Supplemental Digital Content is available in the text
Keywords: acute kidney injury, aortic aneurysm, abdominal, endovascular procedures, hydroxyethyl starch derivatives, patient outcome assessment, vascular surgical procedures
Abstract
Intraoperatively administered hydroxyethyl starch could be a risk indicator for postoperative acute kidney injury (AKI) in vascular surgical patients.
In a single-center retrospective cohort analysis, we assessed the impact of hydroxyethyl starch and other risk indicators on AKI and mortality in 1095 patients undergoing elective open abdominal aneurysm repair (AAA-OR) or endovascular aortic repair (EVAR). We established logistic regression models to determine the effect of various risk indicators, including hydroxyethyl starch, on AKI, as well as Cox proportional hazard models to assess the effect on mortality.
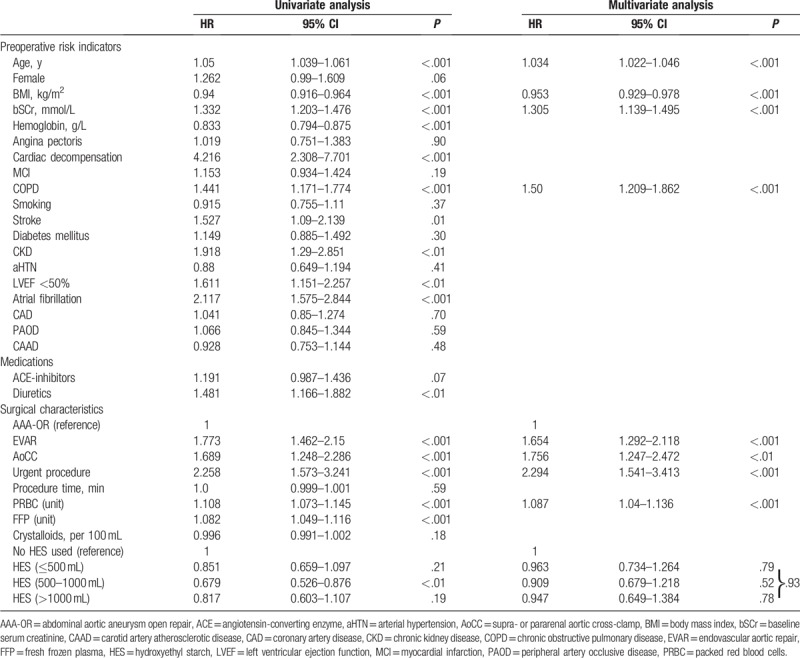
The use of intravenous hydroxyethyl starch was not associated with an increased risk of AKI or mortality. Patients undergoing EVAR were less likely to develop AKI (4% vs 18%). Multivariate risk indicators associated for AKI included suprarenal or pararenal aortic cross-clamp [odds ratio (OR), 4.44; 95% confidence interval (95% CI), 2.538–7.784; P < .001] and procedure length (OR, 1.005; 95% CI, 1.003–1.007; P < .001), and favored EVAR (OR, 0.351; 95% CI, 0.118–0.654; P < .01). Main multivariate risk indicators associated with mortality included patients needing an urgent procedure [hazard ratio (HR), 2.294; 95% CI, 1.541–3.413; P < .001], those with suprarenal or pararenal aortic cross-clamp (HR, 1.756; 95% CI, 1.247–2.472; P < .01), and patients undergoing EVAR (HR, 1.654; 95% CI, 1.292–2.118; P < .001).
We found neither a benefit nor a negative effect of hydroxyethyl starch on the risk of AKI or mortality. Instead, other variables and comorbidities were found to be relevant for the development of postoperative AKI and survival. Nevertheless, clinicians should be aware of the high risk of postoperative AKI, particularly among those undergoing AAA-OR procedures.
1. Introduction
Intravenous fluid management in high-risk surgical patients, such as abdominal aortic aneurysm (AAA) repair, has undergone several paradigm shifts over the last few decades. Type and amount of perioperative fluids to be administered for hemodynamic optimization in surgical patients remains controversial.[1,2] Hydroxyethyl starch (HES) was previously used as intravenous fluid, but has been found to be associated with serious adverse events, including acute kidney injury (AKI) and death.[3]
AKI is an important postoperative complication in vascular surgery patients, with an incidence of up to 19% in both open AAA repair (AAA-OR) and endovascular aneurysm repair (EVAR) cases.[4–6] Various pre- and intraoperative risk factors and mechanisms have been linked to AKI, including cardiovascular risk factors and preoperative impaired renal function,[7] intraoperative contrast agent administration,[8] renal microembolization,[9] dissection of the renal arteries or coverage of the arterial orifice,[10] reperfusion injury,[11] and hypovolemia.[4]
In patients with hypovolemia, various HES products are used, as their specific biochemical features facilitate rapid and lasting circulatory stabilization. However, in critically ill patients[3] and patients undergoing cardiac surgery,[12] HES was found to be a risk factor for AKI; in fact, since 2013, the European Medicines Agency (EMA) has recommended the restricted use of HES and further limitations are expected, though there is still an ongoing controversial debate.[13,14]
To our knowledge, only a few studies have investigated HES administration and its impact on postoperative AKI and mortality in patients undergoing elective AAA-OR or EVAR procedures. Therefore, we performed a study to analyze the impact of the intraoperative administration of HES on the evolution of postoperative AKI in this specific patient group by using the Risk, Injury, Failure, Loss of kidney function and End-stage kidney disease (RIFLE) classification, along with its impact on mortality. Accordingly, we chose a pragmatic research concept analyzing a cohort when no restriction on HES was given, to investigate the influence of the intervention on outcomes that are clinically relevant to patients in a real-life clinical setting.
