Supplemental Digital Content is available in the text
Keywords: fascia iliaca compartment block, hip fracture, meta-analysis, pain control
Abstract
Background:
To assess the effect of fascia iliaca compartment block (FICB) on pain control and morphine consumption in patients with hip fracture.
Methods:
We searched databases (PubMed, Embase, Cochrane Library) for eligible randomized controlled trials (RCTs) published prior to September 12, 2018. We only included hip fracture patients who received FICB versus placebo for pain control. Risk ratios (RRs), standard mean differences (SMD) and 95% confidence intervals (CI) were determined. Stata 12.0 was used for the meta-analysis.
Results:
Eleven trials involving 937 patients underwent hip fracture were retrieved. FICB significantly decreased the pain intensity at 1–8 h (SMD = −1.03, 95% CI [−1.48, −0.58], P = .000), 12 h (SMD = −1.06, 95% CI [−1.36, −0.75], P = .000), 24 h (SMD = −1.14, 95% CI [−1.66, −0.62], P = .000) and 48 h (SMD = −0.96, 95% CI [−1.33, −0.60], P = .000). Moreover, FICB could reduced the total morphine consumption and the occurrence of nausea (P < .05). There was no significant difference between the pain intensity at 72 h (SMD = 0.11, 95% CI [−0.12, 0.34], P = .355).
Conclusions:
FICB has a beneficial role in reducing pain intensity and morphine consumption after hip fracture. Moreover, FICB has morphine-sparing effects when compared with a control group. More high-quality RCTs are needed to identify the optimal technique and volume of injectate for FICB.
1. Introduction
It is reported that nearly 75,000 people suffer a hip fracture each year in the UK.[1] It is predicted that there will be 6.3 million new cases of hip fracture each year.[2] The mortality of hip fracture is increasing in recent years. Though many guidelines reported that early surgery can relieve pain, and reduce the incidence of postoperative complications and mortality. Perioperative pain management is a big challenge for both patients and orthopedists. Systematic administration opioids remain the most commonly used analgesia protocol. However, adverse effects such as nausea and vomiting limited the widely application. FICB, was first described by Sharrock in 1989.[3] FICB involves the fascia iliaca compartment to deliver a large volume of low concentrated local anaesthetic to reduce pain by affecting the femoral and lateral cutaneous nerve of the thigh.
There is no consensus as the real effects of FICB for pain control in hip fracture patients. Several studies have reported that FICB has a beneficial role in reducing pain intensity and morphine consumption after hip fracture.[4] However, some other studies suggest that the use of FICB does not result in improved pain intensity in hip fractures.[5]
Therefore, it is necessary to investigate whether FICB provides better analgesic effects, as well as whether it reduces opioid consumption in hip fracture patients. The purpose of the current meta-analysis was to compare results concerning the efficacy of FICB for pain control in hip fracture patients.
2. Materials and methods
This work has been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
2.1. Search strategy
This meta-analysis was designed on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines.[6] Several electronic databases were searched from inception to December 1, 2018, including PubMed, Embase, and the Cochrane library. No language or other limits were set. The search strategies were listed in Supplement file 1. To ensure the integrity of the search results, reference lists from related reviews were also screened. Before screening, the search task for each database was executed by 2 independent researchers to achieve agreement. A flow diagram is presented in Fig. 1.
Figure 1.
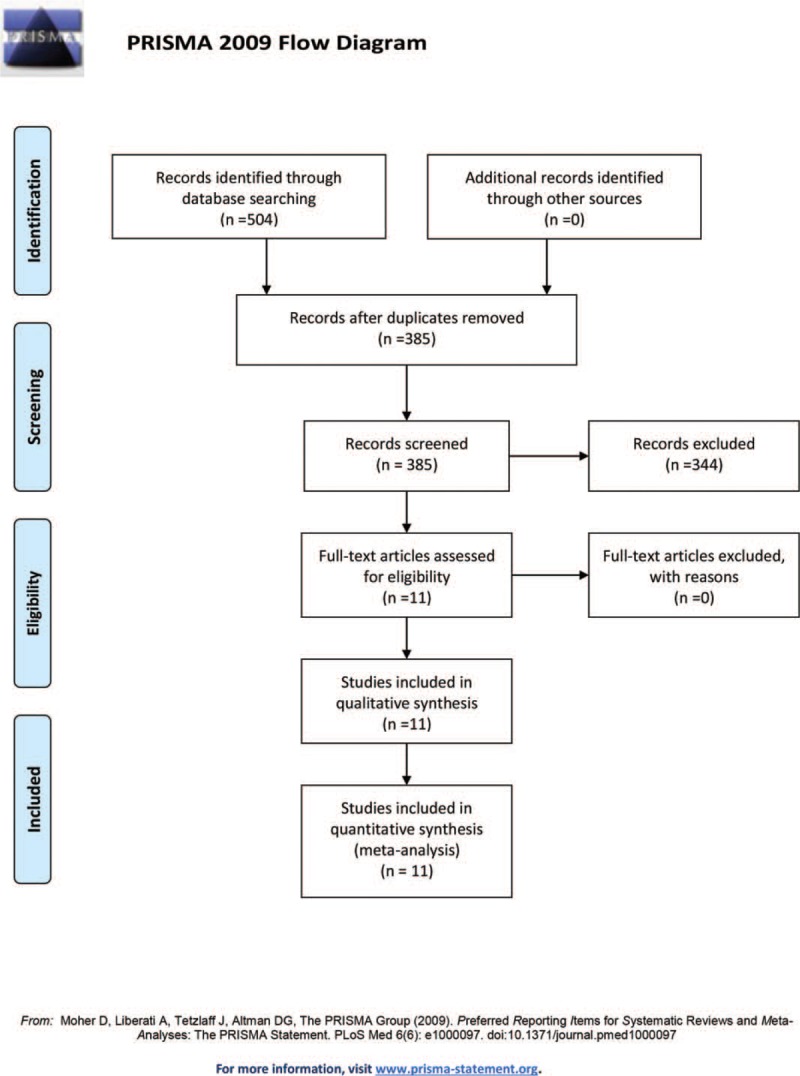
Flow diagram of the study selection process.
