Abstract
The prognostic significance of systemic atherothrombosis in heart failure (HF) with preserved ejection fraction (HFpEF) remains unclear. This study aimed to investigate the relation between the presence of polyvascular disease (PVD) and cardiovascular outcomes in HFpEF patients.
A total of 510 consecutive HFpEF patients were prospectively observed for up to 1500 days or until occurrence of cardiovascular events. PVD was defined as ≥2 coexistence of coronary artery disease, peripheral arterial disease, and cerebrovascular disease.
Overall, 124 cardiovascular events were observed during follow-up (median: 1430 days). Kaplan–Meier curve showed HFpEF with PVD (n = 84) experienced more cardiovascular events than did those without PVD patients (44.0% vs 20.4%, log-rank: P < .001). Multivariable Cox proportional hazards analysis with significant factors from univariate analysis showed the presence of PVD (hazard ratio [HR]: 2.875, 95% [CI]: 1.894–4.365, P < .001), previous HF hospitalization (HR: 1.578, 95% CI: 1.031–2.414, P = .036), hemoglobin (HR: 0.889, 95% CI: 0.805–0.983, P = .021), serum sodium (HR: 0.946, 95% CI 0.896–1.000, P = .048), ln-BNP (per 1.0, HR: 1.255, 95% CI: 1.055–1.494, P = .010), and E/e’ (HR: 1.047, 95% CI: 1.020–1.075, P < .001) significantly predicted future cardiovascular events. Multivariable Cox hazard analysis with 4 established factors (age, BNP, diabetes mellitus, and previous HF hospitalization) from the I-PRESERVE (Irbesartan in HFpEF) study showed PVD was independently associated with cardiovascular events in HFpEF patients (HR: 2.562, 95% CI: 1.715–3.827, P < .001).
The presence of PVD is significantly associated with cardiovascular events in HFpEF, suggesting the importance of screening PVD in HFpEF.
Keywords: atherosclerosis, heart failure with preserved ejection fraction, polyvascular disease, prognosis
1. Introduction
Patients with heart failure (HF) are clinically classified into those with reduced left ventricular ejection fraction (LVEF) (HFrEF) and those with preserved LVEF (HFpEF). More than half of patients with HF belong to the HFpEF group.[1] Recent clinical studies have shown that the mortality in patients with HFpEF is equivalent to that in patients with HFrEF.[1] Therefore, establishing management of HFpEF is urgently required worldwide. The pathophysiology of HFpEF and HFrEF is different, and the precise pathogenesis underlying HFpEF remains unclear. The Irbesartan in patients with Heart Failure with Preserved Ejection Fraction (I-PRESERVE) study clearly demonstrated that atherosclerotic factors, including age and diabetes mellitus, were significantly associated with adverse outcomes in HFpEF.[2] Therefore, HFpEF is characterized by systolic-ventricular and arterial stiffening, resulting in cardiac diastolic dysfunction. Indeed, we previously reported the first evidence for the prognostic significance of pulse pressure,[3] reflecting large arterial stiffness, and brachial-ankle pulse wave velocity[4] (a direct marker of aortic stiffness) in patients with HFpEF. This finding indicates that systemic atherosclerosis is tremendous important for the pathogenesis of HFpEF. However, clinical evidence demonstrating a relationship between the severity of systemic atherosclerosis and HFpEF is insufficient. We previously reported that severe impairment of peripheral microvascular endothelial function is an important pathophysiological component of polyvascular disease (PVD), and that PVD reflects severe systemic atherosclerosis.[5] We conducted the present study to assess the prognostic significance of PVD in HFpEF. We prospectively observed the occurrence of cardiovascular outcomes in patients with HFpEF.
2. Methods
2.1. Study design and patients
We prospectively screened 951 consecutive patients with HF who were hospitalized in Kumamoto University Hospital between January 2007 and September 2013. We recorded the medical history and relevant clinical characteristics of the patients. We excluded 439 patients for the following reasons: severe valvular disease (n = 118), chronic renal failure and undergoing hemodialysis (n = 65), systemic inflammatory disease (autoimmune diseases and rheumatoid diseases required immunosuppression therapies) (n = 5), and not meeting the diagnostic criteria for HFpEF (including reduced LVEF) (n = 251). Two patients had no data for PVD. Finally, 510 patients with HFpEF were analyzed in this study (Fig. 1).
Figure 1.
