Abstract
Background
New approaches are needed to provide care to persons with HIV who do not engage in conventionally organized HIV clinics. The Max Clinic in Seattle, Washington, is a walk-in, incentivized HIV care model located in a public health STD clinic that provides care in collaboration with a comprehensive HIV primary care clinic (the Madison Clinic).
Methods
We compared outcomes in the first 50 patients enrolled in Max Clinic and 100 randomly selected matched Madison Clinic control patients; patients in both groups were virally unsuppressed (viral load [VL] >200 copies/mL) at baseline. The primary outcome was any VL indicating viral suppression (≥1 VL <200 copies/mL) during the 12 months postbaseline. Secondary outcomes were continuous viral suppression (≥2 consecutive suppressed VLs ≥60 days apart) and engagement in care (≥2 medical visits ≥60 days apart). We compared outcomes in the 12 months pre- and postbaseline and used generalized estimating equations to compare changes in Max vs control patients, adjusting for unstable housing, substance use, and psychiatric disorders.
Results
Viral suppression improved in both groups pre-to-post (20% to 82% Max patients; P < .001; and 51% to 65% controls; P = .04), with a larger improvement in Max patients (adjusted relative risk ratio [aRRR], 3.2; 95% confidence interval [CI], 1.8–5.9). Continuous viral suppression and engagement in care increased in both groups but did not differ significantly (continuous viral suppression: aRRR, 1.5; 95% CI, 0.5–5.2; engagement: aRRR, 1.3; 95% CI, 0.9–1.9).
Conclusions
The Max Clinic improved viral suppression among patients with complex medical and social needs.
Keywords: care delivery, high-need patients, HIV care continuum, retention in care, substance use
HIV care and treatment are crucial to reducing morbidity and mortality among people with HIV (PWH), but even with access to care and support services, some PWH are not engaged in care. Several strategies to re-engage out-of-care PWH have been studied, including Data to Care [1], which uses public health surveillance data to guide health department efforts to return patients to care, and a clinic-based patient retracing strategy, which uses clinic data to identify and re-engage patients [2–4]. Although some evaluations have suggested that these strategies are effective in relinking patients to at least 1 medical visit [1, 5–7], controlled studies have shown minimal or no effect on viral suppression [3, 4, 8].
Most re-engagement efforts to date have focused on reconnecting patients to the system of care from which they disengaged, but new approaches are needed to engage the highest-need patients, such as those with co-occurring unstable housing, substance use disorders, and psychiatric disorders [9]. Most clinics funded by the Ryan White HIV/AIDS Program already employ a number of strategies recommended to improve health care delivery for high-need patients [10, 11], and the high level of viral suppression among Ryan White clients (85.9% in 2017) [12] attests to the success of this care system. However, other evidence-based approaches to improve care delivery, such as open access scheduling [13] and financial incentives [14], are not commonly used and could further improve care engagement. Such approaches can be targeted to patients who do not succeed with lower-intensity care and support in order to optimize resource allocation while meeting patient needs [15]. Interventions to improve care and support services for high-need PWH have shown promising outcomes [16, 17], but these could be even more effective if paired with low-barrier clinics tailored to high-need patients.
The Max Clinic in Seattle, Washington, is designed for PWH who are poorly engaged in standard HIV care despite case management services and outreach support. The clinic includes walk-in visits for primary care, incentives for completing visits and achieving viral suppression, and intensive case management support. We developed the clinic in the context of Seattle–King County reaching the UNAIDS 90-90-90 goals in 2015 and in the wake of implementing 2 HIV care re-engagement interventions that had no substantial impact on viral suppression [3, 8]. We reasoned that success in achieving additional improvements in population-level viral suppression would require focusing on the individuals with the most difficulty engaging in care and require structural changes in how our area provides HIV care. The clinic is operated in collaboration between Public Health–Seattle & King County, the Washington State Department of Health, and the Harborview Medical Center (HMC) Madison Clinic, a Ryan White Part C–funded clinic. We previously reported promising outcomes for patients enrolled in the first 2 years [18]. Here we present a retrospective cohort study comparing HIV outcomes among the first 50 patients enrolled in the Max Clinic and 100 contemporary control patients receiving care at the Madison Clinic.
METHODS
Study Population and Design
Criteria for patients to enroll in the Max Clinic are (1) not taking antiretroviral therapy or virally unsuppressed at the time of last viral load (VL) measurement (≥200 cells/mL); (2) poorly engaged in HIV care (multiple no-shows or no visits in the past year); and (3) failed to re-engage in care after outreach attempts from the clinic and/or the health department. Patients are identified through public health outreach programs or referred by case managers, medical providers, jail release planners, or peers.