2.2. Inclusion criteria and exclusion criteria
The following criteria must have been met for the eligible studies:
-
1.
Study design: RCTs;
-
2.
Population: adults undergoing hip fracture repair;
-
3.
Intervention: use of FICB for pain control;
-
4.
Comparison: placebo or no treatment; and
-
5.
Main outcomes: visual analog scale (VAS) at 1–8, 12, 24, and 48 h; total morphine consumption; occurrence of nausea.
Ineligible studies contained the following features:
-
1.
they were retrospective trials or other non-RCTs;
-
2.
they included comparisons with other anesthesia protocols; and
-
3.
they did not report outcomes.
2.3. Study inclusion
Articles found in the databases were imported into reference management software (Endnote X7), and the duplicate articles were removed. The inclusion criteria were applied to the screening of titles and abstracts by 2 independent researchers, and the retained articles were cross-checked. The 58 remaining articles were screened by reading the full articles, and 11 articles ultimately remained in the quantitative synthesis procedure. Agreement was reached by 3 independent researchers by discussion.
2.4. Quality assessment
The methodological quality of each individual study was assessed by 2 independent reviewers according to the Cochrane Handbook for Systematic Reviews of Interventions. The quality and bias assessment had to include the following items: randomization method, allocation concealment, blinding methods, incomplete outcome data, selective reporting and other bias.
2.5. Data extraction
Relevant data from the eligible articles were extracted independently by 2 reviewers, including the author; the data included publication year, country, intervention, size of each group, mean age, proportion of female patients, FICB, technique, anesthesia, outcomes and risk of bias. The corresponding authors of the included RCTs were contacted for the missing data to ensure the integrity of the review if necessary. Consensus was finally reached between 2 reviewers through discussion. When a numerical rating scale (NRS) score was used, it was converted to a VAS score.[7] The 10-point VAS score was converted to a 100-point VAS score.[8] In the absence of a standard deviation (SD), the median value of SDs from other studies of the same comparison were substituted.[9] For data described as the median and range, the mean and SD were estimated according to the previously described manner.[10]
2.6. Data synthesis and statistical methods
Once adequate data were retrieved, weighted mean differences (WMD) were calculated for the following outcomes: visual analog scale (VAS) at 1–8 h, 12 h, 24 h, and 48 h and total morphine consumption. For the dichotomous variables (occurrence of nausea), the risk ratio (RR) with a 95% CI was used. Separate statistics were combined using the inverse variance and Mantel–Haenszel methods. If the values were <0.05, the results were considered statistically significant. Study heterogeneity was estimated by the I2 statistic test and Cochran's Q test in accordance with the values of I2 and P. A fixed-effects model could be used if I2 < 50% and P > .1. Otherwise, a random-effects model was used to pool the effects of the interventions. We performed subgroup analysis for VAS at 1–8 h according to the operative technique (probe parallel to the inguinal ligament or perpendicular to the inguinal ligament), risk of bias (low or unclear/high), volume of injectate (≤30 mL, >30 mL) and anesthesia (general anesthesia or spinal anesthesia). A 2-sided P value of <.05 was considered significant. All statistical analyses were performed using Stata 12.0 (Stata Corp., College Station, TX). Publication bias across studies was examined visually using “funnel plots.”
3. Results
3.1. Search results and general characteristics
A flow chart of the included studies is shown in Fig. 1. The primary database search yielded 504 relevant studies. After duplicates were removed, 385 studies were available for screening. After screening the titles, abstracts and full texts, 344 papers were excluded; 11 RCTs met our inclusion criteria and were included for analyses.[1,5,11–22] The characteristics and demographics for the included studies are presented in Table 1.
Table 1.
General characteristic of the included studies.
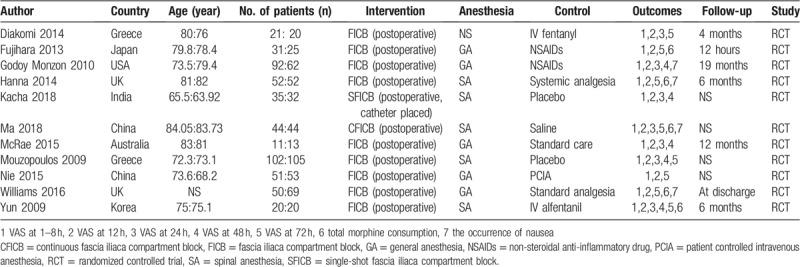
The publication years ranged from 2009 to 2016. These 11 studies originated from 8 countries. The sample sizes in the included trials were from 11 to 105. The mean age of the included patients ranged from 63.92 to 84.05.
3.2. Risk of bias
Details of the risk of bias summary and risk of bias graph are presented in Figs. 2 and 3, respectively. Overall, 3 trials were categorized as being at low risk of bias, 3 as being unclear and 1 as being at high risk of bias.
