Supplemental Digital Content is available in the text
Keywords: acute postoperative pain, ketamine, patient-controlled analgesia, thoracic epidural analgesia, video-assisted thoracic surgery
Abstract
Background:
Thoracic epidural analgesia is the preferred method for postoperative analgesia following thoracic surgery. However, intravenous patient-controlled analgesia (IVPCA) may be an effective alternative. This study was conducted because few scientific reports exist comparing fentanyl-based IVPCA including a low dose of ketamine (fk-IVPCA) with thoracic patient-controlled epidural analgesia (t-PCEA) for the treatment of postoperative pain after video-assisted thoracic surgery (VATS).
Methods:
This prospective, and randomized study included 70 patients randomized into fk-IVPCA and t-PCEA groups. Pain at rest and during movement, successful and unsuccessful triggers after pressing the PCA device button, the need for rescue analgesia, drug-related adverse events, and patient satisfaction were recorded for 48 hours postoperatively.
Results:
No significant differences in the intensity of pain at rest or during movement were observed between the 2 groups within 48 hours postoperatively. The number of unsuccessful PCA triggers in the t-PCEA group 0 to 4 hours after surgery was significantly higher than that in the fk-IVPCA group. However, the numbers of successful PCA triggers in the fk-IVPCA group at 4 to 12 and 0 to 24 hours after surgery were significantly higher than those in the t-PCEA group. The incidence of analgesic-related side effects and patient satisfaction were similar in both groups.
Conclusions:
Compared with t-PCEA, the addition of a subanesthetic dose of ketamine to fentanyl-based IVPCA resulted in similar pain control after VATS with no increase in the incidence of drug-related adverse effects. The results confirm that both multimodal intravenous analgesia and epidural analgesia can provide sufficient pain control and are safe strategies for treating acute post-thoracotomy pain.
1. Introduction
Thoracic surgeries, including open thoracotomy and video-assisted thoracic surgery (VATS), are highly painful.[1–4] Skin incision, intraoperative tissue retraction and dissection, and chest drainage all lead to intense pain after thoracic surgery.[2,4,5] The inadequate control of the pain will affect the quality of life of patients who undergo thoracic surgery and may contribute to postoperative complications[1–4,6] and chronic post-thoracotomy pain.[2–6] VATS has emerged as an established surgical procedure for both oncological and non-oncological lung surgery.[1,5,7–9] Previous studies have shown that VATS with lobectomy for early-stage lung cancer reduces the incidence of morbidities compared with open lobectomy.[4,5,9–12] However, some patients still complain of moderate-to-severe acute pain after surgery,[2,7,8,12,13] especially on the first postoperative day.[11]
Current analgesic options for acute post-thoracotomy pain include thoracic patient-controlled epidural analgesia (t-PCEA), intravenous patient-controlled analgesia (IVPCA), thoracic paravertebral block, and continuous paravertebral infusion with local anesthetics.[5] Though continuous t-PCEA remains the gold standard for treating acute pain after open thoracic surgery,[1–4,6,7,12,14,15] thoracic epidural catheterization is an invasive procedure that may cause pain and takes time to perform.[1] The technique can lead to devastating complications, such as neurological deficits[1,12,16] or epidural hematoma,[1,4,6,12,16,17] and cannot be used with anticoagulated patients or those with severe spinal deformities.[1,2,4,6] Technical failures are also common.[1,6,11,12] In recent years, several authors have commented on the suitability of t-PCEA as a first choice for preventing or treating acute post-thoracotomy pain.[1,7,11–13]
As an alternative, opioid-based IVPCA systems have been demonstrated to be safe for patients undergoing thoracic surgery[2,18] and are widely used because of their technical simplicity and relative effectiveness at pain management.[5,18] Fentanyl has analgesic potency comparable to that of morphine and its high lipid solubility reduces the onset time, making it suitable for IVPCA.[19] Unlike morphine, fentanyl does not produce active metabolites that lead to respiratory depression and has a shorter duration of action.[19] However, the use of IVPCA with fentanyl for acute post-thoracotomy pain management remains uncommon.
Ketamine, known as a non-competitive N-Methyl-D-Aspartate (NMDA) receptor antagonist, plays a role in acute postoperative pain control by reducing the consumption of opioids, decreasing side effects, and improving hemodynamic and respiratory stability.[3,4,20,21] Several studies[3,20,22] demonstrated that a subanesthetic ketamine dose in combination with morphine-based IVPCA (m-IVPCA) may significantly lower opioid consumption, reduce the incidence of opioid-related adverse effects, and improve respiratory function following thoracic surgeries.
To the best of our knowledge, few studies exist comparing fentanyl-based IVPCA containing low-dose ketamine (fk-IVPCA) with t-PCEA. Therefore, this study aims to compare the degree of acute postoperative pain relief, incidence of drug-related adverse events, and patient satisfaction scores with the use of fk-IVPCA or t-PCEA for acute postoperative pain control in adults following VATS.
2. Materials and methods
This prospective randomized study was conducted at Tri-service General Hospital, Republic of China. With the approval of the Institutional Review Board (TSGHIRB No: 1-105-05-110) and registration at the Chinese Clinical Trial Registry (Registration No. ChiCTR1900021103), we enrolled 74 patients scheduled for elective VATS between August 2016 and December 2017 and obtained written informed consent after providing detailed information about the study. All patients were aged between 20 and 80 years old, had a body mass index (BMI) between 18 and 30 kg/m2, and were categorized under II or III in the American Society of Anesthesiology (ASA) classification system. Exclusion criteria included pregnancy, age younger than 20 years old or older than 80 years old, ASA classification of IV or above, emergency, significant coagulopathy, history of chronic pain, conversion to open thoracotomy, inability to immediately extubate, contraindication to t-PCEA/IVPCA techniques or drugs used in the protocol, and thoracic spine deformities or previous thoracic spine surgery. Ultimately, 70 patients completed our standard protocol and were included in the analysis.
