Abstract
Recently, the effectiveness of novel immune checkpoint inhibitors and BRAF-directed therapies has been demonstrated in advanced melanoma trial populations. Limited research, however, has evaluated the impact of these therapies in a real-world setting. The aim of this study was to evaluate treatment patterns and clinical outcomes among advanced melanoma patients treated with modern therapies within community oncology clinics. Adult patients with advanced melanoma who initiated treatment within the US Oncology Network between 1/1/14 and 12/31/16 were included. Data were sourced from electronic healthcare records. Patients were followed through 12/31/17. Descriptive analyses were performed to assess patient and treatment characteristics and Kaplan–Meier methods were used for time-to-event outcomes. In total, 484 patients met eligibility criteria (32.0% with brain metastasis, 12.6% with Eastern Cooperative Oncology Group performance status ≥2). In the first-line (1L) setting during the study period, 37.0% received anti-PD1 monotherapies, 26.4% ipilimumab monotherapy, 19.8% BRAF/MEK combination therapy, 6.4% BRAF or MEK monotherapy, 4.1% ipilimumab/nivolumab combination therapy and 6.2% other regimens. Differences in baseline demographic and clinical characteristics were observed across treatment groups. For the overall study population, the median (95% confidence interval) estimates for overall survival, time to next treatment and progression-free survival were 20.7 (16.0, 26.8), 5.8 (5.3, 6.5), and 4.9 (4.2, 5.7) months, respectively. The results of this study provide real-world insight into advanced melanoma treatment trends and clinical outcomes, including high utilization of immunotherapies and BRAF/MEK combination therapy. Future research can explore underlying differences in patient subpopulations and the sequence of therapies across lines of therapy.
Keywords: cohort studies, demography, immunotherapy, medical record systems computerized, melanoma, survival analysis
1. Introduction
In 2019, it is estimated that 96,480 new melanoma cases will be diagnosed in the United States (US) and approximately 7000 patients will succumb to the disease.[1] Melanoma is the fifth most common cancer in the US and is confined to the primary site in 84% of cases.[1–3] As melanoma spreads to regional lymph nodes or more distant sites, treatment becomes more challenging and the 5-year survival rate decreases to 22.5%, although advancements in treatment options have improved survival rates in the last few years.[2,4,5]
For decades, there had been a lack of effective therapy for advanced melanoma.[6] Commonly used therapies were cytotoxic chemotherapies and cytokine-based immunotherapy (interferon, interleukin-2) which were primarily palliative in nature with no improvements in average survival seen. In the last 10 years, new effective therapies have emerged which focus either on targeting of the BRAF V600E mutation which is commonly seen in melanoma or more precise immune targeting of cytotoxic T-lymphocytes with antibodies against regulatory checkpoints.
The development of molecularly targeted therapies for melanoma was based on the knowledge that mutations in the BRAF gene are commonly seen in this disease.[7–10] This activating BRAF V600 mutation leads to aberrant activation of the mitogen-activated protein kinase (MAPK) pathway, a well-documented cancer cell growth pathway. Multiple drugs targeting BRAF V600 mutations have been approved by the US Food and Drug Administration (FDA), including vemurafenib, dabrafenib, and encorafenib, based on their ability to improve survival outcomes for patients with BRAF-mutant melanoma.[6,11] Additionally, co-targeting of the MEK protein in combination with BRAF inhibition has shown improved efficacy compared to targeting BRAF alone and led to the FDA approval of several BRAF/MEK inhibitor combinations.[11,12] These are dabrafenib/trametinib, vemurafenib/cobimetinib, and encorafenib/binimetinib. Combination BRAF/MEK inhibition therapy has become a standard option for patients with BRAF V600-mutant melanoma, although secondary resistance still manifests with combination therapy for a high proportion of patients.[13,14]
In parallel with the advances seen in molecularly targeted BRAF therapy, novel immunotherapeutics that target T-cell regulation have also shown dramatic benefit for advanced melanoma patients.[6] The discovery of immune checkpoints on cytotoxic T-cell lymphocytes led to therapeutic antibodies that can impact the anticancer immune response. The inhibitory T-cell lymphocyte surface receptors, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1) function to depress T-cell function and have been fruitful targets for anti-body drug development. Ipilimumab is a monoclonal antibody against CTLA-4 was a first in class agent in this new immunotherapy field and showed in phase III clinical trials to have an overall survival (OS) benefit for patients with advanced melanoma.[15]
The PD-1 antibodies, pembrolizumab, and nivolumab, have also demonstrated improved efficacy results with better response rates and survival outcomes compared to ipilimumab and conventional chemotherapy.[18–31] In the KEYNOTE (−001, −002, and −006) and CheckMate (−066, −067, and −069) clinical trials, pembrolizumab, and nivolumab, respectively, were observed to have improved rates of response, OS and progression-free survival (PFS) relative to conventional chemotherapies and ipilimumab.[16–23] A recent Cochrane meta-analyses of 122 randomized control trials confirmed the benefit of anti-PD1 therapies.[24] Compared with either chemotherapies or anti-CTLA4 monoclonal antibodies, anti-PD1 therapy improved OS and PFS with less toxicity.[25,26] BRAF and MEK inhibitors (alone or in combination) performed better than chemotherapy but were not compared in any of the included trials with anti-PD1 monotherapies.
Given the independent outcomes observed with BRAF/MEK inhibitors and immunotherapies in clinical trials, research has begun exploring the potential additive benefit of combination treatment for unresectable and/or metastatic melanoma.[6] Specifically, there is interest in understanding how targeted therapy and immunotherapies may have a synergistic relationship to sensitize the immune system to target tumors and improve clinical outcomes. For example, improved survival and response have been noted among patients who received dual immune checkpoint inhibition with ipilimumab and nivolumab relative to nivolumab or ipilimumab monotherapies.[22,27] While this initial evidence has shown promise, the combination of these treatment has also been associated with increased toxicity.[22,28]
The treatment landscape for advanced melanoma has rapidly evolved with the advent of several novel therapies for the past few years. Whitman et al performed a retrospective analysis of treatment patterns and outcomes among advanced melanoma patients who initiated first-line (1L) treatment in clinical practices.[29] The authors reported that novel therapeutic options for advanced melanoma have resulted in a complex treatment landscape that providers must navigate to identify the most appropriate therapy for their patients. Additionally, the authors reported that ipilimumab-based regimens, anti-PD1 monotherapies, and BRAF/MEK inhibitors were received by the study population, which exhibited favorable clinical outcomes. Overall, limited research has evaluated optimal treatment selection strategies for patients with advanced melanoma and considered reasons for treatment discontinuation.[29] This study aimed to improve understanding of the latest treatment landscape for advanced melanoma in the US real-world clinical practices. Specifically, to provide real-world insight into treatment patterns, reasons for treatment discontinuation, and clinical outcomes, including PFS, of advanced melanoma patients receiving care in an integrated large network of US-based community oncology clinics.