Figure 2.
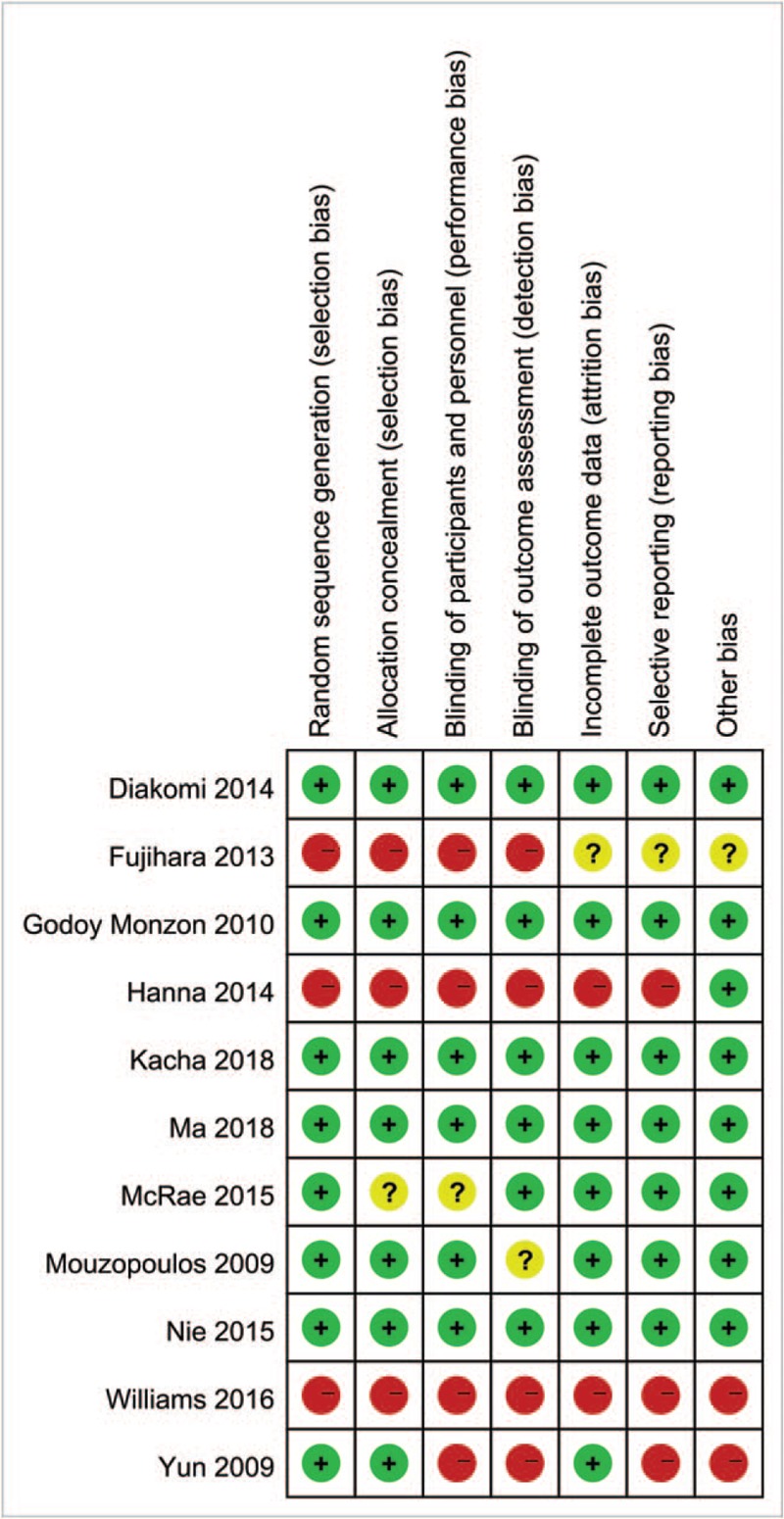
Risk of bias summary.
Figure 3.
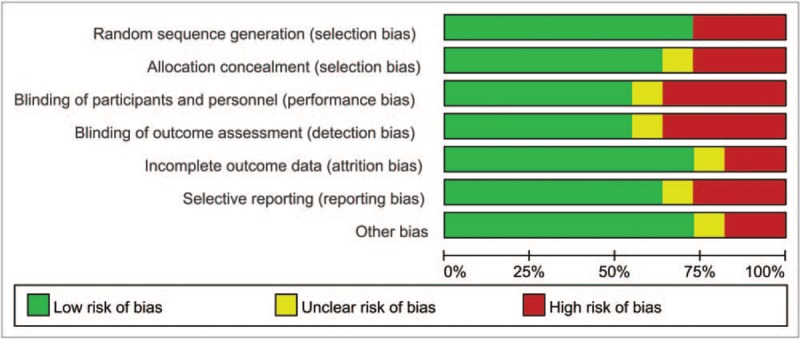
Risk of bias graph.
3.3. Results of meta-analysis
3.3.1. VAS at 1–8 h
Seven RCTs totaling 475 hip fractures reported the VAS at 1–8 h. Compared with the control group, FICB significantly reduced VAS at 1–8 h with high heterogeneity (SMD = −1.03, 95% CI [−1.48, −0.58], P = .000, I2 = 81.7%, Fig. 4).
Figure 4.