2.1. Protocol
Patients were randomly assigned to the fk-IVPCA or t-PCEA group using computer-generated codes that were kept in sequentially numbered opaque envelopes. Standard monitoring, including electrocardiogram, non-invasive blood pressure, and pulse oximetry, was performed. All patients were premedicated with up to 5 mg midazolam intravenously. For patients assigned to the t-PCEA group, a thoracic epidural catheter was inserted at the T4 to T8 epidural space by an experienced anesthesiologist; the patients in the fk-IVPCA group did not undergo this procedure. General anesthesia was induced with intravenous fentanyl at approximately 2 μg/kg and the target-controlled infusion of propofol was initiated at the effect site concentration (Ce) of 3.0 to 4.0 μg/ml. Afterward, patients were intubated using a left-sided double lumen tube or laryngeal mask airway with the appropriate size. If a double lumen tube was indicated, rocuronium at 0.6 to 1.0 mg/kg was administered after the patient lost consciousness. The patient was positioned laterally and anesthesia was maintained using a target-controlled infusion of propofol at Ce 2.0 to 3.0 μg/ml under bispectral index monitoring within the range of 40 to 60. Additional intravenous fentanyl or rocuronium was administered as clinically indicated. The surgeons applied an intercostal block from T1 to T6 for all patients using 1 ml of 0.5% bupivacaine at every nerve. Fifteen minutes before the end of surgery, analgesic treatment was initiated with a loading dose, followed by continuous infusion in accordance with the initial randomization, and was maintained for 48 hours after VATS. At the end of surgery, patients were quickly weaned from mechanical ventilation and extubated. The criteria for discharge from the postanesthetic care unit were stable vital signs and acceptable pain response with a visual analog scale (VAS) <4. At the wards, 30 mg ketorolac was intravenously given as a regular analgesic with a 6-hour interval in the absence of contraindications and tramadol 100 mg was used as a rescue medication if necessary with an 8-hour interval.
2.2. Surgery
VATS was performed with 1 utility incision and 1 or 2 ports according to the surgeon's preference, experience, and procedural requirements. The thoracoscopic port was placed at the 7th or 8th intercostal space (ICS) along the mid-axillary line. A utility incision of approximately 3 to 5 cm in length was made at the 5th or 6th ICS at the mid-clavicular line level. An additional port was placed at the 7th ICS in the post-scapular line if necessary. At the end of the surgery, a chest tube with a drainage device (Pacific Hospital Supply Co., Ltd.) was inserted. Standard postoperative care was provided and the chest tube was removed when air leakage ceased and the amount of drainage was less than 100 mL/day.
2.3. Analgesia delivery
The fk-IVPCA patients received a continuous intravenous infusion containing 10.0 μg/ml fentanyl and 0.5 mg/ml ketamine. After a loading dose of 5 ml, the infusion proceeded at a rate of 1 ml/hour. The demand dose was 1 ml for every successful trigger with a lockout time of 5 minutes. In the t-PCEA group, 1.6 mg/ml bupivacaine combined with 1.0 μg/ml fentanyl was administered with a loading dose of 8 ml and an infusion rate of 2 ml/hour. The analgesic bolus dose was 6 ml for every successful trigger with a lockout time of 15 minutes. In the event of analgesic failure (VAS > 4 lasting more than 15 minutes), 100 mg tramadol was used as a rescue medication with an 8-hour interval.
Pain assessment was conducted twice daily at rest (static) and during movement (dynamic), and drug-related adverse effects were recorded by our acute pain service (APS) team.
2.4. Measurements
Patient demographics (age, sex, height, and weight) and clinical data (ASA, comorbidities, procedure type, surgical and anesthetic time) were recorded. The primary outcome was the pain intensity measured using VAS (0, no pain; 10, worst pain imaginable) at rest (VAS-R) and during movement (VAS-M) at 1, 24, and 48 postoperative hours (POH). Additionally, the number of triggers of the PCA device (“Hospira” Gemstar Seven Therapy and Pain Management Pump), including all successful and unsuccessful attempts, was recorded at different postoperative intervals (T1: 0–4 hours; T2: 4–12 hours; T3: 12–24 hours; T4: 24–48 hours; T24: 0–24 hours; T48: 0–48 hours).
The secondary outcome was the incidence of drug-related adverse effects, such as dizziness, postoperative nausea and vomiting (PONV), pruritus, hallucination, vivid dreams, and others (e.g., hemodynamic instability, sedation, etc), as well as the patients’ postoperative analgesia satisfaction (1 = very unsatisfactory, 2 = unsatisfactory, 3 = neutral, 4 = satisfactory, 5 = very satisfactory). We also recorded total epidural and intravenous analgesic consumption from the PCA device, the administration of intravenous ketorolac, and tramadol rescue postoperatively. All postoperative assessments were performed by our APS team including one on-duty anesthesiologist and one nurse anesthetist with no knowledge of the purpose of the study.
