Abstract
On the basis of the reaction between ketene and alcohol groups to result in an ester linkage and Meldrum’s acid as an effective precursor of ketene group, a bifunctional aliphatic Meldrum’s acid compound (BisMA) is synthesized and used as a monomer to react with ethylene glycol and glycerol for the preparation of linear and cross-linked aliphatic polyesters, respectively. A 62 and 35 wt % of biodegradation fraction have been recorded on the linear and cross-linked aliphatic polyesters after an 8 week biodegradation test, respectively, to demonstrate the good biodegradability of the synthesized polyesters. In addition to conventional linear biodegradable polyesters, this synthetic route also provides a new and convenient method for preparation of cross-linked biodegradable polyesters. The types of the required monomers and reaction methods for preparation of the cross-linked biodegradable polyesters are similar to those used for conventional thermosetting resins. An integration of biodegradable polymers and thermosetting resins in polymer chemistry has been demonstrated.
1. Introduction
In contrast to common polymers, biodegradable polymers are attractive for practical applications and environmental issues.1−3 Biodegradable polymers possessing nontoxic and biocompatible characteristics are attractive biomaterials suitable for biomedical and bioengineering application.4,5 Introduction of hydrolytic linkages, such as esters, anhydrides, carbonates, amides, urethanes, ureas, etc., to the polymer chains could be the basis of molecular designs of synthetic biodegradable polymers.1,6 Nevertheless, this requirement pretty limits the monomers and polymerization routes required for preparation of synthetic polymers possessing biodegradability.
Biodegradable polyesters could be the most studied biodegradable polymers.6 Synthesis of biodegradable polyesters could be achieved with two general approaches.7 One is ring-opening polymerization using cyclic lactones as monomers. Lactides and the corresponding poly(lactide)s have been widely utilized in the commercial markets. This family of biodegradable polyesters has some drawbacks, including limited source of monomers, poor molecular design flexibility of monomers and polymers, and relatively poor thermal stability of polymers. The other approach to prepare biodegradable polyesters is polycondensation using aliphatic diacids and diols as monomers,8 which provides a relatively wide window of structure designs to the biodegradable polyesters. Nevertheless, the low reactivity between aliphatic acids and diols limits the development of biodegradable polyesters prepared with this reaction route. As the diversity of biodegradable polymer structures plays a key factor to their properties, development of new synthesis routes with high molecular design flexibility is attractive for biodegradable polyesters.
Ketene is a highly reactive group toward nucleophiles.9 Reaction between a ketene with a hydroxyl group
(as the nucleophile) forms an ester linkage. This reaction potentially
provides an alternative synthetic route for ester compounds and polyesters.
Nevertheless, ketene compounds could not be directly utilized in the
synthetic method due to their high reactivity and less controllability.
As Meldrum’s acid (MA) group is an effective precursor of ketene,10−14 Hawker et al.15 reported a facile synthesis
route of high-molecular-weight aromatic polyesters using MA derivatives
as monomers based on the abovementioned ketene chemistry.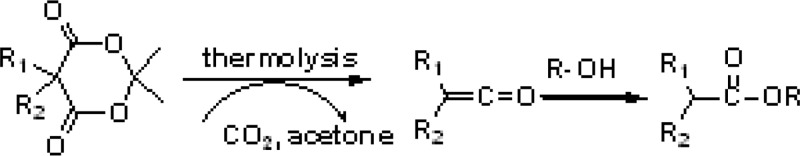
This MA-based synthesis route of polyesters15 inspires our work on synthesis of biodegradable polyesters with MA derivatives as monomers. To target on preparation of aliphatic polyesters with biodegradability, a bifunctional aliphatic MA compound (BisMA) is newly synthesized in this work. BisMA has been utilized as a monomer to be reacted with an aliphatic diol (ethylene glycol (EG)) to result in the corresponding linear aliphatic polyester. We have also demonstrated the synthesis of biodegradable thermosetting resins by reacting BisMA with a triol (glycerol) through a procedure similar to the conventional processes for the preparation of thermosetting resins. On the basis of our best knowledge, this is the first example of preparation of biodegradable thermosetting resins through the conventional thermally induced curing processes. The biodegradability of the obtained polyesters has been evaluated to support the new synthesis route for biodegradable polyesters.