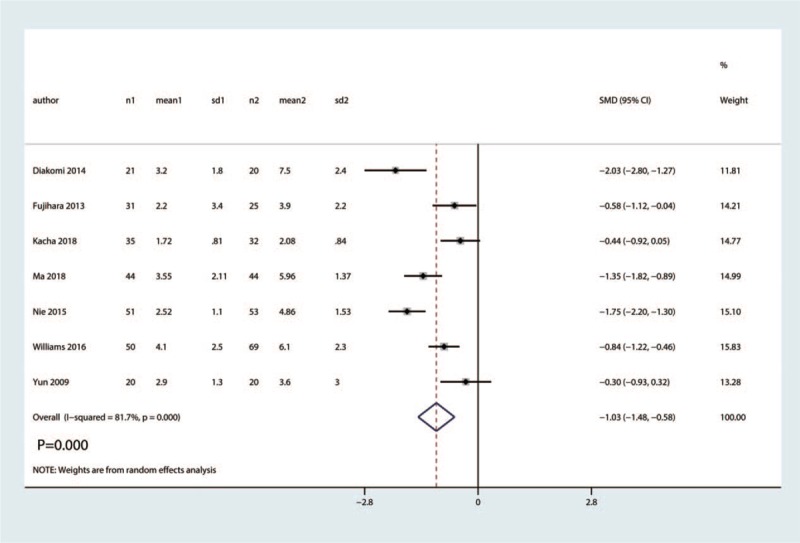
Forest plot for the comparison of VAS at 1–8 h between the FICB group and control group.
3.3.2. VAS at 12 h
Six trials totaling 455 patients provided data on VAS at 12 h. Compared with the control group, FICB significantly reduced VAS at 12 h with moderate heterogeneity (SMD = −1.06, 95% CI [−1.36, −0.75], P = .000, I2 = 51.2%, Fig. 5).
Figure 5.
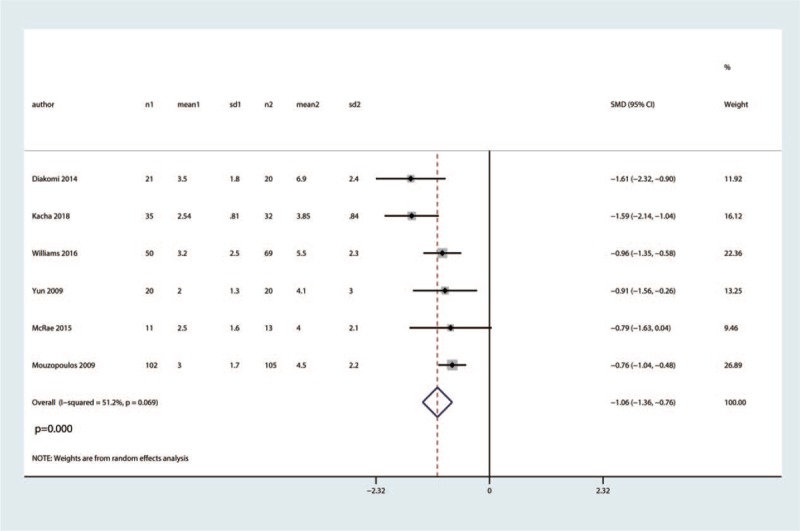
Forest plot for the comparison of VAS at 12 h between the FICB group and control group.
3.3.3. VAS at 24 h
Six studies totaling 556 hip fractures reported the VAS at 24 h. There was high heterogeneity among the included studies (I2 =84.7%, P = .000). FICB has a positive effect on VAS reduction at 24 h after hip fracture when compared with the control group (SMD = −1.14, 95% CI [−1.66, −0.62], P = .000, Fig. 6).
Figure 6.
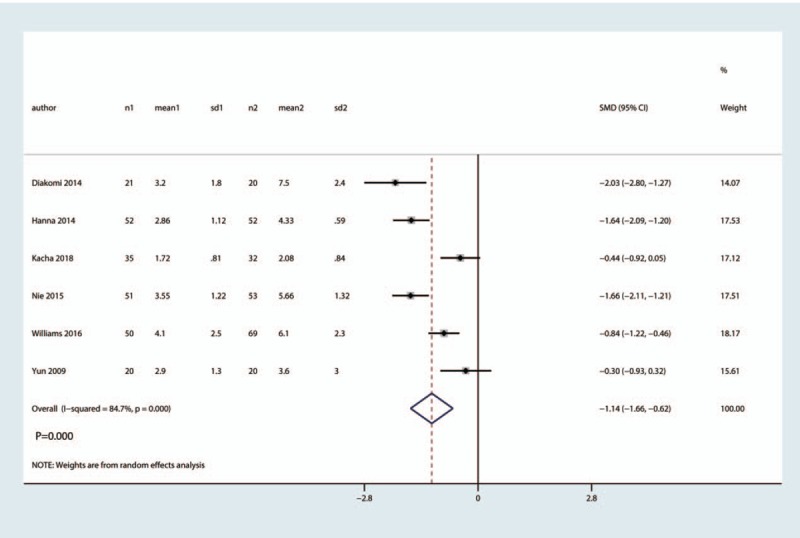
Forest plot for the comparison of VAS at 24 h between the FICB group and control group.
3.3.4. VAS at 48 h
Five trials involving 384 patients reported VAS at 48 h. There was high heterogeneity among the included studies (I2 = 65.0%, P = .022). Compared with the control group, FICB had no benefit on VAS at 48 h (SMD = −0.96, 95% CI [−1.33, −0.60], P = .000, Fig. 7).
Figure 7.
