Abstract
Persistent postsurgical pain (PPP) is defined as the discomfort that lasts >3 months postoperatively. The primary aim of this retrospective study was to estimate the risk of developing moderate-to-severe PPP after primary total knee arthroplasty (TKA). The secondary goal was to explore potential predictors of this outcome.
Data were collected via hospital arthroplasty registry and chart review. The risk of moderate-to-severe PPP, defined as ≥4 on the numerical rating scale (NRS) at minimum of 3 months post-surgery, was calculated. Multivariable logistic regression was used to estimate the association of patient demographics, diagnoses, length of hospital stay, and preoperative NRS with the odds of developing PPP. Exploratory, simple logistic regression was used to estimate the association of perioperative factors with the odds of developing PPP on a subset of patients (n = 72).
The risk of PPP after TKA was 31.3% (95% confidence interval [CI]: 27.5–35.0) (n = 578). Every 2-point increase in baseline NRS was associated with 1.66 (95% CI: 1.37–2.03) times the odds of developing PPP (P < .001). African-Americans (vs whites) had 1.82 (95% CI: 1.03–3.22) times the odds of developing PPP (P = .040). Exploratory analysis suggested that the adductor canal saphenous nerve (vs femoral nerve) blocks were associated with 2.87 (95% CI: 1.00–8.26) times the odds of developing PPP (P = .049).
This study estimated a high risk (31.3%) of moderate-to-severe PPP after primary TKA. This study suggested that higher preoperative pain scores might be associated with greater odds of developing PPP. Moreover, this study suggested the possibility that racial differences and types of peripheral nerve blocks might be associated with greater odds of developing moderate-to-severe PPP after TKA surgery. However, the evidence obtained from our exploratory analysis of limited data certainly requires further exploration in large-scale studies.
Keywords: analgesia, chronic pain, complication, knee arthroplasty, nerve block, persistent postoperative pain
1. Introduction
Total knee arthroplasty (TKA) is a common procedure performed to improve the pain and disability secondary to end-stage knee arthritis when nonsurgical management is no longer effective.[1] However, a significant number of patients undergoing TKA develop disabling long-term postoperative pain despite unremarkable physical examinations and diagnostic studies.[2] Persistent postsurgical pain (PPP) is defined by The International Association for Study of Pain (IASP) as the discomfort that lasts for >3 months after surgery.[3] It is estimated that 10% to 44% of TKA patients develop PPP,[4–6] with the highest-quality studies reporting an incidence of approximately 20%.[4]
The economic impact of long-term, moderate-to-severe, and unexplained pain after TKA is profound.[7] Therefore, preoperative identification of the patients at risk and implementation of better preventive strategies may help to reduce the incidence of PPP and improve outcomes.[8,9]
The primary aim of this retrospective study was to estimate the risk of developing moderate-to-severe PPP after primary TKA, defined as a score of ≥4 on the 11-point numerical rating scale (NRS) at a minimum of 3 months postoperatively. The secondary goal was to explore potential predictors of the development of PPP in this clinical setting.
2. Materials and methods
This retrospective cohort study was approved by the Institutional Review Board (IRB#2015–0167) and was designed to estimate the risk of moderate-to-severe PPP after TKA in a single center specializing in the treatment of musculoskeletal diseases. Data from the IRB-approved Legacy Registry and Collaborative Orthopedic Replacement Registry (CORRe) at the Hospital for Special Surgery were used for this retrospective analysis. The requirement for written consent was waived by the IRB.
Eligibility criteria required patients to have had unilateral or bilateral primary TKA performed between January 3, 2011 and April 13, 2015 recorded in the CORRe registry. Exclusion criteria included >1 operating room admission during the study period, incomplete demographic information, TKA for a reason other than osteoarthritis or inflammatory arthritis, and incomplete NRS data. NRS data were considered complete if the patient had a preoperative score and at least 1 score at minimum of 3 months postoperatively. Data collected from the CORRe and Legacy registries included NRS pain scores (preoperative and 3, 6, and 12 months postoperative), demographics (age, sex, race, American Society of Anesthesiologists [ASA] status), comorbidities (hypertensive disease, ischemic heart disease, pulmonary disease, chronic kidney disease, diabetes mellitus, mood disorder, anxiety disorder, tobacco use), reason for surgery (osteoarthritis vs inflammatory arthritis), laterality (simultaneous bilateral vs unilateral TKA), and length of hospital stay. Comorbidities and reason for surgery were determined via International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes. Chart review was used to obtain preoperative opioid use, tourniquet duration, peripheral nerve block (PNB) type, and perioperative gabapentinoid use for the subset of patients with NRS pain scores available in the database.
