Supplemental Digital Content is available in the text
Keywords: fecal microbiota transplantation, hepatic myelopathy, spastic paraparesis
Abstract
Rationale:
Hepatic myelopathy (HM), also known as portal-systemic myelopathy, is a rare neurological complication that occurs in patients with chronic liver disease. There is no easy and feasible treatment, liver transplantation is the only accepted therapy that may be effective for patients at early stage at present. The pathogenesis of the disease is not clear yet, and the prognosis is poor. Here we describe a reversible HM after fecal microbiota transplantation.
Patient concerns:
In this report, a middle-aged female patient with hepatitis B cirrhosis, occurred HM after transjugular intrahepatic portosystemic shunt, a progressive spastic paraparesis in both legs were the main symptoms.
Diagnosis:
The patient was diagnosed with HM.
Interventions:
The patient received 3 times of fecal microbiota transplantations (FMT).
Outcomes:
The patient's muscle strength of both legs were increased at various degrees, the patient's condition improved from HM2 to HM1.
Lessons:
FMT may be another effective way to treat HM. It is cheaper, more operable, and simpler than the approved treatment and worthy of further research.
1. Introduction
Some patients with decompensated cirrhosis have neurological alterations, the most usual is hepatic encephalopathy (HE), and hepatic myelopathy (HM) is far less common. HE is relatively easy to reverse, but HM is a progressive disease. It is more common after transjugular intrahepatic portosystemic shunt (TIPS) or spontaneous portosystemic shunt of blood. In the beginning, HM is characterized by progressive spastic paraparesis. The symptom is the weakness of legs, and eventually developed into spastic paralysis of lower extremities. Up to now, patients with HM mostly does not respond well to conservative treatment of drugs. And the only accepted treatment that may be effective is liver transplantation.[1,2] However, therapeutic strategies remains an ongoing challenge due to the difficulty of the surgery and the questionable effectiveness. In recent years, there have been some coverages reporting that fecal microbiota transplantation (FMT) has successfully treat some diseases such as hepatitis B,[3] alcoholic hepatopathy,[4]Clostridium difficile infection,[5] alopecia areata,[6] and so on. There are also increasingly studies determining the relation between intestinal flora and brain.[7] Many central nervous system disease are found to have tight connections with intestinal flora such as HE,[8] depression,[9] autism,[10] Parkinson's disease,[11] dementia,[12] sepsis-associated encephalopathy,[13] and so on. Considering that both HM and HE are neurological complications in the decompensated stage of liver cirrhosis, and more and more evidence shows that FMT is effective for HE.[14,15] So, we made FMTs in a patient with HM after informed consent, and achieved good efficacy. This case substantiate that FMT can be a promising treatment for HM.
2. Case report
A 45-year old woman was admitted on April 14, 2015, because of upper abdominal discomfort which aggravated with blood in stool for 1 day. The patient found the hepatitis B surface antigen (HbsAg) were positive 16 years ago. Liver B ultrasonic examination showed liver cirrhosis, portal vein broadening, and splenomegaly. Gastroscopic examination during hospitalization indicated sever esophageal varices and portal hypertensive gastropathy, so she underwent endoscopic variceal ligation. On May 18, 2015, she admitted again because of hematemesis. Gastroscopy revealed the ligation of the esophageal varices and severe varicose gastric fundus veins. She discharged after acid suppression and hemostasis. On July 27, 2015, she was admitted for the third time due to the aggravation of upper abdominal malaise and hematochezia. Emergency gastroscopy showed esophageal varices bleeding. Considered that the patient bled again after ligation, the risk of re-ligation or sclerotherapy is relatively high, and the efficacy is not clear. TIPS can effectively reduce portal pressure, and she had no HE, child-pugh class was B. So, there was a pointer to TIPS, she did TIPS on July 29, 2015.