2. Methods
2.1. Patients and protocols
The study was approved by the Ethics Committee of the Medical University of Vienna, Austria (EK Nr: 964/2011), and was conducted in accordance with the Declaration of Helsinki. The need for informed patient consent was waived due to the retrospective study design.
Between January 1, 1997, and August 1, 2011, all patients who underwent elective AAA-OR or EVAR at the Department of Surgery, Division of Vascular Surgery, Medical University of Vienna, Vienna, Austria, were recorded in a prospectively collected database of the Division of Cardiac Thoracic Vascular Anesthesia and Intensive Care Medicine. For this analysis, we included adult patients (>18 years) who were scheduled to undergo elective surgery.
In the database, we identified 1483 elective abdominal aortic procedures. We excluded 387 patients for various reasons [3 for miscoding, 16 for preoperative dialysis, 204 for missing pre- or postoperative serum creatinine values (SCr), and 165 for missing volume supply data]. Finally, 1095 patients were included in the study.
At the end of the follow-up period, we combined our database with data obtained from the central laboratory and hospital central databases. Mortality data were obtained from the Federal Austrian Statistical Office, which records the death of every individual in Austria. Follow-up was complete in all patients who were included in the study.
2.2. Patient characteristics, acquisition of risk indicator data, and follow-up
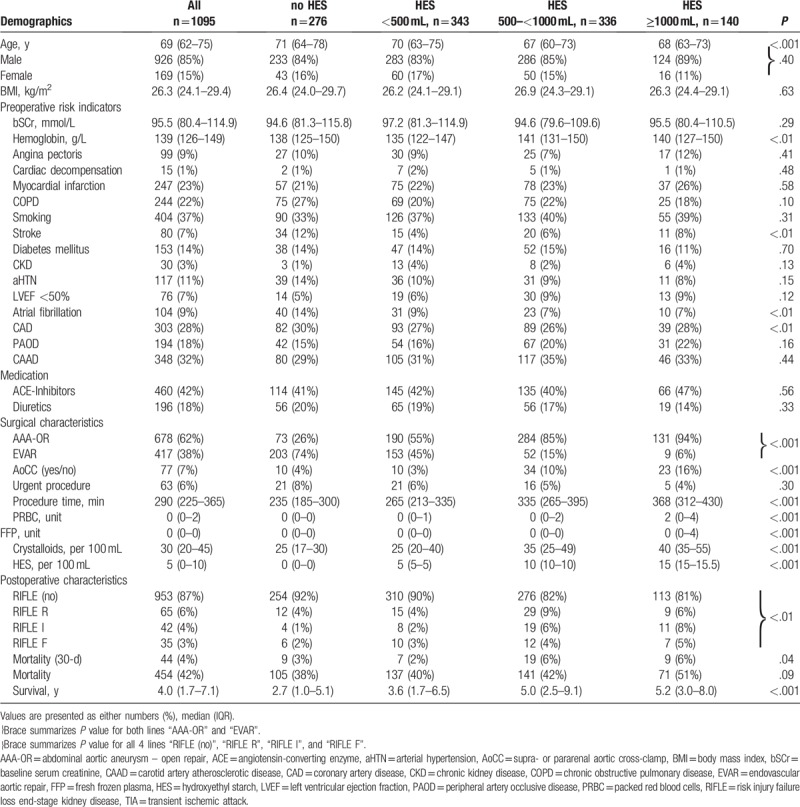
Preoperative patient data were collected prospectively at the time of preoperative anesthesia patient visit. The following risk indicators were recorded in each patient: age, sex, body mass index (BMI, kg/m2), baseline SCr levels (mmol/L), preoperative hemoglobin levels, recent angina pectoris or cardiac decompensation, history of myocardial infarction, chronic obstructive pulmonary disease (COPD), current smoking, stroke, diabetes mellitus, chronic kidney disease (CKD), arterial hypertension (aHTN), reduced left ventricular ejection fraction (LVEF) <50%, atrial fibrillation, coronary artery disease (CAD), peripheral artery occlusive disease (PAOD), carotid artery atherosclerotic disease (CAAD; defined by a history of carotid plaques or carotid stenosis), and treatment with diuretics and/or angiotensin-converting enzyme (ACE) inhibitors.
The procedure-related data included the procedure itself, procedure time (defined as the duration of anesthesia), urgent procedure (defined as patients not been electively admitted for the vascular procedure, but who have been admitted for various medical reasons but underwent a nonemergency AAA-OR or EVAR during the current hospital admission), volume of administered fluids (HES and crystalloids), and volume of packed red blood cells (PRBCs) and fresh frozen plasma (FFP) that were administered to the patients. The presence of renal ischemia (defined as supra- or pararenal aortic cross-clamping) was recorded for further analysis.
2.3. Surgical intervention, anesthesia, and intraoperative fluid management
AAA-OR was performed via a transperitoneal or retroperitoneal approach. Proximal aortic control was achieved with infra-, supra-, or pararenal aortic cross-clamping. The AAA was repaired using tube grafts or bifurcated grafts. EVAR was performed via femoral or iliac access, and a bifurcated endograft was most commonly used.
