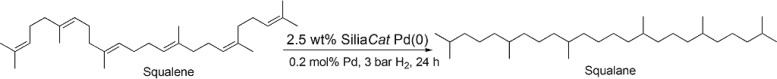Abstract
Squalene is selectively and entirely converted into squalane over the spherical sol–gel-entrapped Pd catalyst SiliaCat Pd(0) under solvent-free and mild reaction conditions of 3 bar H2 and 70 °C. The catalyst was reused successfully in eight consecutive cycles, with palladium leaching values <2 ppm, opening the route to sustainable and less-expensive hydrogenation of phytosqualene with important sustainability consequences.
Introduction
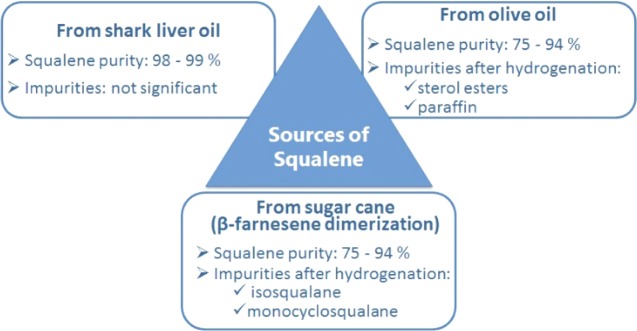
Obtained by full hydrogenation of the highly unsaturated all-(E) linear terpenoid squalene ((6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene), an immune system stimulant and anticarcinogenic and antioxidant agent widely used in traditional medicine and nutraceutical products,1 squalane (C30H62) is a clear and nonirritant oil widely and increasingly employed by the cosmetic, pharmaceutical, and nutraceutical industries.2 For example, thanks to its exceptional capacity to penetrate and impart flexibility to the human skin, as well as to vehiculate and increase the absorption of other active substances,3 squalane is used to formulate top cosmetic products including creams, hair conditioners, lotions, lipsticks, sunscreens, bath oils, and foundations. Today, this valued substance is mainly derived from vegetable sources including olive oil (46% of the overall market share in 2015)4 and biotechnology squalene from sugarcane (10%), even though a significant fraction (40%) is still obtained from deep sea shark liver oil (Scheme 1).
Scheme 1. Squalene Purity and Composition Issues, Depending on Its Origin.
Sugarcane squalene is obtained via Pd-catalyzed dimerization of sesquiterpene (E)-β-farnesene derived from sucrose fermentation over genetically engineered strains of Saccharomyces cerevisiae,5 affording a consistent composition of 94% phytosqualane along with 3.5% isosqualane and 2.5% monocyclosqualane.6Table 1 summarizes the advantages and disadvantages of heterogeneously catalyzed hydrogenation processes carried out in industry over the commonly used supported metal catalysts.
Table 1. Advantages and Disadvantages of Industrial Squalene Hydrogenation over Commonly Used Supported Metal Catalyst.
| heterogeneous catalysts | industrial hydrogenation of squalene | advantage/disadvantage |
|---|---|---|
| Pt catalyst11,12 | shark-liver squalene: in diethyl ether under H2 pressure solvent-free, 1 bar H2, 190 °C | high amount of expensive Pt catalyst toxic solvent |
| Ni-Raney13 | shark-liver squalene: solvent-free, 10 bar H2, 170 °C, 3 h | low-cost Ni catalyst harsher conditions in presence or in absence of solvent |
| Ni-kieselguhr8 | shark-liver squalene: solvent-free, 4 bar H2, 200 °C, 3–4 h | high level of the Ni leached into the squalane product |
| Pricat Ni 61/15P (Ni/alumina-kieselguhr)14 | vegetable squalene: in 2-PrOH, 24 bar H2, 150 °C, 16 h | extensive purification is required to remove most of the Ni leached |
| Pd/C14 | vegetable squalene: in heptane, 58 bar H2, 80 °C, 16 h in 2-PrOH, 150 bar H2, 160 °C | harsher conditions |
Accounting for about 40% of the overall production cost,7 the squalene hydrogenation process is carried out over nickel-kieselguhr catalyst under the solvent-free reaction conditions of 4 bar H2 at 200 °C8 or over more costly Pd/C of 70 bar H2 at 160 °C. In the former case, extensive leaching of Ni requires prolonged purification of the hydrogenated product over silica and other adsorbent materials to meet the maximum acceptable levels of highly toxic nickel compounds (Ni2+ and Ni0) in a cosmetic product (0.2 ppm). In the latter, the surface-derivatized nature of conventional commercial Pd/C catalyst leads to a rapid decrease in the catalytic performance.9 Furthermore, if squalene originating from olive oil is used,10 the inevitable presence of residual waxes requires first a “winterization” step to precipitate wax, followed by hydrogenation in two steps: first under 5 bar of H2 pressure for 3–4 h, followed by another 3 h at 30 bar H2 to afford complete saturation.
We recently reported that the heterogeneous hydrogenation of squalene dissolved in ethanol over SiliaCat Pd(0) selectively affords high yields of squalane using an ultralow amount of palladium loading (0.02 mmol/g of squalene) under remarkably mild conditions (1 bar H2 30 °C, or 50 °C when shorter reaction times are needed).15 Comprising organosilica catalyst doped with Pd(0) nanocrystals, the catalyst is reusable, with hydrogenation of a lesser pure olive oil squalene (82 wt %) requiring somewhat harsher reaction conditions.
Shortly afterwards, Soni and Sharma in India reported the excellent performance of a Pd/clay catalyst under solvent-free conditions at 200 °C under 4 bar H2.16 Complete conversion of squalene to squalane was achieved after 6 h with a Pd loading of approximately 0.06 mmol/g of squalene. Under the said optimized reaction conditions, the catalyst was remarkably stable and an ultralow amount of Pd leached into the product (0.0311 ppm), which established the best squalene hydrogenation catalyst ever reported. Now, we show how the full hydrogenation of all-E linear squalene into valued squalane is achieved over the spherical sol–gel-entrapped Pd catalyst SiliaCat Pd(0) under solvent-free and mild reaction conditions (3 bar H2 and 70 °C) over a 0.2 mol % Pd loading in the form of 2.5 wt % SiliaCat Pd(0) catalyst (Scheme 2) using two squalenes of a different origin and purity.