The control population for this study includes Madison Clinic patients identified retrospectively who met eligibility for Max Clinic enrollment but did not enroll. The Madison Clinic is a comprehensive HIV primary care clinic with on-site medical case management and a pharmacy that is located in a separate building on the same medical center campus as the Max Clinic. We randomly matched control patients to Max Clinic patients in a 2:1 ratio, based on the enrollment date of the Max Clinic patient (“baseline date”), which was defined as the date of the patient’s first completed visit with a medical provider. Control patients completed ≥1 visit with a medical provider in the Madison Clinic within 2 months of the corresponding Max Clinic patient’s enrollment date, were virally unsuppressed at the time of that visit, and in the 12 months before that visit had either no completed visits or multiple no-shows. We included the requirement for ≥1 visit in the Madison Clinic around the time of the matched Max Clinic patients’ enrolment, as many patients with no visits in the prior year had likely moved out of the area or were receiving care elsewhere. It was not feasible to match patients on additional characteristics such as race and gender for this analysis due to the limited number of virally unsuppressed patients in the Madison Clinic (10% of approximately 3000 patients).
Intervention
The components of care at the Max Clinic that differentiate it from standard care are summarized in Table 1. The methods and implementation of the clinic are described in more detail elsewhere [18]. Patients do not always see the same doctor but can choose to attend clinic on a certain day to see a specific provider. The nonmedical case managers are health department disease intervention specialists, front-line public health staff skilled in counseling and care coordination for persons with or at risk for HIV and other sexually transmitted infections. The medical case managers are Master’s-level social workers with HIV-specific training.
Table 1.
Components of the Max Clinic That Differ From the Standard-of-Care Clinic Approach
| Low-barrier access | • Walk-in access to medical care 5 afternoons per week |
| • Walk-in access to medical and nonmedical case management 5 days per week | |
| • Text message and direct phone access to case managers | |
| High-intensity support | • Case managers provide care coordination, navigation, and supporta |
| • Medical case managers have a low case load (~50 patients) compared with standard of care (~150 patients) | |
| Incentives | • Food vouchers worth $10 up to once weekly |
| • Snacks available at each visit | |
| • No-cost bus passes to provide unrestricted transportation support | |
| • Cell phonesb | |
| • Cash incentives for visits with blood drawsc | |
| • Cash incentives for viral suppressiond (HIV RNA < 200 copies/mL) | |
| Intensified care coordination | Case managers serve as primary contacts for patients, providers, and for coordination between Max Clinic and other agencies, including: |
| • Release planning team in King County jails | |
| • Housing and mental health case management agencies | |
| • Day program with medication adherence support | |
| • Office-based opioid treatment nurse managers and methadone providers | |
| Transitional care coordination | • Staff receive automated alerts when patients are seen in the emergency room or admitted to a hospital in the University of Washington Medicine system |
| • Max Clinic staff work with inpatient medical teams to plan transition to outpatient care and day-of-discharge Max Clinic visit |
aPublic health disease intervention specialists who specialize in HIV care re-engagement.
bPatients received cell phones if needed only in the first 2 years of the intervention.
cDuring the period of this analysis: $50 up to once every 2 months; at the time of this report: $25 up to every 2 months.
dDuring the period of this analysis: $100 up to once every 2 months and a 1-time $100 bonus for the third consecutive suppressed viral load; at the time of this report: $50 up to once every 2 months.
Outcomes
We calculated each outcome as the percentage of patients in each group who achieved the metric during the 12 months postbaseline. The primary outcome was viral suppression, defined as ≥1 VL result <200 copies/mL at any time during the 12-month analysis period. Secondary outcomes were continuous viral suppression, defined as ≥2 consecutive suppressed VL results ≥60 days apart, and care engagement, defined as completing ≥2 visits with a medical provider ≥60 days apart. The criteria for a gap of ≥2 months between visits and VL results is consistent with the Department of Health and Human Services consensus metric for engagement in care and the Max Clinic policy of incentivizing patients for bimonthly medical provider visits and VL measurement [19]. The patients who reached continuous viral suppression were a subset of those who reached viral suppression at least once. Patients who did not have a second VL measured after the first suppressed VL or a second completed visit were categorized as not achieving continuous viral suppression.