2. Results and Discussion
2.1. Preparation of Aliphatic Bifunctional MA Derivative
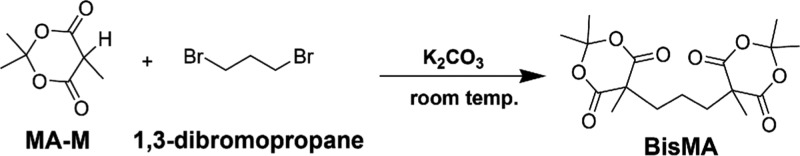
To introduce biodegradability to synthetic polyesters, fully aliphatic polyesters are designed and prepared. The first step is synthesis of an aliphatic bifunctional MA derivative (BisMA) as the monomer. As shown in Scheme 1, BisMA is obtained from the dehydrobromide reaction between dibromopropane (DBP) and 2,2,5-trimethyl-1,3-dioxane-4,6-dione (methyl Meldrum’s acid, MA-M). Spectral characterization of BisMA has been done with Fourier transform infrared (FTIR) spectroscopy, 1H nuclear magnetic resonance spectroscopy (1H NMR), and mass spectroscopy (Figure 1). In FTIR analysis, BisMA exhibits specific absorption peaks of MA ring at 1781 cm–1 (−C=O asymmetric stretching), 1740 cm–1 (−C=O symmetric stretching), and 1050 cm–1 (C–O–C), as well as absorption peaks of −CH2– groups at 2970 cm–1 (stretching) and 1445 cm–1 (bending). The C–Br absorption, which appears in the spectrum of DBP at 590 cm–1, is not observed with BisMA. The above results suggest occurrence of the condensation reaction between DBP and MA-M and the formation of the expected product of BisMA. The 1H NMR spectrum of BisMA provides further support to the successful synthesis of BisMA with the coincidence between the expected chemical structure of BisMA and the resonance peaks at δ = 1.18 ppm (−CH2CH2CH2−), δ = 1.52 ppm (−C(=O)CCH3), δ = 1.65 ppm (−OC(CH3)2), and δ = 1.93 ppm (−CH2CH2CH2−). The molecular weight calculated for BisMA (C17H24O8) is 356.37 g mol–1, which is matched with the value (m/z = 357 Da, (M + 1)+) measured with MS. Moreover, elemental analysis reveals that the obtained BisMA sample has a C and H weight fraction of 57.3 and 6.80%, respectively. Both are highly coincident to the calculated value of 57.3% for C and 6.79% for H. The results support the chemical structure and high purity of the obtained BisMA compound.
Scheme 1. Synthesis of Bifunctional Aliphatic Meldrum’s Acid Compound BisMA.
Figure 1.
Spectral characterization of BisMA with (a) FTIR, (b) 1H NMR, and (c) mass spectroscopies.
BisMA shows a sharp endothermic peak at about 210 °C in differential scanning calorimetry (DSC) measurement (Figure 2), which corresponds to its melting behavior. The sharp melting peak also supports the high purity of the obtained BisMA sample. Moreover, this endothermic peak somewhat overlaps with a relatively small exothermic behavior, corresponding to the thermolysis of MA groups. Generation of ketene groups with MA thermolysis and further ketene dimerization reactions takes place at such high temperatures.15 The MA thermolysis reaction of BisMA evolves one acetone and one CO2 molecule, so as to exhibit a rapid weight loss at about 220 °C in the thermogravimetric analysis (TGA) thermogram of BisMA. The results from both DSC and TGA analysis show good coincidence and support to the proposed reactions. The thermal analysis result of BisMA indicates that this compound possesses reactive MA groups for further reactions.
Figure 2.