In June 2016, she was admitted the fourth time because of a progressive spastic paraparesis in both legs of 2 months duration. First, the symptom was the weakness of the lower extremities, beginning with the right lower extremity, and gradually spreading to the left lower extremity. The right side was more severe than the left side. Gradually, she was unable to walk, ride a bicycle, and go to work. On admission, even though she walked with assistive tools, it was also extremely difficult for her, she was bedridden much at the time of hospitalization, and a significant muscle stiffness appears. The upper limb and sphincter was not involved. There was no history of alcohol consumption. And she had no family history of neurological illness. On neurological examination, she was well orientated and cooperative. The cranial nerve examinations were entirely normal. Muscle strength in the right limb was MRC grade 3. Muscle strength in the left limb was MRC grade 4-. Muscular tension of both legs increased. There was hyperreflexia of double lower limbs. There were no somatosensory symptoms. And there was no other deficit. Hemogram disclosed thrombocytopenia (25∗109), leukocytopenia (2.35∗109), and microcytic hypochromic anemia (3.3∗1012). Liver function tests revealed normal alanine transaminase (ALT) and glutamic oxaloacetic transaminase (AST), minimally raised alkaline phosphatase (ALP) (124U/L), reduced albumin (36.6 g/L) and acetylcholinesterase (AchE) (137U/L). Direct bilirubin (DBIL) was elevated to 21.4 mmol/L, total bilirubin (TBIL) was elevated to 54.7 mmol/L. Anti-HBs was negative, and HBsAg (»225.00 ng/ml), anti-HBe positive (11.55 PEIU/ml). Blood ammonia was elevated to 84.3 mmol/L. Serological test for syphilis was negative. Colony stimulating factor (CSF) examination was not conducted because of thrombocytopenia. The estimation of folate and serum B12 was normal. Computed tomography (CT) (abdomen) showed features of cirrhosis of liver, splenomegaly, the stones-chronic cholecystitis, and the formation of spleen venous thrombosis (Fig. 1A–D). Cranial CT exhibited empty sella (Fig. 1E and F). The magnetic resonance imaging (MRI) of the cervical spine showed a herniated disk in the area of C4-5. MRI of the thoracic spine disclosed no abnormalities. The MRI of the lumbar spine indicated lumbar disc herniation. And there is no evidence of spinal cord compression (Fig. 1G–I). Electromyography (EMG) revealed that the movement conduction velocity, the sensory conduction velocity, and the action potential amplitude were normal. Somatosensory evoked potentials of the lower limbs were normal. Motor evoked potentials (MEPs) studies disclosed MEP abnormalities of double lower limbs.[16] There is no uniform standard for HM diagnosis currently. In view of decompensated cirrhosis, increases of Blood ammonia concentration, history of TIPS, the abnormity of MEPs, free of spinal cord injuries, and excluding other causes of spinal cord disease, her spastic paraparesis was diagnosed with HM secondary to TIPS. Considering splenomegaly and hypersplenism, she was treated with partial spleen embolization. After several weeks of antiviral medications, B Vitamins supplements, liver protection, and nerve-nurturing, the MRC grade and self- care ability had not worsened. One month later, she was discharged. She can walk a few meters with auxiliary tools and she presented obvious scissors gait at that time.
Figure 1.
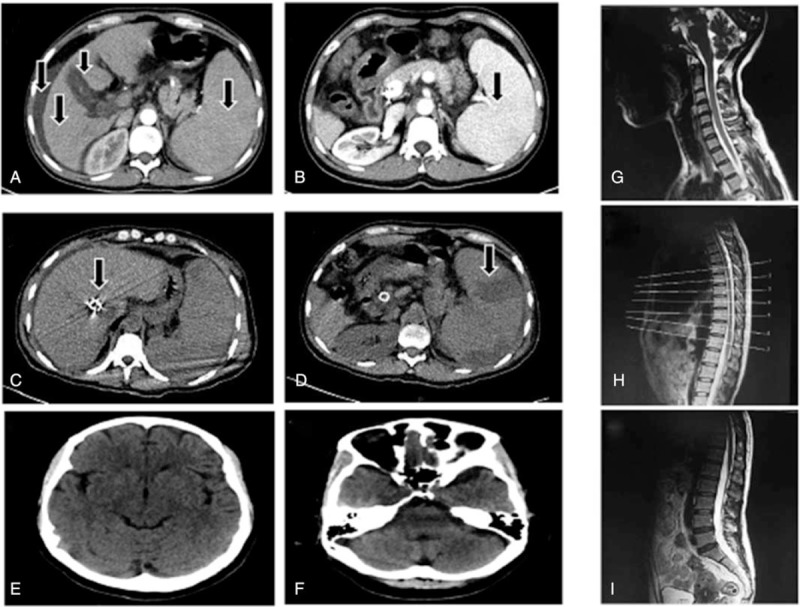
Abdominal CT image of the patient. Arterial phase (A) and venous phase (B) of CT abdomen showing liver cirrhosis, splenomegaly, ascites, cholecystitis. And CT scan (C, D) showing implantation of stent and infarctions of spleen after portacaval shunts. Cranial CT (E, F) indicated the possibility of empty sella, no other abnormality. Sagittal T2-weighted MR images of the cervical (G), thoracic (H), and lumbar (I) spine, there was no pressure on the spinal cord. CT = computed tomography, MR = magnetic resonance.