Scheme 2. Solvent-Free Hydrogenation of Squalene over SiliaCat Pd(0) under Optimized Reaction Conditions.
The catalyst is extensively reusable, with palladium leaching values <2 ppm, opening the route to an easier and low-cost hydrogenation of squalene.
Results and Discussion
SiliaCat Pd(0) is a heterogeneous catalyst obtained via the alcohol-free sol–gel polycondensation of alkoxysilanes such as methyltrimethoxysilane (MTES).17 The hydrophobic character of the organosilica support protects the Pd nanoparticles (NPs) against oxidation, increases the stability against moisture, and increases the catalytic activity of the catalyst under mild conditions.18,19
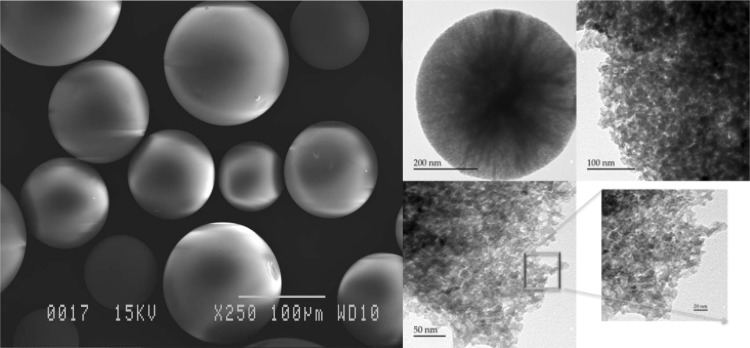
Following advances anticipated in 2011 for which the spherical morphology of the silica microparticles could be beneficial to catalysis,20 the catalyst is now available in spherical morphology (Figure 1, left).
Figure 1.
Scanning electron microscopy (left) and transmission electron microscopy (TEM) (right) images of SiliaCat Pd(0) spherical palladium catalyst used throughout this study.
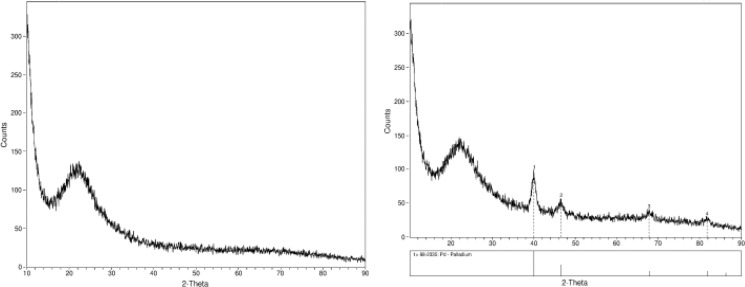
The newly prepared catalyst comprises catalytically active Pd0 NPs encapsulated within a spherical mesoporous organically modified silica matrix. The presence of highly dispersed average 3.1 nm palladium NPs was clearly revealed by the TEM analysis (Figure 1, right). The crystalline nature of the active nanophase is evident from the X-ray diffraction (XRD) pattern of the catalyst powder (Figure 2, right) characteristic of the face-centered cubic (fcc) structure of metallic Pd for which a typical 2.6 nm crystal domain was calculated using the Debye–Scherrer equation21 from the line broadening of majority (111) reflections (Table 2).
Figure 2.
Powder XRD patterns of MeSiO1.5 blank material (left) and the Pd/MeSiO1.5 NP organosilica catalyst SiliaCat Pd(0) (right).
Table 2. Load and Pd NP Size of the Spherical SiliaCat Pd(0).
| catalyst | palladium loading (wt %) | TEM (NP size) | XRD (NP size) |
|---|---|---|---|
| MeSiO1.5 | |||
| SiliaCat Pd(0) | 2.5 | 3.1 nm | 2.6 nm |
| (Pd0/MeSiO1.5) |
The amorphous nature of the spherical 100% MeSiO1.5 undoped material used as entrapping NPs matrix was confirmed by the characteristic wide XRD diffractogram of the blank spherical MeSiO1.5 xerogel devoid of metal NPs (Figure 2, left).
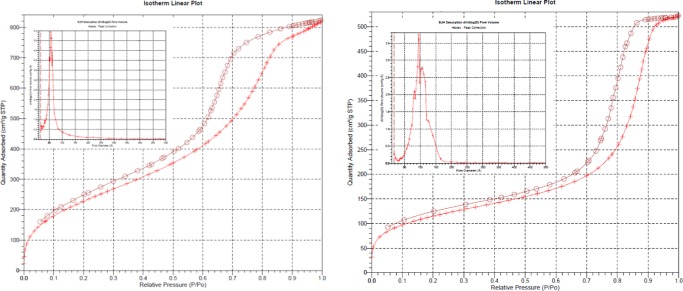
The Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) values of specific surface area, pore size, and pore volume of undoped organosilica microspheres and SiliaCat Pd(0) catalyst are given in Table 3.
Table 3. Textural Properties of Spherical SiliaCat Pd(0) and Blank Support.
| N2 adsorption isotherms |
||||
|---|---|---|---|---|
| catalyst | Pd loading (wt %) | BET surface (m2/g) | pore volume (cm3/g) | pore size (nm) |
| MeSiO1.5 | 835 | 1.27 | 6.1 | |
| SiliaCat Pd(0) | 2.5 | 402 | 0.8 | 8.0 |
| Pd0/MeSiO1.5 | ||||
The type IV N2 adsorption–desorption isotherms of both materials are typical of mesoporous materials.22 The organosilica blank (Figure 3, left) presents a large BET surface area (>800 m2/g) and 6.0 nm average pore diameter with a narrow size distribution of mesopores capable of adsorbing a large volume of cryogenic nitrogen (>1.2 cm3/g). On the other hand, the Pd/MeSiO1.5 catalyst (Figure 3, right) has a lower BET surface area (average 400 m2/g) and a broader pore size distribution centered at 8.0 nm.