The choice of anesthetic technique was dependent on the patient's coexisting diseases, and at the discretion of the anesthesiologist. General anesthesia with or without regional anesthetic techniques was administered in all cases of AAA-OR, whereas regional anesthetic techniques were more frequently used in cases of EVAR. During anesthesia induction, fluid therapy with 250 to 500 mL of Ringer-Lactate “Fresenius” solution (Fresenius Kabi, Bad Homburg, Germany; crystalloid solution) was administered. Intraoperative fluid management was not performed using a strict algorithm, but was left to the discretion of the attending anesthesiologist. Administration of intravenous fluids in our patients was based on institutional standard hemodynamic management to achieve a systolic blood pressure >90 mm Hg and to avoid a decrease in systolic blood pressure >20% compared with baseline, a heart rate <100 beats/min, and to avoid an increase in heart rate of >30% compared with baseline, and normal hemodynamic filling pressures. We routinely performed blood gas analysis to monitor and achieve a central venous oxygen saturation >70% and a serum lactate concentration <2.2 mmol/L. In patients with reduced LVEF, transesophageal echocardiography was used to monitor myocardial performance, as well as the impact of fluid loading and inotropic support on left and right ventricular function. In overt bleeding with threatening hemodynamic instability, 6% HES 130/0.4 in NaCl 0.9% (Voluven; Fresenius Kabi, Bad Homburg, Germany) was given under guidance of our hemodynamic monitoring at the discretion of the anesthesiologist. Intraoperative blood salvage system was used routinely in all AAA-OR cases. Blood transfusion was performed according to the STS-SCA transfusion guidelines.[15,16] In the case of ongoing bleeding, the decision to administer FFP, platelets, fibrinogen, and factor concentrates was based on clinical experience and surgical demand.
Further information on hemodynamics, medication and laboratory values is shown in the supplemental content.
2.4. Outcome variables
The primary outcome of this study was to assess the impact of the intraoperative administration of HES on the evolution of postoperative AKI in elective abdominal aneurysm repair (AAA-OR or EVAR) procedures by using the RIFLE classification. The secondary outcome was to assess the impact of the intraoperative administration of HES on mortality.
We assessed the prognostic potential of SCr elements for the diagnosis of AKI according to the RIFLE–stages (risk, RIFLE R; injury, RIFLE I; or failure, RIFLE F).[17] The increase in SCr levels from baseline to the highest value within 7 postoperative days was used for classification, and the urine output criteria were not considered: RIFLE 0, SCr <1.5-fold; RIFLE R, SCr ×1.5–1.99; RIFLE I, SCr ×2.0–2.99; and RIFLE F, SCr ×3.0 or SCr ≥353.6 mmol/L with an acute increase of >44.2 mmol/L. Baseline SCr (bSCr) levels were defined as the SCr value recorded closest to the time of surgery. For the analysis, a dichotomous variable (“AKI” or “no-AKI”) was created; the variable AKI indicates AKI at any RIFLE-stage. In addition, we investigated the association of risk indicators with mortality.
SCr concentration was measured in a certified laboratory using the Jaffe method with a Hitachi 747 analyzer (Boehringer Diagnostics, Mannheim, Germany) and an Olympus AU5400 (Olympus Europe SE & Co. Kg, Hamburg, Germany) (since 2004); the analyzers and specific tests were extensively evaluated, and the test results were compared before changing the platforms in 2004. No difference between the analyzers was found (r = 0.99).
2.5. Statistical methods
For planning purposes, we assumed a 10% probability for AKI in low-risk patients and we considered an increase to 20% as a minimal relevant effect of a potential risk factor. We calculated the sample size required to detect this effect with 80% power at the 5% significance level with a Chi-squared test, which is asymptotically equivalent to the test employed in the logistic regression models with a binary predictor. If the risk factor has a prevalence of 50% in the population, the required total sample size is approximately 400 patients. If we assume the risk factor has a prevalence of 25%, the required total sample size is approximately 556 patients. We therefore concluded that the study sample size of approximately 1000 patients is sufficient to detect important risk factors with high power.
Demographic and clinical baseline data are summarized as median and interquartile range (IQR) for metric variables or absolute and relative frequencies for categorical variables. The differences between the groups were analyzed using Student t test for continuous variables and the Chi-square test for categorical variables. The Kaplan–Meier method was used to estimate survival functions. Survival curves were compared using the log-rank test.
For the first outcome to analyze the association between the risk of AKI and HES, logistic regression models for potential preoperative risk indicators, medication and surgical characteristics were applied (as summarized in Table 1). First, univariate logistic regression models were fit, explaining the risk for AKI through each risk indicator separately. In addition, a multivariate logistic regression model was fit, including HES as a predictor and further adjusting for risk indicators selected by stepwise forward-backward model selection based on the Bayesian information criterion (BIC). In this model, the risk for AKI is explained jointly through HES and all further selected risk indicators.
Table 1.
Patient characteristics.
Similarly, for the second outcome the association of long-term mortality, the identical potential risk indicators were analyzed using univariate Cox-regression models and a multivariate Cox-regression model. Similar to the logistic regression, the multivariate Cox model included HES as a predictor of main interest and further risk indicators were selected by stepwise forward-backward model selection based on the BIC.
In the model selection procedures for both models, the scope of eligible risk indicators comprised the same variables that were considered in the univariate models for the respective outcome. Furthermore, we also allowed for interactions of risk indicators with surgery type to account for the possibility that some risk indicators might have different effects in the 2 surgery groups (e.g., amount of administered PRBC). The model selection procedure avoids selecting highly correlated risk indicators to the model; instead, out of a set of collinear risk indicators, typically 1 best risk indicator can be expected to be selected. Thus, the chosen analysis avoids potential problems with multicollinearity. Also due to the model selection, problems of overfitting are avoided.[18,19]
Odds ratios (ORs) or hazard ratios (HRs) as well as their respective 95% confidence intervals (95% CIs) were calculated for all the risk indicators in each model. P values of <.05 were considered significant. All statistical analyses were performed with the statistical computing environment R 3.4.1 (https://www.r-project.org).