2.5. Statistical analysis
Data were presented as the mean with standard deviation (SD), standard error of mean, or number (percentage) of patients. Continuous variables were compared using the Student t test or Mann–Whitney U test if the data were not distributed normally. Categorical variables were compared using the chi-square or Fisher exact test based on whether the data presented with normal distribution. Sequential changes in VAS-R and VAS-M in each group from POH1 to POH48 were evaluated using two-way repeated ANOVA with an all-pairwise multiple comparison procedure. Statistical significance was accepted for two-tailed P values of <.05. The statistical analysis was performed using SPSS version 23 statistical software program (SPSS Inc., Chicago, IL).
2.6. Power and sample size
Using the surgical population at our institution, power analysis was performed with the VAS-M values 24 hours postoperatively as the primary variable, with the mean and SD of VAS-M set to 4.0 and 1.25. The power analysis indicated that 31 patients were required in each group to detect a difference of 0.8 points in the VAS score (20% of the non-inferiority margin selected for clinical consideration), with a type I error of 0.05 and power of 80%. Because a 20% dropout rate per group was assumed, 74 patients were to be enrolled.
3. Results
In our study, 76 patients were scheduled for VATS and screened for this study (Fig. 1). Seventy-four patients fulfilled the inclusion criteria and were randomly assigned to the 2 study groups. Four patients were excluded after randomization due to conversion to open thoracotomy: 2 patients from the t-PCEA group, 1 from the fk-IVPCA group, as well as 1 subject from the fk-IVPCA group who failed to extubate immediately after VATS. Ultimately, 35 patients in each group completed the study and were included in the subsequent analysis.
Figure 1.
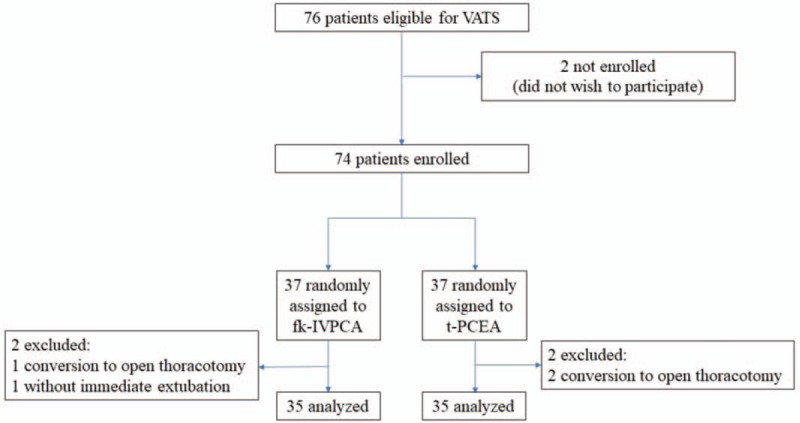
Flow diagram showing patient flow according to the study protocol. VATS = video-assisted thoracic surgery, fk-IVPCA = fentanyl-based IVPCA with addition of low-dose ketamine, t-PCEA = thoracic patient-controlled epidural analgesia.
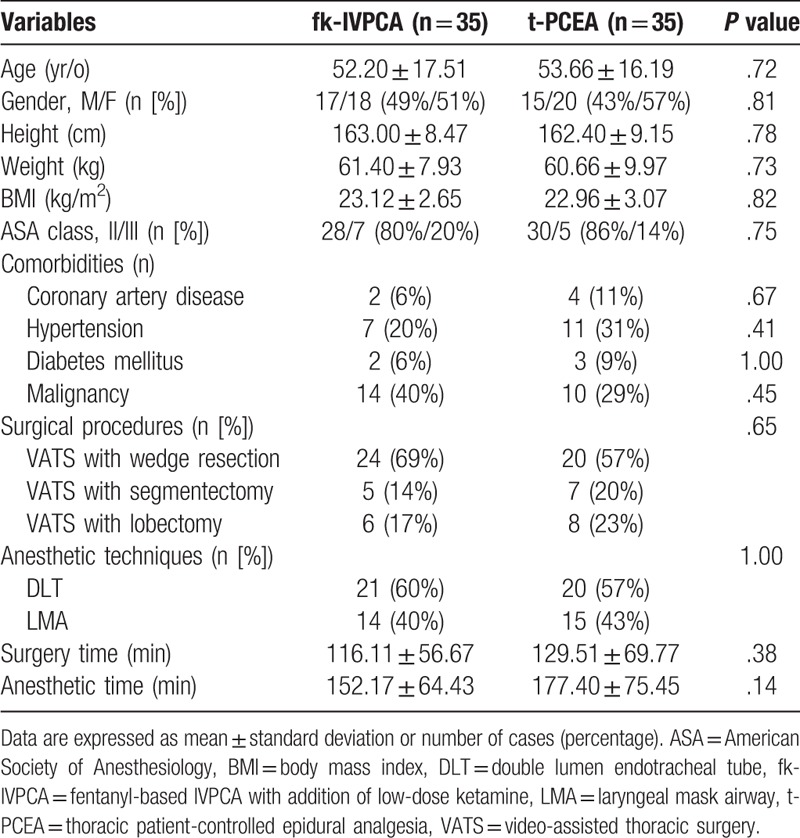
The demographics and major clinical characteristics of the patients are presented in Table 1. There were no significant differences between the 2 groups in age, gender, habitus, ASA physical status, underlying disease, procedure type, anesthetic technique, and surgical and anesthetic time.
Table 1.
Patient demographic characteristics and surgical procedures.