We compared the outcomes in the 12 months postbaseline with the 12 months prebaseline. We reasoned that suppressed VL ascertained in the first 2 weeks after enrollment reflected antiretroviral adherence before enrollment. Thus, for viral suppression in both groups, we defined the prebaseline period as the 365 days before 14 days after the Max Clinic enrollment date and the postbaseline period as the 15–365 days after enrollment (matched visit date for controls). For care engagement, the prebaseline period was 1–365 days before enrollment/matched visit date and the postbaseline period was 0–365 days after enrollment/matched visit date. Although patients were virally unsuppressed at baseline, some had previously been virally suppressed during the prior year.
Sample Size
Our decision to conduct this analysis with the first 50 Max Clinic patients was an a priori analysis plan formulated at the time of the Max Clinic’s inception. We estimated study power before the analysis. Assuming a sample of 50 Max patients and 100 control patients, a type I error rate of 5%, and 90% power, this study was powered to detect a 29% absolute increase in proportion of Max patients who achieved viral suppression compared with control patients.
Data Collection
All study measures were ascertained using data from the electronic health record (EHR), which the Max Clinic shares with the Madison Clinic. We defined the baseline CD4 count and the baseline VL as the last value within the 12 months prebaseline, or for those missing data in the past year, the first value within 14 days of the Max Clinic enrollment date. We conducted a manual chart review of all individuals in the analysis for the following factors because the information was not readily extractable from the EHR: current gender identity, HIV risk factor, substance use, housing status, and history of incarceration.
We categorized HIV transmission risk factors using CDC surveillance definitions. We categorized substance use into mutually exclusive categories, as described by Tegger and colleagues [20] and adapted by Hartzler and colleagues [21]. Because methamphetamine use is more common than opioid use among Max Clinic patients [18] and is associated with poor care continuum outcomes in King County [22], we began the hierarchical categorization with methamphetamine. The categories are shown in Table 2. We categorized psychiatric diagnoses according to the hierarchy validated by Tegger and colleagues [20]. We categorized housing status as stable, transient or unstable, homeless and sleeping in a shelter, or homeless and sleeping outside. History of incarceration included both jail and prison.
Table 2.
Characteristics of Patients Enrolled in the Max Clinic (n = 50) and Standard-of-Care Controls (n = 100)
| Max Clinic (n = 50) | Standard-of-Care Controls (n = 100) | ||
|---|---|---|---|
| No. (%) | No. (%) | P Value | |
| Gender | |||
| Female | 11 (22) | 28 (28) | .073 |
| Male | 36 (72) | 71 (71) | |
| Transgender, genderqueer, or nonbinary | 3 (6) | 0 (0) | |
| Unknown | 0 (0) | 1 (1) | |
| Age, mean (SD), y | 41 (10) | 44 (10) | .042 |
| Race/ethnicity | |||
| White, non-Hispanic | 27 (54) | 44 (44) | .041 |
| Black, non-Hispanic | 12 (24) | 36 (36) | |
| Asian or Pacific Islander | 4 (8) | 1 (1) | |
| American Indian or Alaska Native | 1 (2) | 4 (4) | |
| Multiple | 6 (12) | 8 (8) | |
| Missing | 0 (0) | 7 (7) | |
| Hispanic ethnicity | 3 (6) | 17 (17) | .062 |
| HIV risk factor at the time of HIV diagnosisa | |||
| MSM/IDU | 22 (44) | 19 (19) | .001 |
| IDU (non-MSM) | 12 (24) | 13 (13) | |
| MSM (non-IDU) | 6 (12) | 30 (30) | |
| Heterosexual/presumed heterosexual | 8 (16) | 28 (28) | |
| Unknown | 2 (4) | 10 (10) | |
| Baseline CD4 count, cells/mm3b | |||
| <200 | 28 (56) | 40 (40) | .010 |
| 200–500 | 12 (24) | 43 (43) | |
| >500 | 7 (14) | 17 (17) | |
| No documented tests before enrollment | 3 (6) | 0 (0) | |
| Baseline HIV RNA, median (IQR), copies/mL | 22 695 (4055–122 150) | 1649 (83–23 270) | <.001 |
| Substance use documented in medical recordc | |||
| Methamphetamine (+/- opioids or others) | 29 (58) | 40 (40) | .063 |
| Opioids (+/- crack cocaine, unhealthy alcohol use) | 6 (12) | 8 (8) | |
| Cocaine/crack cocaine (+/- unhealthy alcohol use) | 5 (10) | 10 (10) | |
| Unhealthy alcohol use (+/- marijuana) | 5 (10) | 12 (12) | |
| Marijuana only | 2 (4) | 3 (3) | |
| None of the above | 3 (6) | 27 (27) | |
| Psychiatric illnessc | |||
| Psychotic, bipolar, or personality disorder (+/- depression/anxiety) | 16 (32) | 26 (26) | .682 |
| Depression or anxiety disorder | 23 (46) | 47 (47) | |
| None of the above | 11 (22) | 27 (27) | |
| Housing documented in medical recordc | |||
| Stable | 18 (36) | 61 (61) | <.001 |
| Transient/unstable | 6 (12) | 22 (22) | |
| Homeless, sleeping in a shelter | 7 (14) | 8 (8) | |
| Homeless, sleeping outside | 19 (38) | 6 (6) | |
| Unknown | 0 (0) | 3 (3) | |
| History of incarceration documented in medical record | 34 (68) | 31 (31) | <.001 |
Abbreviations: IDU, Injection drug user; IQR, interquartile range; MSM, man who has sex with men.