Figure 3.
N2 adsorption and desorption isotherms and BJH desorption pore size distribution of MeSiO1.5 blank support (left) and spherical SiliaCat Pd(0) (right).
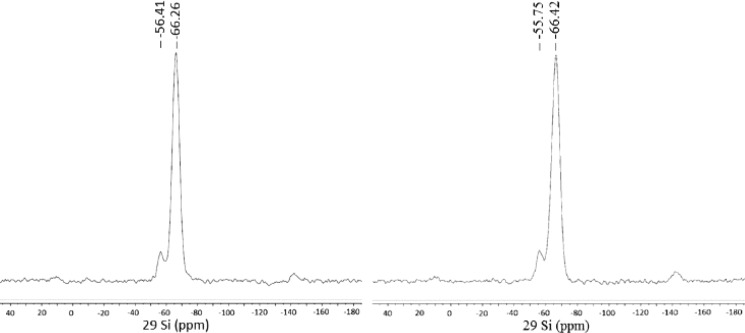
The 29Si magic angle spinning (MAS) NMR spectra of the support and the catalyst in Figure 4 were analyzed according to the chemical shifts of organotrialkoxysilanes for which the Si atoms appear as Tn bands type, where n is the number of siloxane bonds of the Si atom.23 The absence of any signal at −40 ppm related to ethoxy groups derived from nonhydrolyzed MTES and the presence of signals only at −56 ppm (T2, MeSi(OSi)2OH) and −66 ppm (T3, MeSi(OSi)3) correspond to almost complete hydrolysis of the MTES organosiloxane precursor.24
Figure 4.
29Si MAS NMR spectra of MeSiO1.5 blank support (left) and spherical SiliaCat Pd(0) (right).
Effect of Reaction Temperature
Table 4 shows the outcomes of the solvent-free hydrogenation of squalene under the conditions of Scheme 2 using squalene of a different origin and purity (98 wt % from Sigma-Aldrich and 82 wt % from an olive squalene manufacturer).
Table 4. Conversion, Selectivity, and Metal Leaching Values in the Solvent-Free Hydrogenation of Squalene of Different Purities over SiliaCat Pd(0)a.
| leachingc (mg/kg) |
|||||
|---|---|---|---|---|---|
| entry | squalene (purity, %) | T (°C) | conversion/selectivityb (%) | Pd | Si |
| 1 | 98 | 50 | 100/45 | ||
| 2 | 98 | 70 | 100/99 | 1.57 | 1.98 |
| 3 | 82 | 70 | 100/39 | 1.00 | 2.01 |
| 4 | 82 | 80 | 100/51 | 0.99 | 0.59 |
| 5 | 82 | 100 | 100/70 | 0.39 | 0.59 |
| 6 | 82 | 120 | 100/90 | 0.1 | 0.30 |
| 7 | 82 | 150 | 100/98 | 1.27 | 0.39 |
| 8d | 82 | 180 | 100/100 | 0.81 | 1.01 |
Reaction conditions: 3 bar H2 at different temperatures over 0.2 mol % SiliaCat Pd(0) for 24 h.
Squalene conversion/squalane selectivity evaluated by gas chromatography–mass spectrometry (GC–MS) analysis.
Leaching of Pd and Si was assessed by inductively coupled plasma-optical emission spectroscopy (ICP-OES) analysis in 1,1,2-trichloroethane.26
Sixteen hours reaction time.
First, entries 2–8 in Table 4 show that in each case the experimental values of leached Pd (and Si) in the crude product were <2 ppm (threshold value for palladium in oils for cosmetic and food products in the United States).
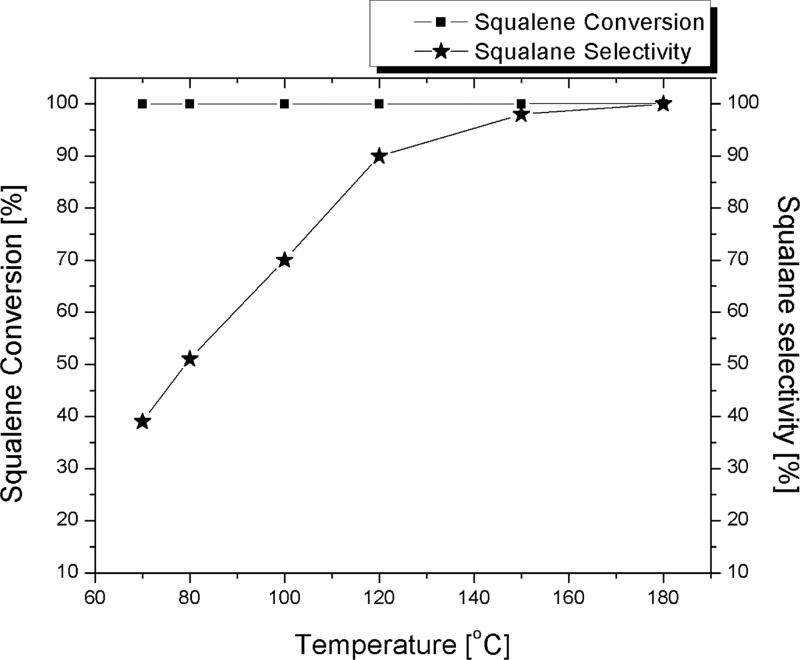
Entries 1 and 2 show that the hydrogenation of high-purity squalene with complete conversion and 99% squalane selectivity was smoothly achieved at 70 °C after 24 h reaction. However, entry 3 shows that when squalene obtained from olive oil in 82 wt % purity was used in place of pure squalene, the substrate conversion into squalane was only 39%. Furthermore, entries 3–8 show evidence that the hydrogenation of olive oil-derived squalene requires harsher conditions than that of pure squalene, with temperature having a crucial effect. Rise in the temperature from 70 to 180 °C increases the selectivity in squalane from 39 to 100%. At 150 °C and under 3 bar H2, the conversion of olive oil squalene to squalane with 98% selectivity was achieved after 24 h (entry 7). At 180 °C, however, complete conversion was obtained after only 16 h (entry 8).
