/L. (E, F) pH = 8.2 ± 0.1, I = 0.1 mol/L NaCl, and contact time: 24 h.
It is well known that seawater is a very complex matrix and alkali metal ions (such as Na(I) and K(I)) and alkaline earth metal ions (such as Ca(II) and Mg(II)) are the predominant cations in seawater. To estimate the effect of ionic strength on PO4/PE adsorption capability for U(VI), the effects of those cations were studied using concentrations of 0.010–1.0 mol/L and are shown in Figure 3B. PO4/PE exhibits high selectivity for U(VI) against Na(I) and K(I) because the adsorption of U(VI) just slightly decreases with an increase in their concentrations. Ca(II) and Mg(II) have a more significant negative effect than that of Na(I) and K(I) on U(VI) adsorption, which can be explained by the complexation reaction among U(VI), CO32–, and alkaline earth metal ions in the solution.2,28−31 Many researchers found that these complexes determine the extraction behavior of U(VI) in seawater because of the high concentrations of Ca(II) and Mg(II).2,32 It is well known that many metal ions, such as Cu(II), Al(III), Fe(III), and V(IV), coexist with U(VI) in seawater and these metal ions result in serious interference in U(VI) separation. Therefore, the selective extraction capability of PO4/PE toward U(VI) was compared to that toward the coexisting metal ions. The results in Figure 3C show that U(VI) adsorption on the surface of PO4/PE is significantly higher than that of other metal ions under same experimental conditions, which reveals the high selectivity of PO4/PE toward U(VI) in seawater in comparison to that toward other metal ions, especially V(IV). A number of researchers1,33,34 reported a similar highly selective retention behavior of U(VI) on −PO4-functionalized adsorbents, and they explained it by the strong complexation of −PO4 groups with U(VI). Furthermore, the competition between U(VI) and V(IV) is one of the most serious challenges for the commercial application of most adsorbents in U(VI) recovery from seawater.3,4,35,36 Our results show that PO4/PE can tolerate V(IV) interference, highlighting the potential application of PO4/PE in the selective extraction of U(VI) from seawater.
The effect of reaction time on the adsorption of U(VI) by PO4/PE was studied because seawater temperature varies widely with the position and season. As depicted in Figure 3D, the adsorption percent increases quickly in the first 6 h and then maintains the level with the increasing reaction time. To further investigate the adsorption kinetics, the pseudo-first-order kinetic models (qt = qe × (1 – exp(−k1t)), where k1 (h–1) is the adsorption rate constant, qe (mg/g) and qt (mg/g) are the equilibrium and experimental adsorption capabilities, respectively) and pseudo-second-order kinetic models (qt = qe·t/(1/(K′·qe) + t), where K′ (g/(mg·h)) is the adsorption rate constant) are used to simulate the experimental data. The related parameters are shown in Table 4. According to correlation parameters (R2), the pseudo-second-order kinetic model provides a better description of adsorption data than the pseudo-first-order kinetic model. It clearly reveals that U(VI) adsorption on PE and PO4/PE can be considered as a chemisorption process through complexation with −PO4 groups under experimental conditions. Many researchers found that U(VI) adsorption on −PO4-functionalized adsorbents followed the pseudo-second-order kinetic model.8,10,11,37−39 Das et al.40 also reported that the adsorption of U(VI) on PAO adsorbents obeyed pseudo-first-order kinetics and pseudo-second-order kinetics at high and low initial concentrations of U(VI), respectively.
Table 4. Parameters for Kinetic Models of U(VI) Adsorption on PE and PO4/PE at pH = 8.2, T = 25 °C, m/V = 0.20 g/L, and C[U(VI)]initial = 50.0 mg/L.