aDefined according to Centers for Disease Control and Prevention surveillance criteria: IDU, MSM.
bUp to 14 days postenrollment.
cDocumented in the medical record (including case management notes) before or on the date of Max Clinic enrollment (or, for controls, the date of the matched Max Clinic patient enrollment).
dIncludes medical motel, staying with friends, “couch surfing.”
Statistical Analysis
The demographics of Max and Madison Clinic patients were compared using a chi-square test for a difference in proportions. This evaluation included 3 primary analyses. The first compared study outcomes in the 12 months postbaseline between Max Clinic and standard-of-care control patients using a chi-square test for a difference in proportions. The second compared study outcomes within each group pre- vs postenrollment using a McNemar chi-square test accounting for repeated measures to assess statistical significance. The third analysis compared the change in post- vs prebaseline study outcomes among Max Clinic patients with the pre–post change in control patients. This analysis adjusted for housing status, substance use, and psychiatric diagnoses. We identified these factors as covariates a priori, based on published literature [23, 24]. We used generalized estimating equations (GEE) to calculate the relative risk of each outcome in the post- vs prebaseline periods within each analysis group (“within-group pre–post comparison”). We also used GEE to calculate the adjusted relative risk ratios comparing pre–post changes between the 2 groups (“between-group pre–post comparison”). The analysis did not account for matching, as the only component matched was time at enrollment and sensitivity analyses demonstrated no effect of matching. We conducted a post hoc analysis to determine the effect of incorporating race/ethnicity, gender, age, baseline CD4 count, and baseline VL into the adjusted models.
All statistical analyses occurred in Stata, version 12.1 (StataCorp, College Station, TX). The University of Washington Human Subjects Division approved this study.
RESULTS
The first 50 Max Clinic patients enrolled between December 2014 and November 2015. As shown in Table 2, most were male (72%). Compared with the control patients, the Max patients were younger (mean age, 41 vs 44 years; P = .04) and differed in race distribution (P = .04). Max patients were more likely to have CD4 counts <200 copies/mL or no CD4 count in the past year (62% vs 40%; P = .001) and higher baseline VLs (median, 22 695 vs 1649 copies/mL; P = .01).
Although the overall distribution of substance use did not vary significantly, Max Clinic patients were more likely than control patients to report illicit stimulant (methamphetamine or cocaine/crack-cocaine) and opioid use (80% vs 58%; P = .008) and to have injection drug use as an HIV risk factor (68% vs 32%; <0.001). Diagnosed psychiatric illness did not differ significantly between the 2 groups. Compared with control patients, Max Clinic patients were less likely to have stable housing (36% vs 61%) and more likely to have a documented history of incarceration (68% vs 31%; P < .001).