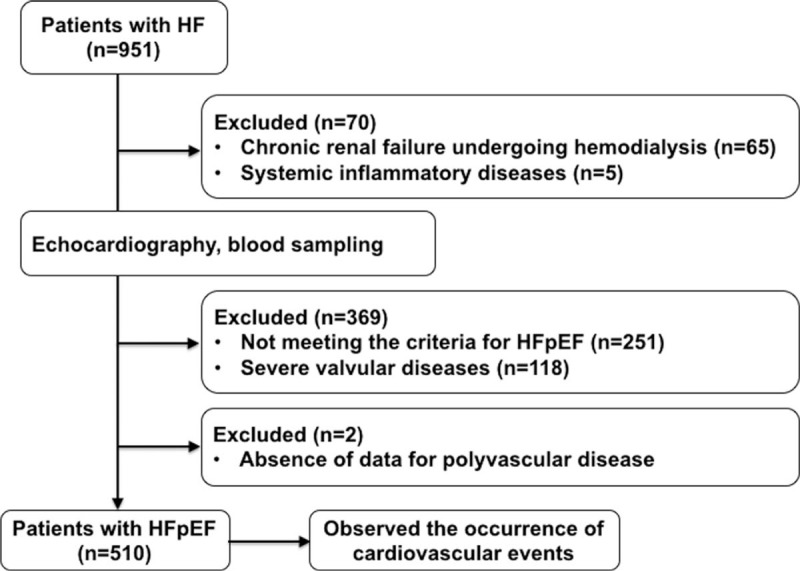
Study flow chart. HF = heart failure, HFpEF = heart failure with preserved left ventricular ejection fraction.
The study protocol conformed to the principles of the Declaration of Helsinki. The study protocol was approved by the Human Ethics Review Committee of Kumamoto University. Written informed consent was obtained from all participating patients. This study is registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN000016385).
2.2. Definition of HFpEF
HFpEF was defined clinically according to the criteria of the European Working Group for HFpEF as follows: symptoms or signs of HF; normal or mildly reduced LVEF (LVEF >50% and left ventricular[2] end-diastolic volume index <97 mL/m2); and evidence of abnormal LV relaxation, filling, diastolic distensibility, and diastolic stiffness.[6] We excluded patients with HFpEF who had shown even a transient reduction in LVEF. Therefore, patients with HFpEF whose LVEF was <50% and was improved by optimal therapy were not included in the present study. In our study, we stratified the ratio of early transmitral flow velocity to early diastolic mitral annular velocity (E/e’) as ≥15 or >8 and <15, and plasma B-type natriuretic peptide (BNP) levels with a cut-off of 100 pg/mL. We confirmed patients with HFpEF according to the New York Heart Association (NYHA) functional class for evaluating the severity of HF under stable conditions after optimal therapy.
2.3. Echocardiographic examinations
Echocardiography was performed by experienced cardiac sonographers who had no knowledge of the study data using Aplio XG [Toshiba, Tokyo, Japan], or Vivid 7, Vivid E9 [GE-Vingmed Ultrasound, Horton, Norway]. Left ventricular (LV) ejection fraction was evaluated by the biplane modified Simpson method in apical 4- and 2-chamber views. The E/e’ ratio was evaluated to evaluate LV diastolic function as the ratio of early diastolic transmitral flow velocity to mitral annular velocity at the septal and lateral wall of the LV.[7,8] LV mass index was calculated by Devereux formula as described previously.[9]
2.4. Definition of PVD
PVD was defined as the presence of >1 affected vascular bed in the coronary, cerebrovascular, or peripheral arteries.[10]
On the basis of quantitative coronary angiography (CAG) analysis (CAAS; Pie Medical Imaging, Maastricht, The Netherlands), we defined coronary artery disease (CAD) as the presence of >50% narrowing of the coronary artery diameter (equivalent to 75% stenosis in the American Heart Association criteria) in at least one major coronary artery at CAG. In some asymptomatic patients who did not consent to CAG, we performed computed tomography (CT) using 64- or 320-low multi-slice CT to detect coronary artery stenosis and plaques. We diagnosed CAD if there was evidence of >70% stenosis of at least 1 major coronary artery. In patients with dense calcification in the proximal parts of coronary arteries in cardiac CT, CAG was undertaken to confirm the presence of CAD.
We primarily diagnosed lower-extremity peripheral artery disease (PAD) in patients with intermittent claudication with a bilateral ankle-brachial index (ABI) <0.9, those with a history of intermittent claudication with previous vascular interventions, and those with intermittent claudication with an ABI >1.4, and significant arterial stenosis or occlusions detected by an imaging examination.[10,11] ABI test was measured on admission or in the outpatient clinic just before admission in a stable condition using an ABI measurement device (BP-203PRE II; Omron Colin, Tokyo, Japan).