| pseudo-first-order model |
pseudo-second-order model |
|||||
|---|---|---|---|---|---|---|
| k1 (h–1) | qe (mg–1) | R12 | K′ (g/(mg·h)) | qe (mg/g) | R22 | |
| PO4/PE | 6.27 | 151.9 | 0.622 | 0.114 | 153.1 | 0.999 |
| PE | 3.02 | 68.5 | 0.762 | 0.104 | 70.0 | 0.961 |
The maximum adsorption capability of PO4/PE for U(VI) was determined via adsorption isotherm. As depicted in Figure 3E, the adsorption isotherms show an increase with an increase in the initial concentration of U(VI) and reaction temperature. The commonly used Langmuir model (Cs = b × Csmax × Ce/(1 + b × Ce), where Csmax (mg/g) and b (L/mg) are the maximum adsorption capability and the Langmuir constant, respectively) and Freundlich model (Cs = K × Ce1/n, where K (mg/g) and 1/n are the constants indicative of the adsorption capability and intensity, respectively) are used to analyze the experimental data. The relative parameters are depicted in Table 5. According to the R2 values, the adsorption process followed the Langmuir model, which indicates that the adsorption of U(VI) on PO4/PE surfaces is localized in a monolayer. According to the analysis results, the maximum adsorption capacities (Csmax) of PO4/PE for U(VI) at pH 8.2 are ∼170.2, ∼173.8, and ∼176.7 mg/g at 288, 298, and 308 K, respectively, which are comparable to those of other adsorbents (Table 6), which highlighted the application of our method in preparing PO4/PE for selectively extracting U(VI) from seawater. The related distribution coefficient (Kd) and thermodynamic parameters are also calculated and are shown in Figure 3F and Table 7, respectively. The negative Gibbs free energy change (ΔG0), the positive value of entropy change (ΔS0), and the positive value of standard enthalpy change (ΔH0) reveal that U(VI) adsorption on the PO4/PE surface is an endothermic and spontaneous process.
Table 5. Parameters Calculated from the Langmuir and Freundlich Models for U(VI) Adsorption on PO4/PE at pH = 8.2.
| Langmuir
model |
Freundlich
model |
|||||
|---|---|---|---|---|---|---|
| Csmax (mg/g) | b (L/mg) | R2 | K (mg/g) | n | R2 | |
| 288 K | 170.2 | 0.296 | 0.990 | 54.3 | 0.313 | 0.945 |
| 298 K | 173.8 | 0.352 | 0.986 | 59.9 | 0.298 | 0.959 |
| 308 K | 176.7 | 0.383 | 0.987 | 62.7 | 0.293 | 0.954 |
Table 6. Comparison of the U(VI) Adsorption Capacity of PO4/PE with That of Other Adsorbents.
| adsorbent | experimental conditions | Csmax (mg/g) | refs |
|---|---|---|---|
| organosilica–phosphonate hybrids | pH = 4, T = 22 °C | 56 | (15) |
| PAO-reduced graphene oxide | pH = 4, T = 20 °C | 872 | (28) |
| mesoporous silica SBA-15 functionalized with phosphonate | pH = 4, T = 25 °C | 217 | (33) |
| phosphonate-functionalized mesoporous silica | pH = 6.9 | 306 | (37) |
| Mesoporous silica SBA-15 functionalized with phosphonate and amino groups | pH = 5.5, T = 11 °C | 240 | (38) |
| PAO-functionalized sorbent | pH = 7.6 | 380.8 | (40) |
| PO4/PE | pH = 8.2, T = 25 °C | 173.8 | this work |
Table 7. Thermodynamic Parameters for U(VI) Adsorption on PO4/PE at pH = 8.2.
| thermodynamic
parameters |
||||
|---|---|---|---|---|
| ln Kd | ΔG0 (kJ/mol) | ΔH0 (kJ/mol) | ΔS0 (J/(mol·K)) | |
| 288 K | 10.62 | –25.4 | 15.4 | 142 |
| 298 K | 10.93 | –27.1 | ||
| 308 K | 11.04 | –28.3 | ||
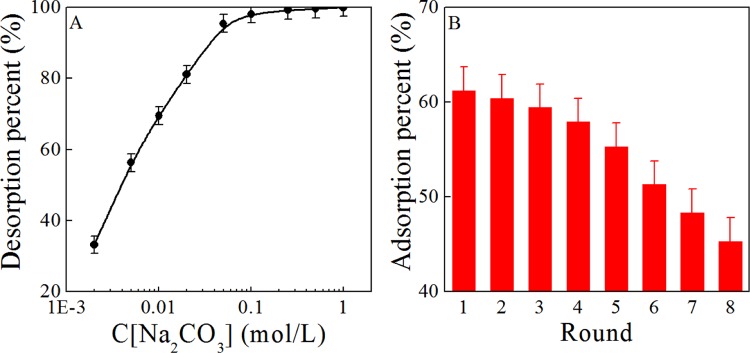
The regeneration–reusing property of PO4/PE was studied to further estimate its potential application. Na2CO3 is selected as the eluting agent, and the results are depicted in Figure 4A. The elution of U(VI) from the PO4/PE surface quickly increases with increasing Na2CO3 concentrations from 0.002 to 0.1 mol/L and then remains at this level. The result demonstrates the effective regeneration of PO4/PE in a 0.1 mol/L Na2CO3 solution. Thereby, the 0.1 mol/L Na2CO3 solution is selected for the regeneration of PO4/PE. The effect of recycling times on the adsorption capability of PO4/PE for U(VI) was studied and is shown in Figure 4B. The regenerated PO4/PE still exhibits high adsorption capability for U(VI) even after regenerating eight times under experimental conditions, which reveals the excellent reusability property of PO4/PE in the extraction of U(VI) from seawater.