Squalene conversion and selectivity to squalane were calculated and plotted against the reaction temperature (Figure 5) revealing the dependence of squalane selectivity on the latter parameter.
Figure 5.
Effect of temperature in hydrogenation of olive squalene (82 wt % purity).
Effect of H2 Pressure
To study the effect of hydrogen pressure on the solvent-free catalytic hydrogenation of olive squalene (82% pure) over 0.2 mol % Pd, the H2 pressure was increased from 3 to 20 bar and the conversion of squalene to squalane at 180 °C was evaluated at different reaction times (Table 5).
Table 5. Conversion and Selectivity of Squalane in the Solvent-Free Hydrogenation of Olive Squalene over SiliaCat Pd(0) under Different Hydrogen Pressurea.
| entry | H2 pressure (bar) | T (h) | conversion/selectivityb (%) |
|---|---|---|---|
| 1 | 3 | 4 | 100/31 |
| 2 | 3 | 7 | 100/54 |
| 3 | 3 | 16 | 100/100 |
| 4 | 6 | 4 | 100/42 |
| 5 | 6 | 7 | 100/76 |
| 6 | 6 | 16 | 100/100 |
| 7 | 10 | 4 | 100/72 |
| 8 | 10 | 7 | 100/90 |
| 9 | 10 | 16 | 100/100 |
| 10 | 20 | 4 | 100/98 |
| 11 | 20 | 7 | 100/100 |
Reaction conditions: 180 °C, H2 pressure as in table entries, 0.2 mol % SiliaCat Pd(0).
Squalene conversion/squalane selectivity evaluated by GC–MS analysis.
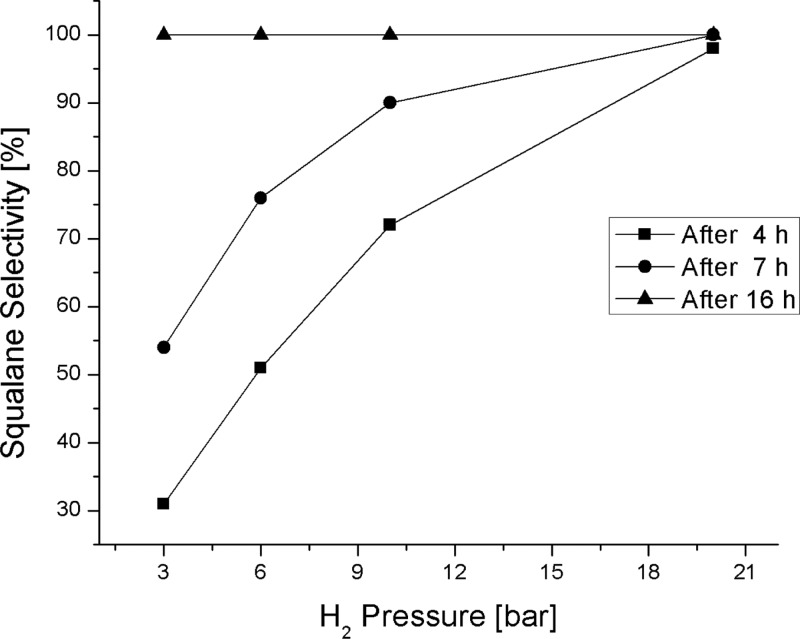
From the data in Table 5, the values for squalane conversion and selectivity after 4, 7, and 16 h were calculated and plotted against the H2 pressure (Figure 6), revealing the dependence of squalane selectivity on the H2 pressure.
Figure 6.
Effect of hydrogen pressure on hydrogenation of 82% pure olive oil squalene.
Figure 6 shows that after 4 h, raising the H2 pressure from 3 to 20 bar improves the squalane selectivity from 30 to 98%. Similarly, after 7 h reaction time, bringing the hydrogen pressure to 20 bar enhances the squalane selectivity from slightly more than 50% to complete selectivity with the full conversion of squalene over a very low Pd loading (0.005 mmol Pd/g of substrate).
After 16 h and at 180 °C, complete conversion of olive oil squalene to squalane was obtained at all of the H2 pressures applied (3, 6, and 10 bar). The effect of the hydrogen pressure suggests that, as expected from a truly heterogeneous reaction involving a solid (the catalyst) and a gaseous reactant (H2), the rate of reaction is strongly dependent on the solubility of the hydrogen in the reaction mixture over the range of pressure studied. The hydrogen pressure impacts directly on the H2 diffusion through the gas and liquid films at the bubble–liquid interface and therefore the diffusion through the bulk liquid phase and the liquid film at the liquid–solid interface, as well as the reaction rate on the catalytic solid surface. A high reaction pressure also affects the kinetics of squalene adsorption and squalane desorption from the Pd0 NPs (the GC–MS analysis clearly showed a fast primary hydrogenation of squalene even after 4 h, revealing the presence of intermediate partially hydrogenated products of squalene).
Effect of Catalyst Loading
Table 6 shows the results obtained by varying the reaction palladium loading using once again 82 wt % olive oil-derived squalene under different reaction conditions. Entry 1 displays that over 0.5 mol % Pd catalyst, corresponding to 0.012 mmol Pd entrapped in SiliaCat Pd(0) per gram of squalene, the substrate conversion to squalane at 120 °C under 3 bar H2 pressure is quantitative after 20 h. At the same H2 pressure and increasing the temperature to 150 °C (entry 2) and 180 °C (entry 3), the conversion becomes quantitative after 16 and 7 h, respectively, showing once again the large impact of the reaction temperature on the rate of squalene hydrogenation.