3.1. The efficacy of pain management
There were no significant differences in VAS-R and VAS-M between the 2 groups at different postoperative time points (Table 2; Fig. 2). However, there were significant differences in these variables at different time points in each group, suggesting the patients who received VATS suffered more pain shortly after surgery. VAS-R was significantly higher at POH1 than at POH24 and POH48, but was not significantly different between POH24 and POH48 for both groups. In addition, VAS-M was not only significantly higher at POH1 in comparison with that at POH24 and POH48, but also differed significantly between POH24 and POH48 in both groups (Table 2).
Table 2.
Mean VAS-R and VAS-M in each group at different postoperative times.
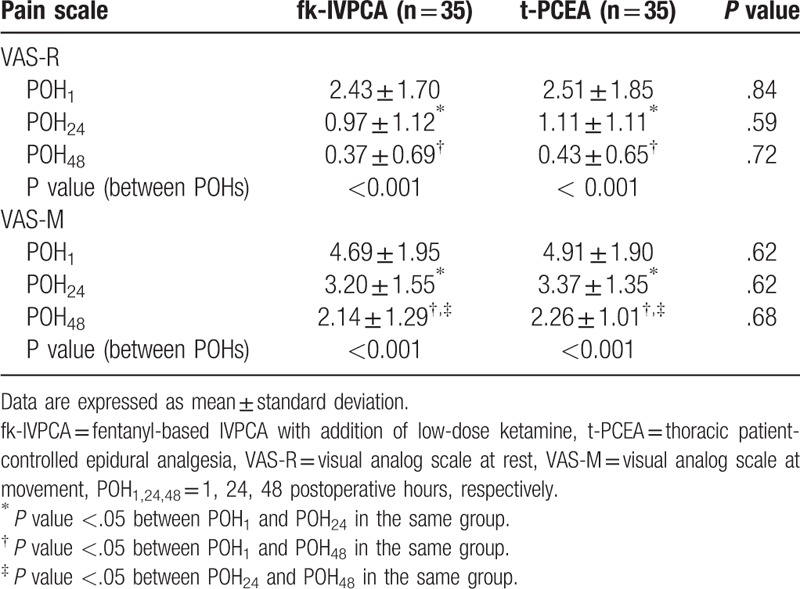
Figure 2.
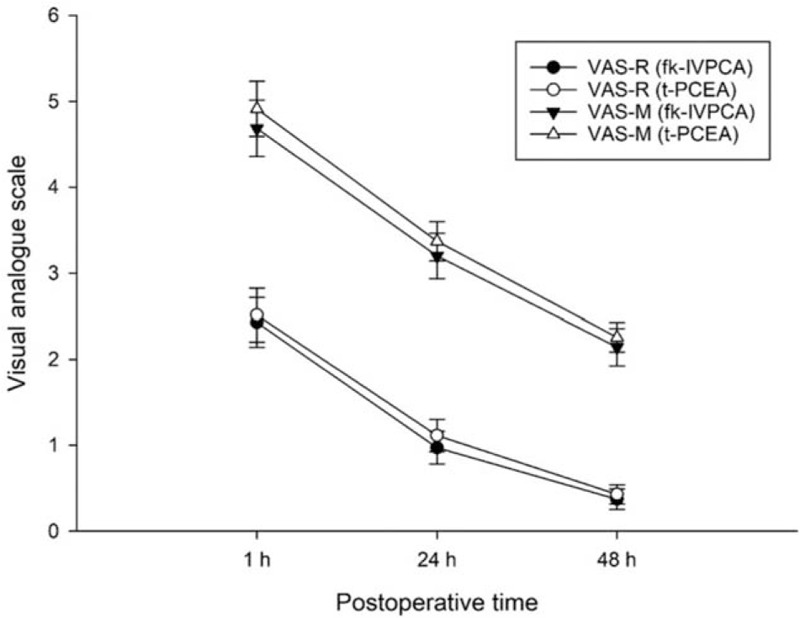
Changes in postoperative pain intensity at rest and during movement over time in each group. Data are expressed as mean ± standard error of mean. fk-IVPCA = fentanyl-based IVPCA with addition of low-dose ketamine, t-PCEA = thoracic patient-controlled epidural analgesia, VAS-R = visual analog scale at rest, VAS-M = visual analog scale during movement.
3.2. The requirement of analgesic triggers
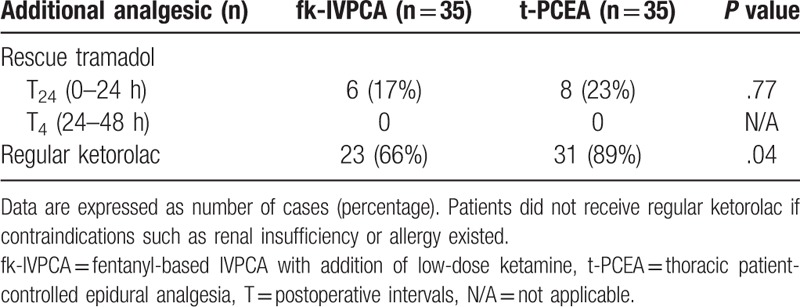
The number of unsuccessful triggers at T1 was significantly greater in the t-PCEA group, whereas the numbers of successful triggers at T2 and T24 were significantly greater in the fk-IVPCA group (Table 3). In addition, the cumulative number of PCA triggers over time showed significant differences in successful triggers at 12 and 24 hours postoperatively and in unsuccessful triggers at 4 hours postoperatively (Fig. 3). There were no significant differences in all attempts, or in successful and unsuccessful triggers between the 2 groups at other intervals. The need for rescue tramadol was similar in both groups, but the ratio of patients administered regular ketorolac showed statistical significance (Table 4).