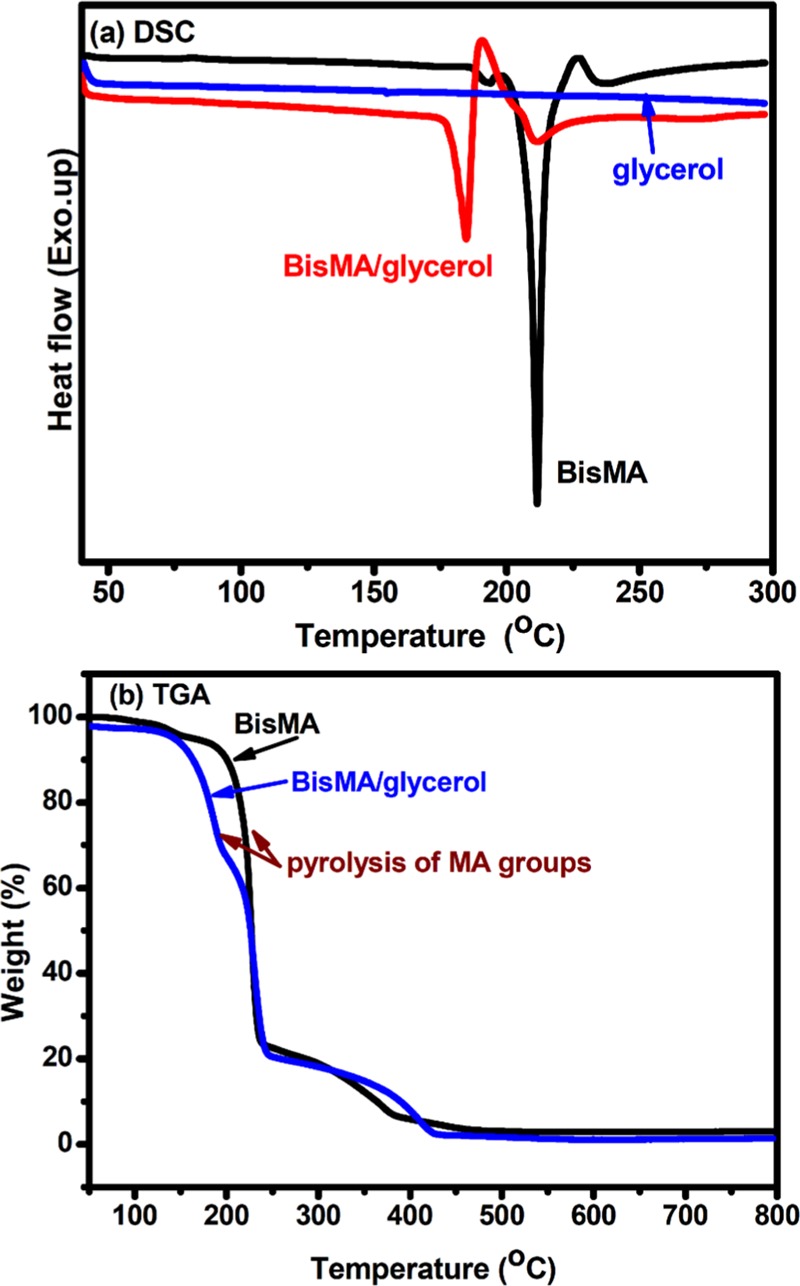
Thermal analysis on BisMA and its mixtures with glycerol using (a) DSC and (b) TGA instruments.
2.2. Preparation of Polyesters with BisMA
BisMA is utilized as a bifunctional monomer to react with multifunctional alcohol compounds for preparation of polyesters. The reaction involves generation of ketene groups through MA thermolysis reaction and formation of ester linkages by means of the addition reaction between ketene and hydroxyl groups.15 As hydroxyl groups have been reported to show effective catalysis effect on MA thermolysis reaction,15,16 the mixture of BisMA and glycerol might show a relatively low reaction temperature compared to the neat BisMA, as demonstrated with the DSC thermograms shown in Figure 2. BisMA/glycerol mixture shows an endothermic peak at about 160 °C and a following exothermic peak at about 184 °C in DSC analysis. A similar result is observed with TGA measurement, in which BisMA/glycerol mixture shows a rapid weight loss at about 160–180 °C, compared to the temperature of 220 °C recorded with neat BisMA. As the weight loss temperature is much lower than the boiling point of glycerol (290 °C), the weight loss is attributed to the occurrence of glycerol-catalytic MA thermolysis reaction. Once the ketene groups form with the MA thermolysis reaction, they react with the hydroxyl groups to result in polyesters.