Table 6. Conversion of Squalene and Squalane Selectivity in the Solvent-Free Hydrogenation of Olive Oil Squalene over Different Amounts of SiliaCat Pd(0).
| experimental conditions |
leachingc (mg/kg) |
||||||
|---|---|---|---|---|---|---|---|
| entry | SiliaCat Pd(0) (wt % catalyst/mol % Pd)a | T (°C) | H2 (bar) | t (h) | conversion/selectivityb (%) | Pd | Si |
| 1 | 5/0.5 | 120 | 3 | 20 | 100/100 | ||
| 2 | 5/0.5 | 150 | 3 | 16 | 100/100 | 0.73 | 1.55 |
| 3 | 5/0.5 | 180 | 3 | 7 | 100/100 | 0.74 | 1.17 |
| 4 | 2/0.2 | 180 | 3 | 16 | 100/100 | 0.81 | 1.01 |
| 5 | 2/0.2 | 180 | 6 | 16 | 100/100 | 0.78 | 0.91 |
| 6 | 2/0.2 | 180 | 10 | 16 | 100/100 | 0.78 | 0.86 |
| 7 | 2/0.2 | 180 | 20 | 4 | 100/98 | 0.92 | 0.67 |
| 8 | 2/0.2 | 180 | 20 | 7 | 100/100 | 0.83 | 0.66 |
| 9 | 1/0.1 | 180 | 10 | 16 | 100/95 | ||
| 10 | 1/0.1 | 180 | 10 | 24 | 100/98 | 0.69 | 0.86 |
| 11 | 1/0.1 | 180 | 20 | 7 | 100/69 | 0.82 | 0.76 |
| 12 | 1/0.1 | 180 | 20 | 16 | 100/100 | 0.72 | 0.70 |
| 13 | 0.5/0.05 | 180 | 10 | 24 | 100/58 | 0.81 | 0.65 |
| 14 | 0.5/0.05 | 180 | 20 | 24 | 100/84 | 0.69 | 0.71 |
Catalyst–squalene mass ratio/palladium–squalene molar ratio.
Squalene conversion/squalane selectivity evaluated by GC–MS.
Leaching of Pd and Si was assessed by ICP-OES analysis in 1,1,2-trichloroethane.
Entries 4–6 in Table 6 show that reactions at 180 °C, with over 0.2 mol % Pd catalyst (corresponding to 0.005 mmol Pd/g of squalene) under 3–10 bar H2 pressure, result in quantitative conversion of squalene to squalane after 16 h. Under higher H2 pressure (20 bar), the rate of the squalene hydrogenation reaction increased with 98% squalane selectivity observed after 4 h only, and the quantitative conversion to squalane after 7 h (entries 7 and 8).
Finally, lowering the SiliaCat Pd(0) load to 0.1 mol % Pd only (corresponding to 0.0024 mmol Pd/g of squalene), the quantitative conversion to squalane could be achieved only at a higher temperature and an H2 pressure (entries 9–12) at 180 °C, whereas over 0.05 mol % Pd (corresponding to 0.0012 mmol Pd/g of squalene), the selectivity to squalane after 24 h was 58% only under 10 bar H2 (entry 13) and 84% under 20 bar H2 pressure (entry 14) despite full conversion of squalene. The relevance of the reaction parameters on the solvent-free squalene hydrogenation to squalane over SiliaCat Pd(0), thus, follows the order: Pd concentration > temperature > pressure.
We ascribe the high catalytic activity of the newly developed spherical SiliaCat Pd(0) catalyst to (i) the large mesoporosity, (ii) hydrophobicity of the organosilica matrix protecting the NPs tightly entrapped within the sol–gel cages (Figure 7), and (iii) the enhanced morphology with its much larger surface-to-volume ratio (S/V) when compared with analogous sol–gel catalyst comprising irregular xerogel microparticles.
Figure 7.
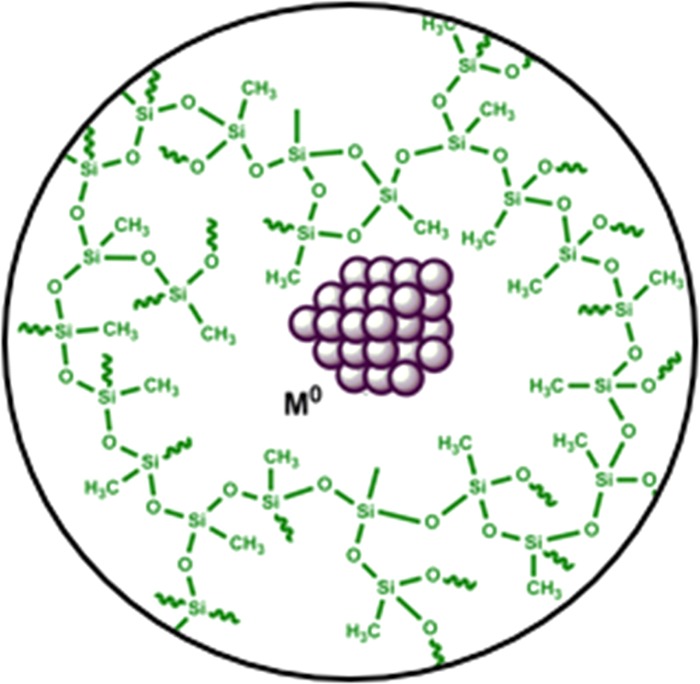
Molecular structure of the SiliaCat Pd(0) catalyst.
The dramatic dependence of the hydrogenation rate on the reaction temperature may be explained by squalene/squalane diffusion limitations through the inner porosity of the solid catalyst and subsequent access to the active sites on the Pd NPs.
