Abstract
The purpose of this study was to explore the diagnostic significance of abdominal sonography (AUS) in infants with Necrotizing enterocolitis (NEC) admitted to a neonatal intensive care unit to better evaluate the ability of AUS to differentiate necrotizing enterocolitis from other intestinal diseases.
All patients diagnosed with NEC at the Department of General Surgery and Neonatal Surgery, Qilu Children‘s Hospital between 1st, Jun, 2010 and 30th, Dec, 2015. The logistic regression analysis and the area under receiver operating characteristic (ROC) curve (AUCs) were also used to identify the sonographic factors for diagnosing NEC.
For the entire cohort of 91 patients, we divided these patients into suspected NEC (n = 35) group and definite NEC (n = 56) group. After adjusting for competing sonographic factors, we identified that thick bowel wall (more than 2.5 mm) (P = .013, OR: 1.246), intramural gas (pneumatosis intestinalis) (P = .002, OR:1.983), portal venous gas (P = .022, OR:1.655) and reduced peristalsis (P = .011, OR:1.667) were independent diagnostic factors associated with NEC. We built a logistic model to diagnose NEC according to the results of multivariable logistic regression analysis. We found the AUROC for thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis), portal venous gas and reduced peristalsis were significantly lower than the AUROC for the logistic model was 0.841 (95% CI: 0.669 to 0.946).
We found that thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis), portal venous gas and reduced peristalsis were independent diagnostic factors associated with NEC. The logistic model was significantly superior to the single sonographic parameter for diagnosing NEC.
Keywords: abdominal sonography, diagnosis, NEC
1. Introduction
Necrotizing enterocolitis (NEC) is the most common and devastating gastrointestinal emergency, and remains one of the dominant cause of death of neonates in neonatal intensive care units (NICU).[1–3] The incidence of NEC varies between 3 and 28%, with an average of 6% to 10% in newborns weighing less than 1500 g.[4,5] High mortality rates among children with NEC are largely due to late diagnosis of early complications, and increasing survival of premature infants with extremely low birth weight who have proportionally increased frequency of severe NEC.[6,7] Many investigators and surgeons developed novel diagnostic methods for this challenging disease and improved strategies for treatment and prevention.[8] Despite these efforts, NEC remains a difficult and elusive disease with the a mortality rate as high as 30%.[9–13]
Traditionally the gold standard for imaging of the neonatal intestine has been the interpretation of the intestinal gas pattern on plain abdominal radiographs. Radiographs are widely available, cheap, can be acquired bedside, and have long been the cornerstone of imaging evaluation in entities such as necrotizing enterocolitis.[14–16] However, the interpretation of abdominal radiographs in infants can be challenging at times and the intestinal gas pattern can be nonspecific.
To date significance of sonographic findings in the diagnosis and prognosis of NEC has been reported in a number of studies.[17–19] Studies have examined the abdominal sonography (AUS) as an adjunctive measure for the diagnosis and management of infants with NEC. AUS is that it enables the direct evaluation of the intestinal wall on grayscale imaging, peristalsis on real-time imaging and bowel-wall perfusion. Moreover, the AUS simultaneously enables evaluation of the peritoneal cavity for free fluid and focal fluid collections, as well as free gas.
The purpose of this study was to explore the diagnostic significance of AUS in infants with NEC admitted to a neonatal intensive care unit to better evaluate the ability of AUS to differentiate necrotizing enterocolitis from other intestinal diseases.
2. Patients and methods
2.1. Patients included
All patients diagnosed with NEC at the Department of General Surgery and Neonatal Surgery, Qilu Children‘s Hospital between 1st, Jun, 2010 and 30th, Dec, 2015. We prospectively analyzed 103 neonates with an early diagnosis of suspected (stage I) or definite (stage II) NEC based on the NEC definition by Walsh and Kliegman.[20] Furthermore, stage II was sub-classified into IIA and IIB according to the systemic signs, radiographic findings and intestinal signs. In brief, patients’ clinical symptoms, radiographic findings, and blood tests were used for NEC classification. After further investigation, 12 patients were excluded from the study because of the incomplete data. Finally, 91 premature neonates were analyzed in this study and we divided the group of neonates into suspected NEC group and definite NEC group.