Total of 8149 TKA cases were identified between January 3, 2011 and April 13, 2015. A total of 5981 patients had single operating room visit and complete demographic information. A total of 5949 patients underwent TKA for osteoarthritis or inflammatory arthritis. A total of 1172 patients had the preoperative NRS score available in the registry database. Five-hundred seventy-eight patients had minimum ≥3-month (up to 12-month) postoperative NRS scores available in the registry database (Fig. 1).
Figure 1.
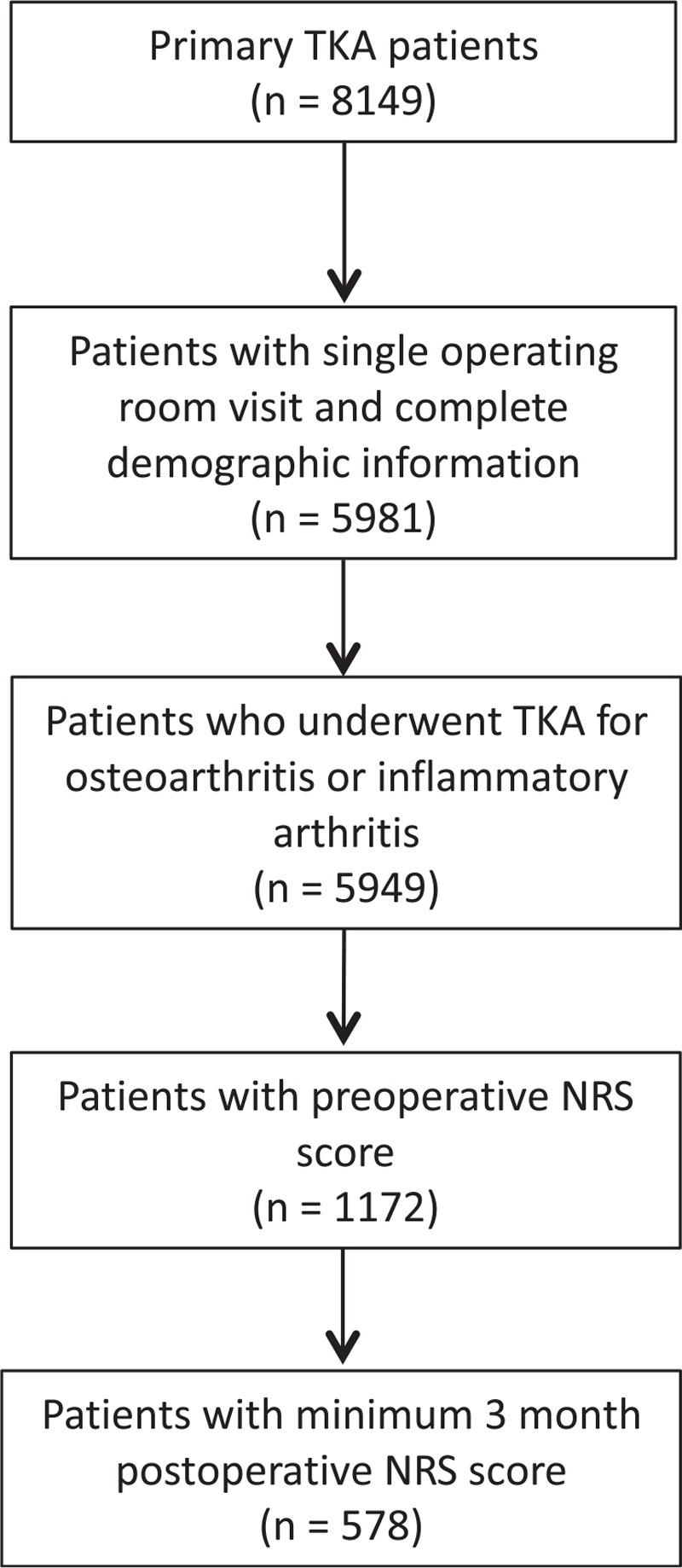
The patient selection flow diagram.
We reviewed the entire data in the registry database during the study time period without any selection bias. Data collectors were blinded to NRS pain scores and were not involved in the data analysis or manuscript preparation.