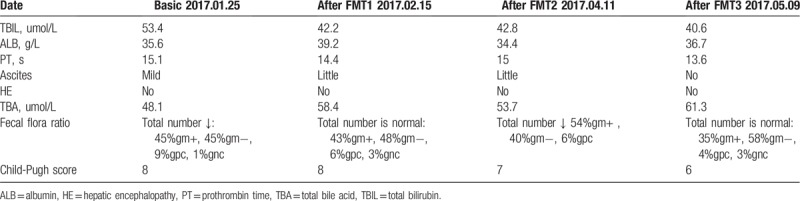
According to the report, the prognosis of HM is poor. In view of the therapeutic effect of FMT on HE,[15] and the relationship between liver disease and gut bacteria.[17] She volunteered to receive FMTs during the follow-up period. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chengdu Medical College and passed the US Clinical Trial Registration Certification (ClinicalTrials.gov ID: NCT03013712). After getting the informed consent of the patient, we performed 3 FMTs from 4 healthy donors. The first time administered by colonoscopy, and the remaining twice by gastroscopy. Because the patient's compliance is not good, the time of FMT is not absolutely regular. There were no adverse events related to FMT during follow-up monitoring. After 3 times FMTs, the appetite improved, the weight increased, the TBIL decreased, the total bile acid slightly increased, and the number of fecal microbiota recovered normal. The score of Child-Pugh decreased to 6 after 3 FMTs (Table 1). More importantly, there arose both subjective and objective changes in spinal conditions. Subjectively, the patient reported that her legs seemed to have toned up since May 2017 (shortly after the third FMT). Objectively, Muscle strength in the right limb was improved to MRC grade 4-. Muscle strength in the left limb was improved to MRC grade 4. The length of her stride became longer. She was able to walk a few meters without auxiliary tools at once and the gait improved. She realized taking care of life for herself the most time. Some scholars have classified HM into the following 4 levels as Supplementary Table 1.[18] This grading classifies the severity of HM in detail, and is particularly suitable as a basis for judging changes in HM conditions. According to this standard, the patient's condition improved from HM2 to HM1. The patient was pleased with this result, and she has provided informed consent for publication of the case.
Table 1.
Several varying indicator after FMT.
Fecal samples were obtained from the patient before FMT and a period of time after FMT. Microbial DNA was extracted using the TIANamp Stool DNA Kit (TIANGEN Biotech, Beijing, China) and using 16S rRNA sequencing. Sequencing was performed using a 2 × 300 paired-end configuration; image analysis and base calling were conducted by the MiSeq Control Software embedded in the MiSeq instrument. Diversity indices were calculated using the QIIME (Version 1.9.1). A principal coordinates analysis (PCoA) based on the unweighted UniFrac distances was conducted to compare patients’ samples. And we used consensus-based clinical case reporting guideline development to construct this paper.
Histograms of stool microbial composition in a several time points were shown in Figure 2A. PCoA analysis based on unweighted unifrac distance (Fig. 2B) found that the distribution of bacteria in samples FMT2 and FMT3 was more similar. And the principal component PC1 distinguished Basic, FMT1 (PC1 >0) from FMT2, FMT3 (PC1 <0). This indicates the microbial composition before FMT is similar to that after the first FMT, and the receivers’ bacteria that after the third FMT had the similar composition to that after the seconded FMT. That is, the composition of gut bacteria was changed after multiple FMTs. The first heat map showed the beneficial bacteria Lactobacillus and Faecalibacterium had a high abundance in the donor samples, which had a low abundance in the recipient samples before FMT, and increased after FMT. Some harmful bacteria, such as Fusobacterium, Veillonella, and Haemophilus, had a lower abundance in the donor samples, but a higher abundance in recipients before FMT and a reduced abundance after serial FMT. This indicated that FMT effectively regulated the flora structure in the recipient (Fig. 3A). The second heat map also indicated that the abundance of Veillonella and Haemophilus were reduced after subsequent FMT (Fig. 3B). Although Faecalibacterium, which played a positive role in human health, had a tendency to decrease in abundance after the initial FMT, the abundance increased after the third FMT. In addition, the abundance of Staphylococcus, sutterella, streptococcus increased after subsequent FMT. Fusobacterium, as a positively correlated bacterium with the risk of inflammatory bowel disease and colorectal cancer,[19] decreased its abundance after the first FMT, increased after the later FMT, but declined overall (Fig. 3B).