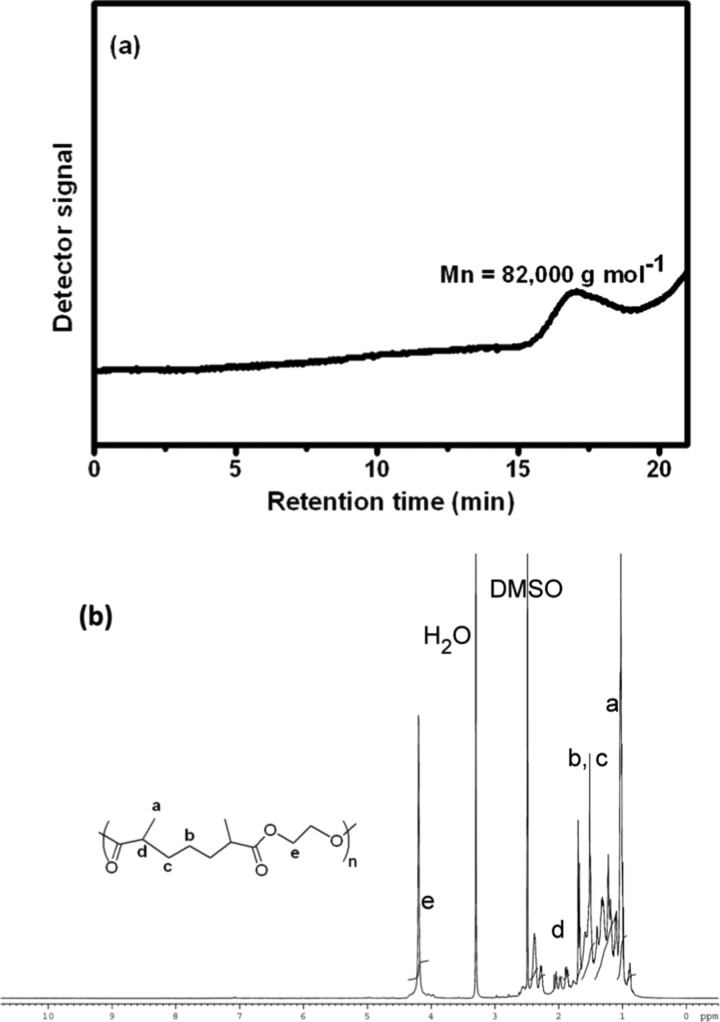
Linear and cross-linked aliphatic polyesters have been prepared through a bulk polymerization process with BisMA/EG and BisMA/glycerol as monomers, respectively (Scheme 2). LP-BisMA/EG shows good solubility in organic solvents and has a number-averaged molecular weight and polydispersity of 82 000 g mol–1 and 2.2, respectively (Figure 3a). On the basis of its solubility in organic solvent, LP-BisMA/EG has been characterized with 1H NMR (Figure 3b). The formation of EG-based ester structure is characterized with the resonance peaks at about δ = 4.2 ppm corresponding to the −C(=O)OCH2– groups. Addition reaction of −OH groups of EG to the ketene groups generated from MA-M thermolysis results in −CH–C(=O) groups, which result in the resonance peaks at about δ = 1.9–2.0 ppm. The resonance peaks at about δ = 1.1 and 1.3–1.6 ppm are assigned to the −CH3 group and the methylene groups of −CH2–CH2–CH2– segments, respectively. Because of possessing a cross-linked structure, CRP-BisMA/glycerol is not soluble in organic solvents. A high gel fraction of 95% has been measured with CRP-BisMA/glycerol in toluene. Moreover, CRP-BisMA/glycerol shows a glass transition temperature of about −16 °C (tan δ) measured with a dynamic mechanical analyzer (DMA).
Scheme 2. Polymerization of BisMA and Alcohol Compounds for Preparation of Linear and Cross-Linked Aliphatic Polyesters.
Figure 3.
(a) Gel permeation chromatogram and (b) 1H NMR spectrum of LP-BisMA/EG.
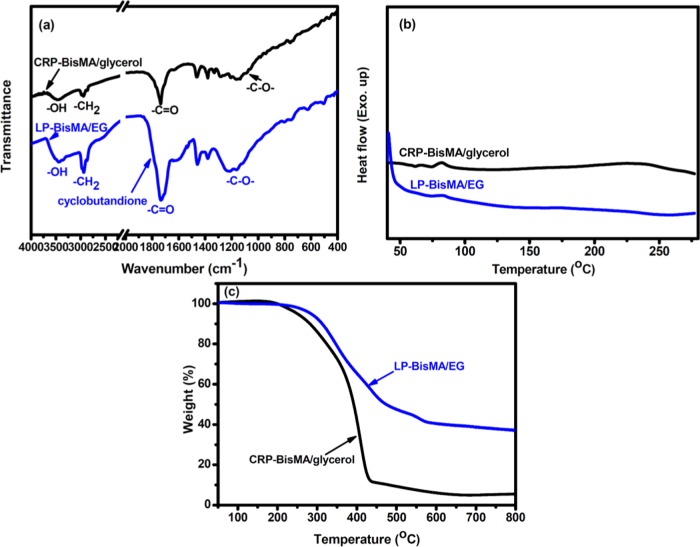
Both polyesters have been characterized with FTIR (Figure 4) and show similar characteristic absorption peaks of ester linkages at 1730 cm–1 and −CH2– groups at 2970 cm–1. The MA absorption is not observed with the polyester samples. Moreover, compared to the linear polyester (LP-BisMA/EG), the cross-linked aliphatic polyester (CRP-BisMA/glycerol) prepared with BisMA and glycerol shows a relatively strong absorption of −OH groups at about 3450 cm–1 (compared to the ester absorption as a reference), indicating that some unreacted −OH groups of glycerol are remained in the sample. On the other hand, LP-BisMA/EG shows an absorption at about 1810 cm–1 corresponding to the C=O groups of 1,3-butandione, which are formed from the ketene dimerization reaction. The results suggest that in the polymerization process of BisMA and EG, ketene might involve in both esterification and dimerization reactions. For the reaction between BisMA and glycerol, the dimerization of ketene becomes minor.