2.2. Data collection and techniques
It was considered suggestive of NEC, if one or more of the following clinical symptoms were present: increased gastric residual before feeding (20% of feeding volume), marked abdominal distension, absence of bowel sounds, abdominal tenderness with or without discoloration and bloody stool without evidence of rectal fissure. The diagnosis of NEC was confirmed by radiographic findings that demonstrated significant effects in univariate analysis for diagnosis of NEC. After adjusting for competing sonographic evidence including thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis) and portal venous gas, clinical findings were evaluated by the same physician and the same pediatric surgeon throughout the study. Once NEC was suspected, plain abdominal radiography was performed immediately. Patients’ AUS and radiographic images were reviewed, as were their clinical, pathological and laboratory archives. The AUS and radiographic findings were reviewed by 2 board-certified radiologists (a pediatric radiology fellow and a pediatric radiologist with more than 15 years of experience). Both were blinded to the clinical outcome and to the findings of other imaging modalities. Decisions were reached by consensus. Sonographic examination was performed by using PHILIPS EPIQ5, PHILIPS IU Elite, with an abdominal 5 to 12 MHz probe transducer. Gray scale images were used to assess bowel wall for thickening (>2.5 mm) and thinning (< 1.1 mm), pneumatosis intestinalis (PI), portal venous gas (PVG), and intraabdominal fluid. This study was approved by the Ethics Committee of Qilu Children‘s Hospital of Shandong University, Shandong, China. The aim, risks, and possible benefits of the study were explained to the parents, and written informed consent was obtained from each and the date was 1st, Jun, 2010.
2.3. Development of logistic regression model
The independent sonographic factors found to be significantly related to diagnosis of NEC at multivariable logistic analysis in all patients were entered into the logistic model. The sum of the odds rate which impact the regression function was used in the logistic model to predict the diagnosis of preterm neonates with NEC.
2.4. Statistical analysis
Continuous variables were expressed as mean ± SD (standard deviation) and compared using a 2-tailed unpaired Student's t test; categorical variables were compared using χ2 or Fisher analysis. The cut-offs of bowel wall was defined by Youden index shown in receiver-operating characteristic (ROC) curve analysis and by other published reports.[21,22] The diagnostic performance of the thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis), portal venous gas and reduced peristalsis were measured using the area under ROC curve (AUC). AUCs were also used to compare the logistic with other single sonographic parameter for diagnosing NEC using the Hanley and McNeil method.[23] The predictions of all models were expressed in sensitivity, specificity, positive and negative predictive values at a particular cut-off value. A value of P < .05 was considered significant in all the analyses. Statistical analysis of continuous and categorical variables and ROC curve analysis were computed using MedCalcV.11.0.3.0 (MedCalc software, Mariakerke, Belgium).