2. Methods
This was a retrospective, observational, descriptive study of advanced melanoma patients who initiated 1L treatment in the US Oncology Network (USON) community setting between 1 January 2014 and 31 December 2016. Data were sourced from the electronic healthcare records (EHR) of the USON, iKnowMed (iKM). iKM is an oncology-specific EHR database that captures outpatient practice encounter history for patients who received care within the USON. A structured data extract of the iKM database was used to address most research questions of the study and a targeted chart review provided supplemental information captured from unstructured fields of the EHR. The Social Security Death Index (SSDI) was used to supplement the data available in iKM on vital status and dates of death.
Patients were eligible if they were at least 18 years of age, diagnosed with advanced melanoma and initiated any 1L treatment during the study identification period between 1 January 2014 and 31 December 2016. Additionally, patients were required to have at least 2 visits during the study period at USON clinics that had fully implemented the iKM EHR. Patients were excluded if they were enrolled in clinical trials and/or had another cancer diagnosis besides basal cell carcinoma, squamous cell carcinoma, bladder carcinoma in situ and cervical carcinoma in situ.
The index date was the start date of 1L anticancer therapy during the patient identification period. The look-back period included patients’ entire medical histories within USON and varied based on the length of disease and treatment time within the USON. Baseline data were measured 30 days prior to the index event and all study variables and outcomes were assessed regardless of maximum follow-up using data available until the end of the study period. All patients were followed until 31 December 2017 or until the date of last visit, or death, whichever came first.
Descriptive analyses were conducted to assess demographic, clinical and treatment characteristics among the overall cohort of patients initiating treatment, stratified by treatment regimens in the 1L, second-line (2L) and third-line setting and beyond (3L+). Patient characteristics assessed included age, race, sex, geographic location, Eastern Cooperative Oncology Group (ECOG) performance status score, body mass index, smoking status, stage at diagnosis, Deyo-adapted Charlson comorbidity score, presence of biomarkers (BRAF, KIT, NRAS, PD-L1), baseline laboratory values and sites of metastases.
Results are presented for the overall study population and for the following treatment subgroups: anti-PD1 monotherapies, BRAF/MEK combination therapy, ipilimumab monotherapy, ipilimumab/nivolumab, BRAF or MEK monotherapy, as well as an “other” category for regimens that were not classified into one of the other groups. Continuous variables were described by mean, standard deviation, median, and range; categorical variables were defined by frequencies and percentages. Demographic and clinical characteristics were compared using Chi-square/Fisher exact test (for categorical variables) and t test/Mann–Whitney U test/ANOVA/Kruskal–Wallis test (for the continuous variables), as applicable.
Kaplan–Meier methods were used to examine time-to-event endpoints, including OS, time-to-next-treatment (TTNT) and physician-assessed PFS with medians and 95%. OS was calculated from the initiation of treatment until date of death from any cause. Patients who did not die were censored for OS on the last visit date available in the database. TTNT was defined as the time from the initiation of the prior treatment until the start of a new treatment. Patients who did not advance to the next treatment or died before receiving the next treatment were censored for TTNT on the last visit date available in the database.
Physician-assessed PFS is the interval from initiation of treatment until the date of physician-documented assessed disease progression. Patients who did not progress and were still alive were censored for PFS on the last visit date available in the database.
Univariate and multivariable Cox regression models were used to assess baseline predictive factors associated with OS and physician-assessed PFS. Select characteristics considered for univariate assessment were based on clinical relevance/and or best-practice. These include baseline characteristics such age, performance status, biomarkers, sites of metastases, as well as prior surgery and first-line treatment (pembrolizumab vs BRAF/MEK combination therapy).
All variables were fitted into a multivariable Cox proportional hazard regression model to assess independent associations between variables of interest and probability of an event adjusting for the influence of other variables within the model. Subsequently, those demonstrating significance using the confounder approach were retained for the final model.
Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). An alpha level of less than .05 was the primary criterion for statistical significance in this study.
The study was reviewed and granted exception and waiver of consent by the US Oncology, Inc. Institutional Review Board.
3. Results
A total of 13,950 adult patients with advanced melanoma and treated at a USON clinic were initially identified in the iKM database (Fig. 1). Of these, 484 met eligibility criteria and were included in the analysis. The median age for all patients was 65 years (range, 26–90+) with nearly half (47.9%) of patients under 65 years at initiation of 1L treatment (Table 1). The majority was male (66.7% vs 33.3% female). Across the study cohort, 93.8% were Caucasian, 1.0% Black/African American and 5.2% other or unknown. At diagnosis of melanoma, 75.8% were diagnosed with Stage III or IV. At initiation of 1L treatment, 66.3% had an ECOG performance status score of 0 or 1. The large majority of melanoma patients did not have a known PD-L1 expression status (95.5%).
Figure 1.