Figure 2.
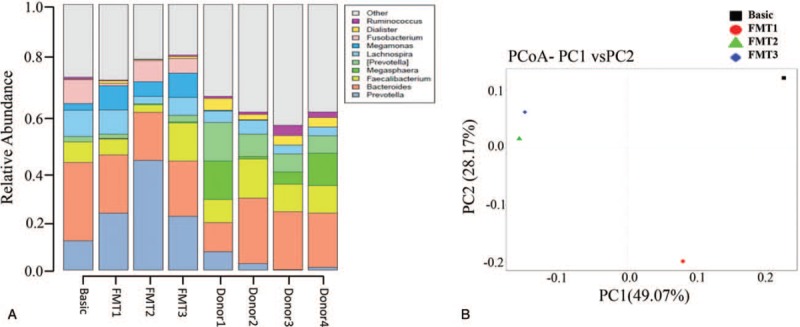
(A) Histograms of stool microbial composition at the genus level. The first 4 columns represented the patient's profile after serial FMT. The remaining columns represented donor profile at 4 different time points of fecal donation. (B) PCoA plots based on unweighted UniFrac metric. Basic: before fecal transplant; FMT1: the first FMT; FMT2: the second FMT; FMT3: the third FMT. Donor 1, Donor 2, Donor 3, Donor 4 referred to 4 healthy donor. FMT = fecal microbiota transplantations, PCoA = principal coordinates analysis.
Figure 3.
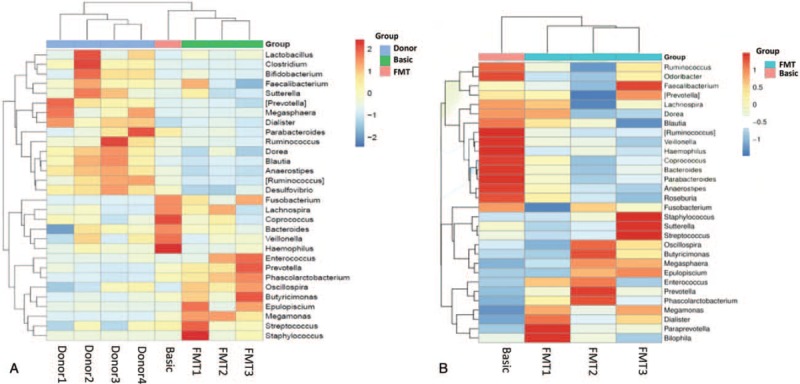
(A) Heat map based on the abundance of stool microbial composition at the genus level, from samples of donors and recipient. (B) Heat map based on the abundance of stool microbial composition at the genus level, from samples of recipient. FMT1: the first FMT; FMT2: the second FMT; FMT3: the third FMT. Donor 1, Donor 2, Donor 3, Donor 4 referred to 4 healthy donor. FMT = fecal microbiota transplantations.
3. Discussion
As far as we know, this is the first report that use FMT to treat HM. The strengths of this case were that it provided a feasible treatment solution for HM. The limitation was that there was no more FMT to see if it has better effect. In this case, the clinical symptoms improved after serial FMT. The composition and diversity analysis of the bacteria found that the microbial composition changed significantly after serial FMTs, mainly including the increase in relative abundance of Faecalibacterium, the reduction in relative abundance of maleficent bacteria like Fusobacterium, Veillonella, and Haemophilus. Faecalibacterium is related to decrease gut permeability and reduce inflammation,[20] affect the effect of anti-tumor therapy,[21] produce of butyrate, exert profound immunometabolic effects that it leads to reduction of its abundance in type 2 diabetes.[22] The pathogenesis of HM is not completely clear. Considering the obvious relationship with the shunt, a common theory is that hepatic dysfunction and portosystemic shunt lead to toxic substances accumulate in the body, and toxic substances produce damage to the spinal cord after passing through the blood-brain barrier (BBB).[23] Interesting, the SCFA butyrate is able to restore BBB integrity. That is, bacterial species which can produce butyrate have an effect on the physiological state of the BBB via metabolic signals.[24] In this case, the lower abundance of Faecalibacterium may increase permeability of the BBB, vanish the selective barrier of serum and lead to many toxins cross the BBB. There is also a theory suggests that HM might associate with ischemia injury of spinal cord and meninges because of the predominant position of the spinal cord lesions.[25] Intriguingly, microbiota is related to plasma concentration of the cytokine granulocyte colony stimulating factor (G-CSF).[26] And G-CSF has a protective effect following ischemic injury.