3. Results
3.1. Description of the study population
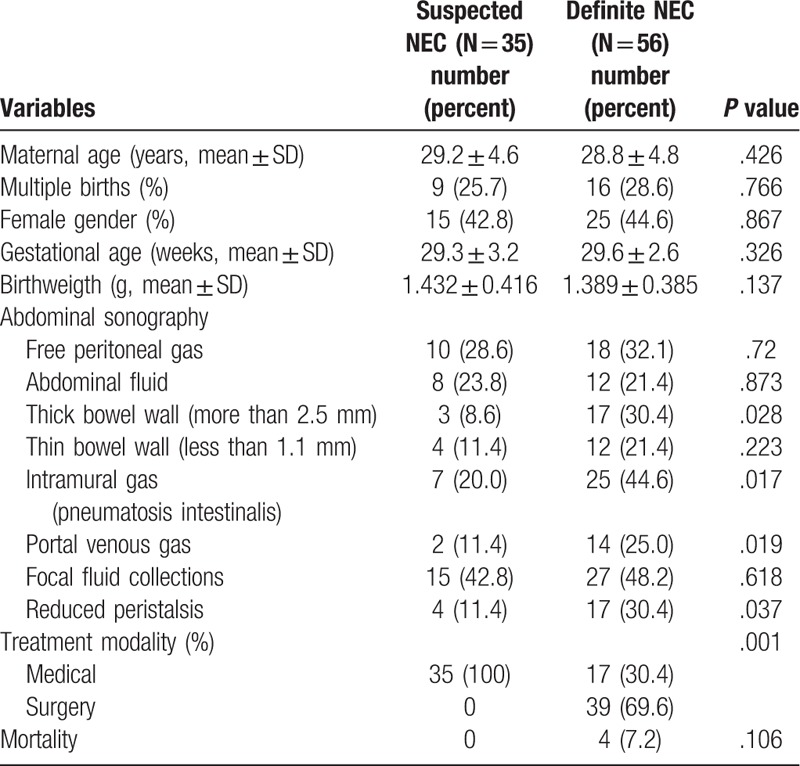
Throughout the study period, 91 preterm neonates were hospitalized with an early diagnosis of suspected NEC (n = 35) or definite NEC (n = 56). We demonstrate AUS findings in Table 1.
Table 1.
Demographic and clinical characteristics of all patients (n = 91).
3.2. Description of abdominal ultrasonography
AUS revealed free peritoneal gas (n = 10), abdominal fluid (n = 8), thick bowel wall (more than 2.5 mm) (n = 3), thin bowel wall (less than 1.1 mm) (n = 4), intramural gas (pneumatosis intestinalis) (n = 7), portal venous gas (n = 2), focal fluid collections (n = 15) and reduced peristalsis (n = 4) in suspected NEC group. While in the definite NEC group, there were free peritoneal gas (n = 18), abdominal fluid (n = 12), thick bowel wall (more than 2.5 mm) (n = 17), thin bowel wall (less than 1.1 mm) (n = 12), intramural gas (pneumatosis intestinalis) (n = 25), portal venous gas (n = 14), focal fluid collections (n = 27) and reduced peristalsis (n = 17). Among these characteristics, there were significant differences of thick bowel wall (more than 2.5 mm) (P = .028), intramural gas (pneumatosis intestinalis) (P = .017), portal venous gas (P = .019) and reduced peristalsis (P = .037) between these 2 groups. Figure 1 illustrates the sonographic findings of different groups of patients.
Figure 1.
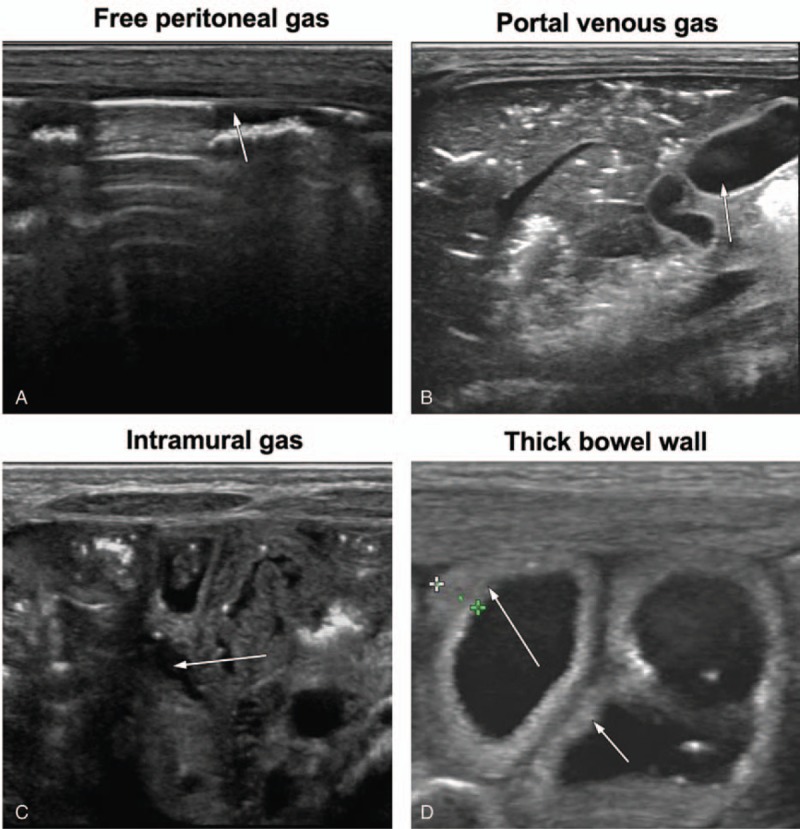
Abdominal sonography scans obtained using a 12 MHz linear tranducer in neonates. The examinations were performed 12 hours after the onset of clinical signs: (A) free peritoneal gas; (B) portal venous gas; (C) intramural gas (pneumatosis intestinalis); (D) thick bowel wall (more than 2.5 mm).