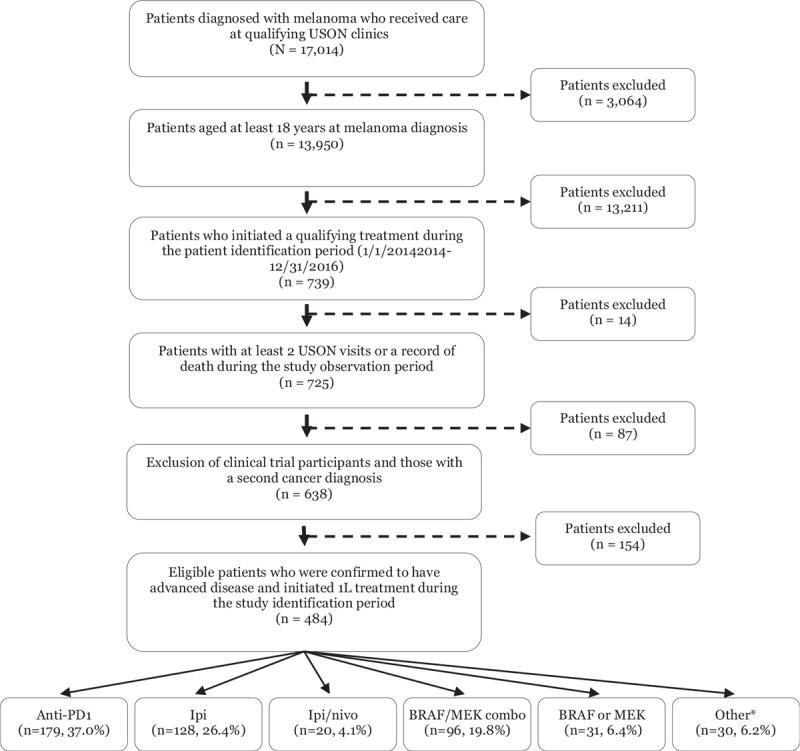
Study attrition. 1L = first-line treatment, anti-PD1 = anti-PD1 monotherapies, BRAF or MEK = BRAF or MEK monotherapies, BRAF/MEK combo = BRAF/MEK combination therapy, ipi/nivo = ipilimumab/nivolumab combination therapy, ipi = ipilimumab, LOT = line of therapy, USON = US Oncology Network. ∗Examples include: dabrafenib/ipilimumab/trametinib, carboplatin/paclitaxel, dabrafenib/ipilimumab and dabrafenib/nivolumab.
Table 1.
Baseline demographic and clinical characteristics of patients with advanced melanoma initiating treatment in the community oncology setting.
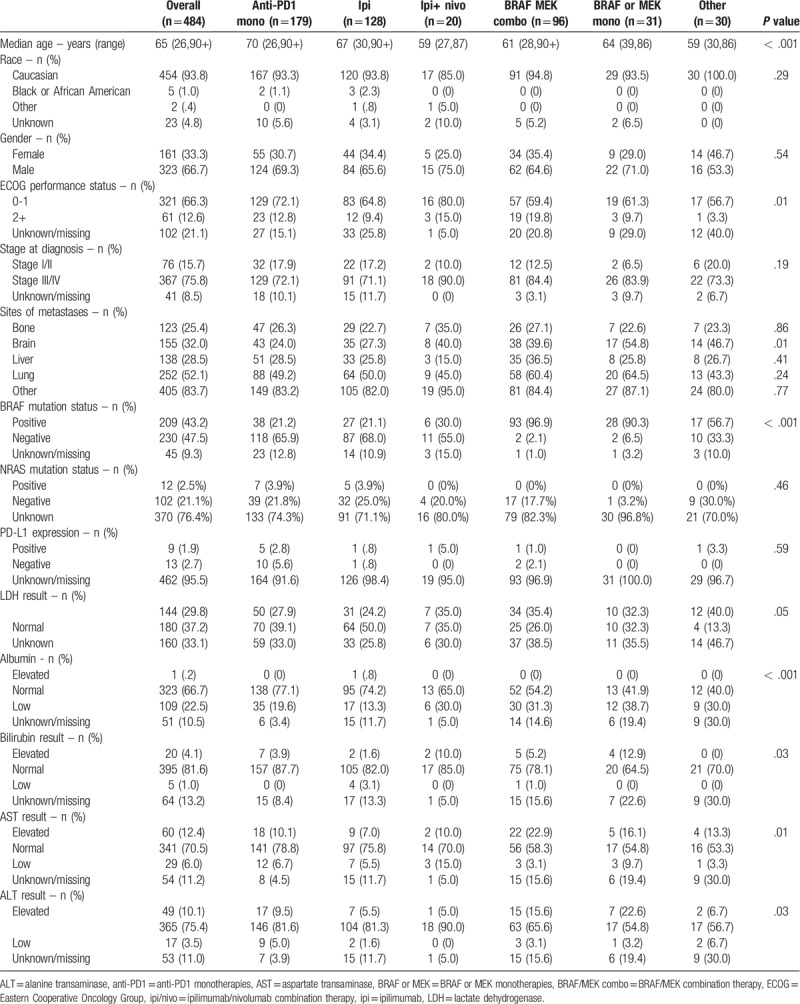
Front-line therapy the patient population was tabulated with a number of various therapies observed (Table 1). In the 1L setting, for the entire cohort, 67.5% of patients received immunotherapy, 26.2% received BRAF-directed therapies, and 6.2%. More granularly, 24.2% received pembrolizumab, 12.8% received nivolumab, 37.0% received anti-PD1 monotherapies, 26.4% ipilimumab monotherapy, 19.8% BRAF/MEK combination therapy, 6.4% BRAF or MEK monotherapy, 4.1% ipilimumab/nivolumab combination therapy and 6.2% other regimens.
Among the 1L treatment regimens, a significant difference in the age at treatment initiation was observed (P < .001 across all stratifications; Table 1). Patients initiating an anti-PD1 monotherapy or ipilimumab in the 1L setting tended to be older compared to other treatment groups (mean age: 68.9 [SD ± 13.5] years for anti-PD1 monotherapy patients, 65.8 [±12.3] years for ipilimumab monotherapy, 63.8 [±11.6] years for BRAF or MEK monotherapy, 60.5 [±12.9] years for BRAF/MEK combination therapy and 58.1 [±13.3] years for ipilimumab/nivolumab).
Across the study cohort, 43.2% were BRAF positive, 47.5% were negative and 9.3% unknown (Table 1). As might be expected, significant difference in BRAF status was observed across treatment subgroups (P < .001), with the highest proportion of BRAF positive status among patients who received BRAF-MEK combination therapy (96.9%), and the lowest among patients who received anti-PD1 monotherapy (21.2%) or ipilimumab monotherapy (21.1%). The proportion of documented BRAF-positive patients in each treatment subgroup were as follows: 21.2% anti-PD1 monotherapies, 21.1% ipilimumab monotherapy, 30.0% ipilimumab/nivolumab, 96.6% BRAF/MEK combination therapy and 90.3% BRAF or MEK monotherapy.