Figure 4.
Effect of Na2CO3 concentration (A) on eluting U(VI) from the PO4/PE surface. Recycling application of PO4/PE in U(VI) adsorption (B). T = 25 ± 1 °C, contact time: 24 h, C[U(VI)]initial = 50.0 mg/L, m/V = 0.20 g/L, pH = 8.2 ± 0.1, and I = 0.1 mol/L NaCl.
Because of the attractive adsorption capability of PO4/PE for U(VI), the real application of PO4/PE in U(VI) extraction from deionized and real waters was studied, and the results are shown in Table 8. First, we studied the performance of PO4/PE in the restoration of deionized water (m/V = 0.20 g/L, pH = 8.2) in which 5–50 mg/L U(VI) was intentionally added. PO4/PE can quantitatively extract mg/L levels of U(VI) from the aqueous solution. Encouraged by the above results, we then studied the adsorption capability of PO4/PE in extraction of U(VI) from real water. The aqueous solution from Chaohu lake was selected and μg/L level of U(VI) was intentionally added. PO4/PE still showed excellent adsorption efficiency for μg/L levels of U(VI). Thereby, the real application of PO4/PE in the extraction of U(VI) from seawater was performed and the seawater from East China Sea was used. The reaction temperature, contact time, PO4/PE mass, and seawater volume were 25 ± 1 °C, 24 h, 200 mg, and 100 mL, respectively. The adsorption efficiency of ∼39% for 3.8 μg/L U(VI) in seawater reveals the excellent adsorption efficiency of PO4/PE in real separation of trace U(VI) from seawater.
Table 8. Selected Results of U(VI) Adsorption on the PO4/PE Surface.
|
C[U(VI)] (μg/L) |
|||||
|---|---|---|---|---|---|
| sample | pH | m/V (g/L) | initial | final | adsorption (%) |
| deionized water | 8.2 | 0.2 | 50 000 | 19 500 | 61.0 |
| 8.2 | 0.2 | 20 000 | 2810 | 86.0 | |
| 8.2 | 0.2 | 10 000 | 1000 | 90.0 | |
| 8.2 | 0.2 | 1000 | 102 | 89.8 | |
| Chaohu lake watera | 8.2 | 0.2 | 1000 | 195 | 80.5 |
| 8.2 | 2.0 | 100 | 31.2 | 68.8 | |
| 8.2 | 2.0 | 10 | 4.14 | 58.6 | |
| contaminated seawaterb | 8.2 | 2.0 | 100 | 37.8 | 62.2 |
| 8.2 | 2.0 | 10 | 5.2 | 48.0 | |
| original seawaterb | 8.2 | 2.0 | 3.8 | 2.3 | 39.4 |
The main composition of Chaohu lake water was 6.6 mg/L Na(I), 1.7 mg/L K(I), 34 mg/L Ca(II), 7.6 mg/L Mg(II), 3.1 mg/L Cl–, 12 mg/L SO42–, and 141 mg/L HCO3–. The pH values of the suspensions were kept at the initial value by adding negligible amount of NaOH solutions.
The main composition of seawater was 9.8 g/L Na(I), 0.36 g/L K(I), 0.42 g/L Ca(II), 0.93 g/L Mg(II), 17 g/L Cl–, 2.1 g/L SO42–, and 0.12 g/L HCO3–. The pH values of the suspensions were kept at the initial value by adding negligible amount of NaOH solutions.