Figure 4.
(a) FTIR, (b) DSC, and (c) TGA measurements on linear (LP-BisMA/EG) and cross-linked (CRP-BisMA/glycerol) aliphatic polyesters.
Both polyesters do not exhibit obvious endothermic and exothermic behaviors in DSC measurements (Figure 4), indicating the high conversion of MA thermolysis reaction in the polymerization processes. Moreover, no crystallization transitions were observed with these two polyesters, which are similar to some polyesters reported in literature.15 In TGA measurements, the temperature of 5% weight loss (Td5) recorded on LP-BisMA/EG and CRP-BisMA/glycerol is 247 and 267 °C, respectively. The degradation patterns of the two polyesters are also different. Compared to CRP-BisMA/glycerol, LP-BisMA/EG shows a multiple weight loss behavior and better thermal stability, although CRP-BisMA/glycerol possesses some cross-linked structure. As a result, the relatively high thermal stability and retarded weight loss behavior of LP-BisMA/EG could be attributed to its cyclic 1,3-butandione structures mentioned in the discussion on FTIR analysis.
2.3. Biodegradation Tests
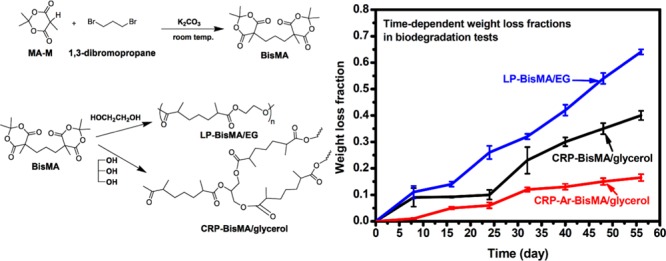
The biodegradability of the prepared aliphatic polyesters is evaluated with a catalytic biodegradation method. For comparison, a cross-linked polyester possessing aromatic structures (CRP-Ar-BisMA/glycerol) is also prepared using Ar-BisMA and glycerol as monomers (Scheme 2). In an 8 week test, the time-dependent weight fractions of the samples have been recorded and shown in Figure 5. Compared to the semiaromatic cross-linked polyester CRP-Ar-BisMA/glycerol, both aliphatic polyesters show relatively high rate and high fractions of weight losses in the biodegradation tests. The result is reasonable and falls into the general knowledge. On the other hand, after 8 weeks, the weight losses found with LP-BisMA/EG and CRP-BisMA/glycerol are 62 and 35 wt %, respectively. Both aliphatic polyesters prepared with BisMA demonstrate sufficient biodegradation characteristics. The biodegradability of the linear sample of LP-BisMA/EG is relatively high compared to the cross-linked CRP-BisMA/glycerol. The weight loss fractions readily increased with increasing the testing time, which is similar to poly(lactide)s17 and suggests both BisMA-based polyesters perform a degradation pattern from surface to inner parts. The scanning electron micrographs (Figure 6) support the results. The smooth surface of CRP-BisMA/glycerol before biodegradation test becomes rough after a 24 day test due to the biodegradation erosion. The erosion also generates some porous structure in the inner part of the sample, indicating the catalytic medium might permeate into the sample in the biodegradation process. This result is coincident to the weight loss pattern shown in Figure 5, in which an increased weight loss rate is observed with CRP-BisMA/glycerol after 24 days. In summary, the biodegradation of CRP-BisMA/glycerol starts at the sample surface and is followed by an erosion effect inside the sample caused by the permeation of the catalytic medium.
Figure 5.