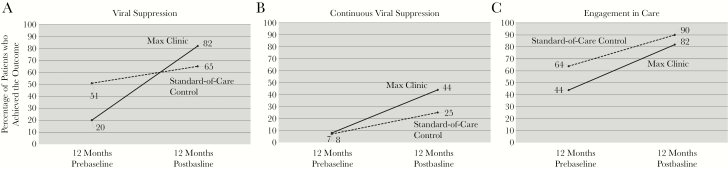
The percentages of patients in each group who achieved each outcome in the 12 months prebaseline and postbaseline are shown in Figure 1. In the year after enrollment, 41 (82%) Max Clinic patients and 65 (65%) patients in the standard-of-care control group achieved viral suppression. Viral suppression in the postenrollment year was significantly higher compared with the pre-enrollment year among both Max Clinic patients (82% vs 20%; P < .001) and standard-of-care control patients (65% vs 51%; P = .04). Among the 50 Max Clinic patients, 22 (44%) reached and maintained continuous viral suppression, 17 (34%) reached viral suppression but did not maintain it (ie, had an unsuppressed VL after the first suppressed VL), 9 (18%) did not reach viral suppression, and 2 (4%) reached suppression but did not have a subsequent VL in the analysis period. Of the 17 who did not maintain continuous viral suppression after reaching it once, 12 (71%) were virally suppressed again at the time of last VL measurement during the analysis period. The level of continuous viral suppression increased significantly after Max Clinic enrollment (8% vs 44%; P < .001). The majority of Max patients engaged in care in the year after enrollment, which was significantly higher than prebaseline engagement (82% vs 44%; P = .011). Among control patients, continuous viral suppression also increased (7% to 25%; P = .002), as did engagement in care (44% to 82%; P < .001).
Figure 1. .
HIV care outcomes among patients enrolled in the Max Clinic (n = 50) and standard-of-care controls (n = 100) in the 12 months pre- and postbaseline.
A, Viral suppression (≥1 HIV RNA result <200 cells/mL). B, Continuous viral suppression (≥2 consecutive HIV RNA results <200 cells/mL ≥2 months apart). C, Care engagement (≥2 visits ≥60 days apart). aWithin-group pre–post comparison, McNemar chi-square test: P < .05. bBetween-group comparison of 12-month postbaseline outcomes, chi-square test: P < .05. Abbreviation: NS, not statistically significant (P > .05).
Table 3 shows the relative risks for within-group comparisons and the adjusted relative risk ratios (aRRRs) for between-group comparisons, adjusted for housing status, substance use, and psychiatric diagnoses. Max Clinic patients had more improvement in viral suppression than control patients (aRRR, 3.2; 95% confidence interval [CI], 1.8–5.9), but the comparative changes were not statistically significant for continuous viral suppression (aRRR, 1.5; 95% CI, 0.5–5.2) or engagement in care (aRRR, 1.3; 95% CI, 0.9–1.9). Additional analyses adjusting for race/ethnicity, gender, age, baseline CD4 count, and baseline VL did not significantly change the point estimates or statistical significance of the results.
Table 3.
Within-Group Comparisons of 12-Month Postbaseline HIV Outcomes Compared With 12-Month Prevaseline Outcomes and Between-Group Comparisons (Max Clinic vs Standard-of-Care Controls) of Pre–Post Changes in HIV Outcomes, Adjusted for Housing, Substance Use, and Psychiatric Diagnoses
| Within Group Pre–Post Comparison | Between Group Pre–Post Comparison | ||
|---|---|---|---|
| Max Clinic (n = 50) | Standard-of-Care Controls (n = 100) | ||
| Clinical outcome | RR (95% CI) | RR (95% CI) | aRRR (95% CI) |
| Viral suppressiona | 4.1 (2.3–7.2) | 1.3 (1.0–1.6) | 3.2 (1.8–5.9) |
| Continuous viral suppressionb | 5.5 (2.2–14.0) | 3.6 (1.6–7.8) | 1.5 (0.5–5.2) |
| Engagement in carec | 1.9 (1.3–2.6) | 1.4 (1.2–1.6) | 1.3 (0.9–1.9) |
Abbreviations: aRRR, adjusted relative risk ratio; CI, confidence interval; RR, relative risk.
aAt least 1 HIV RNA result <200 cells/mL.
bAt least 2 consecutive HIV RNA results <200 cells/mL ≥2 months apart.
cAt least 2 visits ≥60 days apart.
DISCUSSION
In this retrospective cohort study, we found that patients who enrolled in the Max Clinic were substantially more likely to achieve viral suppression compared with patients marginally engaged in a more conventionally organized Ryan White–funded clinic. Although fewer Max patients had ≥1 suppressed VL in the prebaseline year compared with control patients (20% vs 51%), more achieved suppression in the postbaseline year (82% vs 65%). Adjusted for differences in unstable housing, substance use, and psychiatric diagnoses, Max Clinic patients were >3 times as likely to achieve viral suppression. Max Clinic patients also had substantial improvements in continuous viral suppression and engagement in care, but these did not differ significantly from improvements in control patients.