At baseline, documented lactate dehydrogenase (LDH) levels were elevated among 29.8% of the study population (while 37.2% had normal levels and 33.1% no LDH documentation at index; Table 1). Significant differences (P = .005) in the proportion of patients with elevated LDH levels were observed across treatments, with the highest levels observed among patients who received other treatments (such as dabrafenib/ipilimumab/trametinib, carboplatin/paclitaxel, dabrafenib/ipilimumab and dabrafenib/nivolumab) and lowest among those who received ipilimumab monotherapy. In addition, there was a greater prevalence of patients with normal levels of bilirubin (81.6%), aspartate aminotransferase (AST; 70.5%), alanine aminotransferase (ALT; 75.4%) and albumin (66.7%).
Among the study cohort, there was a high proportion of patients with lung metastases (52.1%) at initiation of 1L treatment, followed by brain (32.0%) and liver (28.5%) metastases at index (Table 1). Presence of brain metastases differed by 1L treatment (P = .001). A higher proportion of patients treated with BRAF/MEK inhibitors had brain metastases at initiation of 1L treatment relative to patients treated with anti-PD1 monotherapies (39.6% vs 24.0%, respectively).
Prior to initiation of 1L treatment, 29.5% of patients received radiation and 64.1% surgery. No significant differences in prior treatment were noted across the treatment subgroups.
During the study period, 451 (93.2%) patients discontinued 1L treatment with significant differences noted across treatments (P < .001; Table 2). Across all regimens, 33.0% of patients discontinued due to disease progression, 16.6% for treatment-related toxicities and 12.2% due to death. Within individual 1L regimens, the primary reason for treatment discontinuation was progressive disease, with the exception of toxicity for ipilimumab/nivolumab. Patients who received BRAF/MEK monotherapies had the highest proportion of patients who discontinued 1L treatment due to death (29.0%), whereas none of the ipilimumab/nivolumab patients died during 1L treatment. Similar trends were noted for reasons for 2L treatment discontinuation, with 30.9% discontinuing due to progression, 14.2% due to treatment-related toxicities and 12% due to death.
Table 2.
Treatment patterns of advanced melanoma patients across lines of therapy.
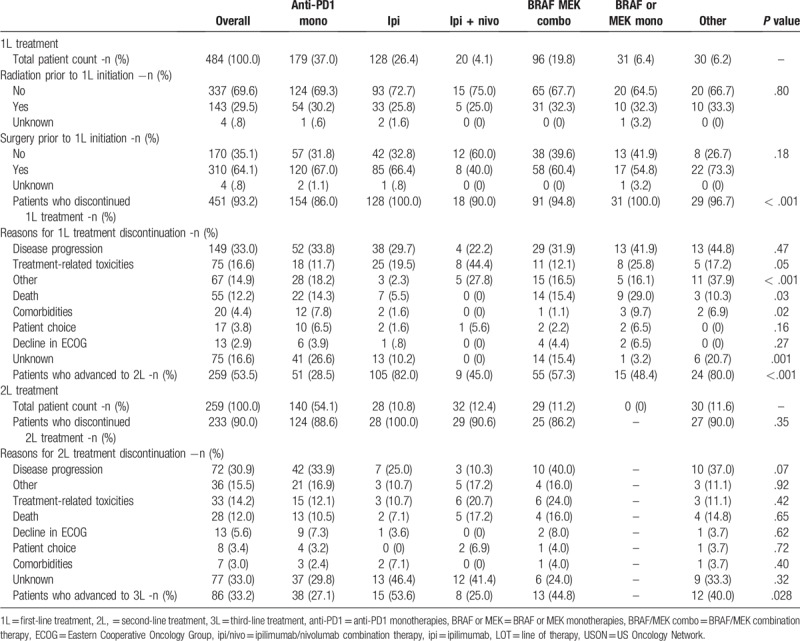
Following 1L discontinuation, 53.5% of all patients advanced to 2L treatment. Within the treatment subgroups, a higher proportion of ipilimumab monotherapy patients received 2L treatment (82.0%), compared with those who received BRAF/MEK combination therapy (57.3%), BRAF or MEK monotherapy (48.4%), ipilimumab/nivolumab (45.0%) and anti-PD1 monotherapies (28.5%; Fig. 2). In the 2L setting immunotherapies were still the predominant regimens, with 54.1% receiving anti-PD1 and 10.8% ipilimumab; 11.2% received BRAF/MEK combination therapy, 12.4% ipilimumab/nivolumab and 11.6% other regimens.
Figure 2.
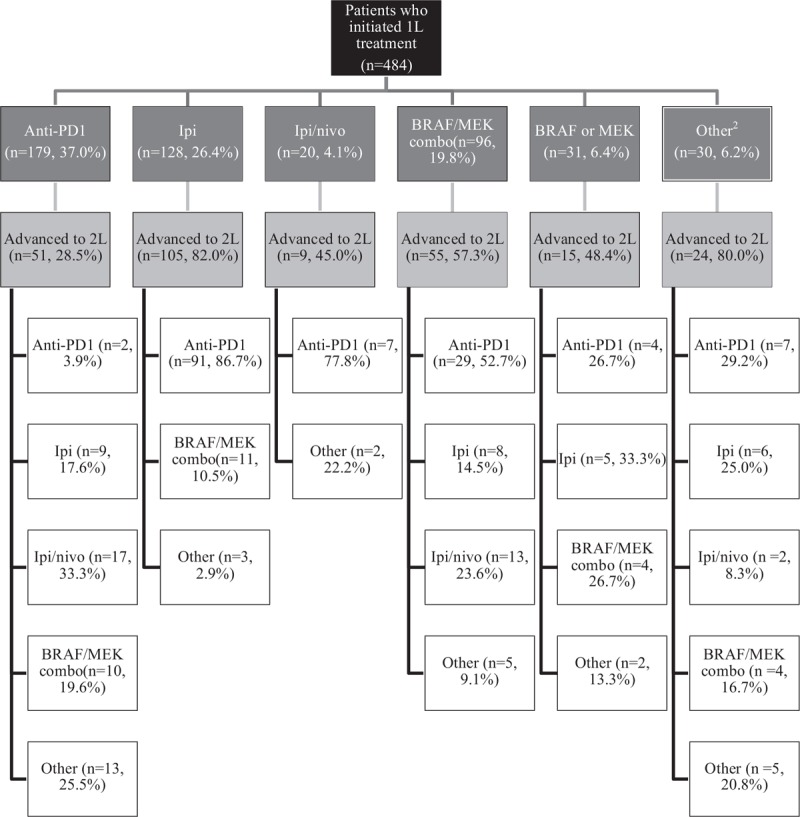
Treatment sequences (1L to 2L) among advanced melanoma patients1. 1Patients advance to the next line of therapy for many reasons, including progression and toxicity. Patients who do not advance may have ongoing treatment, transitioned to another care setting or outside the USON or died. 2Examples include: dabrafenib/ipilimumab/trametinib, carboplatin/paclitaxel, dabrafenib/ipilimumab and dabrafenib/nivolumab.