Conclusions
From the results of PO4/PE characterization and U(VI) adsorption on PO4/PE under different experimental conditions, the following conclusions can be drawn: (1) A PO4/PE adsorbent with high adsorption capacity for U(VI) was synthesized by a simple and efficient Ar-jet plasma treatment technique. (2) A plausible reaction mechanism is proposed and confirmed based on microscopic and spectroscopic characterization. (3) PO4/PE exhibits excellent adsorption capability for U(VI) in seawater (maximum adsorption capacity of 173.8 mg/g at pH 8.2 and 298 K and regeneration–reuse property). The results in this work highlight the application of PO4/PE as an adsorbent in the extraction of U(VI) from seawater.
Experimental Section
Synthesis of PO4/PE
The PE fiber (TYZ Safetex FT-103) was purchased from Beijing Tongyizhong Advanced Material Company. PO4/PE was synthesized by the Ar-jet plasma treatment of PE in H3PO4. Briefly, 5.0 g of PE and 100 mL of 85% H3PO4 were added into a 250 mL round-bottom flask and treated by Ar-jet plasma (high-purity Ar) for 60 min at room temperature and atmospheric pressure under continuous stirring. The Ar-jet plasma conditions were as follows: Ar of 200 sccm, voltage of 5000 V, and electrical current of 1.0 mA. The PE fibers floated on the surface of 85% H3PO4 initially and were gradually dispersed in the H3PO4 solution with the progress of Ar plasma treatment. The obtained material was washed with Milli-Q water after the Ar-jet plasma treatment and dried at 60 °C for 24 h. To evaluate and compare the effects of Ar-jet plasma treatment, H3PO4-dispersed PE was also synthesized by the same method. The diagram of the Ar-jet plasma apparatus used in this work is shown in scheme 1.
Scheme 1. Schematic Diagram of the Synthesis of PO4/PE.

Characterization
The physiochemical properties of PO4/PE were characterized by SEM, element distribution mapping, tensile strength and elongation measurement, powder XRD, Raman spectroscopy, and XPS in detail. SEM images and element distribution mapping were obtained by a field emission-SEM (JSM-6320F; JEOL). The tensile strengths and elongation properties were measured on a tensile tester (MTS criterion model 43-3041E). The powder XRD pattern was collected by XRD (Rigaku D/max 2550) at ambient temperature. Raman spectroscopy analysis was performed by a Raman spectrometer (LabRam HR). XPS spectroscopies were performed by a surface microanalysis system (ESCALab220i-XL; VG Scientific) equipped with an Al Kα (hλ = 1486.6 eV) source at a chamber pressure of 3 × 10–9 mbar. The surface charging effects were corrected with the C 1s peak at 284.4 eV as a reference.
U(VI) Adsorption on PO4/PE and on PE
To evaluate the effect of −PO4 group functionalization by the Ar-jet plasma treatment on the adsorption capability of PE toward U(VI), U(VI) adsorption on PO4/PE and PE was studied by the batch adsorption technique. After the adsorbent and salinity (such as NaCl) were pre-equilibrated for 24 h, the U(VI) solution (UO2CO3) and Milli-Q water were added to achieve the desired components and the pHs of the suspensions were adjusted by the corresponding acid and basic solutions. After shaking for 48 h, the supernatant was filtered by 0.45 μm membrane filters. The final U(VI) concentrations (of mg/L and μg/L levels) in the supernatants were measured on an Optima 2100 DV inductively coupled plasma (ICP) atomic emission spectroscopy system (Perkin Elmer) and on an ICP mass spectroscopy (ICP-MS, Thermo Scientific X-Series II) system, respectively.
Regeneration–Reuse of PO4/PE
Na2CO3 was selected for the regeneration of PO4/PE. About 25 mg of U-laden PO4/PE was regenerated in 50 mL of Na2CO3 solution for 24 h followed by rinsing with water and drying at 60 °C. Thus, regenerated PO4/PE was obtained and reused in following experiments.
Acknowledgments
Financial supports from the NSAF (U1530131), the National Natural Science Foundation of China (11675210), the Radiochemistry 909 Project in the China Academy of Engineering Physics, the Science and Technology Development Foundation of China Academy of Engineering Physics (2014B0301034), and the China Postdoctoral Science Foundation (2015M582769XB and 2016T90872) are acknowledged.
The authors declare no competing financial interest.