The published literature contains relatively little with which to compare our results. Several approaches to modify structures of medical care for high-needs patients have been described, but these have been primarily outside of the HIV care setting [25–27]. Our results are generally consistent with findings from those studies of improved clinical outcomes with reduced barriers to care access and addressing nonmedical needs such as food and shelter in addition to medical care. Ongoing efforts to provide low-barrier HIV care tailored to high-need patients in Vancouver, British Columbia, San Francisco, California, and Detroit, Michigan (authors’ personal experience) suggest that the dearth of peer-reviewed literature on this topic does not reflect current real-world practice. Previous studies have described alternate approaches to improving care engagement, specifically among high-need PWH, in cooperation between the health department and HIV care providers, such as projects in Los Angeles [17] and New York City [16, 28, 29], but these did not include low-barrier clinics or financial incentives. The concept of the Max Clinic is consistent with the idea of differentiated HIV care, which aligns resources with the level of patient need and is under study in Sub-Saharan Africa [15] but has been understudied in the United States to date.
The key implication of our findings is that an alternate structure of HIV care can improve outcomes in hard-to-reach patients. We cannot determine which components of our intervention were responsible for the effect observed, as we implemented it as a multicomponent intervention. Our impression, based on experience working with patients and the clinic’s staff, is that 3 elements of a clinic for high-need patients are essential: walk-in primary care visits, some type of incentive to encourage care attendance (not necessarily cash), and intensive case management. None of these components of the intervention would likely have had the magnitude of effect we observed if implemented in isolation.
Our findings confirm longstanding clinical experience that maintaining viral suppression over time is more difficult for patients than achieving it once. Cycling in and out of viral suppression was common among patients in the Max Clinic. Long-acting antiretroviral medications could help patients maintain viral suppression, and the high levels of patient engagement in the clinic would support this intervention. Our finding that engagement in care did not improve in Max patients more than in control patients reflects both the high levels of engagement among control patients and the way we measured engagement, using the standard metric of completed provider visits. Most patient encounters in the Max Clinic involve medical and nonmedical case managers, not physicians, including a once-weekly meal voucher and a visit with the Max team. For example, in the first quarter of 2019, with approximately 160 patients actively enrolled in the Max Clinic, provider visits accounted for only 17% (180 of 1029) of the total encounters.
This study leads to additional questions about the Max Clinic approach. Foremost among them is the cost-effectiveness and scalability of this strategy. We have not yet analyzed cost data, but we expect that the Max Clinic has a relatively high cost per patient enrolled. For those inclined to dismiss our approach as achievable only in relatively resource-rich environments, we urge consideration of several points. First, it is possible that low-intensity interventions simply do not work for some populations. Our prior, less intensive, Data to Care activities yielded minimal or no effect, so they could not have been cost-effective [3, 8]. Second, the Max Clinic may have been associated with savings elsewhere in the health care system and thus be more cost-effective than is immediately apparent. Third, it may be possible to implement care strategies like Max Clinic as part of broader changes in HIV care delivery that reduce care intensity for stable, low-need patients, lessening the burden on the health care system and rationalizing the use of existing health care resources. Fourth, although the Max Clinic approach in its entirety may not be exportable to all areas, the basic elements of the strategy could be adapted to a variety of settings depending on local resources. Finally, the high-intensity intervention we describe must be viewed the context of efforts to end the HIV/AIDS epidemic. Though success is uneven, the United States is making significant progress in the fight against HIV. In places like Seattle, where 85% of diagnosed PWH are virally suppressed [30], achieving the next increment of progress will not come easily. An effective response to control and prevent HIV will inevitably involve investing more to improve outcomes in harder-to-reach patients.
The primary strength of our study is inclusion of a control population. However, the study was not randomized, and Max Clinic enrollment depended on clinician and case manager decisions to refer patients. The control population was less complex, with lower levels of unstable housing and illicit opioids and stimulant use. As these characteristics are typically associated with poor clinical outcomes [23, 24], these differences should have biased our study toward the null, and we might have underestimated the effect size. Our reliance on EHR to ascertain some cofactors may have led us to underestimate some variables, or conversely, we may have differentially ascertained factors such as homelessness, substance use, and incarceration among Max Clinic patients. We did not collect data on the date of HIV diagnosis or no-show visits during the pre-intervention period. Conducting this analysis with the first 50 patients enrolled may have biased the study to overestimate the effect of the intervention if those enrolled earlier had less complex barriers and were more likely to succeed than patients who enrolled later. Finally, our study was performed in 1 geographic area with a relatively well-resourced HIV care and prevention system, making generalizability uncertain. In this study of a real-world programmatic intervention, we were limited in our options for identifying a control population by the retrospective data available for this analysis and the relatively small size of the virally unsuppressed population in the Madison Clinic. Our study provides evidence that the walk-in, incentivized clinic approach is effective, but prospective studies in areas outside of Seattle are necessary to determine the true effect of this type of intervention.