2.1. Statistical analysis
2.1.1. Sample size calculation
Assuming a 20% to 40% risk of developing PPP[4–6] at 95% Wilson score confidence interval with (±2%) precision, an estimated sample size of 1535 to 2300 patients would be required respectively. We were able to identify 578 patients with the complete data available for our study in the registry database. We analyzed the data for 578 patients, which were less than the estimated sample size, but still had validity from the statistical point of view, indicating that the calculated result would be less precise than projected. Henceforth, assuming a 20% to 40% risk of developing PPP with a sample size of 578, at 95% Wilson score confidence interval, the estimation precession is ±4%, as opposed to the anticipated ±2%.
2.1.2. Statistical methods
Continuous variables are summarized as means with standard deviations (SD) or medians with 1st and 3rd quartiles. Categorical variables are summarized as frequencies and percentages. Continuous variables were compared between patients with and without preoperative NRS pain scores using 2-sample t tests or Wilcoxon rank-sum tests, depending upon the distribution of the data. Categorical variables were compared between patients with and without preoperative NRS pain scores using χ2, Fisher exact, or Cochran-Armitage trend test, as appropriate. The risk of moderate-to-severe PPP is presented as a point estimate with 95% Wilson score confidence interval. The association between 18 a priori selected predictors and the odds of developing moderate-to-severe PPP was assessed using a Firth's penalized multivariable logistic regression model. Variance inflation factor test was performed, and no multicollinearity problem was detected. Normality of distribution was determined by Shapiro-Wilk testing wherein significance of >.05 indicates normally distributed data. For the subgroup of patients with NRS pain scores available at all time points (preoperative, 3 months, 6 months, and 12 months; n = 72), additional data were collected. Given the small size of this subgroup, separate simple logistic regression models were used to explore the association between each predictor and the odds of developing PPP. Statistical analyses were performed with SAS Version 9.3 (SAS Institute, Cary, NC).
3. Results
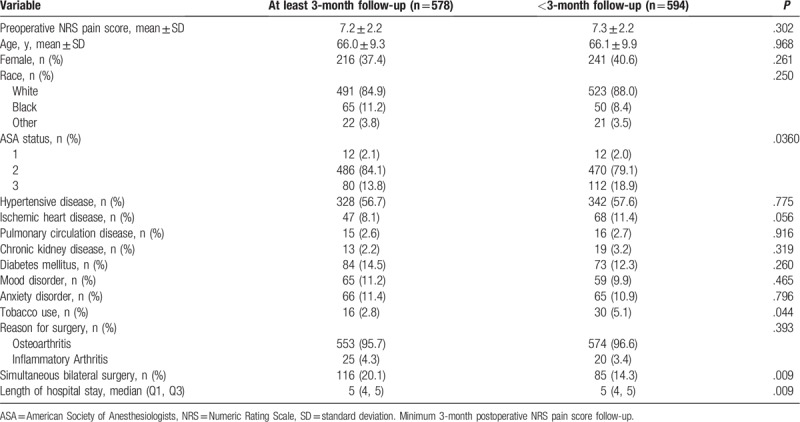
The patient selection flow diagram is shown in Figure 1. Patients with and without minimum a 3-month postoperative NRS pain score were mostly similar with respect to baseline NRS pain scores, demographics, and comorbidities. Exceptions included that patients with postoperative follow-up data had better ASA status, were less likely to use tobacco, more likely to have had simultaneous bilateral TKA, and had slightly longer lengths of hospital stay (Table 1).
Table 1.
Patient and surgery characteristics.
The risk of moderate-to-severe PPP after primary TKA was calculated as 31.3% (95% confidence interval [CI]: 27.5–35.0).
Multivariable logistic regression revealed associations between baseline NRS pain score and the odds of developing moderate-to-severe PPP (Table 2). Specifically, every 2-point increase in baseline NRS pain score was associated with 1.66 (95% CI: 1.37–2.03) times the odds of developing moderate-to-severe PPP (P < 0.001).
Table 2.
Multivariable logistic regression model of moderate-to-severe persistent postoperative pain at least 3 months after the surgery. The patient was categorized as having PPP if NRS ≥4 at any of the 3 time-points (3, 6, 12 months).