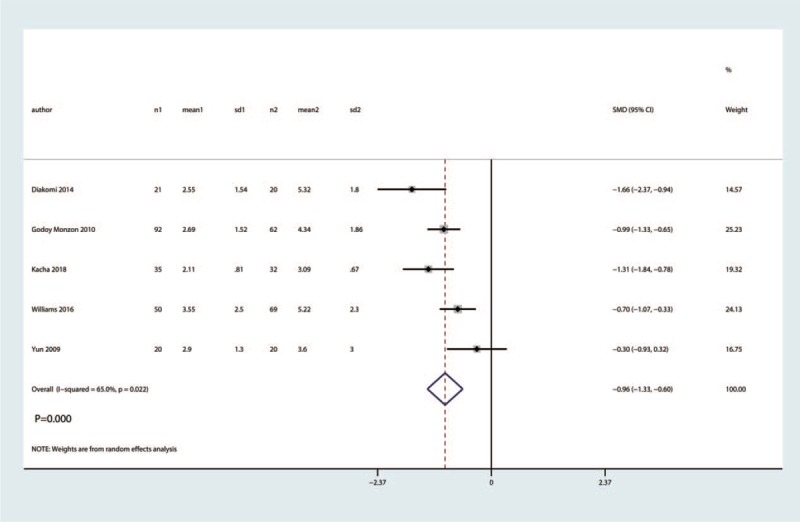
Forest plot for the comparison of VAS at 48 h between the FICB group and control group.
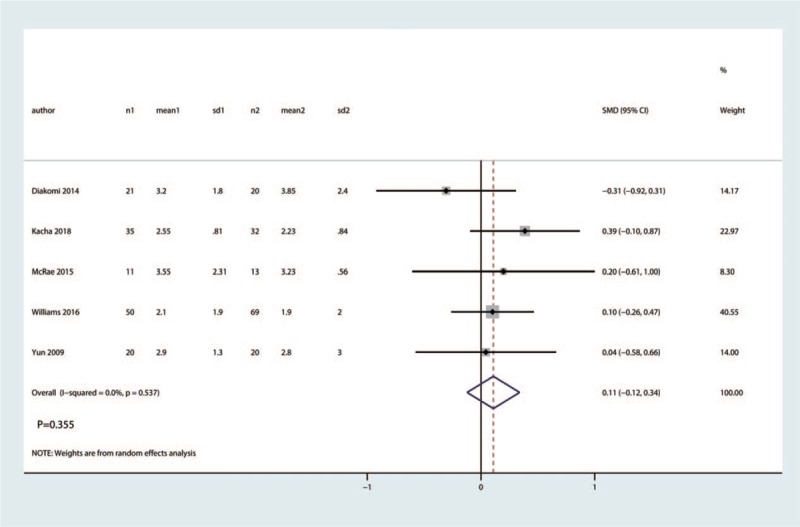
3.3.5. VAS at 72 h
Five trials involving 406 patients reported VAS at 72 h. There was no heterogeneity among the included studies (I2 = 0.0%, P = .537). Compared with the control group, FICB had no benefit on VAS at 72 h (SMD = 0.11, 95% CI [−0.12, 0.34], P = .355, Fig. 8).
Figure 8.
Forest plot for the comparison of total morphine consumption between the FICB group and control group.
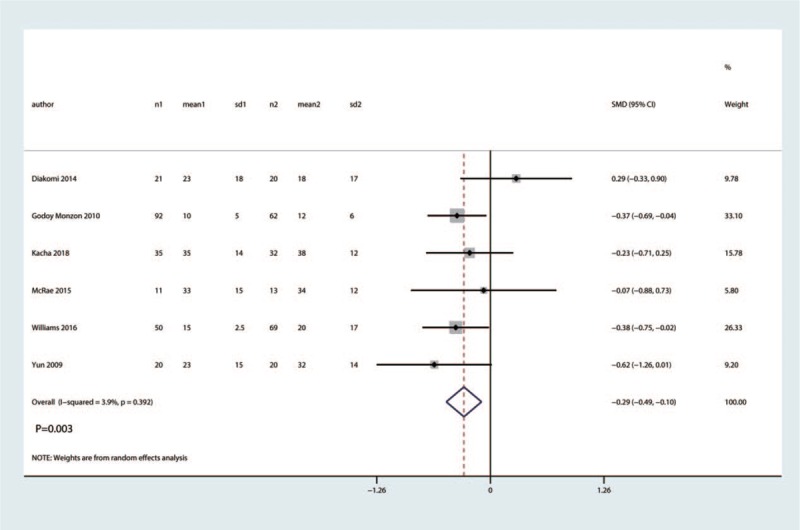
3.4. Total morphine consumption
A fixed-effects model was used to pool the total morphine consumption data, since the heterogeneity across the four studies was low (I2 = 3.9%, P = .392). Pooled analysis demonstrated clinical inferiority of the efficacy of FICB compared with placebo for total morphine consumption (SMD = −0.29, 95% CI [−0.48, −0.11], P = .002, Fig. 9).
Figure 9.
Forest plot for the comparison of the occurrence of nausea between the FICB group and control group.