Cerebrovascular disease (CVD) was diagnosed by clinical symptoms, or a history of ischemic stroke or transient ischemia attack. For patients with a neurological deficit without a relevant stroke history or symptoms, the attending physicians performed carotid artery ultrasound and magnetic resonance imaging (MRI). CVD was diagnosed when there was evidence of ischemic stroke, >50% stenosis of a carotid artery on vascular ultrasound, or >75% stenosis of a cerebral artery on MRI.
2.5. Atherosclerotic risk factors
We defined atherosclerotic risk factors as diabetes mellitus (diabetic symptoms and casual plasma glucose concentration ≥200 mg/dL, fasting plasma glucose concentration ≥126 mg/dL, 2-hour plasma glucose concentration ≥200 mg/dL during 75 g oral glucose tolerance test or taking hypoglycemic medications), hypertension (>140/90 mmHg or taking antihypertensive medications), dyslipidemia (high-density lipoprotein [HDL]-cholesterol <40 mg/dL, low-density lipoprotein [LDL]-cholesterol ≥140 mg/dL, triglycerides ≥150 mg/dL or taking lipid lowering medications), and current smoking (within 1 year) from an interview. Chronic kidney disease was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. eGFR was calculated by following formula: male, eGFR (mL/min/1.73m2) = 194 × Cr−1.094 × age−0.287; Female, eGFR(mL/min/1.73m2) = 194 × Cr−1.094 × age−0.287 × 0.739 that the Japanese Society of Nephrology defined.[12]
2.6. Biomarkers measurement
Blood samples were obtained in the stable and fasting condition in the early morning. Plasma or serum samples were kept frozen at −80°C until measurement. Plasma BNP levels and other parameters were measured in our institutes.
2.7. Follow-up and outcomes
Patients were followed up prospectively at our outpatient clinics or by the primary care physician every month until July 2015 or until occurrence of a cardiovascular event, including the following: cardiovascular death, hospitalization for HF decompensation, nonfatal myocardial infarction (MI), unstable angina pectoris, coronary revascularization for a new diagnosis of angina or in-stent restenosis after percutaneous coronary intervention, and nonfatal ischemic stroke. Cardiovascular death was defined as death within 30 days of documented sudden death without apparent non-cardiovascular causes, MI, death from HF, or death from stroke. Hospitalization for HF decompensation was defined if the patient was admitted for at least an overnight stay in hospital because of HF with typical symptoms and had objective signs of worsening HF requiring intravenous drug administration. MI was diagnosed by an increase or decrease in cardiac biomarkers (plasma creatine kinase-MB or cardiac troponin) above the 99th percentile of the upper limit of the normal range together with evidence of myocardial ischemia and at least 1 of the following symptoms: electrocardiographic changes (new ST-T changes, left bundle branch block, or pathological Q wave) or imaging evidence of new viable myocardial loss, or a new regional wall motion abnormality.[13] Unstable angina pectoris was diagnosed by new or accelerating symptoms of myocardial ischemia accompanied by new ischemic ST-T-wave changes. Ischemic stroke was diagnosed by the documented a focal neurological deficit with radiological evidence of brain infarction excluding intracranial hemorrhage. Cardiovascular events were ascertained from review of the medical records and confirmed by direct contact with the patients, their families, and physicians, or by annual telephone interview with each patient. An Events Committee comprising at least 3 independent physicians reviewed all events to avoid intraobserver biases.
2.8. Statistical analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed variables according to the Shapiro–Wilk test. Variables with a non-normally distributions are expressed as the median value with interquartile range. Categorical variables are presented as frequencies and percentages. Differences between groups were determined using Fisher exact test for categorical variables. Differences in continuous variables were analyzed by the unpaired t test or the Mann–Whitney U test, as appropriate. Missing data were excluded from the analyses. The Kaplan–Meier method was used to estimate the probability of HF-related events up to 3 years after enrollment. The log-rank test was used to compare distributions of event-free times between the PVD group and the non-PVD group. The Cox proportional hazards model was used to estimate HF-related event hazard ratios (HRs) by univariate and multivariable analyses with forced inclusion modeling. HRs and 95% confidence intervals (CIs) are presented. A P value < .05 was considered statistically significant. Statistical analyses were performed using SPSS 23 for Macintosh (SPSS Inc, Tokyo, Japan).