[27] This may be 1 reason why FMT works, Faecalibacterium or other microbial taxa may have the competent to contribute to the production of G-CSF. Another theory is that immune damage may play a role in the pathogenesis of HM, since viral infection and replication can cause cellular immune responses in the extrahepatic spinal cord and nerves, and there is an effective case of antiviral treatment for hepatitis c virus-related HM.[28] Gut bacteria can affect autoimmune diseases like systemic sclerosis (SSc). It is worth noting that patients with SSc also have less abundance of Faecalibacterium and increased abundance of Fusobacterium.[29] The gut bacteria of this disease changes roughly similar to this HM case, which confirms the possibility that gut microbiota control neurological function through the immune system in this HM case. Microbial-associated molecular pattern can prompt innate immune cells to produce pro-inflammatory cytokines, which can cross BBB and act on receptors expressed by microglia.[30] An animal study has shown that intestinal flora of laboratory mice can control the period at which microglial cells attains maturity and its functions.[31] Besides, M2 microglia can enhance remyelination and resist demyelination.[32] And a symmetric loss of myelin in the lateral corticospinal tracts was the specific feature of HM.[33] The above concepts also set up a bridge between intestinal flora and HM. In previous coverage, patients with HM had poor prognosis, and the damage was almost irreversible.[34] So, FMT may affect HM in many aspects. The only well-established treatment method is liver transplantation at the early stage, it can improve the symptoms of legs.[2] But the extremely high operation fee, limited liver source, and complex process of liver transplantation make the feasibility of treating HM with liver transplantation limit. Previous studies about microbiome-gut-brain axis provide us new enlightenment for the therapy of HM. This case indicated that FMT may have a good therapeutic effect for HM and deserve further investigation.
Acknowledgment
The authors thank the National Science Foundation for Young Scholars of China (Grant No. 81702446); the State Key Clinical Specialty Construction Project (Grant No. CYFY2017GLPXH002; CYFY2018GLPXH01; CYFY2018GLPXH06); the Science and Technology Plan Project of Sichuan Province (Grant No. 2018JY0654); the Scientific Research Project of Sichuan Education Department (Grant No. 18Z030) for the support.
Author contributions
Conceptualization: Lin Sun, Li-Li Lan, Xiao-An Li.
Data curation: Lin Sun.
Formal analysis: Jun Li.
Funding acquisition: Xiao-An Li.
Investigation: Lin Sun, Jun Li, Li-Li Lan.
Project administration: Lin Sun, Jun Li, Xiao-An Li.
Resources: Jun Li.
Software: Jun Li.
Supervision: Lin Sun, Jun Li, Xiao-An Li.
Validation: Lin Sun.
Visualization: Lin Sun.
Writing – original draft: Lin Sun.
Writing – review and editing: Lin Sun, Jun Li.
Supplementary Material
Footnotes
Abbreviations: BBB = blood-brain barrier, FMT = fecal microbiota transplantation, G-CSF = granulocyte colony stimulating factor, HbsAg = hepatitis B surface antigen, HE = hepatic encephalopathy, HM = hepatic myelopathy, MEPs = motor evoked potentials, SSC = systemic sclerosis, TIPS = transjugular intrahepatic portosystemic shunt.