References
- Wei Y.; Zhang L.; Shen L.; Hua D. Positively charged phosphonate-functionalized mesoporous silica for efficient uranium sorption from aqueous solution. J. Mol. Liq. 2016, 221, 1231–1236. 10.1016/j.molliq.2015.04.056. [DOI] [Google Scholar]
- Endrizzi F.; Leggett C. J.; Rao L. Scientific basis for efficient extraction of uranium from seawater. I: understanding the chemical speciation of uranium under seawater conditions. Ind. Eng. Chem. Res. 2016, 55, 4249–4256. 10.1021/acs.iecr.5b03679. [DOI] [Google Scholar]
- Mehio N.; Johnson J. C.; Dai S.; Bryantsev V. S. Theoretical study of the coordination behavior of formate and formamidoximate with dioxovanadium(V) cation: implications for selectivity towards uranyl. Phys. Chem. Chem. Phys. 2015, 17, 31715–31726. 10.1039/C5CP06165B. [DOI] [PubMed] [Google Scholar]
- Mehio N.; Ivanov A. S.; Ladshaw A. P.; Dai S.; Bryantsev V. S. Theoretical study of oxovanadium(IV) complexation with formamidoximate: implications for the design of uranyl-selective adsorbents. Ind. Eng. Chem. Res. 2016, 55, 4231–4240. 10.1021/acs.iecr.5b03398. [DOI] [Google Scholar]
- Kim J. S.; Han K. S.; Kim S. J.; Kim S. D.; Lee J. Y.; Han C.; Kumar J. R. Synergistic extraction of uranium from Korean black shale ore leach liquors using amine with phosphorous based extractant systems. J. Radioanal. Nucl. Chem. 2016, 307, 843–854. 10.1007/s10967-015-4327-7. [DOI] [Google Scholar]
- Zheng T.; Wu Q. Y.; Gao Y.; Gui D.; Qiu S.; Chen L.; Sheng D.; Diwu J.; Shi W. Q.; Chai Z.; Albrecht-Schmitt T. E.; Wang S. Probing the influence of phosphonate bonding modes to uranium(VI) on structural topology and stability: a complementary experimental and computational investigation. Inorg. Chem. 2015, 54, 3864–3874. 10.1021/acs.inorgchem.5b00024. [DOI] [PubMed] [Google Scholar]
- Knope K. E.; Cahill C. L. Synthesis and characterization of 1-, 2-, and 3-dimensional bimetallic UO22+/Zn2+ phosphonoacetates. Eur. J. Inorg. Chem. 2010, 2010, 1177–1185. 10.1002/ejic.200901080. [DOI] [Google Scholar]
- Budnyak T. M.; Strizhak A. V.; Gładysz-Płaska A.; Sternik D.; Komarov I. V.; Kołodyńska D.; Majdanc M.; Tertykh V. A. Silica with immobilized phosphinic acid-derivative for uranium extraction. J. Hazard. Mater. 2016, 314, 326–340. 10.1016/j.jhazmat.2016.04.056. [DOI] [PubMed] [Google Scholar]
- Abney C. W.; Das S.; Mayes R. T.; Kuo L. J.; Wood J.; Gill G.; Piechowicz M.; Lin Z.; Lin W.; Dai S. A report on emergent uranyl binding phenomena by an amidoxime phosphonic acid co-polymer. Phys. Chem. Chem. Phys. 2016, 18, 23462–23468. 10.1039/C6CP04772F. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Yang S.; Zhang L.; Hua D. Phosphonate-functionalized polystyrene microspheres with controlled zeta potential for efficient uranium sorption. RSC Adv. 2016, 6, 74110–74116. 10.1039/C6RA16219C. [DOI] [Google Scholar]
- Wang R.; Ye J.; Rauf A.; Wu X.; Liu H.; Ning G.; Jiang H. Microwave-induced synthesis of pyrophosphate Zr1–xTixP2O7 and TiP2O7 with enhanced sorption capacity for uranium(VI). J. Hazard. Mater. 2016, 315, 76–85. 10.1016/j.jhazmat.2016.03.092. [DOI] [PubMed] [Google Scholar]
- Liu X.; Li J.; Wang X.; Chen C.; Wang X. High performance of phosphate-functionalized graphene oxide for the selective adsorption of U(VI) from acidic solution. J. Nucl. Mater. 2015, 466, 56–64. 10.1016/j.jnucmat.2015.07.027. [DOI] [Google Scholar]
