Abstract
Background.
In the US, benzodiazepine overdose deaths increased at an alarming rate in the past two decades. Benzodiazepines were also the most common drugs involved in prescription opioid overdose deaths. Benzodiazepine prescribing has been monitored by Prescription Drug Monitoring Programs (PDMPs), but little was known about whether PDMPs reduced drug overdose deaths involving benzodiazepines.
Design and Methods.
This study used a difference-in-difference design with state-quarter aggregate data on drug overdose deaths. The primary data source was Mortality Multiple Cause Files in 1999–2016. Three age-adjusted rates of drug overdose deaths were examined, including those involving benzodiazepines, those involving prescription opioids, and those involving both benzodiazepines and prescription opioids. The policy variables included PDMP data access for benzodiazepines and mandatory use of PDMP data for benzodiazepines. Linear multivariable regressions were used to assess the associations of PDMP policies specific to benzodiazepines with drug overdose death rates, controlling for other state-level policy and socioeconomic factors, state and time fixed effects, and state-specific time trends.
Results.
No significant associations were found between PDMP data access for benzodiazepines and changes in drug overdose death rates involving benzodiazepines and/or prescription opioids. Similarly, no significant associations were found between mandatory use of PDMP data for benzodiazepines and changes in drug overdose death outcomes.
Discussion and Conclusions.
This study suggested no evidence that PDMP policies specific to benzodiazepines were associated with reduction in benzodiazepine overdose death rates. Future research is warranted to examine detailed features of PDMPs and continuously monitor the impacts of PDMP policies on benzodiazepine-related consequences.
Keywords: Prescription Drug Monitoring Programs, benzodiazepines, prescription opioids, drug overdose
1. Introduction
Benzodiazepines are a class of psychoactive drugs widely used in the treatment of anxiety, insomnia, and seizures. They are Schedule IV drugs under the Controlled Substances Act in the US. Inappropriate and long-term use of benzodiazepines is associated with detrimental or even life-threatening consequences, such as drug dependence, cognitive decline, reduced mobility, and increased risks of dementia, falls, and car crashes, especially among the elderly. [1–3] Co-use of benzodiazepines and opioids is particularly dangerous as co-use sedates users, impairs cognitive functions, and may cause overdose deaths by suppressing breathing. [4–8]
In the past two decades, the US has seen an alarmingly increasing trend of benzodiazepine use. During 1996–2013, the number of US adults filling a benzodiazepine prescription grew by 67% from 8.1 million to 13.5 million and the quantity of benzodiazepines filled rose from 1.1 kg to 3.6 kg lorazepam-equivalents per 100,000 adults. [9] In parallel with the increasing trend of benzodiazepine use, drug overdose deaths involving benzodiazepines increased nearly 10 fold from 1,135 to 10,684 during 1999–2016. [10] Co-prescription of benzodiazepines and opioids was common in clinical practice and increased significantly in the past two decades. [11–15] Benzodiazepines were involved in approximately 30% of opioid overdose deaths as the most common concurrent drugs. [16, 17]
In the US, Prescription Drug Monitoring Programs (PDMPs) were designed to monitor prescribing and dispensing information of prescription drugs which are also controlled drugs. PDMPs could help providers identify patients with excessive use of drugs and inappropriate co-use of drugs (e.g., benzodiazepines and prescription opioids). The elements of PDMP varied across states and over time in terms of intended users, included drugs, law enforcement, and mandates on prescriber registration, clinical circumstances, and frequency of data queries. As of 2019, 49 states and the District of Columbia have implemented PDMPs in some form. Because of the long-standing concern about the deterioration of the opioid epidemic, PDMPs were considered a major effort to curb the overprescribing of prescription opioids (mostly Schedules II and III drugs in the US). Most existing studies evaluating the impacts of PDMPs focused on prescription opioids related outcomes. Although the effects of the implementation of PDMPs or access to PDMP data were mixed, recent studies suggested that the mandatory use of PDMP data was promising in reducing opioid prescribing and related mortality. [18–32]