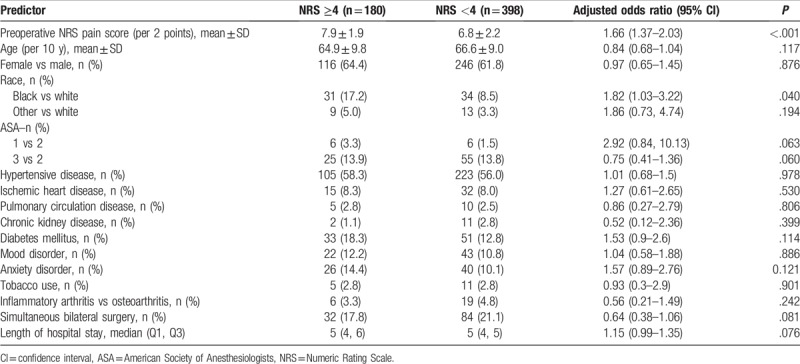
Regression also suggested an association between race and the odds of developing moderate-to-severe PPP. African-American patients had 1.82 (95% CI: 1.03–3.22) times the odds of developing persistent postoperative pain compared to white patients (P = .040) (Table 2).
There was no evidence of an association between mood disorders and the odds of developing moderate-to-severe PPP (P = .465) (Table 2).
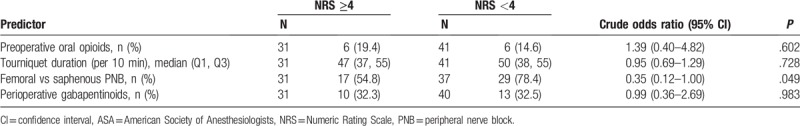
Exploratory, simple logistic regression analyses suggested that adductor canal blocks (ACB) may be associated with greater odds of developing moderate-to-severe PPP than femoral blocks when used for postoperative analgesia (P = .049) (Table 3). Tourniquet duration and perioperative gabapentinoid use were not associated with the odds of developing moderate-to-severe PPP (P = .728 and P = .921, respectively) (Table 3).
Table 3.
Simple logistic regression models of moderate-to-severe persistent postoperative pain in a subgroup of 72 patients with pain data at all time points.
4. Discussion
A Numeric Rating Scale (NRS) score of ≥4 is considered as moderate-to-severe pain, and is recommended as a trigger for a more comprehensive assessment and treatment.[10] Previous studies in this area of research have reported that approximately 25% of patients had complaints of significant pain (equivalent to NRS >4) at 3 months after TKA, despite normal clinical evaluation and radiographic work-up,[2] and the incidence of chronic pain was ≥20% at 3 to 24 months postoperatively.[11] In one study, up to 44% of TKR patients reported PPP of any severity.[6]
Some of the causes of long-term postsurgical pain after TKA include: mechanical pain secondary to poor prosthetic design and positioning, component loosening, periprosthetic fractures, ligament imbalances, patellofemoral disorders; inflammatory pain, which may occur due to a prolonged inflammatory response for implanted prosthesis or infection, and 3) neuropathic pain that arises after nerve injuries and sensitization of pain pathways.[3,12–14]
We used the IASP definition of PPP, defined as a NRS score ≥4 at minimum of 3 months post-surgery.[3] Our analysis of the available registry data showed that the incidence of PPP after primary TKA was 31.3% (95% CI: 27.5–35.0).
Although our estimate of the risk of PPP in primary TKA patients was within the range of other results reported in the literature,[4,6,14–16] the results should be interpreted with caution because of some of the limitations of present study; in our sample, the number of patients with complete data was less than estimated (N = 578 instead of 1535 for 20% risk and 2300 for 40% risk of developing PPP). Therefore, our estimate of the persistent postoperative pain risk was less precise than projected (ie, a precision of ±4% as opposed to the anticipated ±2%). There is also a possibility of overestimation, likely attributable to follow-up bias. It is possible that patients with PPP were more likely to return for a follow-up visit and report NRS scores than those without PPP. Our registry database did not include the amount of analgesic medication use after 3 months postoperatively. Therefore, the patients who were actively taking analgesic medications and reported their pain levels as low (NRS <4) at 3-, 6-, and 12-month time points were not included in our analysis. This might have led to underestimation of our calculated result of risk of PPP development after TKA.
Preoperative identification of high-risk patients and the implementation of better preventive strategies including patient education, analgesia, and physical therapy may serve as a strategy to reduce poor outcomes after TKA.[2,8,9] In one meta-analysis, it was reported that variables such as severe baseline preoperative pain and catastrophizing were the strongest independent predictors for development of PPP after TKA.[8] Our analysis showed that every 2-point increase in baseline NRS was associated with 1.66 (95% CI: 1.37–2.03) times the odds of developing PPP (P < .001). Our results were consistent with other studies reported in the literature in that the baseline preoperative pain score was an important predictor of increased risk for development of PPP. There is evidence that sensitization of pain pathways owing to severe uncontrolled preoperative pain may play a role in the development of neuropathic pain in osteoarthritic knee, in addition to mechanical and inflammatory pain.[17] This may be one of the possible explanations by which the incidence of PPP increases if patients present with higher baseline preoperative pain levels before TKA surgery.