- Alexandratos S. D.; Zhu X. Bifunctional coordinating polymers: auxiliary groups as a means of tuning the ionic affinity of immobilized phosphate ligands. Macromolecules 2005, 38, 5981–5986. 10.1021/ma050057b. [DOI] [Google Scholar]
- Trochimczuk A. W. Synthesis of functionalized phenylphosphinic acid resins through Michael reaction and their ion-exchange properties. React. Funct. Polym. 2000, 44, 9–19. 10.1016/S1381-5148(99)00072-3. [DOI] [Google Scholar]
- Lebed P. J.; Savoie J. D.; Florek J.; Bilodeau F.; Larivière D.; Kleitz F. Large pore mesostructured organosilica-phosphonate hybrids as highly efficient and regenerable sorbents for uranium sequestration. Chem. Mater. 2012, 24, 4166–4176. 10.1021/cm3023709. [DOI] [Google Scholar]
- Canniccioni B.; Monge S.; David G.; Robin J. J. RAFT polymerization of dimethyl(methacryloyloxy)-methyl phosphonate and its phosphonic acid derivative: a new opportunity for phosphorus-based materials. Polym. Chem. 2013, 4, 3676–3685. 10.1039/c3py00426k. [DOI] [Google Scholar]
- Qiu Q.; Liu G.; An Z. Efficient and versatile synthesis of star polymers in water and their use as emulsifiers. Chem. Commun. 2011, 47, 12685–12687. 10.1039/c1cc15679a. [DOI] [PubMed] [Google Scholar]
- Pan X.; Cao J.; Wang Y.; Huang W.; Hua D.; Zhu X.; Liang H. Synthesis of surface-functionalized polystyrene sub-micron spheres using novel amphiphilic comonomer. Polymer 2012, 53, 3508–3513. 10.1016/j.polymer.2012.05.058. [DOI] [Google Scholar]
- Hua D.; Tang J.; Jiang J.; Zhu X.; Bai R. A facile approach for preparation of phenylphosphinic acid-functionalized PST microspheres by emulsion polymerization using amphiphilic Macro-RAFT agent as emulsifier. Macromolecules 2009, 42, 8697–8701. 10.1021/ma9018334. [DOI] [Google Scholar]
- Xing Z.; Hu J.; Wang M.; Zhang W.; Li S.; Gao Q.; Wu G. Properties and evaluation of amidoxime-based UHMWPE fibrous adsorbent for extraction of uranium from seawater. Sci. China: Chem. 2013, 56, 1504–1509. 10.1007/s11426-013-5002-x. [DOI] [Google Scholar]
- Alamo R. G.; Jeon K.; Smith R. L.; Boz E.; Wagener K. B.; Bockstaller M. R. Crystallization of polyethylenes containing chlorines: precise Vs random placement. Macromolecules 2008, 41, 7141–7151. 10.1021/ma801152p. [DOI] [Google Scholar]
- Romero G.; Estrela-Lopis I.; Zhou J.; Rojas E.; Franco A.; Espinel C. S.; Fernández A. G.; Gao C.; Donath E.; Moya S. E. Surface engineered poly(lactide-co-glycolide) nanoparticles for intracellular delivery: uptake and cytotoxicitysa confocal raman microscopic study. Biomacromolecules 2010, 11, 2993–2999. 10.1021/bm1007822. [DOI] [PubMed] [Google Scholar]
- Kim M. J.; Jeon I. Y.; Seo J. M.; Dai L.; Baek J. B. Graphene phosphonic acid as an efficient flame retardant. ACS Nano 2014, 8, 2820–2825. 10.1021/nn4066395. [DOI] [PubMed] [Google Scholar]
- Puziy A. M.; Poddubnaya O. I.; Socha R. P.; Gurgul J.; Wisniewski M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. 10.1016/j.carbon.2008.09.010. [DOI] [Google Scholar]
- Siow K. S.; Britcher L.; Kumar S.; Griesser H. J. Deposition and XPS and FTIR analysis of plasma polymer coatings containing phosphorus. Plasma Processes Polym. 2014, 11, 133–141. 10.1002/ppap.201300115. [DOI] [Google Scholar]
- Gao L.; Yang Z.; Shi K.; Wang X.; Guo Z.; Wu W. U(VI) sorption on kaolinite: effects of pH, U(VI) concentration and oxyanions. J. Radioanal. Nucl. Chem. 2010, 284, 519–526. 10.1007/s10967-010-0510-z. [DOI] [Google Scholar]
- Jerden J. L. Jr.; Sinha A. K. Geochemical coupling of uranium and phosphorous in soils overlying an unmined uranium deposit: Coles Hill, Virginia. J. Geochem. Explor. 2006, 91, 56–70. 10.1016/j.gexplo.2005.12.003. [DOI] [Google Scholar]