Catalyst Stability
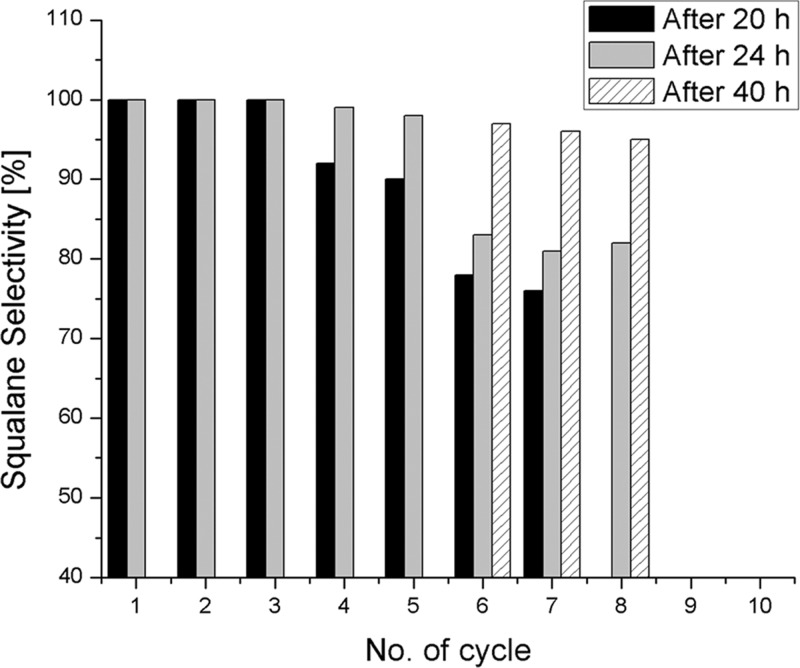
The reusability of the catalyst was evaluated in several consecutive multigram hydrogenation reactions of 82% pure olive oil squalene (50 mL, 43.13 g, 105 mmol) using 0.5 mol % SiliaCat Pd(0) under solvent-free conditions at 150 °C under 3 bar hydrogen pressure. In each cycle, once the maximum conversion of squalene to squalane was achieved, the catalyst was collected by filtration, washed with tetrahydrofuran (three times during 10 min) to remove the wax impurities present in the olive oil squalene, dried at room temperature and reused in the subsequent cycle. Figure 8 shows that the spherical SiliaCat Pd(0) heterogeneous catalyst was reused successfully in eight consecutive cycles.
Figure 8.
Reusability of SiliaCat Pd(0) in squalene hydrogenation.
In the first three consecutive reaction tests, complete conversion was obtained in 20 h, after which the squalane selectivity progressively decreased to reach high values after 24 h (cycles 4 and 5) and after 40 h (cycles 6–8). The longer reaction time was applied to ensure a high squalane conversion.
In brief, the SiliaCat Pd(0) heterogeneous catalyst was stable and robust, which is a crucially important factor for practical applications in place of conventional Pd/C and Ni-kieselguhr commercial catalysts.
The amounts of Pd and Si leached from the SiliaCat Pd(0) during catalysis were assessed by analyzing via ICP-OES the metal content in the isolated crude product after the filtration of the catalyst.a Values reported in Tables 4 and 6 show that in each case the values of leached Pd and Si found in the crude product are <2 ppm.
Table 7 summarizes the outcomes of squalene hydrogenation reaction using different heterogeneous catalysts under optimized solvent-free conditions or with the substrate dissolved in alcohol or n-heptane. Entry 2 in Table 7 shows that SiliaCat Pd(0) affords the highest yield and selectivity under the mildest reaction conditions.
Table 7. Conversion and Squalane Selectivity in the Hydrogenation of Squalene with Different Purities under Optimized Reaction Conditions over Different Catalysts under Solvent-Free Conditions or Carried Out with the Substrate Dissolved in Solventa.
| experimental
conditions |
|||||||
|---|---|---|---|---|---|---|---|
| entry | catalyst (mol % M)b | purity (%) | squalene solution/solvent-free | conversion/selectivityc(%) | T (°C) | H2 (bar) | t (h) |
| 1 | 2.5 wt % SiliaCat Pd(0) (0.2) | 98 | EtOH (1.00 M) | 100/99 | 50 | 3 | 24 |
| 2 | 82 | EtOH (0.70 M) | 100/94 | 70 | 3 | 24 | |
| 3 | 2.5 wt % SiliaCat Pd(0) (0.2) | 98 | solvent-free | 100/99 | 70 | 3 | 24 |
| 4 | 82 | solvent-free | 100/98 | 150 | 3 | 24 | |
| 5 | 82 | solvent-free | 100/100 | 180 | 3 | 16 | |
| 6d | 5 wt % Pd/C (0.2) | 82 | solvent-free | 100/51 | 150 | 3 | 16 |
| 7d | 82 | solvent-free | 100/69 | 150 | 3 | 24 | |
| 8e | 5 wt % Pd/C14 (1.1) | >80 | 2-PrOH/heptane (0.64 M) | 100/89 | 85 | 70 | 16 |
| 9e | 5 wt % Pd/C14 (0.5) | >80 | 2-PrOH (0.70 M) | 100/99 | 160 | 150 | |
| 10e | 10 wt % Pd/C14 (0.4) | >90 | heptane (0.50 M) | 100/97.5 | 80 | 20 | 16 |
| 11e | 10 wt % Pd/C14 (1.1) | >90 | heptane (0.70 M) | 100/99 | 85 | 60 | 16 |
| 12 | 6 wt % Pd/clay16 (0.6) | 98 | solvent-free | 100/100 | 300 | 10 | 10 |
| 13 | Ni-Raney15 (14.5) | 98 | solvent-free | 100/99 | 170 | 10 | 4 |
Experimental and literature data.
Palladium/squalene molar ratio.
Squalene conversion/squalane selectivity evaluated by GC–MS.
Pd/C (5 wt %) from Sigma-Aldrich tested in the same conditions as entry 4.
Squalene reaction mixture obtained after dimerization of (E)-β-farnesene was passed to hydrogenation step without work-up.