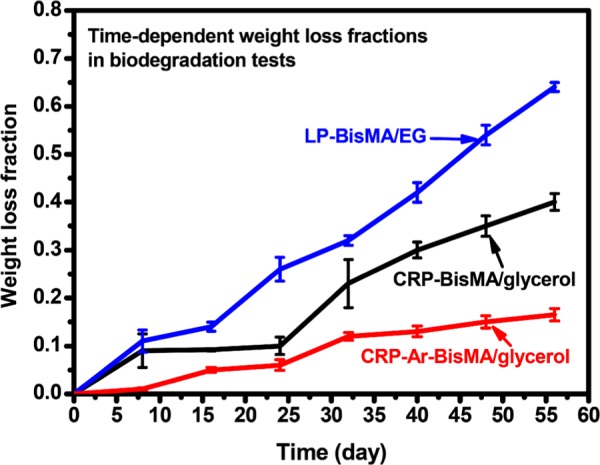
Time-dependent weight loss fractions of the prepared polyesters in biodegradation tests.
Figure 6.
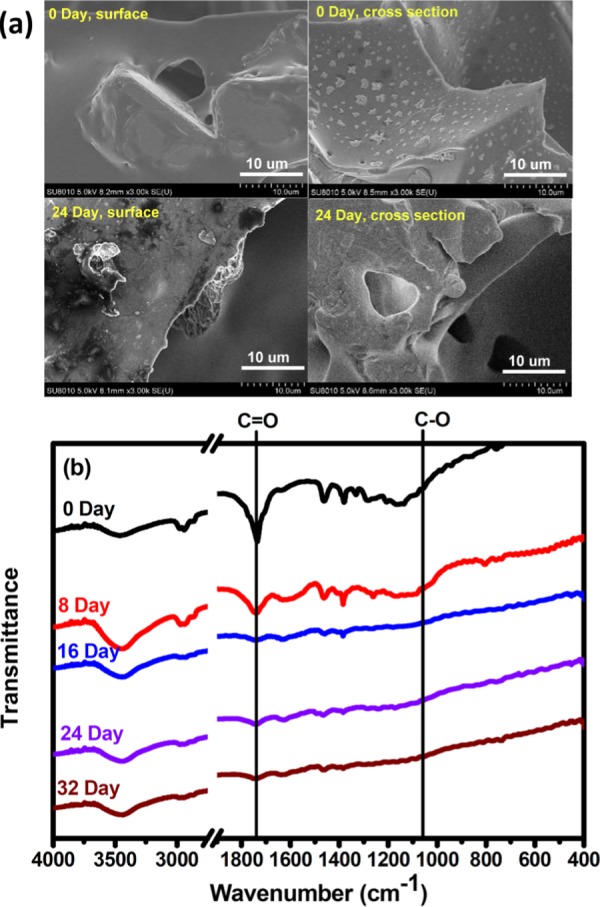
(a) Scanning electron micrograms and (b) FTIR spectra tracing the morphological and chemical structural changes of CRP-BisMA/glycerol in biodegradation tests, respectively.
3. Methods and Materials
3.1. Materials
MA-M (97%), DBP (99%), ethylene glycol (EG, 99%), and glycerol (99%) were purchased from Sigma-Aldrich Chemical Co. and used as received. Potassium carbonate (K2CO3) and hydrochloride acid were received from Showa Chemical Co. The bifunctional MA compound possessing aromatic group was prepared in our laboratory according to the reported method13 and coded as Ar-BisMA.
3.2. Instrumental Methods
FTIR spectra were recorded with a PerkinElmer Spectrum Two FTIR instrument with a resolution of 4 cm–1 and 16 scans. 1H NMR spectra were obtained with a Varian Unity INOVA 500 NMR (500 MHz) spectrometer using CDCl3 as a solvent. Elemental analysis was conducted with a Heraeus CHN-O rapid elemental analyzer using acetanilide as a standard. Mass spectra were recorded with a JEOL AccuTOF GCX mass analyzer using a field desorption atomizer. DSC measurements were taken with a differential scanning calorimeter Q-100 instrument from Thermal Analysis (TA) Instrument Company with a nitrogen flow at 50 mL min–1 and a heating rate of 10 °C min–1. TGA thermograms were recorded with a TA TGA Q-500 instrument at a heating rate of 10 °C min–1 and a nitrogen flow rate of 100 mL min–1. Dynamic mechanical analysis was carried out with a TA DMA Q-800 instrument using a three-point bending mode with an applied force of 0.1 N, a frequency of 1 Hz, and an amplitude of 10 μm.