3. Results
The data of 1095 patients were analyzed. The median age of these patients was 69 (IQR, 62–75) years. The proportion of female patients was 15%. Forty-four (4%) patients died within 30 days, overall, within the 14-year observation period, 454 (42%) patients died. Among the patients who survived, the mean follow-up period was 4.0 (IQR, 1.7–7.1) years.
Overall, 678 patients (62%) underwent AAA-OR and 417 patients (38%) underwent EVAR. Tube graft implantation via an abdominal or retroperitoneal approach was performed in 223 patients (33%), whereas implantation of a bifurcated graft was conducted in 455 patients (67%). Most of patients (N = 963, 88%) received general anesthesia. The mean procedure time was 338 ± 101 minutes for AAA-OR and 248 ± 87 minutes for EVAR procedures. An aortic cross clamp (AOX) was placed in the infrarenal position in 601 patients (89%), in the suprarenal position in 64 patients (9%), and in the pararenal position in 13 patients (2%).
Overall, AKI was found in 142 patients (13%), whereas 125 AAA-OR patients (18%) and 17 EVAR-patients (4%) developed AKI.
HES was not used in 276 (25%) patients, 343 (31%) patients received <500 mL HES, 336 (31%) patients received between 500 and 1000 mL of HES, and 140 (13%) patients received >1000 mL of HES. In the AAA-OR group, 73 (11%) patients did not receive HES, 190 (28%) patients received <500 mL of HES, 284 (42%) patients received between 500 and 1000 mL of HES, and 131 (19%) patients received >1000 mL of HES. In the EVAR group, 203 (40%) patients did not receive HES, 153 (37%) received <500 mL of HES, 52 (13%) patients received between 500 and 1000 mL of HES, and 9 (2%) patients received >1000 mL of HES. Patients undergoing AAA-OR received HES significantly more frequently (P < .001) than patients undergoing EVAR. Survival was not found to be associated with the amount of administered HES. Detailed patient characteristics are shown in Table 1 and Fig. 1.
Figure 1.
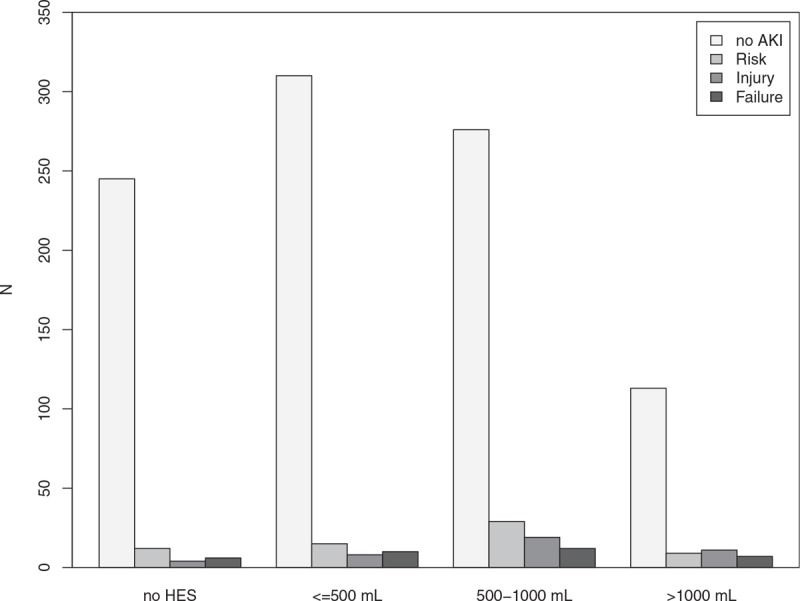
Distribution of different RIFLE-stages to the amount of hydroxyethyl starch. Barplots showing acute kidney injury (AKI) according to the RIFLE–stages and the patients’ distribution to the amount of hydroxyethyl starch (HES) used. Shades of grey indicate the patients different RIFLE-stages: no AKI, Risk, Injury and Failure.
3.1. Association of risk indicators with AKI
Univariate analysis indicated significant associations between 10 risk indicators and AKI. The largest effects were observed in patients with a suprarenal or pararenal aortic cross-clamp (OR, 8.043; 95% CI, 4.925–13.14; P < .001), in patients receiving >1000 mL of HES, as compared to those not receiving HES (OR, 2.759; 95% CI, 1.506–5.052; P = .001), and those receiving between 500 and 1000 mL of HES (OR, 2.51; 95% CI, 1.496–4.211; P < .001). Patients undergoing an EVAR procedure had a lower risk of AKI (OR, 0.188; 95% CI, 0.111–0.317; P < .001).
Multivariate analysis indicated significant associations between 3 risk indicators and AKI: suprarenal or pararenal aortic cross-clamp (OR, 4.44; 95% CI, 2.538–7.784; P < .001), procedure length per minute (OR, 1.005; 95% CI, 1.003–1.007; P < .001), and use of EVAR (OR, 0.351; 95% CI, 0.118–0.654; P < .01). Detailed results are summarized in Table 2.
Table 2.
Regression analysis on AKI.