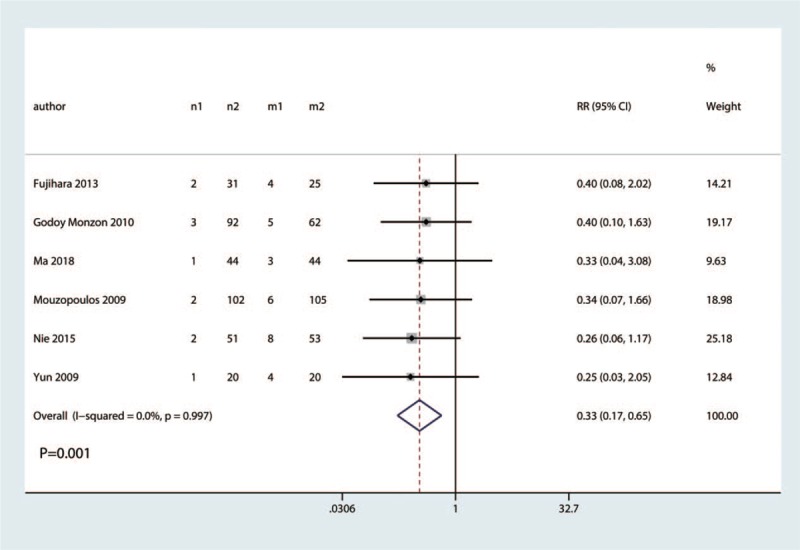
3.5. The occurrence of nausea
Six studies involving 224 hip fractures were available for analysis of the occurrence of nausea. FICB led to significantly less occurrence of nausea than in the control group (RR = 0.33, 95% CI 0.17–0.65, P = .001; I2 = 0.0%, P = .997, Fig. 10). Thus, we used a fixed-effects model to pool the relevant data.
Figure 10.
Funnel plot for the comparison of the VAS at 1–8 h between the FICB group and control group.
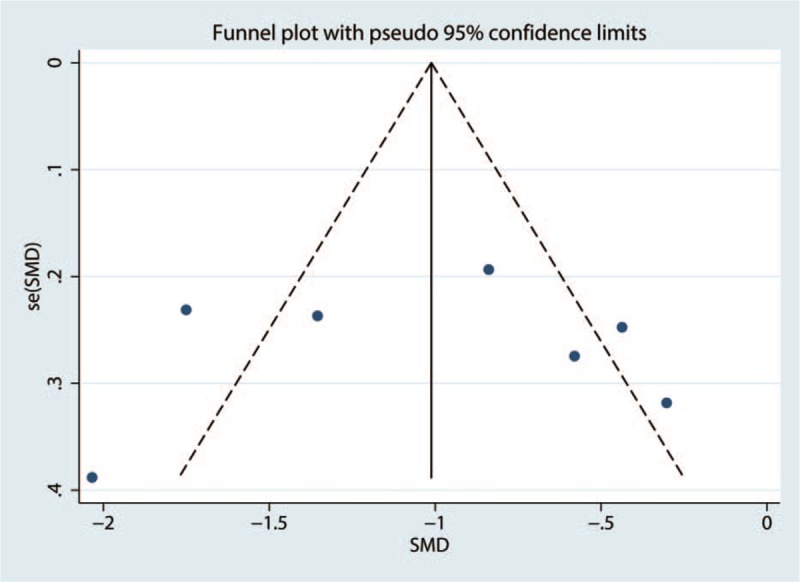
3.6. Publication bias, sensitivity analysis and subgroup analysis
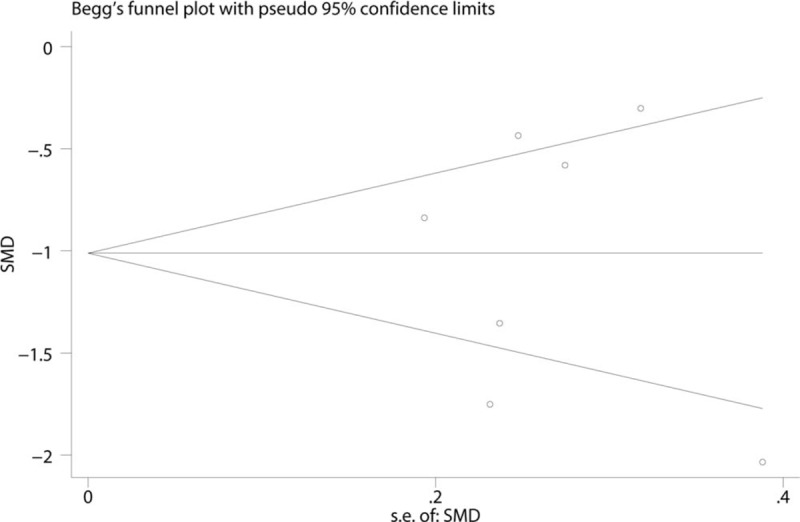
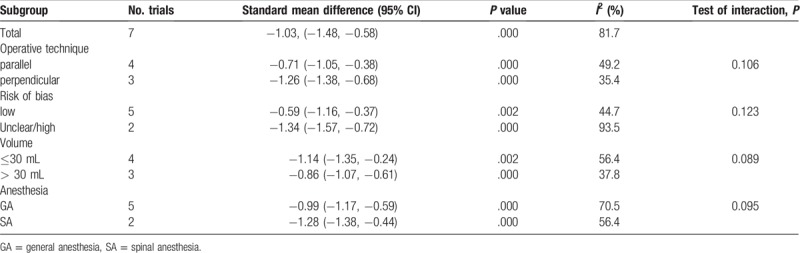
To address publication bias, we created funnel plots for VAS at 1–8 h. No asymmetric patterns were observed (Fig. 10). Begg's test indicated that there was no publication bias between the included studies (Fig. 11). Sensitivity analysis was performed by excluding one trial at a time and recalculating the pooled WMD for the remaining trials, which showed that none of the studies affected the results (Fig. 12). Subgroup analysis results can be seen in Table 2. The findings of the VAS at 1–8 h were consistent in all subgroup analyses.