3. Results
3.1. Clinical characteristics of enrolled patients with HFpEF
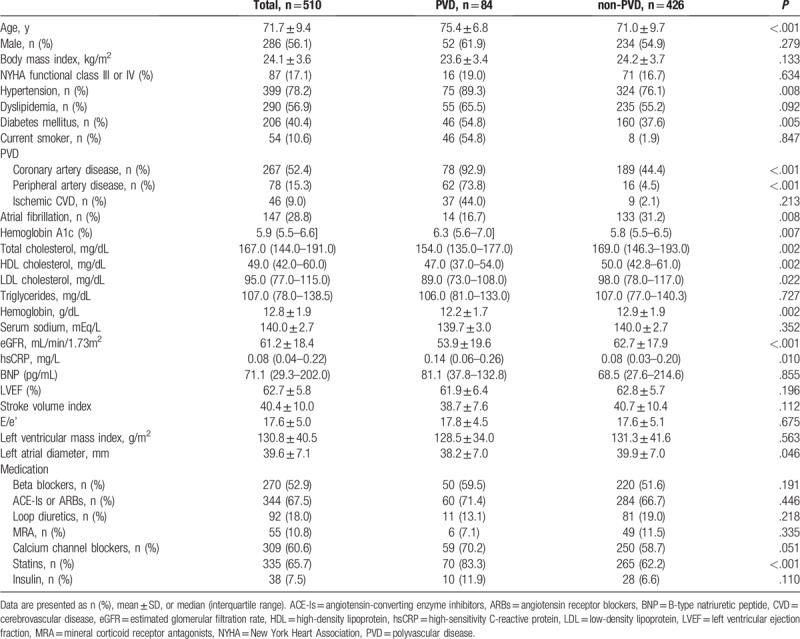
A total of 439 patients met the exclusion criteria and 2 patients were lost to follow-up. No missed data were missing for the baseline. Finally, 510 patients with HFpEF (mean age: 71.7 ± 9.4 years old, men: 56.1%, prevalence of PVD: 16.5%) were analyzed. The baseline characteristics of patients with HFpEF are shown in Table 1. HFpEF patients with PVD had a significantly higher prevalence of hypertension, diabetes mellitus, CAD, PAD, and ischemic CVD, and use of statins (Table 1) than did those without PVD. The prevalence of atrial fibrillation was higher in non-PVD compared with PVD. HbA1c and high-sensitivity C-reactive protein (hsCRP) levels were significantly higher, and HDL-cholesterol levels, LDL cholesterol levels, and the eGFR were significantly lower in HFpEF patients with PVD compared with those without PVD patients. Importantly, the proportions of NYHA class III or IV, BNP levels, and transthoracic echocardiographic parameters, including LVEF, E/e’, stroke volume index, and left atrial diameter, were not significantly different between the 2 groups. This finding indicated equivalent severity of HF between the PVD and the non-PVD groups at baseline.
Table 1.
Baseline characteristics of enrolled patients.
3.2. Cardiovascular events at follow-up
Overall, 124 cardiovascular events were recorded during the follow-up period (median: 1430 days, 95% CI: 815–1500 days). Table 2 shows the details of cardiovascular events during follow-up. HFpEF patients with PVD experienced a significantly higher rate of unstable angina pectoris (6.0% vs 1.4%, P = .022) and coronary revascularization (11.9% vs 3.8%, P = .005) compared with those without PVD. The rates of cardiovascular death, hospitalization for HF decompensation, non-fatal MI, and nonfatal ischemic stroke were similar between the PVD and the non-PVD groups. Kaplan–Meier curve showed that HFpEF patients with PVD had a significantly higher rate of cardiovascular events than those without PVD (44.0% vs 20.4%, log-rank test: P < .001, Fig. 2A).
Table 2.
Details of cardiovascular events.
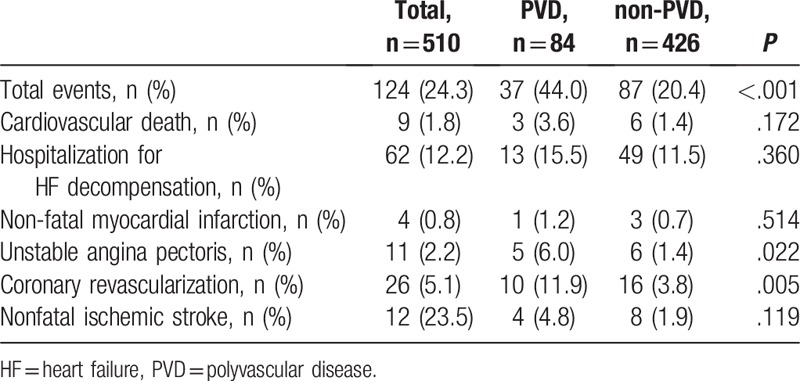
Figure 2.