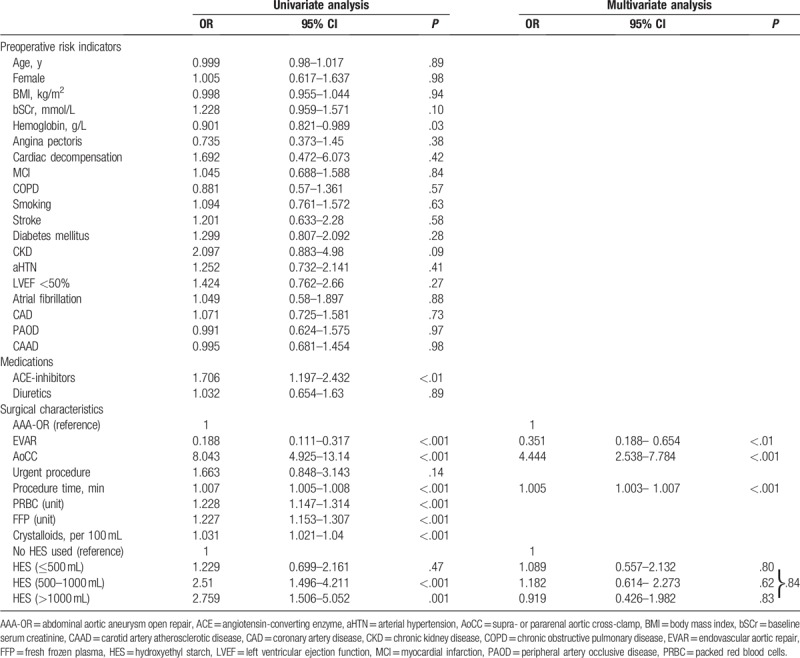
After adjusting for these 3 risk indicators, the estimated risk for AKI was not different in patients not receiving HES compared with patients receiving up to 500 mL of HES (OR, 1.089; 95% CI 0.557–2.132; P = .80), or compared with patients receiving between 500 and 1000 mL HES (OR, 1.182; 95% CI, 0.614– 2.273; P = .62) or compared with patients receiving more than 1000 mL (OR, 0.919; 95% CI 0.426–1.982; P = .83).
Renal ischemia, due to the need for a supra- or pararenal aortic cross-clamp, only found in AAA-OR procedures, was a rare condition overall with 77 cases.
We did not observe a difference in the relative frequencies of AKI per year during the observational period (P = .33).
3.2. Risk indicators and association with long-term mortality
Univariate analysis indicated significant associations between 17 risk indicators and mortality. The highest HRs were observed in patients with recent cardiac decompensation (HR, 4.216; 95% CI, 2.308–7.701; P < .001), those needing an urgent procedure (HR, 2.258; 95% CI, 1.573–3.241; P < .001), and those with atrial fibrillation (HR, 2.117; 95% CI, 1.575–2.844; P < .001). In patients receiving 500 to 1000 mL of HES, univariate analysis indicated a survival benefit compared with patients where no HES was given (HR, 0.679; 95% CI, 0.526–0.876; P < .01; Fig. 2).
Figure 2.
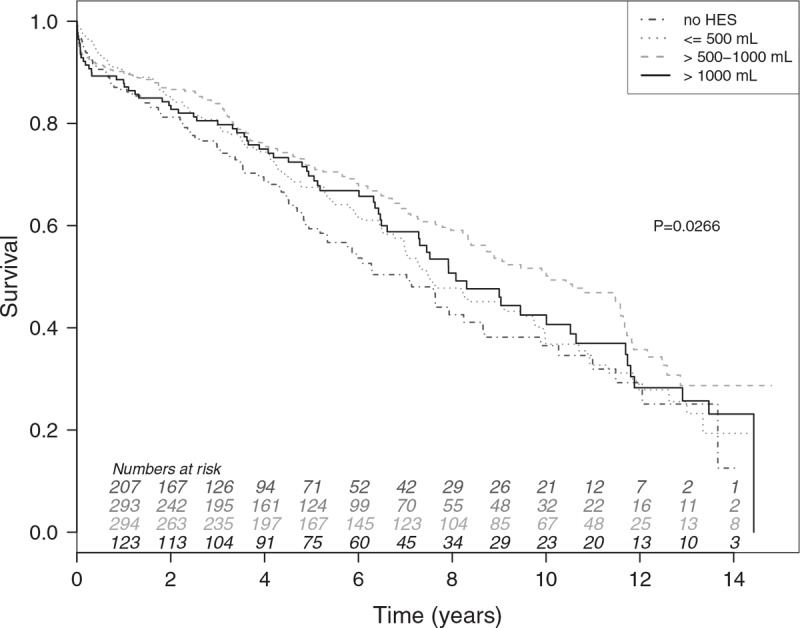
Survival stratified by amount of hydroxyethyl starch. Univariate Kaplan–Meier survival curves for the complete patient cohort, stratified by the amount of hydroxyethyl starch (HES) used. The dashed dotted grey line indicates the patients receiving no HES, the dotted grey line indicates the patients receiving ≤500 mL of HES, the dashed grey line indicates the patients receiving >500 and ≤1000 mL of HES, and the solid black line indicates the patients receiving >1000 mL of HES.
Multivariate analysis indicated a significant association between 8 risk indicators and mortality. The highest HRs were observed in patients needing an urgent procedure (HR, 2.294; 95% CI, 1.541–3.413; P < .001), in patients with a suprarenal or pararenal aortic cross-clamp (HR, 1.756; 95% CI, 1.247–2.472; P < .01), and in patients undergoing EVAR (HR, 1.654; 95% CI, 1.292–2.118; P < .001). After adjusting for these risk indicators, the HRs between different HES groups were highly similar for patients not receiving HES and patients receiving HES and were close to one. Detailed results are summarized in Table 3.
Table 3.
Regression analysis of long-term mortality.