3.3. Preparation of BisMA
MA-M (2.0 g, 12.66 mmol) and K2CO3 (2.62 g, 19.0 mmol) were dissolved in dried N,N-dimethylformamide (DMF, 15 mL). The solution was charged into a 25 mL round-bottom flask. A solution of DBP (1.22 g, 6.0 mmol) in 3 mL of DMF was added dropwise. The mixture was then reacted at room temperature (about 25 °C) for 20 h. After being added 2.0 mL of HCl (aq) (10 wt %), the solution was extracted with chloroform (20 mL) for three times. The collected organic phase was dried over anhydrous MgSO4. After removal of chloroform with a rotary evaporator, the product was obtained with precipitation from excess toluene (yield: 20%)
3.4. Preparation of Polyesters
Polymerization of BisMA (Ar-BisMA) and alcohol compounds was carried out in a similar manner as reported in the literature.15 For BisMA and EG, a stoichiometric amount of BisMA and EG was charged into an ampoule. The reaction was carried out at 190 °C for 20 min. The product (LP-BisMA/EG) was dissolved in 6F-isopropanol and precipitated from excess methanol. For BisMA/glycerol, a stoichiometric amount of BisMA and glycerol was mixed and poured into a Teflon-coated stainless mold. The product (CRP-BisMA/glycerol) was obtained after a reaction at 180 °C for 0.5 h and 200 °C for 0.5 h. CRP-Ar-BisMA/glycerol was prepared in the same manner using Ar-BisMA and glycerol as the monomers.
3.5. Measurement of Gel Fraction
A dried CRP-BisMA/glycerol sample (weight: Wo) was immersed in toluene for 24 h. After being drawn out the wetted sample was treated with an oil-absorbing paper to remove the liquid on the sample surface and then weighed (Wr). The gel fraction (%) of the sample was calculated from Wr/Wo × 100%.
3.6. Biodegradation Test
Polyester thin films with a thickness of about 500 μm were punched into disklike samples with a diameter of 7.0 mm. A solution of lipase II (from porcine pancreas) in Dulbecco’s phosphate-buffered saline (10 U mL–1) was utilized as the degradation medium. Polyester samples were incubated in the degradation medium at 37 °C for 8 weeks. The used medium was freshly changed every 2 days. The biodegradation was evaluated with weight measurements on the samples every week. Samples (three pieces) were washed with distilled deionized (dd) water, immersed in ethanol and dd water for 24 h, and dried for weight measurement.
4. Conclusions
Multifunctional aliphatic Meldrum’s acid compounds are effective monomers for preparation of aliphatic polyesters showing biodegradability similar to the aliphatic polyesters from other polymerization methods. The synthetic route demonstrates a high flexibility of molecular designs of biodegradable polyesters. Moreover, with multifunctional aliphatic Meldrum’s acid compound and aliphatic triols as monomers, this preparation method also provides a direct synthesis of biodegradable cross-linked aliphatic polyesters.18 As the reaction routes are similar to those for conventional thermosetting resins, the developed synthesis method shows a good integration between biodegradable polymers and thermosetting resins.
Acknowledgments
This work was supported by the Ministry of Science and technology of Taiwan (MOST, Taiwan) under a research grant of MOST 106-221-E-007-098-MY3.