In summary, we found that patients who enrolled in a walk-in, incentivized HIV clinic were more likely to achieve viral suppression than control patients enrolled in the standard-of-care clinic. The walk-in, incentivized care approach needs further study, but our findings support the idea that clinicians, public health leaders, and HIV clinic administrators need to consider how the HIV medical system—including that system’s interaction with health departments—might be modified to meet the needs of the most difficult-to-treat patients.
Acknowledgments
The authors would like to acknowledge Chris Bell for data management assistance, the University of Washington/Fred Hutch Center for AIDS Research Biostatistics Core, Megan Touhey for chart review, other members of the Max Clinic and HIV care relinkage team (Rachel Patrick, Mark Fleming, Angela Nunez, Courtney Large, Mike Nicholson, Katherine Lincicum, Xico Ceballos, Andy Duarte, Nordia Shackelford), and our many collaborators in Seattle & King County working with Max Clinic patients. We would also like to acknowledge the Seattle Transitional Grant Area (TGA) Planning Council, the Ryan White Part A & B programs in Seattle & Washington State, and the WA State Department of Health, and Madison Clinic and pharmacy leadership (Pegi Fina, Gwen Barker, Reid Branson, Ji Lee, Bob Loeffelbein), who have been instrumental in the Max Clinic.
Financial support. This work was supported by a grant to J.C.D. from the National Institute on Drug Abuse (R03 DA 042668); by the University of Washington Center for AIDS Research (CFAR), an National Institutes of Health–funded program (P30AI027757); and by a CFAR supplement grant (P30 AI 027757-28S1).
Potential conflicts of interest. J.C.D. and M.R.G. have participated in research supported by grants to the University of Washington from Hologic. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Sweeney P, Hoyte T, Mulatu MS, et al. . Implementing a data to care strategy to improve health outcomes for people with HIV: a report from the care and prevention in the United States demonstration project. Public Health Rep 2018; 133:60S–74S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sitapati AM, Limneos J, Bonet-Vázquez M, et al. . Retention: building a patient-centered medical home in HIV primary care through PUFF (Patients Unable to Follow-up Found). J Health Care Poor Underserved 2012; 23:81–95. [DOI] [PubMed] [Google Scholar]
- 3. Bove JM, Golden MR, Dhanireddy S, et al. . Outcomes of a clinic-based surveillance-informed intervention to relink patients to HIV care. J Acquir Immune Defic Syndr 2015; 70:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bershetyn A, Odeny TA, Lyamuya R, et al. ; East Africa International Epidemiologic Databases to Evaluate AIDS (EA-IeDEA) Consortium The causal effect of tracing by peer health workers on return to clinic among patients who were lost to follow-up from antiretroviral therapy in Eastern Africa: a “Natural Experiment” arising from surveillance of lost patients. Clin Infect Dis 2017; 64:1547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hart-Malloy R, Brown S, Bogucki K, Tesoriero J. Implementing data-to-care initiatives for HIV in New York state: assessing the value of community health centers identifying persons out of care for health department follow-up. AIDS Care 2018; 30:391–6. [DOI] [PubMed] [Google Scholar]
- 6. Tesoriero JM, Johnson BL, Hart-Malloy R, et al. . Improving retention in HIV care through New York’s expanded partner services data-to-care pilot. J Public Health Manag Pract 2017; 23:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchacz K, Chen MJ, Parisi MK, et al. . Using HIV surveillance registry data to re-link persons to care: the RSVP project in San Francisco. PLoS One 2015; 10:e0118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dombrowski JC, Hughes JP, Buskin SE, et al. . A Cluster randomized evaluation of a health department data to care intervention designed to increase engagement in HIV care and antiretroviral use. Sex Transm Dis 2018; 45:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teasdale CA, Yuengling K, Preko P, et al. . Persons living with HIV with advanced HIV disease: need for novel care models. J Int AIDS Soc 2018; 21:e25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shaw S, Modi R, Mugavero M, et al. . HIV standard of care for ART adherence and retention in care among HIV medical care providers across four CNICS clinics in the US. AIDS Behav. 2019; 23:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Figueroa JF, Jha AK. Approach for achieving effective care for high-need patients. JAMA Intern Med 2018; 178:845–6. [DOI] [PubMed] [Google Scholar]