4. Discussion
In the present single-center retrospective cohort study, we investigated a large group of patients undergoing elective AAA repair. We focused on identifying whether the use of HES was associated with postoperative AKI. Accordingly, we examined the impact of potential risk indicators, including the volume of administered HES, on postoperative AKI as well as mortality.
We found that the volume of infused HES was not associated with postoperative AKI and mortality after adjusting for the procedural risk indicators. However, in our patients, the total amount of HES infused was below the maximum recommended dose of 50 mL/kg/day, which might have limited the side effects of HES on renal function. Although the potential of HES to cause AKI is known and dose-dependent, the mechanisms underlying the manner in which HES causes AKI remain unclear.[20]
The morphological changes caused by tubular swelling and decreasing filtration pressure due to the oncotic force of HES have been previously discussed as related factors.[21] Also, results from experimental and clinical studies strongly suggest that the toxicity of HES is attributed to tissue storage.[22] In humans, HES administration leads to decreased cell viability in the proximal tubular cells, and in a rat model, HES administration caused kidney function impairment even under healthy conditions.
On the contrary, Margraf et al[14] demonstrated recently in a mice model that HES exerts protective effects on glycocalyx integrity during systemic inflammation. Also, in a recently published meta-analysis, HES was found to have no impact on AKI or renal replacement therapy in elective surgical patients resuscitated with HES; however, the authors concluded that the data were not sufficient to identify a difference in the outcomes associated with crystalloid and HES use.[23]
Nevertheless, due to evidence from high-quality, investigator-initiated clinical trials,[24] which have shown that HES use is associated with serious adverse effects with no patient benefits, the EMA suspended the marketing authorizations of HES solutions across the European Union on January 26, 2018.[25]
The topic of possible harms due to HES administration in major surgical patients is still a controversial debate.[25–27] Publications continue to document the absence of increased risk[28,29] and even the improved outcomes[30] associated with the use of HES under defined clinical circumstances.[26] Maybe a currently ongoing prospective trial on elective abdominal surgery (PHOENICS trial, NCT03278548) provides clinically relevant information regarding the safety and efficacy of HES.[26]
We identified operative risk indicators, procedure time, and aortic cross-clamp in supra- or pararenal position, to be the main risk indicator associated with an increased risk of AKI. Hence, we found that AAA-OR was the most important risk indicator for postoperative AKI. In particular, we observed an almost 5 times higher rate of AKI (in various levels of impairment) in AAA-OR patients, although contrast media could be a risk factor for AKI.[31] But in our study, contrast media was only used in EVAR patients.
In contrast to other studies, we could not identify the well-known risk factors for AKI, such as diabetes mellitus and the need of PRBC[32–34] in AAA-OR, which may be a result of low variability in these risk indicators.
In the present study, we assessed the effect of different risk indicators on mortality after aortic surgery. We found that nonmodifiable risk indicators such as procedure-related renal ischemia or urgent procedure, as well as nonmodifiable patient factors such as age or comorbidities reduced the likelihood of long-term survival. Also, the need of PRBCs was identified as potentially modifiable operative risk indicators. Patients who underwent EVAR were less likely to have postoperative renal impairment, but had increased mortality. EVAR patients are in general older and exhibit higher rates of baseline comorbidities; the willingness of surgeons to perform less-invasive EVAR in these patients is also higher.[35] Therefore, age could represent a source for selection bias in the present study, as the EVAR cohort was significantly older as compared to the AAA-OR patients. Moreover, EVAR is a less invasive procedure and could explain the lower morbidity, as compared to the more complex AAA-OR. In a recent study, Liang et al[36] compared perioperative and short-term outcomes in younger (<65 years) EVAR and AAA-OR patients, and found that the propensity-weighted procedural mortality rates of EVAR and AAA-OR are fairly comparable.
4.1. Limitations
Although we included a broad range of relevant acute and chronic risk indicators to minimize bias, we did not obtain any information on aneurysm-specific data (size/morphology), renal microembolization, procedure-related harm to the renal arteries or reperfusion injury, and the amount of contrast agents used. In a recent study, the quantity of contrast agent did not bias the outcome of AKI.[31] Also, we do not have consistently data on blood loss or intraoperative hemodynamics. Estimation of blood loss is inaccurate and unreliable[37]; we can only estimate blood loss during the operation, not even the amount of blood sucked into suction devices is a precise volume, often diluted with flushing water. Also, the amount of intraoperative cell salvage reflects just a part of blood loss. Moreover, we cannot quantify blood loss on tapes, sponges, cover, coat and the floor, which can be an extensive amount. Therefore, we used the amount of PRBC and FFP as a surrogate parameter for blood loss.
Moreover, changes in surgical and anesthetic management during the long observational period were not considered in our study. Furthermore, we did not assess a more recent time period because since 2011, we stopped using synthetic HES in our vascular surgical patients. In addition, we used the RIFLE criteria for staging AKI and not the currently recommended Kidney Disease–Improving Global Outcome stages (KDIGO), because we do not have consistent information regarding postoperative renal replacement therapy in our patients. Moreover, 18 patients died within the 7-day observational period required for the RIFLE classification. Of these patients, RIFLE stage F was noted in 3 patients; in the others, the severity or occurrence of AKI was unknown. Finally, owing to the difficulties in determining the cause of death for many patients, we analyzed overall mortality, but not surgery-related mortality.