Table 3.
The number of analgesic triggers in each group at different postoperative intervals.
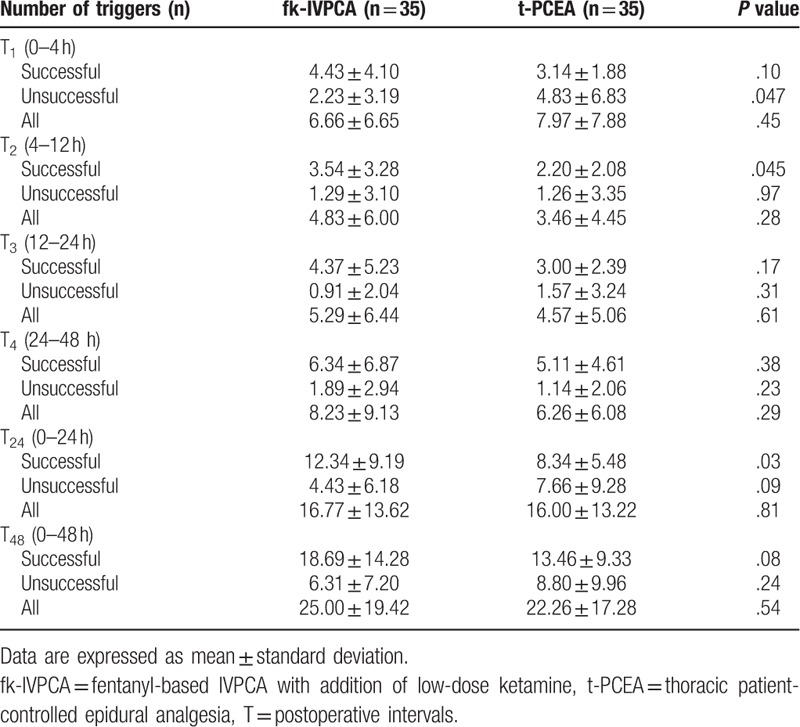
Figure 3.
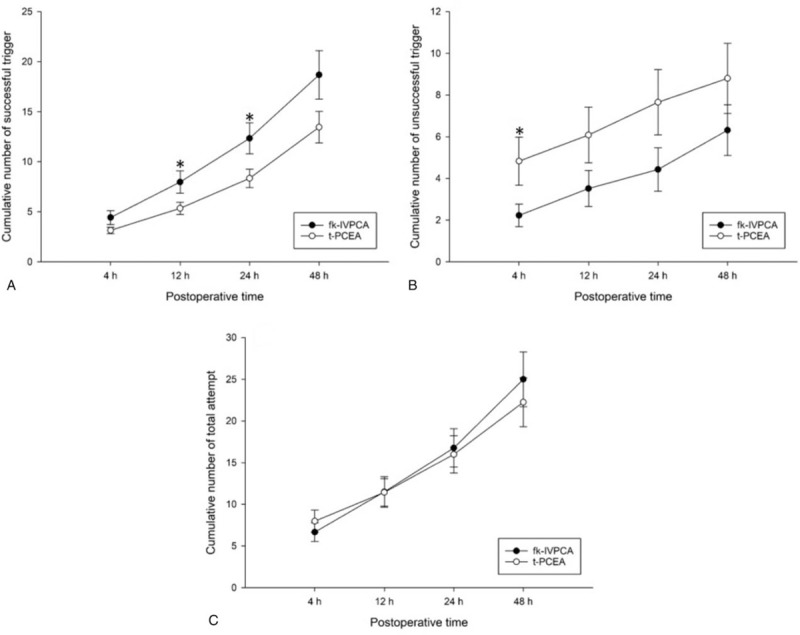
Cumulative numbers of successful (A), unsuccessful triggers (B), and total attempts (C) over time in each group. Data are expressed as mean ± standard error of mean. fk-IVPCA = fentanyl-based IVPCA with addition of low-dose ketamine, t-PCEA = thoracic patient-controlled epidural analgesia. ∗indicating P < .05.
Table 4.
The number of cases that required rescue tramadol in each group at different postoperative intervals and the number that received regular ketorolac.
3.3. Assessment of analgesic-related side effects and patient satisfaction
The incidence of analgesic-related side effects was similar between the 2 groups (Table 5). Only 1 patient in the fk-IVPCA group experienced ketamine-related vivid dreams, while 1 patient in the t-PCEA group complained of numbness in the lower limbs. None of the patients in either group experienced severe or repetitive drug-related side effects resulting in the discontinuation of analgesic infusion.
Table 5.
Drug-related adverse effects, analgesic consumption, and overall postoperative analgesia satisfaction in each group.