In 3L+ setting, 34.9% received anti-PD1 monotherapies, 16.3% BRAF/MEK combination therapy and 48.8% received other regimens.
Across the study population, the median OS was 20.7 months (95% CI, 16.0–26.8; Fig. 3a). The estimated OS at 12 and 24 months were 62.7% and 47.6%, respectively. The median physician-assessed PFS was 4.9 months (95% CI, 4.2–5.7), with estimated PFS at 12 and 24 months 29.1% and 18.2% (Fig. 3b). The median TTNT was 5.8 months (95% CI, 5.3, 6.5; Fig. 3c).
Figure 3.
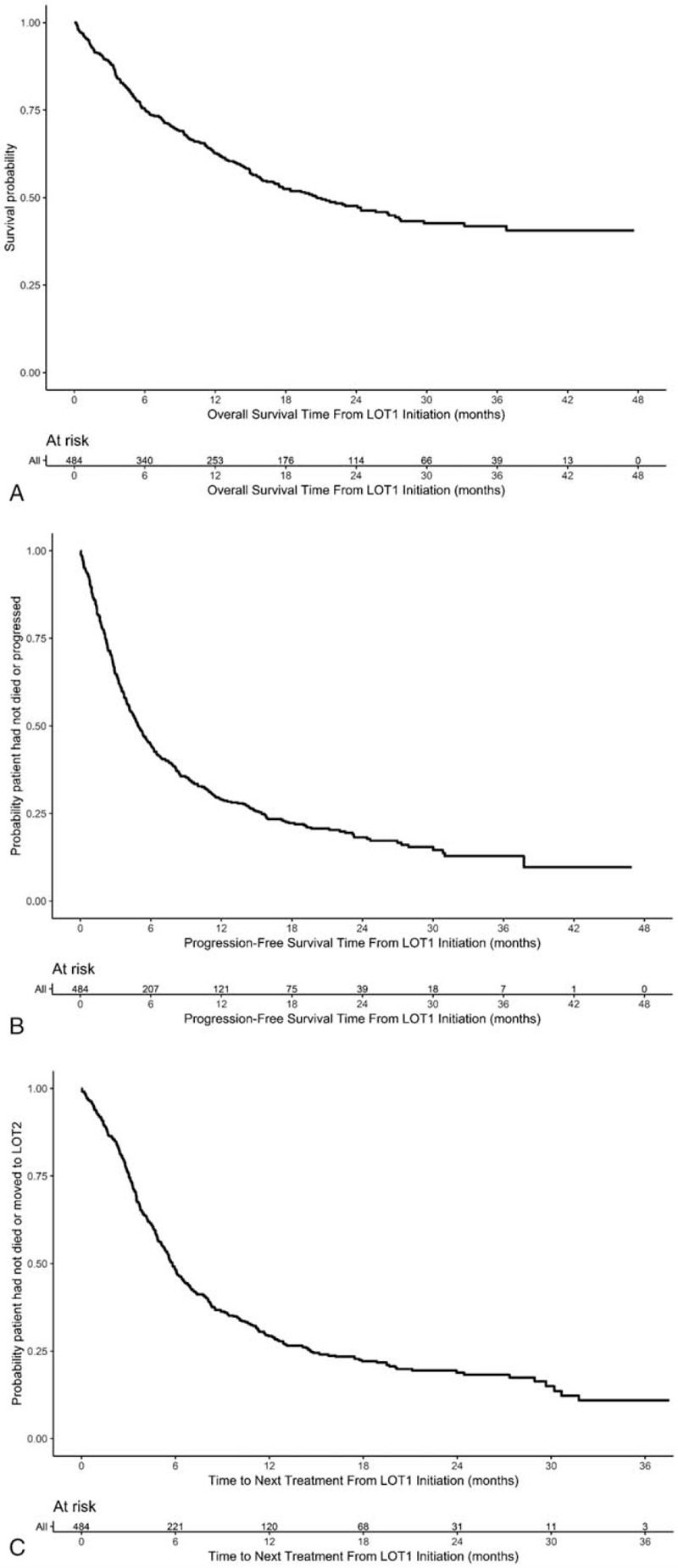
a: Kaplan–Meier estimates of overall survival. b: Kaplan–Meier estimates of progression-free survival. c: Kaplan–Meier estimates of time to next line of treatment.