Less attention has been given to the relationships between PDMPs and benzodiazepine-related consequences. A few existing studies found no evidence that the implementation of PDMPs or access to PDMP data was associated with benzodiazepine-related emergency department visits, substance abuse treatment admissions, or overdose death rates. [20, 33, 34] Only three studies examined the impacts of the mandatory use of PDMP data on benzodiazepine prescribing or benzodiazepine-related morbidity and mortality and the results were mixed. A study reported that the mandatory use of PDMPs was associated with a statistically significant reduction in the quantity of benzodiazepines dispensed in a single state (Ohio). [35] Two studies using national data suggested that the mandatory use of PDMPs had no associations with substance abuse treatment admissions related to benzodiazepines, [33] but was associated with decreased overdose deaths involving benzodiazepines. [20]
Among the studies reporting the associations between PDMPs and benzodiazepine-related outcomes, only two studies examined PDMP policies specific to benzodiazepines [34, 35] whereas others focused on PDMP policies specific to prescription opioids. Within a state, benzodiazepines and prescription opioids may have very different dates when the associated PDMP data became available to prescribers and very different dates when prescribers were mandated to check the associated PDMP records. Most states included Schedule IV drugs (benzodiazepines are Schedule IV drugs) in PDMPs later than Schedule II/III drugs (prescription opioids are mostly Schedules II/III drugs). The states also often mandated PDMP data use for benzodiazepines later than that for prescription opioids. Failure to make distinctions between PDMP policies specific to benzodiazepines and PDMP policies specific to prescription opioids will generate measurement errors in policy variables of interest.
In this study, we tested two hypotheses about state PDMP policies specific to benzodiazepines. First, we examined whether PDMP policies, including both PDMP data access and mandatory use of PDMP data specific to benzodiazepines, were associated with reduction in drug overdose deaths involving benzodiazepines. Second, we examined whether these PDMP policies specific to benzodiazepines were associated with reduction in drug overdose deaths involving prescription opioids and those involving both benzodiazepines and prescription opioids. Because co-use of benzodiazepines and prescription opioids was not recommended in clinical practice, [36, 37] PDMP policies specific to benzodiazepines may have the potential to influence drug overdose deaths involving both drugs.
2. Methods
2.1. Data
The primary data source was the National Vital Statistics System’s (NVSS) restricted use Mortality Multiple Cause Files that provided information on nearly all deaths occurring within the US. The data were based on death certificates each of which contained a single underlying cause of death, up to twenty multiple causes of death, and demographic data of the deceased. Causes of death have been classified in accordance with the International Classification of Diseases, Ninth Revision (ICD-9) before 1999 and Tenth Revision (ICD-10) since 1999. The study period in this study was restricted to 1999–2016 to ensure coding consistency.
2.2. Measures
We analyzed three state-quarter level drug overdose death outcomes: (i) drug overdose deaths involving benzodiazepines, (ii) drug overdose deaths involving prescription opioids, and (iii) drug overdose deaths involving both benzodiazepines and prescription opioids. The drug overdose death cases were aggregated to state-quarter level and age-adjusted to obtain rates per 100,000 population in each state and quarter.