- Shao D.; Li J.; Wang X. Poly(amidoxime)- reduced graphene oxide composites as adsorbents for the enrichment of uranium from seawater. Sci. China: Chem. 2014, 57, 1449–1458. 10.1007/s11426-014-5195-7. [DOI] [Google Scholar]
- Shao D.; Ren X.; Wen J.; Hu S.; Xiong J.; Jiang T.; Wang X.; Wang X. Immobilization of uranium by biomaterial stabilized FeS nanoparticles: Effects of stabilizer and enrichment mechanism. J. Hazard. Mater. 2016, 302, 1–9. 10.1016/j.jhazmat.2015.09.043. [DOI] [PubMed] [Google Scholar]
- Endrizzi F.; Rao L. Chemical speciation of uranium(VI) in marine environments: complexation of calcium and magnesium ions with [(UO2)(CO3)3]4– and the effect on the extraction of uranium from seawater. Chem. - Eur. J. 2014, 20, 14499–14506. 10.1002/chem.201403262. [DOI] [PubMed] [Google Scholar]
- Dong W.; Brooks S. C. Determination of the formation constants of ternary complexes of uranyl and carbonate with alkaline earth metals (Mg2+, Ca2+, Sr2+, and Ba2+) using anion exchange method. Environ. Sci. Technol. 2006, 40, 4689–4695. 10.1021/es0606327. [DOI] [PubMed] [Google Scholar]
- Baghdadi S.; Bouvier-Capely C.; Ritt A.; Peroux A.; Fevrier L.; Rebiere F.; Agarande M.; Cote G. Impact of the uranium(VI) speciation in mineralized urines on its extraction by calix6 arene bearing hydroxamic groups used in chromatography columns. Talanta 2015, 144, 875–882. 10.1016/j.talanta.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhu L.; Guo B.; Chen S.; Wu W. Mesoporous silica SBA-15 functionalized with phosphonate derivatives for uranium uptake. New J. Chem. 2014, 38, 3853–3861. 10.1039/C3NJ01494K. [DOI] [Google Scholar]
- Chi F.; Wang X.; Xiong J.; Hu S. Polyvinyl alcohol fibers with functional phosphonic acid group: synthesis and adsorption of uranyl(VI) ions in aqueous solutions. J. Radioanal. Nucl. Chem. 2013, 296, 1331–1340. 10.1007/s10967-012-2303-z. [DOI] [Google Scholar]
- Das S.; Pandey A. K.; Vasudevan T.; Athawale A. A.; Manchanda V. K. Adsorptive preconcentration of uranium in hydrogels from seawater and aqueous solutions. Ind. Eng. Chem. Res. 2009, 48, 6789–6796. 10.1021/ie801912n. [DOI] [Google Scholar]
- Kelley S. P.; Barber P. S.; Mullins P. H.; Rogers R. D. Structural clues to UO22+/VO2+ competition in seawater extraction using amidoxime-based extractants. Chem. Commun. 2014, 50, 12504–12407. 10.1039/C4CC06370H. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Liu Y.; Shi W.; Lv Y.; Lan J.; Zhao Y.; Chai Z. High performance of phosphonate-functionalized mesoporous silica for U(VI) sorption from aqueous solution. Dalton Trans. 2011, 40, 7446–7453. 10.1039/c1dt10085h. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yuan L.; Wang Y.; Li Z.; Lan J.; Liu Y.; Feng Y.; Zhao Y.; Chai Z.; Shi W. Mesoporous silica SBA-15 functionalized with phosphonate and amino groups for uranium uptake. Sci. China: Chem. 2012, 55, 1705–1711. 10.1007/s11426-012-4625-7. [DOI] [Google Scholar]
- Cao Q.; Liu Y.; Wang C.; Cheng J. Phosphorus-modified poly(styrene-co-divinylbenzene)-PAMAM chelating resin for the adsorption of uranium(VI) in aqueous. J. Hazard. Mater. 2013, 263, 311–321. 10.1016/j.jhazmat.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Das S.; Pandey A. K.; Athawale A. A.; Manchanda V. K. Exchanges of uranium(VI) species in amidoxime-functionalized sorbents. J. Phys. Chem. B 2009, 113, 6328–6335. 10.1021/jp8097928. [DOI] [PubMed] [Google Scholar]