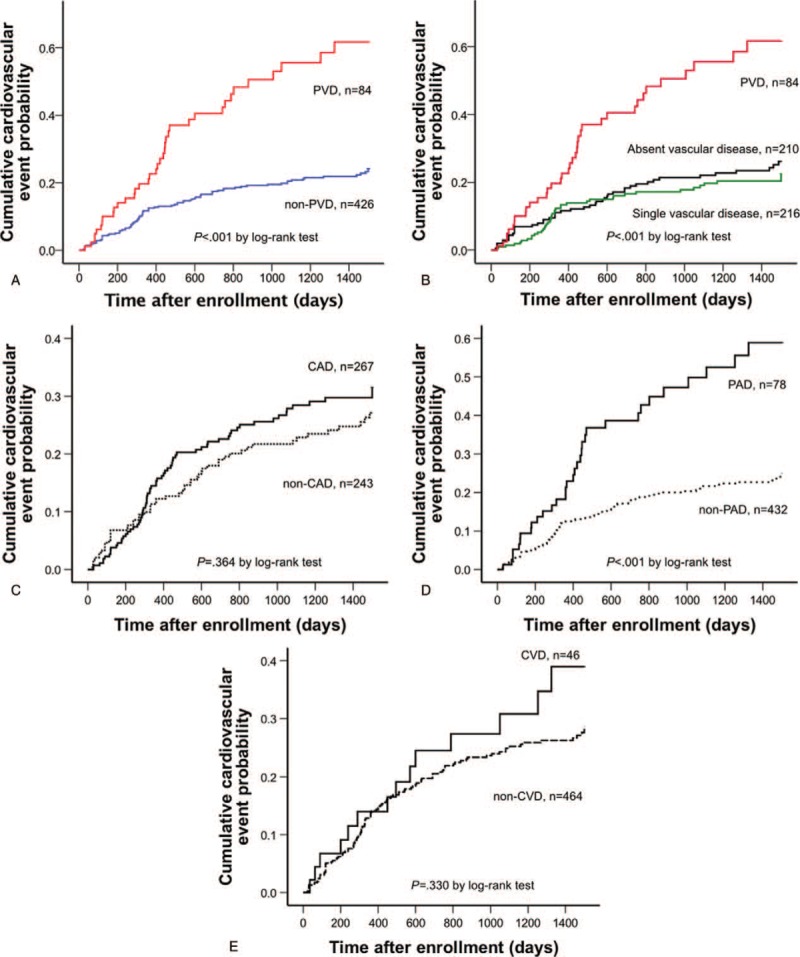
Kaplan–Meier curve. The probability of cardiovascular events in patients with preserved left ventricular ejection fraction complicated with and without PVD (A), and that in patients with absent vascular disease, single vascular disease, and PVD (B). The probability of cardiovascular events in patients with preserved left ventricular ejection fraction complicated with and without CAD (C), PAD (D), and CVD (E). CAD = coronary artery disease, CVD = cerebrovascular disease, PAD = peripheral artery disease, PVD = polyvascular disease.
Figure 2B shows the cumulative probability of cardiovascular events among 3 groups (absent vascular disease, single vascular disease, and PVD). Kaplan–Meier curve showed that the rates of absent vascular disease and single vascular disease were similar. By contrast, the PVD group had a remarkably higher rate of cardiovascular events than did the other 2 groups (log-rank test: P < .001, Fig. 2B).
The relation between each vascular disease (CAD, PAD, and CVD) and cardiovascular outcome was shown in Figure 2C–E. CAD and CVD did not increase cardiovascular events compared with their counterparts, respectively (CAD, 25.8% vs 22.6%, P = .364; CVD, 30.4% vs 23.7%, P = .330 by log-rank test, Fig. 2C and E). PAD had significantly higher rate of cardiovascular events than those with counterparts (42.3% vs 21.1%, P < .001 by log-rank test, Fig. 2D).