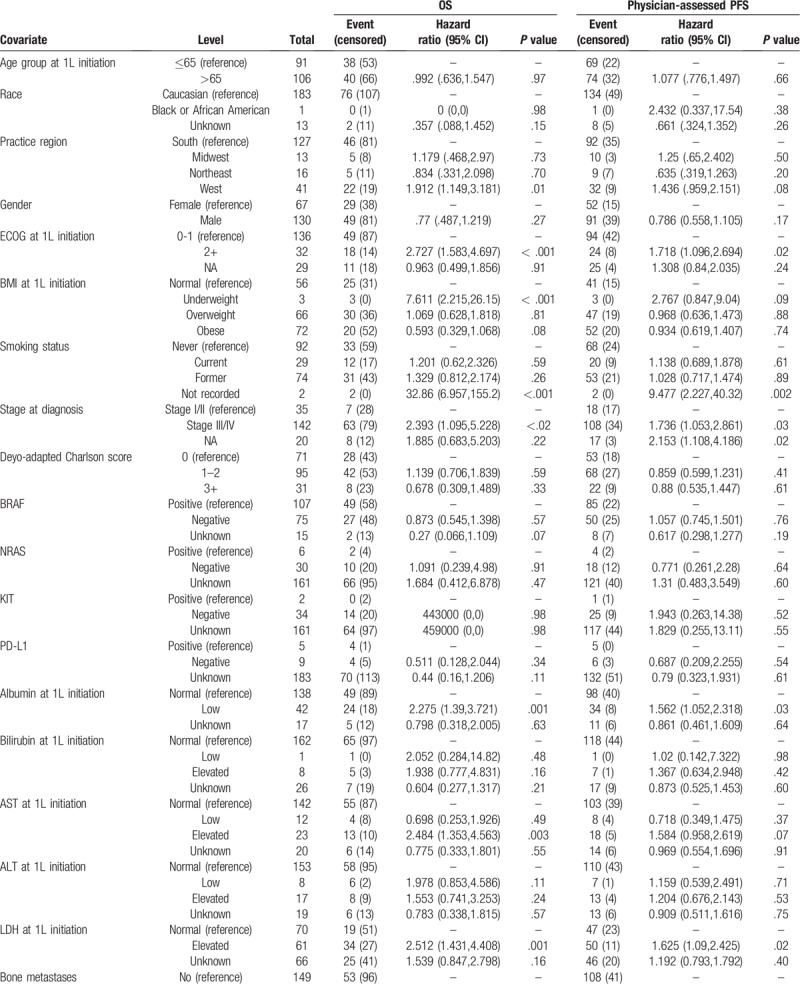
A univariate analysis identified the following predictors of OS: ECOG performance status at 1L treatment initiation, BMI at 1L treatment initiation, smoking status, albumin level, AST level, LDH level, bone metastases, brain metastases, liver metastases, prior surgery and 1L treatment (Table 3 ). Likewise, ECOG performance status, smoking status, brain metastases, liver metastases, prior radiation therapy were predictive of PFS in the univariate analysis.
Table 3.
Univariate Cox proportional hazard models on overall survival (OS) and physician-assessed progression-free survival (PFS) among advanced melanoma patient initiating first-line treatment.
Multivariable Cox model found presence of brain metastases to be predictive of both worse OS and PFS (hazard ratio [HR] for OS: 2.888 [95% CI 1.801, 4.632], P < .001; HR for PFS: 2.262 [95% CI 1.564, 3.272], P < .001; Table 4). No other variables, including BRAF mutation status, were found to be predictive in both multivariable models. For OS, patients with brain metastases at index were more likely to die during the study period compared to patients without brain metastases (HR = 2.888, 95% CI = 1.801–4.632).
Table 3 (Continued).
Univariate Cox proportional hazard models on overall survival (OS) and physician-assessed progression-free survival (PFS) among advanced melanoma patient initiating first-line treatment.
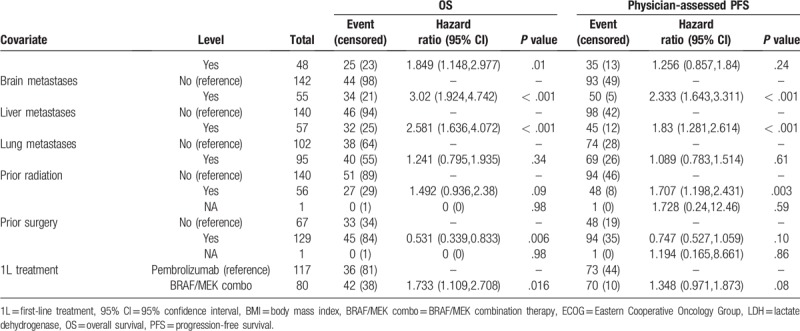
Table 4.
Multivariable Cox proportional hazard models on overall survival and physician-assessed PFS among advanced melanoma patient initiating first-line treatment.
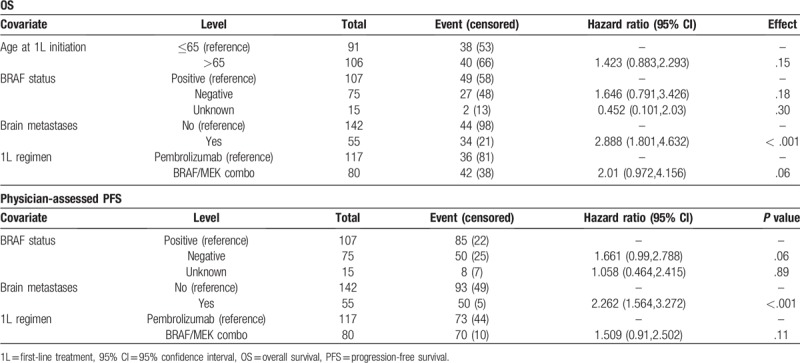
For physician-assessed PFS, patients with brain metastases were more likely to progress during the study period relative to patients without brain metastases (HR = 2.1, 95% CI = 1.5–2.8, P < .001). Additionally, patients with low albumin were more likely to progress during the study period (HR = 1.8, 95% CI = 1.3–2.5, P = .002) adjusting for other covariates in the model.
4. Discussion
In this study, we examined the real-world treatment of advanced melanoma in patients treated in a network of US-based community oncology clinics. Much of the existing research on advanced melanoma patients has been conducted in clinical trials setting among patients who meet stringent inclusion and exclusion criteria. As a result, much of the existing clinical evidence for modern advanced melanoma treatments has not included patients with active brain metastases, poor performance status, and restrictive comorbid conditions. The results of this study, in contrast, presents information on patient profiles, treatment patterns and outcomes of patients treated in the community oncology setting.
Immunotherapies and BRAF-directed therapies were the most commonly observed regimens across the study population. Treatment patterns and clinical outcomes differed from those published in recent studies, likely due to a non-clinical trial population of patients. Included in this study cohort are patients that would have been excluded from clinical trial based on comorbid conditions and other factors such as poorer performance status and presence of brain metastases. In addition to real-world treatment patterns and clinical outcomes for patients with modern therapies, several observations regarding treatment selection were made.