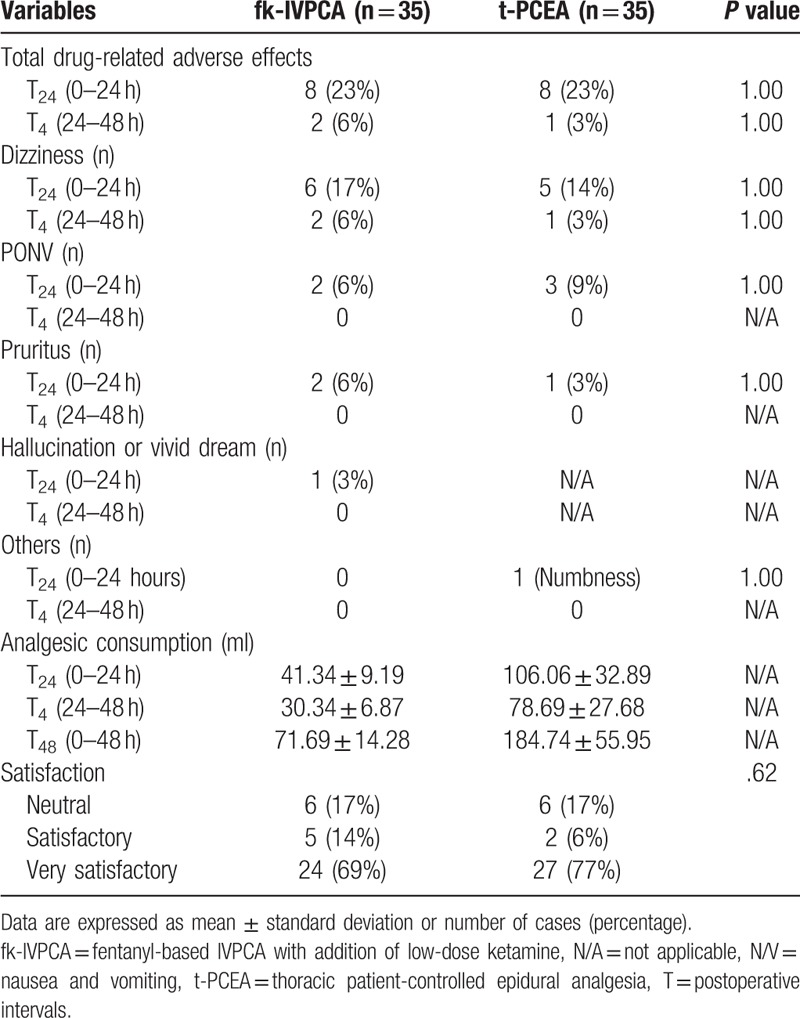
In terms of patient satisfaction, 1 (very unsatisfactory) or 2 (unsatisfactory) points were not noted and there was no significant difference between the groups (Table 5).
3.4. Administration of nonsteroidal anti-inflammatory drugs
The fk-IVPCA group contained 2 subgroups, separated according to the administration of regular ketorolac (Supplement 1). The mean VAS-R and VAS-M in the subgroup that received regular ketorolac were slightly lower, but there was no statistically significant difference (Supplement 2). Additionally, the number of unsuccessful triggers was significantly higher in the subgroup that did not receive regular ketorolac at T1, T24, and T48 (Supplement 3). There were no significant differences between the 2 subgroups in analgesic-related adverse effects, analgesic consumption, need for rescue tramadol, and postoperative analgesia satisfaction (Supplement 4).
4. Discussion
Though 1 of the advantages of VATS compared with open thoracotomy is the reduction in postoperative pain,[1,4,7,8,10–13,15] VATS is still associated with moderate-to-severe acute postoperative pain.[2,7,8,12,13] Therefore, adequate pain management for VATS is necessary.
The main finding in the present study was that the addition of a subanesthetic dose of ketamine to fentanyl-based IVPCA provides pain control similar to that of t-PCEA for acute post-thoracotomy pain after VATS. This result was consistent with the results of a previous study by Kim et al[12] showing that the benefits of IVPCA in terms of the pain score, consumption of analgesics, restoration of pulmonary function, and satisfaction were equal to those of t-PCEA for patients undergoing VATS lobectomy. Yie et al[11] agreed with the above viewpoints and suggested that the necessity of epidural analgesia under minimally invasive thoracic surgery should be reevaluated.
El-Tahan[15] emphasized that t-PCEA usage could be associated with favorable postoperative pulmonary function, a low incidence of postoperative pulmonary complications, fast return of normal bowel function, and a shorter hospital stay after thoracic surgery. Zejun et al[14] also conducted a prospective randomized study, which showed that when compared with IVPCA, t-PCEA resulted in not only lower postoperative pain scores, but also earlier restoration of bowel function after VATS lobectomy. Tiippana et al[6] agreed that t-PCEA with local anesthetics and opioids was superior to IVPCA with opioids for post-thoracotomy pain and the recovery of pulmonary function. A meta-analysis study found that compared with opioid-based IVPCA, local anesthetic-based PCEA with or without opioids could provide significantly superior postoperative analgesia both at rest and with activity for up to 3 days after all types of surgery.[23] These findings supported the superiority of epidural analgesia with a combination of local anesthetic and opioids over systemic opioid analgesia as an analgesic technique for thoracic surgeries.