3.3. Cox proportional hazard analysis for cardiovascular events
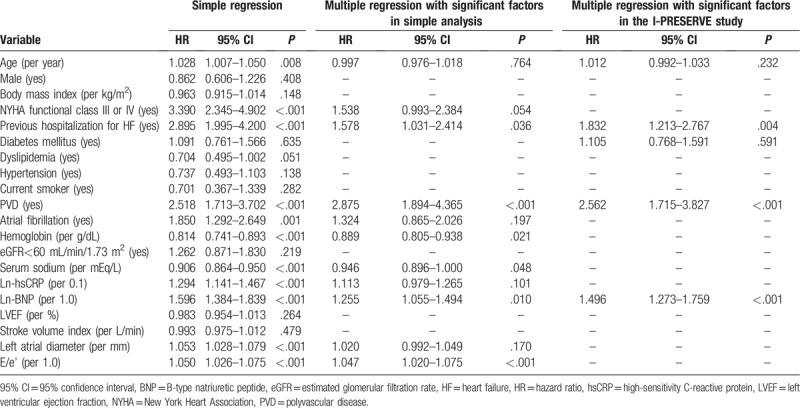
Table 3 shows the results of univariate and multivariable Cox proportional hazards analyses for cardiovascular events. Univariate Cox proportional hazards analysis identified age (HR: 1.028, 95% CI: 1.007–1.050, P = .008), NYHA class III or IV (HR: 3.390, 95% CI: 2.345–4.902, P < .001), previous hospitalization for HF (HR: 2.895, 95% CI: 1.995–4.200, P < .001), atrial fibrillation (HR: 1.850, 95% CI: 1.292–2.649, P = .001), hemoglobin (HR: 0.814, 95% CI: 0.741–0.893, P < .001), serum sodium (HR: 0.906, 95% CI 0.864–0.950, P < .001), ln-hsCRP (per 0.1, HR: 1.294, 95% CI: 1.141–1.467, P < .001), ln-BNP (per 1.0, HR: 1.596, 95% CI: 1.384–1.839, P < .001), left atrial diameter (HR: 1.053, 95% CI: 1.028–1.079, P < .001), E/e’ (HR: 1.050, 95% CI: 1.026–1.075, P < .001), and the presence of PVD (HR: 2.518, 95% CI: 1.713–3.702, P < .001) as significant factors associated with cardiovascular events. Multivariable Cox proportional hazards analysis using the above-mentioned 11 significant factors from the univariate analysis identified that the presence of PVD (HR: 2.875, 95% CI: 1.894–4.365, P < .001), previous hospitalization for HF (HR: 1.578, 95% CI: 1.031–2.414, P = .036), hemoglobin (HR: 0.889, 95% CI: 0.805–0.983, P = .021), serum sodium (HR: 0.946, 95% CI 0.896–1.000, P = .048), ln-BNP (per 1.0, HR: 1.255, 95% CI: 1.055–1.494, P = .010), and E/e’ (HR: 1.047, 95% CI: 1.020–1.075, P < .001) as the independent predictors for cardiovascular events in patients with HFpEF (Table 3). Furthermore, multivariable Cox proportional hazards analysis that incorporated 4 factors (age, BNP, diabetes mellitus, and previous hospitalization for HF which were identified in the I-PRESERVE study) identified the presence of PVD as an independent predictor for cardiovascular events in patients with HFpEF (HR: 2.562, 95% CI: 1.715–3.827, P < .001).
Table 3.
Results of univariate and multivariable Cox proportional hazards analyses for cardiovascular events in patients with heart failure with preserved ejection fraction.
4. Discussion
Although increased aortic stiffness significantly contributes to cardiovascular events in patients with HF, the clinical significance of PVD in these patients, especially in the subgroup with HFpEF, is unclear. To the best of our knowledge, this is the first report to clearly demonstrate the clinical impact of the presence of PVD on future cardiovascular events in patients with HFpEF. CAD, one of the components of PVD, is a great prognostic predictor in HF. In our study, the prevalence of CAD in patients with HFpEF was 52.4%. The Chronic Heart Failure Analysis and Registry in Tohoku District 2 (CHART-2) study was a recent, large, prospective, multicenter cohort study on HF in Japan. The CHART-2 study showed that the prevalence of CAD was markedly increased to approximately 50%,[14,15] and accompanied by an increase in the proportion of men and older patients. The findings from the CHART-2 study suggest that the recent changes in lifestyle and developments in percutaneous coronary intervention or coronary bypass grafting for acute MI may have caused an increase in HFpEF in CAD. Furthermore, the proportions of men, mean age, and prevalence of CAD in patients with HFpEF in our study were similar to those in the CHART-2 study. Therefore, the clinical characteristics of patients with HFpEF in our study reflect the real-world practice of HFpEF in Japan in a relative recent period, but could be different from those in Western countries. Many previous reports have demonstrated the clinical impact of the presence of CAD on patients with HFpEF. However, the prognostic association between PVD and HFpEF is still unclear. In this study, we firstly showed the prognostic significance of PVD in patients with HFpEF, and this significance remained after adjustment by several prognostic significant factors from the I-PRESERVE study.[2] In the Reduction of Atherothrombosis for Continued Health (REACH) registry, the prevalence of patients with PVD among those with established and symptomatic CAD, CVD, and lower-extremity PAD was 16% in outpatients with atherosclerotic risk factors.[10] The authors of this previous report speculated that the prevalence of PVD would have been higher if a fuller assessment of asymptomatic patients with vascular disease could have been undertaken. Furthermore, Imori et al prospectively and rigorously examined patients with asymptomatic and symptomatic carotid artery stenosis, renal artery stenosis, lower-extremity PAD, and CAD scheduled for non-emergent diagnostic CAG to establish the prevalence of concomitant lesions and their severity.[16] In 1734 patients with suspected CAD, these authors found that the prevalence of carotid artery stenosis was 6%, renal artery stenosis was 7%, lower-extremity PAD was 13%, and CAD was 72%. In our high-risk population, the prevalence of CVD was 9.0%, lower-extremity PAD was 15.3%, and CAD was 52.4% in patients with HFpEF, which are consistent with those rates in Imori et al.'s study.[12] Furthermore, the present study showed that HFpEF patients with PVD had a worse prognosis than did those with CAD, CVD, or only lower-extremity PAD. This finding indicates that the incremental atherosclerotic risk worsens the prognosis of HFpEF.