3.3. Diagnostic value of sonography
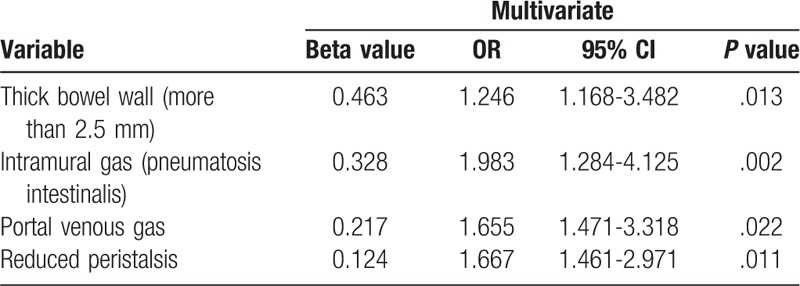
A multivariable logistic regression analysis was performed to assess these sonographic factors that demonstrated significant effects in univariate analysis for diagnosis of NEC. After adjusting for competing sonographic factors, we identified that thick bowel wall (more than 2.5 mm) (P = .013, OR: 1.246), intramural gas (pneumatosis intestinalis) (P = .002, OR:1.983), portal venous gas (P = .022, OR:1.655) and reduced peristalsis (P = .011, OR:1.667) were independent diagnostic factors associated with NEC (Table 2).
Table 2.
Multivariate logistic regression for diagnosis of premature neonates with NEC.
3.4. The role of sonographic parameters in diagnosing NEC
The performance of sonographic parameters to diagnose NEC was measured in term of AUROC. The AUROC for thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis), portal venous gas and reduced peristalsis were 0.595 (95% CI: 0.442 to 0.736) (Fig. 2A), 0.687 (95% CI: 0.500 to 0.839) (Fig. 2B), 0.708 (95% CI: 0.522 to 0.855) (Fig. 2C) and 0.667 (95% CI: 0.479 to 0.823) (Fig. 2D), respectively. Table 3 shows the predictive accuracies of these parameters in diagnosing NEC. Moreover, we built a logistic model to diagnose NEC according to the results of multivariable logistic regression analysis. We found the AUROC for the logistic model was 0.841 (95% CI: 0.669 to 0.946) (Fig. 3), which was significant higher than the single sonographic parameter for diagnosing NEC.
Figure 2.