5. Conclusion
In our large cohort of patients undergoing abdominal aneurysm repair, the perioperative infusion of low volumes of HES was not associated with postoperative AKI. We found neither a benefit nor a negative effect of HES. Instead, other variables and comorbidities were found to be relevant for the development of postoperative AKI and survival. The large effect of procedural and patient-related risk indicators may mask either the small benefit or risk associated with the use of HES. The findings of this study are important to the still ongoing debate concerning the use of HES in major surgery and also for official regulatory limitations.
Acknowledgments
We thank all the medical staff from the Division of Cardiac Thoracic Vascular Anesthesia and Intensive Care Medicine, for their contributions to the database. Special thanks go to Thomas Neugebauer for his invaluable help in database processing.
Author contributions
Substantial contributions to the conception or design off the work:
MHB, DGH, CMD, and AL
Acquisition, analysis, or interpretation of data for the work:
MHB, CMD, RR, MH, MaH, MJH and AL
Drafting the work:
MHB, CMD, and AL
Revising it critically for important intellectual content:
MHB, DGH, CMD, RR, MH, MaH, MJH and AL
Final approval of the version to be submitted:
MHB, DGH, CMD, RR, MH, MaH, MJH and AL
Conceptualization: Martin Hermann Bernardi, Dominik G. Haider, Christoph M. Domenig, Michael J. Hiesmayr, Andrea Lassnigg.
Data curation: Martin Hermann Bernardi, Markus Haisjackl, Michael J. Hiesmayr, Andrea Lassnigg.
Formal analysis: Martin Hermann Bernardi, Robin Ristl, Michael Hagmann, Markus Haisjackl, Michael J. Hiesmayr, Andrea Lassnigg.
Investigation: Martin Hermann Bernardi, Dominik G. Haider, Christoph M. Domenig, Markus Haisjackl, Michael J. Hiesmayr, Andrea Lassnigg.
Methodology: Martin Hermann Bernardi, Dominik G. Haider, Christoph M. Domenig, Robin Ristl, Michael Hagmann, Markus Haisjackl, Michael J. Hiesmayr, Andrea Lassnigg.
Project administration: Martin Hermann Bernardi, Andrea Lassnigg.
Resources: Andrea Lassnigg.
Software: Robin Ristl, Michael Hagmann.
Supervision: Michael J. Hiesmayr, Andrea Lassnigg.
Validation: Martin Hermann Bernardi, Dominik G. Haider, Christoph M. Domenig, Robin Ristl, Michael Hagmann, Markus Haisjackl, Michael J. Hiesmayr, Andrea Lassnigg.
Visualization: Martin Hermann Bernardi, Robin Ristl.
Writing – original draft: Martin Hermann Bernardi, Markus Haisjackl, Andrea Lassnigg.
Writing – review & editing: Martin Hermann Bernardi, Dominik G. Haider, Christoph M. Domenig, Robin Ristl, Michael Hagmann, Markus Haisjackl, Michael J. Hiesmayr, Andrea Lassnigg.
Supplementary Material
Footnotes
Abbreviations: AAA-OR = abdominal aortic aneurysm – open repair, ACE = angiotensin-converting enzyme, aHTN = arterial hypertension, AKI = acute kidney injury, AoCC = supra- or pararenal aortic cross-clamp, BIC = Bayesian information criterion, BMI = body mass index, bSCr = baseline serum creatinine, CAAD = carotid artery atherosclerotic disease, CAD = coronary artery disease, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, EMA = European Medicines Agency, EVAR = endovascular aortic repair, FFP = fresh frozen plasma, HES = hydroxyethyl starch, HR = hazard ratio, KDIGO = Kidney Disease–Improving Global Outcome, LVEF = left ventricular ejection fraction, OR = odds ratio, PAOD = peripheral artery occlusive disease, PRBC = packed red blood cells, RIFLE = risk injury failure loss end-stage kidney disease, SCr = serum creatinine, TIA = transient ischemic attack.
Departmental funding only was received for this study.
The manuscript was reviewed by a professional English-speaking editor (Oxford Science Editing Ltd.).
Parts of the work were presented in 2015 as a poster at the annual meeting of the European Association of Cardiothoracic Anesthesiologists.
The data that support the findings of this study are available in an anonymized form from the corresponding author on reasonable request and after agreement with the local ethics committee.
Supplemental Digital Content is available for this article.
The authors of this work have nothing to disclose and have no conflicts of interest.