Conclusions
The newly developed sol–gel-entrapped Pd catalyst SiliaCat Pd(0) in spherical morphology added in small amount of 0.2 mol % smoothly mediates the chemoselective full hydrogenation of squalene into valued squalane under solvent-free, mild reaction conditions (3 bar H2 at 70 °C). Catalysis is truly heterogeneous, with values of leached palladium <2 ppm. The catalyst is easily recovered and reused for several consecutive reaction runs with practically no loss of catalytic activity, opening the route to a cleaner and less-expensive hydrogenation of squalene. Compared with numerous other commercial catalysts employed in industry, lower temperatures and pressures are generally required due to several synergistic factors that include easier accessibility of the Pd0 NPs entrapped in the inner huge porosity of the hydrophobic organosilica spherical microparticles of enhanced surface/volume ratio. Like organosilica irregular xerogel catalysts of the SiliaCat series,25 the spherical SiliaCat Pd(0) catalyst does not swell or stick to the reactor surface and can be easily handled in air, showing no tendency to ignite upon exposure to air, as happens with conventional Pd/C and related catalysts.
Squalane is an extremely important emollient and bioactive substance with multiple health benefits and its production cost is significantly (40%) impacted by the cost of the hydrogenation process.7 Now that phytosqualene biotechnologically derived from sugarcane or extracted from olive oil milling residues is replacing squalene of animal origin,2 these findings will further contribute to lowering the cost and increasing the availability of high-quality squalane.
Materials and Methods
The squalene samples with different purities (98% from Sigma-Aldrich, 82 wt % from an olive squalene manufacturer) and 5% Pd/C (from Sigma-Aldrich) were used as received. Multigram hydrogenation tests of squalene under maximum pressure of 3 bar H2 at 120 °C were carried out in an Ace Glass 6437 system equipped with a batch glass reactor. The 500 mL glass reactor was charged with 250 mL olive squalene (82 wt % purity; 214.5 g, 522.25 mmol) and 4.43 g SiliaCat Pd(0) (2 wt %) for 0.2 mol % Pd. Mechanical stirring was then set at 850 rpm and the reaction mixture was degassed five times, replacing three times the vacuum by Ar and twice Ar with H2. The hydrogenation reactions conducted at H2 pressure higher than 3 bar were carried out in a Parr 4848 high-pressure reactor controller equipped with a steel container. The 100 mL steel reactor was charged with 50 mL olive squalene (43.13 g, 105 mmol, 82 wt % purity) and 0.89 g SiliaCat Pd(0) (2.5 wt %) for 0.2 mol % Pd loading. The mixture stirred at 700 rpm was purged with H2 at 20 bar five times, after which the desired pressure was maintained. The reaction temperature was then raised from 22 °C to the desired reaction temperature and maintained for several hours, after which the reaction mixture was allowed to reach 50 °C and the solid catalyst recuperated by filtration. The reaction conversion and squalane selectivity were assessed by GC–MS.
The GC–MS analysis showed that in cases where the conversion of squalene (molecular weight (MW): 410) to squalane (MW: 422) was incomplete, a large number of intermediate partially hydrogenated products (MW: 412, 414, 416, 418, and 420) were present in the reaction mixture. We thus explored the effects of the reaction temperature, hydrogen pressure, and catalyst loading on conversion and selectivity.
The physical properties of SiliaCat Pd(0) catalyst were determined by nitrogen adsorption, transmission XRD, TEM, 29Si MAS NMR spectroscopy, and ICP-OES. Nitrogen adsorption and desorption isotherms at 77 K were measured using a Micromeritics TriStar II 3020 system. The resulting data were analyzed with the TriStar II 3020 version 3.02 software. Both BJH adsorption and desorption branches were used to calculate the pore size distribution. The XRD analysis were performed on a Siemens D-5000 X-ray diffractometer equipped with a monochromatic Cu Kα radiation source (λ = 1.5418). The spectra were recorded in the 2θ range of 0–30° for the undoped support and of 10–90° for SiliaCat Pd(0) catalyst at a scan speed of 1°/min and a step scan of 0.02°. The powder diffraction file of the International Centre for Diffraction Data was used to identify the diffraction peaks characteristic of crystalline Pd(0) with a fcc lattice.
The TEM photographs were taken with a JEOL-2010 microscope equipped with a LaB6 electron gun source operated at 200 kV. Solid-state 29Si NMR spectra were recorded on a Bruker Avance spectrometer (Milton, ON, Canada) at a Si frequency of 79.5 MHz. Each time the solid sample was spun at 8 kHz in a 4 mm ZrO rotor. A Hahn echo sequence synchronized with the spinning speed was used while applying a TPPM15 composite pulse decoupling during acquisition. A total of 2400 acquisitions were recorded with a recycling delay of 30 s.
The leaching of Pd and Si was assessed by ICP-OES analysis of the squalane crude product in 1,1,2-trichloroethane (concentration 100 mg/mL) using a PerkinElmer Optima 2100 DV system. The values measured are reported as milligram of Pd and as milligram of Si per kilogram of squalane oil.
Acknowledgments
This article is dedicated to Professor Valentine Anakinov, Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences, for his eminent contributions to the progress of organometallic and metal nanoparticle catalysis.
The authors declare no competing financial interest.
Footnotes
Leaching values are given in mg/kg active pharmaceutical ingredients. ICP-OES limit of detection: LODPd, Si = 0.05 ppm in solution or 0.50 mg/kg in the crude product.
References
- Spanova M.; Daum G. Squalene – biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. 10.1002/ejlt.201100203. [DOI] [Google Scholar]
- Laserson F.Neossance, 3rd Generation Squalane. Expression Cosmetique, 2013; pp 325–328.
- Huang Z.-R.; Lin Y.-K.; Fang J.-Y. Biological and Pharmacological Activities of Squalene and Related Compounds: Potential Uses in Cosmetic Dermatology. Molecules 2009, 14, 540–554. 10.3390/molecules14010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand View Research . Squalene Market Analysis by Raw Material (Vegetable, Synthetic, Animal), by Application (Food, Pharmaceuticals, Cosmetics) and Segment Forecasts to 2024; Pune, India, 2016.