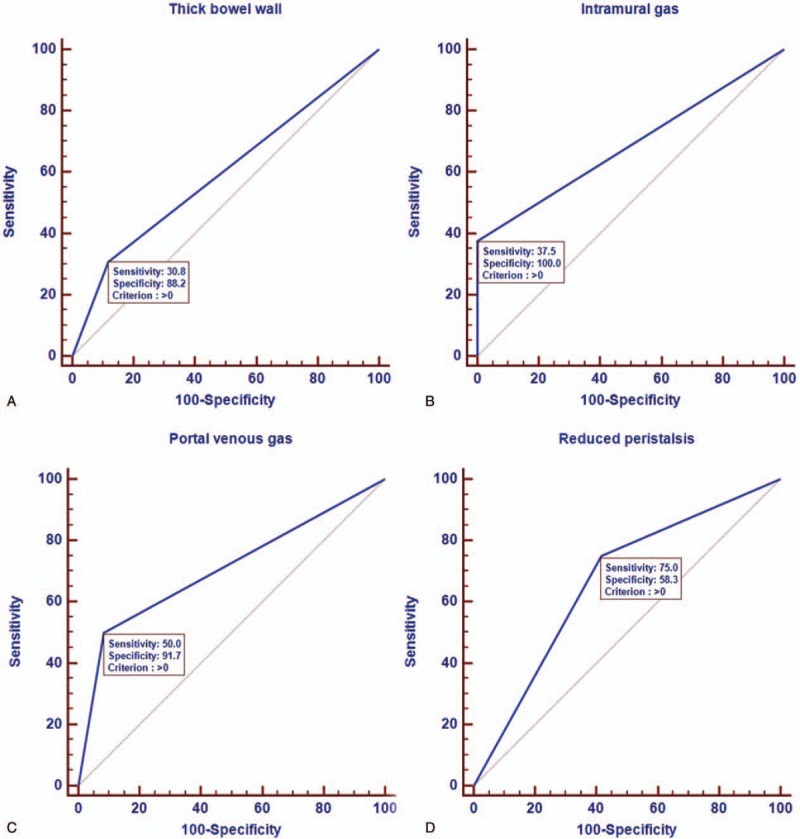
Receiver operating curve of 4 sonographic features seen in neonates with necrotizing enterocolitis. The sonographic parameters were: thick bowel wall (more than 2.5 mm)(A), intramural gas (pneumatosis intestinalis) (B), portal venous gas (C) and reduced peristalsis (D).
Table 3.
Predictive accuracies of AUS characteristics in diagnosing patients with NEC.
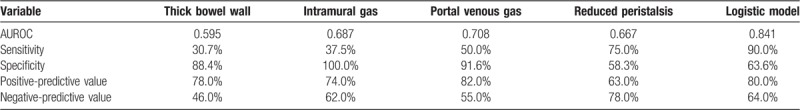
Figure 3.
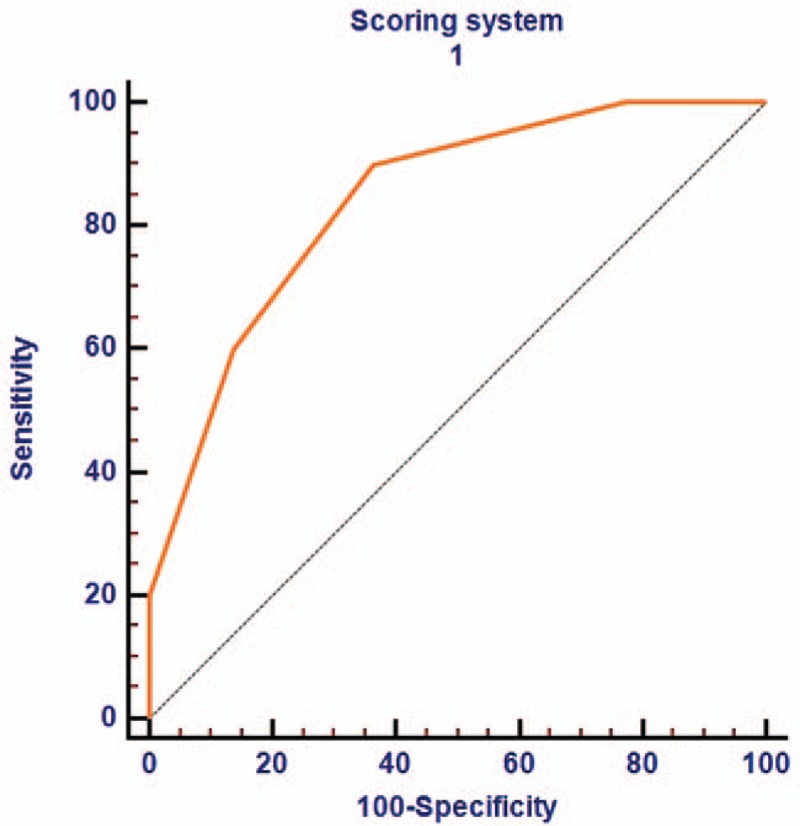
Receiver operating curve of logistic model in neonates for diagnosing necrotizing enterocolitis.