Across the study population, demographic and clinical characteristics were consistent with expectations of a melanoma population receiving care in the US community oncology setting. Prior real-world research has demonstrated that most patients (56.8%) in the US are diagnosed with metastatic melanoma after the age of 55 and the malignancy is more common among male (62.5%) patients.[30] These trends were reflected in our results, which found that, among patients who initiated 1L treatment, nearly half of patients were under 65 years, with majority being Caucasian and/or male.
In the 1L setting, only about 20% of patients who received anti-PD1 or ipilimumab monotherapy were BRAF positive, suggesting a physician preference for immunotherapy in patients that did not harbor the BRAF mutation. This is despite clinical trial data showing equal benefit of CTLA-4 and PD-1 inhibition in metastatic melanoma with or without BRAF mutation. While insight into physician thinking regarding treatment choice among two available options (BRAF-focused vs immune-based therapies) is difficult to ascertain in a study such as this, it is possible that choice between these treatments is being driven by the BRAF test result alone. Other explanation may be patient tumor burden and the desire for rapid response with BRAF-focused therapy, but again this is challenging to understand in a retrospective review such as this and represents a limitation of our study.
Across all treatments, the median OS was observed to be 20.7 months, with 47.6% of patients surviving at 24 months. Following initiation of 1L treatment, the median PFS and TTNT durations were 4.9 and 5.8 months, respectively. The only factor found to be predictive of both worse OS and PFS was brain metastases. This finding highlights the aggressive nature of melanoma when it involves the brain and the dire need for further drug development that is effective at treating, controlling and preventing brain metastases.
As would be expected with the recent drug advances in the field, immune checkpoint inhibitors and BRAF/MEK combination treatment were the most common regimens across the different lines of therapy. In a retrospective claims analysis, Ma et al found that among metastatic melanoma patients treated in the US between January 2011 and August 2013, most patients (39%) received ipilimumab, 35% vemurafenib, 19% temozolomide and 7% dacarbazine.[30] By analyzing a more recent EHR dataset from Flatiron Health, Whitman et al reported that the most common 1L regimens observed in their retrospective EHR-based analysis of advanced melanoma patients treated through February 2017 were ipilimumab-based regimens (34%), anti-PD1 monotherapy (26%) and BRAF/MEK inhibitors (20%).[29] The greater utilization of anti-PD1 monotherapies and BRAF/MEK combination therapy found in this study relative to the Ma et al and Whitman et al studies likely reflects advancements in the treatment landscape for melanoma, particularly recent approvals of anti-PD1 therapies in 2014. In our real-world population, we observed that patients are still receiving conventional chemotherapy, although this has become a small minority of the treatment usage landscape.
Disease progression was the most common reason for 1L treatment discontinuation across treatment subgroups, with the exception of ipilimumab/nivolumab combination therapy. For the ipilimumab/nivolumab subgroup, a higher proportion of patients discontinued due to toxicity than progression (44.4% vs 22.2%, respectively). Of note, however, none of the 20 ipilimumab/nivolumab patients died during 1L treatment as observed during the study observation period. In a randomized, double-blind phase 3 study, patients who received ipilimumab/nivolumab combination therapy more frequently reported treatment-related adverse events grade 3 or 4 than those who received either treatment as a monotherapy (55% of ipilimumab/ nivolumab patients, 16.3% of nivolumab patients and 27.3% of ipilimumab patients).[23]
The treatment subgroup sample sizes observed in this study limited comparisons of clinical outcomes across regimens. Prior research, however, demonstrated that anti-PD1 monotherapies are associated with improved clinical outcomes relative to chemotherapy and anti-CTLA4 monotherapy for advanced melanoma.[16,17,19–21,24] In a German registry of melanoma patients treated between 2011 and 2014, patients without brain metastases that received first-line immunotherapy (reported to be mostly ipilimumab) had the longest OS (median 35 months).[31] Among patients with brain metastases, the longest OS was observed among those who received a targeted therapy (median 14 months). The median OS observed in this study, 20.7 weeks, is consistent with this range.
The duration of follow-up may have limited comparisons that could be made across treatment groups. Specifically, the study observation period spanned from 2014 through 2017 and anti-PD1 monotherapies were approved in 2014. A potential delay in adoption of these therapies in routine clinical care may have meant PD-1 monotherapies patients were more likely to have ongoing therapy at the end of the study period. Future research can continue to follow these patients to more fully assess clinical outcomes.
The results of this study should be considered in the context of the strengths and limitations of the data source and study design. The standardization of the data collection methods and instruments, and the reporting practices of the physicians might have been impeded by the iKM system which is used for clinical practice reasons and not solely for research purposes. The iKM EHR contains information on patients only when they are seen by USON physicians. Services and procedures provided outside of the USON (e.g., hospitalizations, bone marrow transplants, and radiation therapies) are not captured by the database. The USON may be different from other community oncology practices in the patient population that is seen or the prescribing practices of the physicians. Therefore, results cannot be generalized to the U.S. population or to all community oncology practices without further evaluation.
The treatment landscape for advanced melanoma has evolved with the advent of novel agents, particularly anti-PD1 therapies. By retrospectively assessing how these changes manifested across a large network of US-based community oncology practices, the results of this study provide insight into real-world treatment trends and clinical outcomes in the era of immunotherapies. A rapid adoption of modern immune checkpoint inhibitors and BRAF targeted therapies has been observed with treatment selection in the community setting. There do appear to be some variation in treatment selection among different populations of patients including those with BRAF mutations, older age, elevated LDH, and brain metastases. Treatment outcomes in the community setting are within expected ranges compared to clinical trial outcomes. As might be expected, patients with poor performance status or brain metastases due more poorly than patients without these features, making their exclusion from clinical trials reasonable. Future comparative outcomes research can expand upon these findings to explore differences between treatment subgroups, as well as factors that influence treatment-decision making for advanced melanoma, including underlying differences in patient subpopulations and the sequence of therapies across lines of therapy.
Acknowledgments
The authors would like to acknowledge Lina Asmar (McKesson Life Sciences) for medical writing assistance with the preparation of this manuscript.
Author contributions
Conceptualization: C. Lance Cowey, Frank Xiaoqing Liu, Marley Boyd, Clemens Krepler.