HFpEF is considered to be characterized by arterial and ventricular stiffening beyond that related to aging and hypertension, resulting in cardiac diastolic dysfunction.[17] Arterial stiffness reflects systemic atherosclerosis, resulting in increased LV afterload. We previously reported that arterial stiffness, indicated by pulse pressure, was closely associated with prognosis in patients with HFpEF.[3] Furthermore, elevated pulse pressure could affect cardiac structural changes, leading to eccentric LV hypertrophy and diastolic dysfunction.[18] We also demonstrated significant associations of high pulse pressure with E/e’ and BNP levels, which are established indicators of cardiac overload in HF. This suggests a close involvement of pulse pressure in the pathophysiology of HFpEF. Therefore, stiffness of relatively large arteries contributes to the cause of HFpEF. Progression of arterial stiffness can progress systemic atherosclerosis, resulting in PVD. Furthermore, we previously demonstrated that peripheral endothelial dysfunction, as assessed by reactive hyperemia peripheral arterial tonometry, was independently correlated with future cardiovascular events, adding incremental clinical significance for risk stratification in patients with HFpEF.[19] Because vascular endothelial dysfunction reflects an early systemic atherosclerotic change, this study supports the association between the early phase of systemic atherosclerosis and HFpEF. Taken together these data from our previous and present studies, we provided a novel insight of the clinical impact of systemic atherosclerotic change on the pathophysiology of HFpEF. However, the further study is required to demonstrate the details of the mechanism of how systemic atherosclerotic progression affects the pathophysiology of HFpEF.
This study has several limitations. First, this was a single-center study with a relatively small number of patients. We undertook post hoc power analyses with adjustment of multiple comparisons with the log-rank test to compare the prevalence of each event (data not shown). We found that the sample size was sufficient for comparisons regarding total cardiovascular events. In contrast, the power was <80% for all comparisons regarding each event (cardiovascular death, hospitalization for HF decompensation, coronary-related events, and non-fatal ischemic stroke) because the number of patients was dispersed in analyses of these events. Second, all of the patients in our study were Japanese, which might limit regional factors of our findings to other cohorts. Third, we cannot conclude whether the prognosis of HFpEF is improved by the treatment for PVD because this study was not intervention study. Therefore, large studies involving many patients and many races will be required to address these issues. Fourth, we do not have the data about the etiology of hospitalizations owing to decompensated HF at follow-up. Therefore, we could not conclude what kind of decompensated HF was induced by PVD. Fifth, in this study, the HFpEF patients with PVD showed a significant higher rate of both CAD and PAD. Because the risk of CAD and PAD is equivalent, it is hard to distinguish whether the increased cardiovascular events observed in HFpEF patients with PVD are related to preexisted CAD or PVD. Furthermore, the significance of this study might be attenuated because of quite limited number of HFpEF patients with PVD. Sixth, we cannot mention the impact on cardiovascular outcomes whether subclinical or silent vascular disease affects the results in patients with HFpEF because this study included only clinical vascular diseases.
In conclusion, the prevalence of PVD is related to cardiovascular events in HFpEF, indicating the clinical importance of screening PVD in patients with HFpEF for risk stratification. Assessment of not only CAD but also of systemic atherosclerotic diseases is required to treat patients with HFpEF.
Author contributions
Conceptualization: Koichiro Fujisue and Eiichiro Yamamoto.
Data curation: Koichiro Fujisue, Takanori Tokitsu, Daisuke Sueta, Masanori Takae, Taiki Nishihara, Fumi Oike, Miwa Ito, Hisanori Kanazawa, Hiroki Usuku, Satoshi Araki, and Kota Motozato.