4. Discussion
Necrotising enterocolitis (NEC) is a serious disorder in preterm infants with a mortality of up to 60%. In order to reduce the mortality, early and precise diagnosis and rapid initiation of proper treatment are essential.[24,25] The etiology of NEC is yet incompletely understood, although several fundamental factors in the pathogenesis, such as prematurity, enteral feeding, intestinal colonization by bacteria and bowel ischemia are known.[26] The clinically suspected diagnosis was usually confirmed by abdominal radiography (AR) which was also used for developing a well-known staging system of NEC by Bell.[1] Bell's original classification system, as well as its modified version by Walsh and Kliegman[20] are still widely applied. Although many reports have been published on ultrasound use for diagnosing NEC,[27,28] Plain AR continues to be used as the current standard imaging modality for diagnosing NEC.
Ultrasound offers some potential advantages over plain X-ray films, in that it can depict bowel wall thickness and echogenicity, free and focal fluid collections, peristalsis, and the presence or absence of bowel wall perfusion using Doppler imaging.[19,29] An additional benefit is the absence of ionizing radiation. Recent data suggest that abdominal US can identify or exclude infants with NEC who may need surgery by detecting bowel necrosis (prior to the development of perforation or clinical deterioration) with high sensitivity and specificity. Surgically intervening earlier in the clinical pathway of NEC may lead to improved outcomes.[30] Finally, ultrasound may be useful in helping to decide the appropriate time to re-initiate and advance feeding. Consistent with previous research results, our findings showed that several sonographic factors that demonstrated significant effects in univariate analysis for diagnosis of NEC were independent diagnostic factors after multivariable logistic regression analysis.
The significant usefulness of AUS in the diagnosis of NEC, known since 1984, was reported in a number of studies.[17–19,31] Studies have examined the AUS as an adjunctive measure for the diagnosis and management of infants with NEC. This imaging modality allows for an earlier detection of typical NEC signs, with more rapid disease management. When comparing with the AR in predicting NEC, studies showed that AR can depict bowel distension, to some extent bowel wall thickness, pneumatosis intestinalis (PI) portal venous gas (PVG) and free abdominal air, while AUS could easily depict as well. Moreover, AUS provides important additional information regarding bowel wall viability and free abdominal fluid, which might be helpful in diagnosis and management of NEC.[32,33] The great value of AUS is that it enables the direct evaluation of the intestinal wall on grayscale imaging, peristalsis on real-time imaging and bowel-wall perfusion with Doppler interrogation. Moreover, the AUS simultaneously enables evaluation of the peritoneal cavity for free fluid and focal fluid collections, as well as free gas. It is generally considered a “real-time” and the radiologists could not objectively and accurately evaluate the ultrasound examinations in retrospect. This study identified thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis), portal venous gas and reduced peristalsis were significant in diagnosing NEC. Thickening of bowel wall during NEC occurs due to mucosal hemorrhage and edema, and its finding is proposed to reflect the earliest pathologic feature in NEC prior to the formation of PI. Thickening of the bowel wall usually is accompanied by an increase in echogenicity. Meanwhile, some case reports were published to show that .a thickness below 1.0 mm indicated an abnormal thinning resulting from ischemia or necrosis, while in this study, it showed no independent significance. Intramural gas emerges due to the passage of presence of intramural air, delivered by bacterial fermentation of intestinal contents, into the injured bowel wall. PVG is believed to originate from the absorption of intramural gas into the intestinal venous system travelling into the portal vein thereafter, thus PVG is as early a finding as PI.[34,35] After that, we explored the performance of sonographic parameters to diagnose NEC in term of AUROC. We found the AUROC for the logistic model was 0.841 (95% CI: 0.669 to 0.946), which was significantly higher than the single sonographic parameter for diagnosing NEC.