- Tabur P.; Dorin G.. US 20120040396 A1, 2012.
- de Vries E.Industrial Bioscience for Cosmetic Ingredients. How Open Innovation Advances Science. California Chapter of the Society of Cosmetic Chemists; San Francisco, 22 March, 2016.
- Ciriminna R.; Pandarus V.; Béland F.; Pagliaro M. Catalytic Hydrogenation of Squalene to Squalane. Org. Process Res. Dev. 2014, 18, 1110–1115. 10.1021/op5002337. [DOI] [Google Scholar]
- Wu C.-S.; Tsay Y.-J.; Liou H.-J. Studies on the content and hydrogenation condition of squalene from the liver oil of deep sea sharks. J. Fish. Soc. Taiwan 1980, 7, 43–55. [Google Scholar]
- Bonrath W.; Medlock J.; Schütz J.; Wüstenberg B.; Netscher T.. Hydrogenation in the Vitamins and Fine Chemicals Industry – An Overview. In Hydrogenation; Karamé I., Ed.; InTechOpen: Zagreb, 2012; pp 69–90. [Google Scholar]
- Naziri E.; Mantzouridou F.; Tsimidou M. Z. Squalene resources and uses point to the potential of biotechnology. Lipid Technol. 2011, 23, 270–273. 10.1002/lite.201100157. [DOI] [Google Scholar]
- Tsujimoto M. A Highly Unsaturated Hydrocarbon in Shark Liver Oil. J. Ind. Eng. Chem. 1916, 8, 889–896. 10.1021/i500010a005. [DOI] [Google Scholar]
- Chapman A. C. Spinacene: a new hydrocarbon from certain fish liver oils. J. Chem. Soc., Trans. 1917, 111, 56–69. 10.1039/CT9171100056. [DOI] [Google Scholar]
- Dale J.; Årtun T.; Waterman H. I.; Eliasson N. A.; Thorell B. Double Bond Migration and Dehydrogenation of Squalene on Hydrogenation Catalysts. Acta Chem. Scand. 1956, 10, 439–444. 10.3891/acta.chem.scand.10-0439. [DOI] [Google Scholar]
- Fisher K.; Schofer S. J.; Kanne D. B.. US 20110287988 A1, 2011.
- Pandarus V.; Ciriminna R.; Kaliaguine S.; Béland F.; Pagliaro M. Heterogeneously Catalyzed Hydrogenation of Squalene to Squalane under Mild Conditions. ChemCatChem 2015, 7, 2071–2076. 10.1002/cctc.201402668. [DOI] [Google Scholar]
- Soni V. K.; Sharma R. K. Palladium-Nanoparticles-Intercalated Montmorillonite Clay: A Green Catalyst for the Solvent-Free Chemoselective Hydrogenation of Squalene. ChemCatChem 2016, 8, 1763–1768. 10.1002/cctc.201600210. [DOI] [Google Scholar]
- Pagliaro M.; Pandarus V.; Béland F.; Ciriminna R.; Palmisano G.; Carà P. D. A new class of heterogeneous Pd catalysts for synthetic organic chemistry. Catal. Sci. Technol. 2011, 1, 736–739. 10.1039/c1cy00119a. [DOI] [Google Scholar]
- Pandarus V.; Gingras G.; Béland F.; Ciriminna R.; Pagliaro M. Selective Hydrogenation of Alkenes under Ultramild Conditions. Org. Process Res. Dev. 2012, 16, 1230–1234. 10.1021/op300079z. [DOI] [Google Scholar]
- Pandarus V.; Gingras G.; Béland F.; Ciriminna R.; Pagliaro M. Selective Hydrogenation of Vegetable Oils over SiliaCat Pd(0). Org. Process Res. Dev. 2012, 16, 1307–1311. 10.1021/op300115r. [DOI] [Google Scholar]
- Ciriminna R.; Sciortino M.; Alonzo G.; de Schrijver A.; Pagliaro M. From Molecules to Systems: Sol-Gel Microencapsulation in Silica-Based Materials. Chem. Rev. 2011, 111, 765–789. 10.1021/cr100161x. [DOI] [PubMed] [Google Scholar]
- Sangeetha P.; Seetharamulu P.; Shanthi K.; Narayanan S.; Rama Rao K. S. Studies on Mg-Al oxide hydrotalcite supported Pd catalysts for vapor phase hydrogenation of nitrobenzene. J. Mol. Catal. A: Chem. 2007, 273, 244–249. 10.1016/j.molcata.2007.03.020. [DOI] [Google Scholar]
- Leofanti G.; Padovan M.; Tozzola G.; Venturelli B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. 10.1016/S0920-5861(98)00050-9. [DOI] [Google Scholar]
- Glaser R. H.; Wilkes G. L.; Bronnimann C. E. Solid-state 29Si NMR of TEOS-based multifunctional sol-gel materials. J. Non-Cryst. Solids 1989, 113, 73–87. 10.1016/0022-3093(89)90320-7. [DOI] [Google Scholar]
- Grunenwald A.; Ayral A.; Albouy P.-A.; Licitra C.; Gergaud P.; Quemener D.; Deratani A.; Rouessac V.; Zenasni A.; Jousseaume V. Hydrophobic mesostructured organosilica-based thin films with tunable mesopore ordering. Microporous Mesoporous Mater. 2012, 150, 64–75. 10.1016/j.micromeso.2011.09.007. [DOI] [Google Scholar]
- Ciriminna R.; Pandarus V.; Fidalgo A.; Ilharco L. M.; Béland F.; Pagliaro M. SiliaCat: A Versatile Catalyst Series for Synthetic Organic Chemistry. Org. Process Res. Dev. 2015, 19, 755–768. 10.1021/acs.oprd.5b00137. [DOI] [Google Scholar]