References
- [1].Funk DJ, HayGlass KT, Koulack J, et al. A randomized controlled trial on the effects of goal-directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Crit Care 2015;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Toomtong P, Suksompong S. Intravenous fluids for abdominal aortic surgery. Cochrane Database Syst Rev 2010;CD000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 2013;309:678–88. [DOI] [PubMed] [Google Scholar]
- [4].Saratzis A, Melas N, Mahmood A, et al. Incidence of acute kidney injury (AKI) after endovascular abdominal aortic aneurysm repair (EVAR) and impact on outcome. Eur J Vasc Endovasc Surg 2015;49:534–40. [DOI] [PubMed] [Google Scholar]
- [5].Giles KA, Pomposelli FB, Hamdan AD, et al. Comparison of open and endovascular repair of ruptured abdominal aortic aneurysms from the ACS-NSQ I2005-07. J Endovasc Ther 2009;16:365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kauvar DS, Martin ED, Givens MD. Thirty-day outcomes after elective percutaneous or open endovascular repair of abdominal aortic aneurysms. Ann Vasc Surg 2016;31:46–51. [DOI] [PubMed] [Google Scholar]
- [7].Brooks MJ, Brown LC, Greenhalgh RM. Defining the role of endovascular therapy in the treatment of abdominal aortic aneurysm: results of a prospective randomized trial. Adv Surg 2006;40:191–204. [DOI] [PubMed] [Google Scholar]
- [8].Barrett BJ, Parfrey PS. Clinical practice. Preventing nephropathy induced by contrast medium. N Engl J Med 2006;354:379–86. [DOI] [PubMed] [Google Scholar]
- [9].Boules TN, Stanziale SF, Chomic A, et al. Predictors of diffuse renal microembolization following endovascular repair of abdominal aortic aneurysms. Vascular 2007;15:18–23. [DOI] [PubMed] [Google Scholar]
- [10].Karmacharya J, Parmer SS, Antezana JN, et al. Outcomes of accessory renal artery occlusion during endovascular aneurysm repair. J Vasc Surg 2006;43:8–13. [DOI] [PubMed] [Google Scholar]
- [11].Edrees WK, Lau LL, Young IS, et al. The effect of lower limb ischaemia-reperfusion on intestinal permeability and the systemic inflammatory response. Eur J Vasc Endovasc Surg 2003;25:330–5. [DOI] [PubMed] [Google Scholar]
- [12].Min JJ, Cho HS, Jeon S, et al. Effects of 6% hydroxyethyl starch 130/0.4 on postoperative blood loss and kidney injury in off-pump coronary arterial bypass grafting: a retrospective study. Medicine (Baltimore) 2017;96:e6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124–34. [DOI] [PubMed] [Google Scholar]
- [14].Margraf A, Herter JM, Kuhne K, et al. 6% Hydroxyethyl starch (HES 130/0.4) diminishes glycocalyx degradation and decreases vascular permeability during systemic and pulmonary inflammation in mice. Crit Care 2018;22:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ferraris VA, Brown JR, Despotis GJ, et al. Society of Thoracic Surgeons Blood Conservation Guideline Task F. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944–82. [DOI] [PubMed] [Google Scholar]
- [16].Ferraris VA, Ferraris SP, Saha SP, et al. Society of Thoracic Surgeons Blood Conservation Guideline Task F. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg 2007;835 suppl:S27–86. [DOI] [PubMed] [Google Scholar]
- [17].Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative w. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schwarz G. Estimating the dimension of a model. Ann Statist 1978;6:461–4. [Google Scholar]
- [19].Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC). Psychol Methods 2012;17:228–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kashy BK, Podolyak A, Makarova N, et al. Effect of hydroxyethyl starch on postoperative kidney function in patients having noncardiac surgery. Anesthesiology 2014;121:730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schortgen F, Brochard L. Colloid-induced kidney injury: experimental evidence may help to understand mechanisms. Crit Care 2009;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sossdorf M, Marx S, Schaarschmidt B, et al. HES 130/0.4 impairs haemostasis and stimulates pro-inflammatory blood platelet function. Crit Care 2009;13:R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Raiman M, Mitchell CG, Biccard BM, et al. Comparison of hydroxyethyl starch colloids with crystalloids for surgical patients: a systematic review and meta-analysis. Eur J Anaesthesiol 2016;33:42–8. [DOI] [PubMed] [Google Scholar]
- [24].Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med 2012;367:1901–11. [DOI] [PubMed] [Google Scholar]
- [25].Roberts I, Shakur H, Bellomo R, et al. Hydroxyethyl starch solutions and patient harm. Lancet 2018;391:736. [DOI] [PubMed] [Google Scholar]
- [26].Priebe HJ. Should hydroxyethyl starch be banned? Lancet 2018;392:117–8. [DOI] [PubMed] [Google Scholar]
- [27].Annane D, Fuchs-Buder T, Zoellner C, et al. EMA recommendation to suspend HES is hazardous. Lancet 2018;391:736–8. [DOI] [PubMed] [Google Scholar]
- [28].Kammerer T, Brettner F, Hilferink S, et al. No differences in renal function between balanced 6% hydroxyethyl starch (130/0.4) and 5% albumin for volume replacement therapy in patients undergoing cystectomy: a randomized controlled trial. Anesthesiology 2018;128:67–78. [DOI] [PubMed] [Google Scholar]
- [29].Oh HW, Lee JH, Kim HC, et al. The effect of 6% hydroxyethyl starch (130/0.4) on acute kidney injury in paediatric cardiac surgery: a prospective, randomised trial. Anaesthesia 2018;73:205–15. [DOI] [PubMed] [Google Scholar]
- [30].Joosten A, Delaporte A, Ickx B, et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology 2018;128:55–66. [DOI] [PubMed] [Google Scholar]
- [31].Saratzis A, Nduwayo S, Sarafidis P, et al. Renal function is the main predictor of acute kidney injury after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg 2016;31:52–9. [DOI] [PubMed] [Google Scholar]
- [32].Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of Veterans Health Administration Data. Am J Kidney Dis 2015;67:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].James MT, Grams ME, Woodward M, et al. A meta-analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis 2015;66:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tang Y, Chen J, Huang K, et al. The incidence, risk factors and in-hospital mortality of acute kidney injury in patients after abdominal aortic aneurysm repair surgery. BMC Nephrol 2017;18:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chang DC, Parina RP, Wilson SE. Survival after endovascular vs open aortic aneurysm repairs. JAMA Surg 2015;150:1160–6. [DOI] [PubMed] [Google Scholar]
- [36].Liang NL, Reitz KM, Makaroun MS, et al. Comparable perioperative mortality outcomes in younger patients undergoing elective open and endovascular abdominal aortic aneurysm repair. J Vasc Surg 2018;67:1404–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rothermel LD, Lipman JM. Estimation of blood loss is inaccurate and unreliable. Surgery 2016;160:946–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