However, there are limitations of this study:
-
(1)
the sample size is too small in this study, and further larger sample studies are needed to confirm the present results;
-
(2)
whether the logistic model have the optimal specificity and sensitivity for NEC diagnosis and prognosis also needs future confirmation.
In conclusion, we found that thick bowel wall (more than 2.5 mm), intramural gas (pneumatosis intestinalis), portal venous gas and reduced peristalsis were independent diagnostic factors associated with NEC. The logistic model was significant superior to the single sonographic parameter for diagnosing NEC.
Author contributions
Conceptualization: Shuai Chen, Kelai Wang.
Data curation: Shuai Chen, Yuanjun Hu, Xiaoying Li, Aihua Zhang.
Formal analysis: Shuai Chen, Qinghua Liu, Aihua Zhang.
Investigation: Qinghua Liu.
Methodology: Shuai Chen.
Project administration: Qinghua Liu.
Resources: Yuanjun Hu, Xiaoying Li.
Software: Yuanjun Hu, Xiaoying Li, Hefeng Wang.
Validation: Hefeng Wang, Aihua Zhang.
Visualization: Hefeng Wang.
Writing – original draft: Shuai Chen, Kelai Wang.
Writing – review & editing: Kelai Wang, Aihua Zhang.
Footnotes
Abbreviations: AUS = abdominal sonography, NEC = Necrotizing enterocolitis, NICU = neonatal intensive care units, PI = pneumatosis intestinalis, PVG = portal venous gas, ROC = receiver operating characteristic.
The authors have no conflicts of interest to disclose.
References
- [1].Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg 1978;187:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Datab Syst Rev. 2014. (4): Cd005496. [DOI] [PubMed] [Google Scholar]
- [3].Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40:27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Uauy RD, Fanaroff AA, Korones SB, et al. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 1991;119:630–8. [DOI] [PubMed] [Google Scholar]
- [5].Sankaran K, Puckett B, Lee DS, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr 2004;39:366–72. [DOI] [PubMed] [Google Scholar]
- [6].Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 2002;110(1 Pt 1):143–51. [DOI] [PubMed] [Google Scholar]
- [7].Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol 2013;40:135–48. [DOI] [PubMed] [Google Scholar]
- [9].Llanos AR, Moss ME, Pinzon MC, et al. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol 2002;16:342–9. [DOI] [PubMed] [Google Scholar]
- [10].Guthrie SO, Gordon PV, Thomas V, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003;23:278–85. [DOI] [PubMed] [Google Scholar]
- [11].Luig M, Lui K. Epidemiology of necrotizing enterocolitis--Part II: Risks and susceptibility of premature infants during the surfactant era: a regional study. J Paediatr Child Health V 41 2005;174–9. [DOI] [PubMed] [Google Scholar]
- [12].Kasivajjula H, Maheshwari A. Pathophysiology and current management of necrotizing enterocolitis. Indian J Pediatr 2014;81:489–97. [DOI] [PubMed] [Google Scholar]
- [13].Henry MC, Moss RL. Current issues in the management of necrotizing enterocolitis. Semin Perinatol 2004;28:221–33. [DOI] [PubMed] [Google Scholar]
- [14].Rehan VK, Seshia MM, Johnston B, et al. Observer variability in interpretation of abdominal radiographs of infants with suspected necrotizing enterocolitis. Clin Pediatr 1999;38:637–43. [DOI] [PubMed] [Google Scholar]
- [15].Baird R, Tessier R, Guilbault MP, et al. Imaging, radiation exposure, and attributable cancer risk for neonates with necrotizing enterocolitis. J Pediatr Surg 2013;48:1000–5. [DOI] [PubMed] [Google Scholar]