In our study, African-American race was associated with 1.82 (95% CI: 1.03–3.22) times the odds of developing PPP compared to white patients (P = .040). There is conflicting and limited evidence regarding this topic in the medical literature. Although one meta-analysis reported that racial background had limited influence on predictors of persistent pain at >3 months after TKA,[8] there were studies indicating that African-Americans had higher rates of pain scores when compared to other races.[18–20] Our study was limited to only 65 patients who reported their racial background as African-American in our data sample (white = 513 vs African-American = 65). Therefore, the authors of this manuscript believe that further studies are required to better explore racial differences in the development of PPP after TKA.
A total of 68 patients included in our sample analysis with 46 patients were given femoral nerve block (FNB) and 22 patients were given ACB for adjunct postoperative analgesia. The majority of the patients in our dataset had combined spinal-epidural anesthesia, with continuation of the epidural catheter for postoperative analgesia through the first postoperative day, along with adjunct postoperative analgesia using either FNB or ACB performed before surgical incision.
Interestingly, our exploratory simple logistic regression analyses suggested that ACB might be associated with higher odds of developing persistent postoperative pain than FNB (P = .049).
Review of literature in regards to relationship of ACB and nerve injury showed one study that was designed to determine the risk of saphenous nerve injury in postoperative TKA patients when ACB is also used for analgesia.[21] This study did not specifically question PPP, but only tested the sensory abnormalities in certain areas of the operated limb 3 months postoperatively. Their conclusion was that the sensory changes were frequent, but mostly confined to the distribution of the infrapatellar branch of the saphenous nerve, which has a known anatomical susceptibility to injury during TKA. The cutaneous area innervated by the medial crural branch of the saphenous nerve on the leg was relatively spared. This study concluded that a serious risk of nerve injury related to ACB was unlikely,[21] which was not consistent with our findings of potentially increased PPP with ACB. Therefore, the authors of this manuscript believe that further studies are required to better explore risks associated with peripheral nerve blocks in the development of PPP after TKA.
Multivariable logistic regression analysis of our entire sample did not detect an association of mood or anxiety disorders with the odds of developing persistent postoperative pain (P = .886 and .121, respectively). Simple logistic regression analyses on a subsample of patients (N = 72) did not detect an association of perioperative gabapentinoid use or tourniquet duration with the odds of developing persistent postoperative pain (P = .921 and .728, respectively).
4.1. Limitations
This was a retrospective review of data from a limited time period at a single institution. In our sample, the number of patients with complete data was less than estimated (N = 578 instead of 1535 and 2300, for 20% and 40% risk of developing PPP respectively). Therefore, our estimate of the persistent postoperative pain risk was less precise than projected (ie, a precision of ±4% as opposed to the anticipated ±2%). There is also a possibility of overestimation, likely attributable to follow-up bias. It is possible that patients with PPP were more likely to return for a follow-up visit and report NRS scores than those without PPP.
Our registry database did not include the amount of analgesic medication use after 3 months postoperatively. Therefore, the patients who were actively taking analgesic medications and reported their pain levels as low (NRS <4) at 3-, 6-, and 12-month time points were not included in our analysis. This might have led to underestimation of our calculated result of risk of PPP development after TKA. Also, we did not have the data for various validated tests to differentiate the pain types as neuropathic versus other types in the registry database. As the data about the body mass index of patients and acute postoperative pain scores were inadequate in the orthopedic registry database, these items could not be included in our retrospective analysis.
5. Conclusions
This study estimated a high risk (31.3%) of moderate-to-severe PPP development in patients undergoing primary TKA from a sample of 578 patients. This study suggested that higher preoperative pain scores may be associated with greater odds of developing moderate-to-severe PPP after TKA surgery.
Moreover, this study suggested the possibility that racial differences and types of peripheral nerve blocks might be associated with greater odds of developing moderate-to-severe PPP after TKA surgery. However, the evidence obtained from our exploratory analysis of limited data certainly requires further exploration in large-scale studies.