LS and JL contributed equally to this study and share the first authorship.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
This study is supported by the National Science Foundation for Young Scholars of China (Grant No. 81702446); the State Key Clinical Specialty Construction Project (Grant No. CYFY2017GLPXH002; CYFY2018GLPXH01; CYFY2018GLPXH06); the Science and Technology Plan Project of Sichuan Province (Grant No. 2018JY0654); the Scientific Research Project of Sichuan Education Department (Grant No. 18Z030).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Weissenborn K, Tietge UJ, Bokemeyer M, et al. Liver transplantation improves hepatic myelopathy: evidence by three cases. Gastroenterology 2003;124:346–51. [DOI] [PubMed] [Google Scholar]
- [2].Baccarani U, Zola E, Adani GL, et al. Reversal of hepatic myelopathy after liver transplantation: fifteen plus one. Liver Transpl 2010;16:1336–7. [DOI] [PubMed] [Google Scholar]
- [3].Ren YD, Ye ZS, Yang LZ, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology 2017;65:1765–8. [DOI] [PubMed] [Google Scholar]
- [4].Ferrere G, Wrzosek L, Cailleux, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol 2017;66:806–15. [DOI] [PubMed] [Google Scholar]
- [5].Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med 2016;165:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rebello D, Wang E, Yen E, et al. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep J 2017;4:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015;17:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 2015;125:2841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kazemi A, Noorbala AA, Azam K, et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr 2019;38:522–8. [DOI] [PubMed] [Google Scholar]
- [10].Strati F, Cavalieri D, Albanese D, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun MF, Zhu YL, Zhou ZL, et al. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson's disease mice: gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun 2018;70:48–60. [DOI] [PubMed] [Google Scholar]
- [12].Zhuang ZQ, Shen LL, Li WW, et al. Gut microbiome is altered in patients with Alzheimer's disease. J Alzheimers Dis 2018;63:1337–46. [DOI] [PubMed] [Google Scholar]
- [13].Li S, Lv J, Li J, et al. Intestinal microbiota impact sepsis associated encephalopathy via the vagus nerve. Neurosci Lett 2018;662:98–104. [DOI] [PubMed] [Google Scholar]
- [14].Mullish BH, McDonald J, Thursz MR, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1354–5. [DOI] [PubMed] [Google Scholar]
- [15].Kao D, Roach B, Park H, et al. Fecal microbiota transplantation in the management of hepatic encephalopathy. Hepatology 2016;63:339–40. [DOI] [PubMed] [Google Scholar]
- [16].Nardone R, Orioli A, Holler Y, et al. Central motor and sensory conduction in patients with hepatic myelopathy. Spinal Cord 2014;52:439–43. [DOI] [PubMed] [Google Scholar]
- [17].Sanduzzi ZM, Rocco A, Compare D, et al. The gut microbiota: a new potential driving force in liver cirrhosis and hepatocellular carcinoma. United European Gastroenterol J 2017;5:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shuyao R, Hui C, Yong L. Hepatic myelopathy after transjugular intrahepatic portosystemic shunt: natural course, survival analysis, and treatment. J Clin Hepatol 2016;06:1112–7. [Google Scholar]
- [19].Hussan H, Clinton SK, Roberts K, et al. Fusobacterium's link to colorectal neoplasia sequenced: a systematic review and future insights. World J Gastroenterol 2017;23:8626–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morkl S, Lackner S, Meinitzer A, et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr 2018;57:2985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Routy B, Gopalakrishnan V, Daillere R, et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol 2018;15:382–96. [DOI] [PubMed] [Google Scholar]
- [22].Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut 2014;63:1513–21. [DOI] [PubMed] [Google Scholar]
- [23].O’Brien J, Staples C, Florin T. Trouble with a shunt: alcohol and spastic paraparesis. Hepatic myelopathy. Gastroenterology 2010;139:1099. [DOI] [PubMed] [Google Scholar]
- [24].Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 2014;6:158r–263r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Giangaspero F, Dondi C, Scarani P, et al. Degeneration of the corticospinal tract following portosystemic shunt associated with spinal cord infarction. Virchows Arch A Pathol Anat Histopathol 1985;406:475–81. [DOI] [PubMed] [Google Scholar]
- [26].Deshmukh HS, Liu Y, Menkiti OR, et al. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 2014;20:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shyu WC, Lin SZ, Yang HI, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation 2004;110:1847–54. [DOI] [PubMed] [Google Scholar]
- [28].di Biase L, Picillo M, Freitas ME, et al. Hepatitis C virus-related hepatic myelopathy after treatment with sofosbuvir and ribavirin: a case report. Ann Intern Med 2017;166:379–80. [DOI] [PubMed] [Google Scholar]
- [29].Bellocchi C, Volkmann ER. Update on the gastrointestinal microbiome in systemic sclerosis. Curr Rheumatol Rep 2018;20:49. [DOI] [PubMed] [Google Scholar]
- [30].Dantzer R, Konsman JP, Bluthe RM, et al. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci 2000;85:60–5. [DOI] [PubMed] [Google Scholar]
- [31].Erny D, Hrabe DAA, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yu Z, Sun D, Feng J, et al. MSX3 switches microglia polarization and protects from inflammation-induced demyelination. J Neurosci 2015;35:6350–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ben AS, Saied MZ, Harzallah MS, et al. Hepatic myelopathy with spastic paraparesis: report of two cases and review of the literature. Eur Spine J 2014;23 Suppl 2:167–71. [DOI] [PubMed] [Google Scholar]
- [34].Utku U, Asil T, Balci K, et al. Hepatic myelopathy with spastic paraparesis. Clin Neurol Neurosurg 2005;107:514–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