The difference in surgical trauma between open and thoracoscopic surgery raises the question whether t-PCEA should be considered the golden standard for VATS. While Obuchi et al[13] reported that t-PCEA sufficiently reduced postoperative pain after open thoracotomy, they believed that other methods of analgesia can substitute for epidural analgesia for patients undergoing VATS. In addition, Kamiyoshihara et al [1] demonstrated that routine t-PCEA is not always necessary after VATS and suggested the use of simpler postoperative pain management for less invasive surgical approaches. The authors of a systematic review concluded that no clear gold standard regarding the optimal regional analgesia for VATS could currently be demonstrated, hinting that other alternatives could be as effective as thoracic epidural analgesia for minimally invasive thoracic surgery.[7]
The most commonly used drug for IVPCA in the past was morphine.[2,19,23] Morphine has a good antinociceptive effect, but is associated with some well-known adverse effects, including respiratory depression, excessive sedation, nausea, vomiting, ileus, and constipation,[2,5,19–21] all of which preclude its continuous use. Despite the use of different components in IVPCA, such as oxycodone or sufentanil rather than fentanyl, some studies[2,14] still showed that t-PCEA provided superior analgesia and less opioid-related complications compared to IVPCA in thoracic surgeries. In our hospital, fentanyl is routinely used in IVPCA for postoperative pain control in all kinds of surgeries, as it has a quicker onset and results in less sedation than morphine.[19] In addition, fentanyl is suitable for patients with renal insufficiency because it does not rely on renal excretion for elimination.[19] The continuous infusion of fentanyl-based IVPCA improves postoperative pain compared to an intermittent bolus and also increases patient satisfaction.[18]
The current preference for the optimal perioperative analgesic strategy is multimodal analgesia, which improves antinociception, decreases side effects, and accelerates postoperative recovery. Part of the pathophysiologic mechanism of acute postoperative pain involves the activation of the NMDA receptor by nociceptive stimulation followed by hyperexcitability.[20] Therefore, ketamine, as a known NMDA antagonist, may play a crucial role in the management of acute postoperative pain. In a recent Cochrane review,[24] perioperative intravenous ketamine was found to reduce postoperative analgesic consumption, pain intensity, and even PONV after different types of surgery. Carstensen et al[20] reported that the addition of ketamine to m-IVPCA resulted in superior effects compared to m-IVPCA alone in thoracic surgeries, with a significant reduction in the pain score, cumulative morphine consumption, and postoperative desaturation. The combination did not significantly increase the side effects of ketamine. The conclusion by Mathews et al[22] was in accord with the aforementioned authors’ findings. However, 1 systematic review and meta-analysis reported that the reduction in the intensity of postoperative pain in the ketamine plus morphine or hydromorphone IVPCA was low, but still statistically significant compared with the effect of morphine or hydromorphone IVPCA alone.[21] In addition, the authors believed that adjunctive ketamine could reduce the incidence of PONV without an increase in the incidence of neuropsychiatric effects, similar to the result obtained by Brinck et al.[24]
In our study, the pain management methods used provided sufficient analgesia for not only static, but also dynamic pain control in the first 2 days postoperatively, suggesting that fk-IVPCA may promote early mobilization and physiotherapy to prevent postoperative pulmonary complications. In addition, the intensity of postoperative pain in both groups improved significantly over time. The significantly higher number of unsuccessful triggers only at T1 in the t-PCEA group may imply that epidural analgesics have a longer onset time than intravenous analgesics for acute postoperative pain. In contrast, there were significantly more successful triggers at T2 and T24 in the fk-IVPCA group, indicating that intravenous analgesics with shorter duration may require more frequent triggers to maintain the optimal level of acute pain control. However, the numbers of successful, unsuccessful, and total triggers of the PCA device at all intervals were similar for the two groups. Hence, both analgesic techniques were efficacious for managing the acute postoperative pain of VATS. The incidence of common drug-related adverse effects in the fk-IVPCA group was no higher than that in the t-PCEA group. Previous researchers[20–22,24] speculated that the addition of ketamine reduced the requirement of opioids along with the incidence of adverse events inherent in opioids.
Although Tiippana et al [6] regarded thoracic epidural analgesia as the preferred pain management method for acute thoracotomy pain, they also declared that m-IVPCA combined with adjuvant analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs), and strict follow-up may be a valuable alternative. Our results showed that patients with regular ketorolac treatment in the fk-IVPCA group had significantly fewer unsuccessful triggers of the PCA device in the immediately postoperative period, although the intensity of pain was not significantly different. This result was also in accordance with the suggestion of multimodal analgesia as an optimal strategy for acute pain management mentioned previously.
There were some limitations in this study. First, for medical and logistic reasons, double blinding was not performed because it seemed neither feasible nor realistic for this study. However, the protocol for this study did not differ from that used in our routine practice and the APS team did not know the details of our study. Second, our study focused on the short-term outcomes after VATS, but we did not observe the long-term outcomes, such as the development of chronic pain, cancer recurrence, or mortality. Third, the same team of surgeons performed the surgeries, yet the individual characteristics of patients and anatomical conditions necessitated some modifications of the surgical techniques used and this can be associated with slight differences in the extent of surgical injuries.
In conclusion, the key message of this prospective study is that the combination of fentanyl-based IVPCA with low-dose ketamine, compared with t-PCEA, may provide similar analgesia for acute post-thoracotomy pain after VATS with no increased risk of adverse effects. Notably, multimodal analgesic management with pharmacologically distinct mechanisms should be taken into account to treat acute pain, especially for post-thoracotomy pain after VATS.
Author contributions
Conceptualization: Wei-Lin Lin, Hou-Chuan Lai, Tsai-Wang Huang, Zhi-Fu Wu.
Data curation: Wei-Cheng Tseng, Pin-Hsuan Chen.
Formal analysis: Wei-Cheng Tseng, Hou-Chuan Lai, Pin-Hsuan Chen, Zhi-Fu Wu.
Project administration: Wei-Lin Lin, Tsai-Wang Huang.
Resources: Wei-Lin Lin, Tsai-Wang Huang.
Writing – original draft: Wei-Cheng Tseng, Pin-Hsuan Chen.