Data curation: Marley Boyd.
Formal analysis: Marley Boyd.
Methodology: C. Lance Cowey, Frank Xiaoqing Liu, Marley Boyd, Clemens Krepler.
Project administration: Kathleen M. Aguilar.
Supervision: C. Lance Cowey, Frank Xiaoqing Liu, Clemens Krepler.
Visualization: Marley Boyd, Kathleen M. Aguilar.
Writing – original draft: Kathleen M. Aguilar.
Writing – review & editing: C. Lance Cowey, Frank Xiaoqing Liu, Marley Boyd, Kathleen M. Aguilar, Clemens Krepler.
Kathleen M. Aguilar orcid: 0000-0002-2467-8663.
Footnotes
Abbreviations: 1L = first-line treatment, 2L = second-line treatment, 3L = third-line treatment, 95% CI = 95% confidence interval, ALT = alanine transaminase, Anti-PD1 = anti-PD1 monotherapies, AST = aspartate transaminase, BMI = body mass index, BRAF or MEK = BRAF or MEK monotherapies, BRAF/MEK combo = BRAF/MEK combination therapy, CTLA-4 = Cytotoxic T-lymphocyte-associated protein 4, ECOG = Eastern Cooperative Oncology Group, EHR = electronic healthcare records, FDA = Food and Drug Administration, HR = hazard ratio, Ipi = Ipilimumab, Ipi/nivo = Ipilimumab/nivolumab combination therapy, LDH = lactate dehydrogenase, LOT = line of therapy, MAPK = mitogen-activated protein kinase, OS = overall survival, PD-1 = programmed cell death protein-1, PFS = progression-free survival, SSDI = Social Security Death Index, TTNT = time-to-next-treatment, USON = US Oncology Network.
McKesson Life Sciences received funding from Merck and Co. Inc., Kenilworth, NJ, for conducting this study and the development of this manuscript.
Dr Cowey received research funding and provided research consulting services to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Drs. Liu and Krepler were employees and stockowners of Merck Sharp & Dohm Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ at the time of the study.
Mr. Boyd and Ms. Aguilar are employed by McKesson Life Sciences and provided research consulting services to Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
The authors report no conflicts of interest
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].National Cancer Institute. Cancer stat facts: Melanoma of the Skin. Surveillance, Epidemiology and End Results Program. 2018. https://seer.cancer.gov/statfacts/html/melan.html. [Accessed 15 May, 2018]. [Google Scholar]
- [3].Mounessa JS, Caravaglio JV, Dellavalle RP. Comparison of regional and state differences in melanoma rates in the United States: 2003 vs 2013. JAMA Dermatol 2017;153:345–7. [DOI] [PubMed] [Google Scholar]
- [4].Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res 2007;13(18 Pt 1):5256–61. [DOI] [PubMed] [Google Scholar]
- [5].Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res 2012;72:3125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].NCCN Guidelines for Melanoma V2.2018. Clinical practice guidelines in oncology: melanoma version 2.2018. Available at: https://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf [accessed 15 May, 2018]. [Google Scholar]
- [7].Amanuel B, Grieu F, Kular J, et al. Incidence of BRAF p.Val600Glu and p.Val600Lys mutations in a consecutive series of 183 metastatic melanoma patients from a high incidence region. Pathology 2012;44:357–9. [DOI] [PubMed] [Google Scholar]
- [8].Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011;29:1239–46. [DOI] [PubMed] [Google Scholar]
- [9].Willmore-Payne C, Holden JA, Tripp S, et al. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol 2005;36:486–93. [DOI] [PubMed] [Google Scholar]
- [10].Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54. [DOI] [PubMed] [Google Scholar]
- [11].Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:603–15. [DOI] [PubMed] [Google Scholar]
- [12].Ascierto PA, McArthur GA, Dreno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol 2016;17:1248–60. [DOI] [PubMed] [Google Scholar]
- [13].Simeone E, Grimaldi AM, Festino L, et al. Combination treatment of patients with braf-mutant melanoma: a new standard of care. BioDrugs 2017;31:51–61. [DOI] [PubMed] [Google Scholar]
- [14].Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107–14. [DOI] [PubMed] [Google Scholar]
- [15].Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109–17. [DOI] [PubMed] [Google Scholar]
- [17].Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521–32. [DOI] [PubMed] [Google Scholar]
- [19].Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- [20].Ribas A, Wolchok JD, Robert C, et al. Updated clinical efficacy of the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in 411 patients with melanoma. Eur J Cancer 2015;51(S2):e24. [Google Scholar]
- [21].Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, et al. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev 2018;2:CD011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chicago, IL, Hamid O, Robert C, Daud A, et al. 5-year survival outcomes in patients (pts) with advanced melanoma treated with pembrolizumab (pembro) in KEYNOTE-001. Paper presented at: ASCO Annual Meeting; June 4. 2018. [Google Scholar]
- [26].Chicago, IL, Long GV, Schachter J, Ribas A, et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. Paper presented at: ASCO Annual Meeting; June 4. 2018. [Google Scholar]
- [27].Larkin J, Sileni VC, Gonzalez R, et al. Overall Survival Results From a Phase III Trial of Nivolumab Combined With Ipilimumab in Treatment-naïve Patients With Advanced Melanoma (CheckMate 067). American Association for Cancer Research; April 1–5, Washington, DC. Cancer Res 2017;7713 Suppl: [Google Scholar]
- [28].Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Whitman ED, Liu FX, Cao X, et al. Treatment patterns and outcomes for patients with advanced melanoma in US oncology clinical practices. Future Oncol 2019;15:459–71. [DOI] [PubMed] [Google Scholar]
- [30].Ma Q, Chen YJ, Hines DM, et al. Patterns of use of systemic therapies among patients with metastatic melanoma: a retrospective claims database analysis in the United States. J Dermatolog Treat 2017;28:549–53. [DOI] [PubMed] [Google Scholar]
- [31].Forschner A, Eichner F, Amaral T, et al. Improvement of overall survival in stage IV melanoma patients during 2011-2014: analysis of real-world data in 441 patients of the German Central Malignant Melanoma Registry (CMMR). J Cancer Res Clin Oncol 2017;143:533–40. [DOI] [PubMed] [Google Scholar]