Acknowledgments
The authors thank Haoyan Zhong, Biostatistician from Healthcare Research Institute at Hospital for Special Surgery, for reviewing and commenting on this version of manuscript.
Author contributions
Conceptualization: Semih Gungor, Alejandro Gonzalez DellaValle, Edwin Su.
Data curation: Kara Fields.
Formal analysis: Semih Gungor, Kara Fields.
Investigation: Semih Gungor, Rohit Aiyer.
Methodology: Semih Gungor.
Project administration: Semih Gungor.
Resources: Semih Gungor, Kara Fields, Alejandro Gonzalez DellaValle, Edwin Su.
Software: Semih Gungor, Kara Fields, Rohit Aiyer, Alejandro Gonzalez DellaValle, Edwin Su.
Supervision: Semih Gungor.
Validation: Semih Gungor, Kara Fields.
Writing – original draft: Semih Gungor, Kara Fields, Rohit Aiyer.
Writing – review & editing: Semih Gungor, Kara Fields, Rohit Aiyer, Alejandro Gonzalez DellaValle, Edwin Su.
Semih Gungor orcid: 0000-0002-4484-9183.
Footnotes
Abbreviations: ACB = adductor canal block, BMI = body mass index, FNB = femoral nerve block, IASP = International Association for Study of Pain, NRS = Numeric Rating Scale, PNB = peripheral nerve block, PPP = persistent postsurgical pain, TKA = total knee arthroplasty.
The authors report no conflicts of interest.
References
- [1].Hawker G, Wright J, Coyte P, et al. Health-related quality of life after knee replacement. J Bone Joint Surg Am 1998;80:163–73. [DOI] [PubMed] [Google Scholar]
- [2].Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res 2003;27–36. [DOI] [PubMed] [Google Scholar]
- [3].IASP Press, Mersky H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed1994. [Google Scholar]
- [4].Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Searle RD, Simpson KH. Chronic post-surgical pain. Continuing Education in Anaesthesia, Critical Care & Pain 2009;101:12–4. [Google Scholar]
- [6].Wylde V, Hewlett S, Learmonth ID, et al. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011;152:566–72. [DOI] [PubMed] [Google Scholar]
- [7].Cram P, Lu X, Kates SL, et al. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA 2012;308:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lewis GN, Rice DA, McNair PJ, et al. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth 2015;114:551–61. [DOI] [PubMed] [Google Scholar]
- [9].Noiseux NO, Callaghan JJ, Clark CR, et al. Preoperative predictors of pain following total knee arthroplasty. J Arthroplasty 2014;29:1383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oldenmenger WH, de Raaf PJ, de Klerk C, et al. Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage 2013;45:1083–93. [DOI] [PubMed] [Google Scholar]
- [11].Baker PN, van der Meulen JH, Lewsey J, et al. National Joint Registry for E, Wales. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br 2007;89:893–900. [DOI] [PubMed] [Google Scholar]
- [12].Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81. [DOI] [PubMed] [Google Scholar]
- [13].Graven-Nielsen T, Wodehouse T, Langford RM, et al. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum 2012;64:2907–16. [DOI] [PubMed] [Google Scholar]
- [14].Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- [15].Ip HY, Abrishami A, Peng PW, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 2009;111:657–77. [DOI] [PubMed] [Google Scholar]
- [16].Puolakka PA, Rorarius MG, Roviola M, et al. Persistent pain following knee arthroplasty. Eur J Anaesthesiol 2010;27:455–60. [DOI] [PubMed] [Google Scholar]
- [17].Petersen KK, Simonsen O, Laursen MB, et al. Chronic postoperative pain after primary and revision total knee arthroplasty. Clin J Pain 2015;31:1–6. [DOI] [PubMed] [Google Scholar]
- [18].Allen KD, Helmick CG, Schwartz TA, et al. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage 2009;17:1132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kamath AF, Horneff JG, Gaffney V, et al. Ethnic and gender differences in the functional disparities after primary total knee arthroplasty. Clin Orthop Relat Res 2010;468:3355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rahavard BB, Candido KD, Knezevic NN. Different pain responses to chronic and acute pain in various ethnic/racial groups. Pain Manag 2017;7:427–5321. [DOI] [PubMed] [Google Scholar]
- [21].Henningsen MH, Jaeger P, Hilsted KL, et al. Prevalence of saphenous nerve injury after adductor-canal-blockade in patients receiving total knee arthroplasty. Acta Anaesthesiol Scand 2013;57:112–7. [DOI] [PubMed] [Google Scholar]