- 12. Health Resources and Services Administration. Ryan White HIV/AIDS Program annual client-level data report 2017. 2018. Available at: https://hab.hrsa.gov/sites/default/files/hab/data/datareports/RWHAP-annual-client-level-data-report-2017.pdf. Accessed 12 February 2019. [Google Scholar]
- 13. Ansell D, Crispo JAG, Simard B, Bjerre LM. Interventions to reduce wait times for primary care appointments: a systematic review. BMC Health Serv Res 2017; 17:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Sadr WM, Donnell D, Beauchamp G, et al. ; HPTN 065 Study Team Financial incentives for linkage to care and viral suppression among HIV-positive patients: a randomized clinical trial (HPTN 065). JAMA Intern Med 2017; 177:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barnabas RV, Celum C. Closing the gaps in the HIV care continuum. PLoS Med 2017; 14:e1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irvine MK, Chamberlin SA, Robbins RS, et al. . Come as you are: improving care engagement and viral load suppression among HIV care coordination clients with lower mental health functioning, unstable housing, and hard drug use. AIDS Behav 2017; 21:1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garland WH, Oksuzyan S, Mejia M, Kulkarni S.. Medical care coordination services for persons living with HIV in Los Angeles County: a robust strategy to strengthen the HIV care continuum. Los Angeles County Department of Public Health. Available at: http://publichealth.lacounty.gov/dhsp/Reports/HIV/MCC_Year-1_EvaluationReport-FINAL.pdf. Accessed 12 February 2019. [Google Scholar]
- 18. Dombrowski JC, Ramchandani M, Dhanireddy S, et al. . The Max Clinic: medical care designed to engage the hardest-to-reach persons living with HIV in Seattle and King County, Washington. AIDS Patient Care STDS 2018; 32:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep 2013; 128:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tegger MK, Crane HM, Tapia KA, et al. . The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS 2008; 22:233–43. [DOI] [PubMed] [Google Scholar]
- 21. Hartzler B, Dombrowski JC, Williams JR, et al. . Influence of substance use disorders on 2-year HIV care retention in the United States. AIDS Behav 2018; 22:742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hood JE, Buskin SE, Golden MR, et al. . The changing burden of HIV attributable to methamphetamine among men who have sex with men in King County, Washington. AIDS Patient Care STDS 2018; 32:223–33. [DOI] [PubMed] [Google Scholar]
- 23. Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of adult retention in HIV care: a systematic review. AIDS Behav 2018; 22:752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aidala AA, Wilson MG, Shubert V, et al. . Housing status, medical care, and health outcomes among people living with HIV/AIDS: a systematic review. Am J Public Health 2016; 106:e1–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Academy of Medicine. Effective care for high-need patients. Available at: https://nam.edu/wp-content/uploads/2017/06/Effective-Care-for-High-Need-Patients.pdf. Accessed 12 February 2019. [Google Scholar]
- 26. O’Toole TP, Johnson EE, Aiello R, et al. . Tailoring care to vulnerable populations by incorporating social determinants of health: the Veterans Health Administration’s “Homeless Patient Aligned Care Team” program. Prev Chronic Dis 2016; 13:E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCarthy D, Ryan J, Klein S. Models of care for high-need, high-cost patients: an evidence synthesis. Issue Brief (Commonw Fund) 2015. Available at: https://www.commonwealthfund.org/publications/issue-briefs/2015/oct/models-care-high-need-high-cost-patients-evidence-synthesis. Accessed 12 February 2019 [PubMed] [Google Scholar]
- 28. Robertson MM, Waldron L, Robbins RS, et al. . Using registry data to construct a comparison group for programmatic effectiveness evaluation: The New York City HIV care coordination program. Am J Epidemiol 2018; 187:1980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robertson MM, Penrose K, Irvine MK, et al. . Impact of an HIV care coordination program on durable viral suppression. J Acquir Immune Defic Syndr 2019; 80:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Public Health – Seattle & King County and Washington State Department of Health. HIV/AIDS epidemiology report, 2018. Available at: https://www.kingcounty.gov/depts/health/communicable-diseases/hiv-std/patients/epidemiology/~/media/depts/health/communicable-diseases/documents/hivstd/2018-hiv-aids-epidemiology-annual-report.ashx. Accessed 12 February 2019. [Google Scholar]