- [16].Coursey CA, Hollingsworth CL, Gaca AM, et al. Radiologists’ agreement when using a 10-point scale to report abdominal radiographic findings of necrotizing enterocolitis in neonates and infants. AJR Am J Roentgenol 2008;191:190–7. [DOI] [PubMed] [Google Scholar]
- [17].Raboisson MJ, Huissoud C, Lapointe A, et al. Assessment of uterine artery and aortic isthmus Doppler recordings as predictors of necrotizing enterocolitis. Am J Obstet Gynecol 2012;206:232.e1–6. [DOI] [PubMed] [Google Scholar]
- [18].Akin MA, Yikilmaz A, Gunes T, et al. Quantitative assessment of hepatic blood flow in the diagnosis and management of necrotizing enterocolitis. J Matern Fetal Neonatal Med 2015;28:2160–5. [DOI] [PubMed] [Google Scholar]
- [19].Yikilmaz A, Hall NJ, Daneman A, et al. Prospective evaluation of the impact of sonography on the management and surgical intervention of neonates with necrotizing enterocolitis. Pediatr Surg Int 2014;30:1231–40. [DOI] [PubMed] [Google Scholar]
- [20].Walsh MC, Kliegman RM, Fanaroff AA. Necrotizing enterocolitis: a practitioner's perspective. Pediatr Rev 1988;9:219–26. [DOI] [PubMed] [Google Scholar]
- [21].Shiraki K, Takase K, Tameda Y, et al. A clinical study of lectin-reactive alpha-fetoprotein as an early indicator of hepatocellular carcinoma in the follow-up of cirrhotic patients. Hepatology (Baltimore, Md) 1995;22:802–7. [PubMed] [Google Scholar]
- [22].Tateishi R, Yoshida H, Matsuyama Y, et al. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008;2:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging 1989;29:307–35. [PubMed] [Google Scholar]
- [24].AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2014;9:584–671. [DOI] [PubMed] [Google Scholar]
- [25].Wu SF, Caplan M, Lin HC. Necrotizing enterocolitis: old problem with new hope. Pediatr Neonatol 2012;53:158–63. [DOI] [PubMed] [Google Scholar]
- [26].Morgan JA, Young L, McGuire W. Pathogenesis and prevention of necrotizing enterocolitis. Curr Opin Infect Dis 2011;24:183–9. [DOI] [PubMed] [Google Scholar]
- [27].Faingold R, Daneman A, Tomlinson G, et al. Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology 2005;235:587–94. [DOI] [PubMed] [Google Scholar]
- [28].Aliev MM, Dekhqonboev AA, Yuldashev RZ. Advantages of abdominal ultrasound in the management of infants with necrotizing enterocolitis. Pediatr Surg Int 2017;33:213–6. [DOI] [PubMed] [Google Scholar]
- [29].Silva CT, Daneman A, Navarro OM, et al. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol 2007;37:274–82. [DOI] [PubMed] [Google Scholar]
- [30].Taylor HG, Klein N, Schatschneider C, et al. Predictors of early school age outcomes in very low birth weight children. J Dev Behav Pediatr 1998;19:235–43. [DOI] [PubMed] [Google Scholar]
- [31].Bohnhorst B. Usefulness of abdominal ultrasound in diagnosing necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F445–50. [DOI] [PubMed] [Google Scholar]
- [32].Higashizono K, Yano H, Miyake O, et al. Postoperative pneumatosis intestinalis (PI) and portal venous gas (PVG) may indicate bowel necrosis: a 52-case study. BMC Surg 2016;16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nevins EJ, Moori P, Ward CS, et al. A rare case of ischaemic pneumatosis intestinalis and hepatic portal venous gas in an elderly patient with good outcome following conservative management. Int J Surg Case Rep 2016;25:167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bomelburg T, von Lengerke HJ. Sonographic findings in infants with suspected necrotizing enterocolitis. Eur J Radiol 1992;15:149–53. [DOI] [PubMed] [Google Scholar]
- [35].Lindley S, Mollitt DL, Seibert JJ, et al. Portal vein ultrasonography in the early diagnosis of necrotizing enterocolitis. J Pediatr Surg 1986;21:530–2. [DOI] [PubMed] [Google Scholar]