Formal analysis: Koichiro Fujisue, and Eiichiro Yamamoto.
Investigation: Koichiro Fujisue, Takanori Tokitsu, Daisuke Sueta, Masanori Takae, Taiki Nishihara, Fumi Oike, Miwa Ito, Hisanori Kanazawa, Hiroki Usuku, Satoshi Araki, and Kota Motozato, Yasuhiro Izumiya, Satoru Suzuki, Kenji Sakamoto, and Koichi Kaikita.
Methodology: Koichiro Fujisue.
Project administration: Koichiro Fujisue, Eiichiro Yamamoto, Kenichi Tsujita.
Supervision: Kenichi Tsujita.
Writing – original draft: Koichiro Fujisue.
Writing – review & editing: Koichiro Fujisue, Eiichiro Yamamoto, Seiji Takashio, Yuichiro Arima, Yasuhiro Izumiya, Kenji Sakamoto, Koichi Kaikita, Kenichi Tsujita.
Footnotes
Abbreviations: ABI = bilateral ankle-brachial index, BNP = B-type natriuretic peptide, CAD = coronary artery disease, CAG = coronary angiography, CVD = cerebrovascular disease, E/e’ = early transmitral flow velocity to early diastolic mitral annular velocity, eGFR = estimated glomerular filtration rate, HFpEF = heart failure with preserved left ventricular ejection fraction, hsCRP = high-sensitivity C-reactive protein, LVEF = left ventricular ejection fraction, NYHA = New York Heart Association, PAD = peripheral artery disease, PVD = polyvascular disease.
KF is the first author.
The authors report no conflicts of interest.
References
- [1].Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- [2].Komajda M, Carson PE, Hetzel S, et al. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE). Circ Heart Fail 2011;4:27–35. [DOI] [PubMed] [Google Scholar]
- [3].Tokitsu T, Yamamoto E, Hirata Y, et al. Clinical significance of pulse pressure in patients with heart failure with preserved left ventricular ejection fraction. Eur J Heart Fail 2016;18:1353–61. [DOI] [PubMed] [Google Scholar]
- [4].Tokitsu T, Yamamoto E, Oike F, et al. Clinical significance of brachial-ankle pulse-wave velocity in patients with heart failure with preserved left ventricular ejection fraction. J Hypertens 2018;36:560–8. [DOI] [PubMed] [Google Scholar]
- [5].Maeda H, Sugiyama S, Jinnouchi H, et al. Advanced peripheral microvascular endothelial dysfunction and polyvascular disease in patients with high cardiovascular risk. J Cardiol 2016;67:455–62. [DOI] [PubMed] [Google Scholar]
- [6].Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–50. [DOI] [PubMed] [Google Scholar]
- [7].Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30:1527–33. [DOI] [PubMed] [Google Scholar]
- [8].Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93. [DOI] [PubMed] [Google Scholar]
- [9].Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- [10].Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. Jama 2006;295:180–9. [DOI] [PubMed] [Google Scholar]
- [11].Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007;45suppl S:S5–67. [DOI] [PubMed] [Google Scholar]
- [12].Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–92. [DOI] [PubMed] [Google Scholar]
- [13].Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–53. [DOI] [PubMed] [Google Scholar]
- [14].Ushigome R, Sakata Y, Nochioka K, et al. Temporal trends in clinical characteristics, management and prognosis of patients with symptomatic heart failure in Japan—report from the CHART Studies. Circ J 2015;79:2396–407. [DOI] [PubMed] [Google Scholar]
- [15].Takada T, Sakata Y, Miyata S, et al. Impact of elevated heart rate on clinical outcomes in patients with heart failure with reduced and preserved ejection fraction: a report from the CHART-2 Study. Eur J Heart Fail 2014;16:309–16. [DOI] [PubMed] [Google Scholar]
- [16].Imori Y, Akasaka T, Ochiai T, et al. Co-existence of carotid artery disease, renal artery stenosis, and lower extremity peripheral arterial disease in patients with coronary artery disease. Am J Cardiol 2014;113:30–5. [DOI] [PubMed] [Google Scholar]
- [17].Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 2003;107:714–20. [DOI] [PubMed] [Google Scholar]
- [18].Boutouyrie P, Laurent S, Girerd X, et al. Common carotid artery stiffness and patterns of left ventricular hypertrophy in hypertensive patients. Hypertension 1995;25:651–9. [DOI] [PubMed] [Google Scholar]
- [19].Akiyama E, Sugiyama S, Matsuzawa Y, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 2012;60:1778–86. [DOI] [PubMed] [Google Scholar]