Figure 11.
Begg's test for the comparison of the VAS at 1–8 h between the FICB group and control group.
Figure 12.
Sensitivity analysis of the VAS at 1–8 h between the FICB group and control group.
Table 2.
Subgroup analysis for the VAS at 1–8 h.
4. Discussion
4.1. Main findings
This review and meta-analysis aimed to investigate the efficacy and safety of FICB comparing with placebo as the control group. The existing body of evidence demonstrated that FICB was evidently effective for hip fracture patients. What's more, FICB could also reduce the total morphine consumption and the occurrence of nausea.
4.2. Relationship to other systematic reviews
Only one relevant systematic review and meta-analysis comparing FICB and placebo for hip fracture patients has been published.[4] In this meta-analysis, several problems existed. First, this meta-analysis included FICB versus other anesthesia method (3-in-1 block or femoral nerve block)[12,19] for pain control in hip fracture patients. Second, subgroup analysis and sensitivity analysis were not performed. In contrast, the current meta-analysis included 11 RCTs and only focused on FICB versus placebo for hip fracture patients. Thus, our current meta-analysis was the latest and most comprehensive meta-analysis.
4.3. Implications for clinical practice
Hip fractures are painful and pain left untreated can result in a host of complications that may delay operative intervention and complicate hospital stay. Previously, systemic analgesia and 3-in-1 block were the routine method for pain control in hip fracture patients. However, systemic analgesia was associated with an increase of the complications such as nausea and vomiting. 3-in-1 block require of a nerve stimulator to locate the femoral nerve. FICB is proposed as an alternative anterior approach to the lumber plexus.
Several studies reporting FICB has a good outcome when compared to non-steroidal anti-inflammatory drug[14] and alfentanil.[22] We identified VAS after hip fracture as outcomes, results shown that FICB has a beneficial role in reducing pain intensity up to 48 h. There was no significant difference between 72 h after hip fracture. Yang L et al[23] conducted a meta-analysis about FICB versus no block for pain control in lower limb surgery. Results shown that, FICB is an effective and safe method for alleviating the pain in lower limb surgery. Liu et al[24] conducted a network meta-analysis and also found that FICB was superior than placebo in hip arthroplasty patients. We performed a subgroup analysis for VAS at 1–8 h, results shown that FICB has a beneficial role in reducing pain scores at 1–8 h at any subgroup (operative technique, risk of bias, volume, and anesthesia).
Next, we measured total morphine consumption in the FICB group and control group. The results showed that FICB could significantly reduce morphine consumption compared with the control group. Foss et al[12] reported that median total morphine consumption was 0 mg in the FICB group and 6 mg in the control group. Moreover, we measured the occurrence of nausea between FICB and control group. Results have shown that FICB could significantly be reduced the occurrence of nausea. Klukowski et al[25] performed a retrospective study and found that FICB has significantly less need for analgesics than non-block patients.
In addition, FICB is easy and safe to administer for surgeons and anesthetists. FICB only requires ultrasound guidance for placing a catheter. Reports have shown that FICB can generally be performed with minimal training[26–28] and by nonmedical practitioners.[29] The fascia iliaca compartment is located away from the femoral nerve, femoral artery, and femoral vein.
4.4. Limitations
Our study has several limitations. Of the 11 studies, 3 were with high risk of bias, resulting in some inherent heterogeneity due to uncontrolled bias. Due to this heterogeneity, we applied the random-effect model for meta-analysis, but there have still been many considerations to be emphasized. Additionally, postoperative pain control protocol was not unanimous in all of the included studies, which can also affect pain intensity after hip fracture. The drugs and dose of regional anesthesia was not comparable and thus need for more studies to identify the optimal drug and dose for FICB.
5. Conclusion
Compared with placebo, FICB is a safe and effective method to reduce postoperative pain scores, morphine consumption, and the occurrence of nausea in patients after hip fracture. Furthermore, more RCTs concerning FICB are needed to identify the optimal technique and volume of injectate for FICB.
Author contributions
HHK and MY conceived of the design of this meta-analysis. HHK and MY performed the literature retrieval and article writing. MY contributed to the data extraction, and HHK revised the manuscript. All authors read and approved the final manuscript.
Formal analysis: Hui-kan Hong.
Visualization: Yi Ma.
Writing – Original Draft: Hui-kan Hong, Yi Ma.
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, FICB = fascia iliaca compartment block, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCTs = randomized controlled trials, RR = risk ratio, VAS = visual analogue scale, WMD = weighted mean difference, .
Declarations
Funding: None.
Availability of data and materials: As a meta-analysis, there are no patient data sets.
Ethics approval and consent to participate: Not applicable. This meta-analysis does not involve research on humans.
Consent for publication: Not applicable.
Competing interests: The authors declare that they have no competing interests.
Supplemental Digital Content is available for this article.