Writing – review & editing: Hou-Chuan Lai, Zhi-Fu Wu.
Wei-cheng Tseng orcid: 0000-0003-3528-8633.
Supplementary Material
Footnotes
Abbreviations: APS = acute pain service, ASA = American Society of Anesthesiology, BMI = body mass index, NMDA = N-Methyl-D-Aspartate, PCA = patient-controlled analgesia, PCEA = patient-controlled epidural analgesia, PONV = postoperative nausea and vomiting, SD = standard deviation, VAS = visual analog scale, VATS = video-assisted thoracic surgery.
The authors have no funding and conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kamiyoshihara M, Nagashima T, Ibe T, et al. Is epidural analgesia necessary after video-assisted thoracoscopic lobectomy? Asian Cardiovasc Thorac Ann 2010;18:464–8. [DOI] [PubMed] [Google Scholar]
- [2].Bialka S, Copik M, Daszkiewicz A, et al. Comparison of different methods of postoperative analgesia after thoracotomy-a randomized controlled trial. J Thorac Dis 2018;10:4874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rodriguez-Aldrete D, Candiotti KA, Janakiraman R, et al. Trends and new evidence in the management of acute and chronic post-thoracotomy pain-an overview of the literature from 2005 to 2015. J Cardiothorac Vasc Anesth 2016;30:762–72. [DOI] [PubMed] [Google Scholar]
- [4].Elmore B, Nguyen V, Blank R, et al. Pain management following thoracic surgery. Thorac Surg Clin 2015;25:393–409. [DOI] [PubMed] [Google Scholar]
- [5].Lee CY, Narm KS, Lee JG, et al. A prospective randomized trial of continuous paravertebral infusion versus intravenous patient-controlled analgesia after thoracoscopic lobectomy for lung cancer. J Thorac Dis 2018;10:3814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tiippana E, Nelskyla K, Nilsson E, et al. Managing post-thoracotomy pain: epidural or systemic analgesia and extended care – a randomized study with an “as usual” control group. Scand J Pain 2014;5:240–7. [DOI] [PubMed] [Google Scholar]
- [7].Steinthorsdottir KJ, Wildgaard L, Hansen HJ, et al. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg 2014;45:959–66. [DOI] [PubMed] [Google Scholar]
- [8].Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836–44. [DOI] [PubMed] [Google Scholar]
- [9].Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602–9. [DOI] [PubMed] [Google Scholar]
- [10].Laursen LO, Petersen RH, Hansen HJ, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg 2016;49:870–5. [DOI] [PubMed] [Google Scholar]
- [11].Yie JC, Yang JT, Wu CY, et al. Patient-controlled analgesia (PCA) following video-assisted thoracoscopic lobectomy: comparison of epidural PCA and intravenous PCA. Acta Anaesthesiol Taiwan 2012;50:92–5. [DOI] [PubMed] [Google Scholar]
- [12].Kim JA, Kim TH, Yang M, et al. Is intravenous patient controlled analgesia enough for pain control in patients who underwent thoracoscopy? J Korean Med Sci 2009;24:930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Obuchi T, Yoshida Y, Moroga T, et al. Postoperative pain in thoracic surgery: re-evaluating the benefits of VATS when coupled with epidural analgesia. J Thorac Dis 2017;9:4347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zejun N, Wei F, Lin L, et al. Improvement of recovery parameters using patient-controlled epidural analgesia for video-assisted thoracoscopic surgery lobectomy in enhanced recovery after surgery: a prospective, randomized single center study. Thoracic Cancer 2018;9:1174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El-Tahan MR. Role of thoracic epidural analgesia for thoracic surgery and its perioperative effects. J Cardiothorac Vasc Anesth 2017;31:1417–26. [DOI] [PubMed] [Google Scholar]
- [16].von Hosslin T, Imboden P, Luthi A, et al. Adverse events of postoperative thoracic epidural analgesia: a retrospective analysis of 7273 cases in a tertiary care teaching hospital. Eur J Anaesthesiol 2016;33:708–14. [DOI] [PubMed] [Google Scholar]
- [17].Kupersztych-Hagege E, Dubuisson E, Szekely B, et al. Epidural hematoma and abscess related to thoracic epidural analgesia: a single-center study of 2,907 patients who underwent lung surgery. J Cardiothorac Vasc Anesth 2017;31:446–52. [DOI] [PubMed] [Google Scholar]
- [18].McNicol ED, Ferguson MC, Hudcova J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2015;Cd003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grass JA. Patient-controlled analgesia. Anesth Analg 2005;101:S44–61. [DOI] [PubMed] [Google Scholar]
- [20].Carstensen M, Moller AM. Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: a qualitative review of randomized trials. Br J Anaesth 2010;104:401–6. [DOI] [PubMed] [Google Scholar]
- [21].Wang L, Johnston B, Kaushal A, et al. Ketamine added to morphine or hydromorphone patient-controlled analgesia for acute postoperative pain in adults: a systematic review and meta-analysis of randomized trials. Can J Anaesth 2016;63:311–25. [DOI] [PubMed] [Google Scholar]
- [22].Mathews TJ, Churchhouse AM, Housden T, et al. Does adding ketamine to morphine patient-controlled analgesia safely improve post-thoracotomy pain? Interact Cardiovasc Thorac Surg 2012;14:194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology 2005;103:1079–88. quiz 1109-1010. [DOI] [PubMed] [Google Scholar]
- [24].Brinck EC, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev 2018;12:Cd012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