Author Contributions
L.-K.L. and Y.-L.L. gave the original concepts of this work and designed the experiments; L.-K.L. carried out the experiments on synthesis and characterization. L.-K.L. and J.W. worked on the biodegradation tests. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Doppalapudi S.; Jain A.; Khan W.; Domb A. J. Biodegradable Polymers - An Overview. Polym. Adv. Technol. 2014, 25, 427–435. 10.1002/pat.3305. [DOI] [Google Scholar]
- Kijchavengkul T.; Auras R.; Rubino M.; Selke S.; Ngouajio M.; Fernandez R. T. Biodegradation and Hydrolysis Rate of Aliphatic Aromatic Polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. 10.1016/j.polymdegradstab.2010.07.018. [DOI] [Google Scholar]
- Rabnawaz M.; Wyman I.; Auras R.; Cheng S. A Roadmap Towards Green Packaging: The Current Status and Future Outlook for Polyesters in The Packaging Industry. Green Chem. 2017, 19, 4737–4753. 10.1039/C7GC02521A. [DOI] [Google Scholar]
- Piskin E. Biodegradable Polymers as Biomaterials. J. Biomater. Sci., Polym. Ed. 1995, 6, 775–795. 10.1163/156856295X00175. [DOI] [PubMed] [Google Scholar]
- Nair L. S.; Laurencin C. T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- Li S. Hydrolytic Degradation Characteristics of Aliphatic Polyesters Derived from Lactic and Glycolic Acids. J. Biomed. Mater. Res. 1999, 48, 342–353. . [DOI] [PubMed] [Google Scholar]
- Okada M. Chemical Synthesis of Biodegradable Polymers. Prog. Polym. Sci. 2002, 27, 87–133. 10.1016/S0079-6700(01)00039-9. [DOI] [Google Scholar]
- Rai R.; Tallawi M.; Grigore A.; Boccaccin A. R. Synthesis, Properties and Biomedical Applications of Poly(glycerol sebacate) (PGS): A Review. Prog. Polym. Sci. 2012, 37, 1051–1078. 10.1016/j.progpolymsci.2012.02.001. [DOI] [Google Scholar]
- Tidwell T. T. Ketene Chemistry after 100 Years: Ready for A New Century. Eur. J. Org. Chem. 2006, 2006, 563–576. 10.1002/ejoc.200500452. [DOI] [Google Scholar]
- Leibfarth F. A.; Kang M.; Ham M.; Kim J.; Campos L. M.; Gupta N.; Moon B.; Hawker C. J. A Facile Route to Ketene-Functionalized Polymers for General Materials Applications. Nat. Chem. 2010, 2, 207–212. 10.1038/nchem.538. [DOI] [PubMed] [Google Scholar]
- Leibfarth F. A.; Schneider Y.; Lynd N. A.; Schultz A.; Moon B.; Kramer E. J.; Bazan G. C.; Hawker C. J. Ketene Functionalized Polyethylene: Control of Cross-Link Density and Material Properties. J. Am. Chem. Soc. 2010, 132, 14706–14709. 10.1021/ja1060643. [DOI] [PubMed] [Google Scholar]
- Miyamura Y.; Park C.; Kinbara K.; Leibfarth F. A.; Hawker C. J.; Aida T. Controlling Volume Shrinkage in Soft Lithography through Heat-Induced Cross-Linking of Patterned Nanofibers. J. Am. Chem. Soc. 2011, 133, 2840–2843. 10.1021/ja110901h. [DOI] [PubMed] [Google Scholar]
- Wu J.; Iacono S. T.; McCandiess G. T.; Smith D. W. Jr.; Novak B. M. Utilization of a Meldrum’s Acid towards Functionalized Fluoropolymers Possessing Dual Reactivity for Thermal Crosslinking and Post-Polymerization Modification. Chem. Commun. 2015, 51, 9220–9222. 10.1039/C5CC02382C. [DOI] [PubMed] [Google Scholar]
- Lin L.-K.; Hu C.-C.; Su W.-C.; Liu Y.-L. Thermosetting Resins with High Fractions of Free Volume and Inherent Low Dielectric Constants. Chem. Commun. 2015, 51, 12760–12763. 10.1039/C5CC03899E. [DOI] [PubMed] [Google Scholar]
- Wolffs M.; Kade M. J.; Hawker C. J. An Energy Efficient and Facile Synthesis of high Molecular Weight Polyesters Using Ketenes. Chem. Commun. 2011, 47, 10572–10574. 10.1039/c1cc14055h. [DOI] [PubMed] [Google Scholar]
- Leibfarth F. A.; Wolffs M.; Campos L. M.; Delany K.; Treat N.; Kade M. J.; Moon B.; Hawker C. J. Low-Temperature Ketene Formation in Materials Chemistry through Molecular Engineering. Chem. Sci. 2012, 3, 766–771. 10.1039/C2SC00841F. [DOI] [Google Scholar]
- Teramoto N.; Urata K.; Ozawa K.; Shibata M. Biodegradation of Aliphatic Polyester Composites Reinforced by Abaca Fiber. Polym. Degrad. Stab. 2004, 86, 401–409. 10.1016/j.polymdegradstab.2004.04.026. [DOI] [Google Scholar]
- Luo Q.; Xiao X.; Dai X.; Duan Z.; Pan D.; Zhu H.; Li X.; Sun L.; Luo K.; Gong Q. Cross-Linked and Biodegradable Polymeric System as a Safe Magnetic Resonance Imaging Contrast Agent. ACS Appl. Mater. Interfaces 2018, 10, 1575–1588. 10.1021/acsami.7b16345. [DOI] [PubMed] [Google Scholar]