References
- [1].Reavley P, Montgomery AA, Smith JE, et al. Randomised trial of the fascia iliaca block versus the ’3-in-1’ block for femoral neck fractures in the emergency department. Emerg Med J 2015;32:685–9. [DOI] [PubMed] [Google Scholar]
- [2].Watters CL, Moran WP. Hip fractures—a joint effort. Orthop Nurs 2006;25:157–65. quiz 166-7. [DOI] [PubMed] [Google Scholar]
- [3].Sharrock E, Inadvertent N. ‘3 in 1’ block following injection of the lateral cutaneous nerve of the thigh 1980;Vol.59:887–8. [PubMed] [Google Scholar]
- [4].Steenberg J, Moller AM. Systematic review of the effects of fascia iliaca compartment block on hip fracture patients before operation. Br J Anaesth 2018;120:1368–80. [DOI] [PubMed] [Google Scholar]
- [5].Mouzopoulos G, Vasiliadis G, Lasanianos N, et al. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. J Orthop Traumatol 2009;10:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [7].Wang C, Cai XZ, Yan SG. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [8].Sun X-L, Zhao ZH, Ma JX, et al. Continuous local infiltration analgesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine 2015;94:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Furukawa TA, Barbui C, Cipriani A, et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7–10. [DOI] [PubMed] [Google Scholar]
- [10].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diakomi M, Papaioannou M, Mela A, et al. Preoperative fascia iliaca compartment block for positioning patients with hip fractures for central nervous blockade: a randomized trial. Reg Anesth Pain Med 2014;39:394–8. [DOI] [PubMed] [Google Scholar]
- [12].Foss NB, Kristensen BB, Bundgaard M, et al. Fascia iliaca compartment blockade for acute pain control in hip fracture patients: a randomized, placebo-controlled trial. Anesthesiology 2007;106:773–8. [DOI] [PubMed] [Google Scholar]
- [13].Fujihara Y, Fukunishi S, Nishio S, et al. Fascia iliaca compartment block: its efficacy in pain control for patients with proximal femoral fracture. J Orthop Sci 2013;18:793–7. [DOI] [PubMed] [Google Scholar]
- [14].Godoy Monzon D, Vazquez J, Jauregui JR, et al. Pain treatment in post-traumatic hip fracture in the elderly: regional block vs. systemic non-steroidal analgesics. Int J Emerg Med 2010;3:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hanna L, Gulati A, Graham A. The role of fascia iliaca blocks in hip fractures: a prospective case-control study and feasibility assessment of a junior-doctor-delivered service. ISRN Orthop 2014;2014:191306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kacha NJ, Jadeja CA, Patel PJ, et al. Comparative study for evaluating efficacy of fascia iliaca compartment block for alleviating pain of positioning for spinal anesthesia in patients with hip and proximal femur fractures. Indian J Orthop 2018;52:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ma Y, Wu J, Xue J, et al. Ultrasound-guided continuous fascia iliaca compartment block for pre-operative pain control in very elderly patients with hip fracture: A randomized controlled trial. Exp Ther Med 2018;16:1944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McRae PJ, Bendall JC, Madigan V, et al. Paramedic-performed fascia iliaca compartment block for femoral fractures: a controlled trial. J Emerg Med 2015;48:581–9. [DOI] [PubMed] [Google Scholar]
- [19].Newman B, McCarthy L, Thomas PW, et al. A comparison of pre-operative nerve stimulator-guided femoral nerve block and fascia iliaca compartment block in patients with a femoral neck fracture. Anaesthesia 2013;68:899–903. [DOI] [PubMed] [Google Scholar]
- [20].Nie H, Yang YX, Wang Y, et al. Effects of continuous fascia iliaca compartment blocks for postoperative analgesia in patients with hip fracture. Pain Res Manag 2015;20:210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Williams H, Paringe V, Shenov S, et al. Standard preoperative analgesia with or without fascia iliaca compartment block for femoral neck fractures. J Orthop Surg (Hong Kong) 2016;24:31–5. [DOI] [PubMed] [Google Scholar]
- [22].Yun MJ, Kim YH, Han MK, et al. Analgesia before a spinal block for femoral neck fracture: fascia iliaca compartment block. Acta Anaesthesiol Scand 2009;53:1282–7. [DOI] [PubMed] [Google Scholar]
- [23].Yang L, Li M, Chen C, et al. Fascia iliaca compartment block versus no block for pain control after lower limb surgery: a meta-analysis. J Pain Res 2017;10:2833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu P, Wu Y, Liang Z, et al. Comparing the efficacy of pain managements after total hip arthroplasty: a network meta-analysis. J Cell Biochem 2018;Epub 2018 Oct 9. [DOI] [PubMed] [Google Scholar]
- [25].Klukowski M, Kowalczyk R, Górniewski G, et al. Iliac fascia compartment block and analgesic consumption in patients operated on for hip fracture. Ortop Traumatol Rehabil 2017;19:451–9. [DOI] [PubMed] [Google Scholar]
- [26].Hauritz RW, Gerlif C, Ronholm E. Fascia iliaca block performed by emergency department physician trainees in hip fractures. Ugeskr Laeger 2009;171:515–8. [PubMed] [Google Scholar]
- [27].Hogh A, Dremstrup L, Jensen SS, et al. Fascia iliaca compartment block performed by junior registrars as a supplement to pre-operative analgesia for patients with hip fracture. Strategies Trauma Limb Reconstr 2008;3:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Groot L, Dijksman LM, Simons MP, et al. Single fascia iliaca compartment block is safe and effective for emergency pain relief in hip-fracture patients. West J Emerg Med 2015;16:1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dochez E, van Geffen GJ, Bruhn J, et al. Prehospital administered fascia iliaca compartment block by emergency medical service nurses, a feasibility study. Scand J Trauma Resusc Emerg Med 2014;22:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