The 3 drug overdose death outcomes were constructed in the following steps as recommended in previous research [10]. First, we identified drug overdose deaths by underlying cause death with ICD-10 codes of unintentional drug poisoning (X40-X44), suicide drug poisoning (X60-X64), homicide drug poisoning (X85), or drug poisoning of undetermined intent (Y10-Y14) [10]. Second, we further identified drug overdose deaths involving specific drugs using multiple causes of death. Specifically, an overdose death involving benzodiazepines was identified if the multiple causes of death contained benzodiazepines (ICD-10 code T42.4). An overdose death involving prescription opioids was identified if the multiple causes of death contained prescription opioids (ICD-10 codes T40.2–40.3). Other synthetic narcotics (other than methadone) (ICD-10 code T40.4) were not included, as this category was dominated by fentanyl-related overdoses, especially in recent years [10]. An overdose death involving both benzodiazepines and prescription opioids was identified if the multiple causes of death contained both benzodiazepines (ICD-10 code T42.4) and prescription opioids (ICD-10 codes T40.2–40.3). Third, the overdose death cases identified above were aggregated to the state-quarter level. For each state-quarter pair, age-adjusted death rates per 100,000 population were calculated by applying age-specific death rates to the year 2000 US standard population by age groups to facilitate comparisons over time. Following previous studies, [20, 30] Florida was excluded from this study. Florida implemented multiple policies (e.g., pill mill laws) along with PDMP data access at the same time [38] in response to its escalating opioid overdose crisis [39]. The changes in drug overdose death rates involving prescription opioids therefore could not be solely attributed to PDMPs [40].
The policy variables of interests were 2 PDMP policies specific to benzodiazepines. The first policy variable was access to PDMP records for benzodiazepines, a dichotomous indicator taking value of 1 if PDMP records for benzodiazepines were accessible in that state-quarter and 0 otherwise. The second policy variable was mandatory use of PDMP data for benzodiazepines, a dichotomous indicator taking value of 1 if the state mandated prescribers to query PDMP data before prescribing benzodiazepines under certain clinical circumstances in that state-quarter and 0 otherwise. We also included a third dichotomous variable indicating the enactment of PDMP laws. All the policy dates related to PDMPs were extracted from namsdl.org and pewtrusts.org (Table S1). By the end of the study period, all states but Missouri had passed PDMP laws. Except for Nevada and Utah which made benzodiazepine records available earlier than 1999, all other states with PDMPs made benzodiazepine records accessible during the study period. Among these states, a total of 18 states further implemented mandates on PDMP data use for benzodiazepines during the study period.
2.3. Statistical Analyses
The analysis used a difference-in-difference design with observations at state-quarter pair level. As a quasi-experimental method, difference-in-difference design is widely used in policy evaluation studies to reduce bias from confounding factors when randomized controlled trials are not feasible [41]. It compares the changes in outcomes over time between states with and without a policy exposure, such that time-invariant confounding factors and certain time-variant confounding factors can be controlled for. It has been the most commonly adopted method for studies evaluating PDMP impacts [18, 26, 28, 30, 42–45]. After excluding Florida, the study sample included 3,600 state-quarter pairs. Specifically, linear multivariable regressions were used to assess the associations of PDMP data access and mandatory use of PDMP data with age-adjusted drug overdose deaths involving benzodiazepines, prescription opioids, and both drugs, respectively. The outcomes were log transformed to obtain normal distributions of errors. To retain observations with zero values after log transformation, 0.01 was added to all outcomes following previous research [20].
We estimated two model specifications. Model 1 included the presence of PDMP data access for benzodiazepines as the primary policy predictor. Model 2 further added the indicator for mandatory use of PDMP data for benzodiazepines to Model 1. The standard errors in all regression models were clustered at the state level. Models 1 and 2 had the identical number of state-quarter observations (n=3600).
Both models 1 and 2 controlled for the enactment of PDMP laws and other state-level policy and socioeconomic covariates, which might confound the relationships between PDMP policies and overdose deaths. The following covariates were included: a dichotomous indicator for the implementation of Medicaid expansion to provide insurance to all adults with income up to 138% of the federal poverty level, the number of active physicians per 1,000 population, poverty rate (the percentage of residents with household income below the federal poverty level), median household income in 2016 constant dollars (in thousands), and unemployment rate.
Both models also included the following regressors: state indicators to control for unobserved time-invariant state-level fixed effects; year indicators and quarter indicators to control for national-level shocks applying to all the states at the same time; and state-specific linear time trends to control for state-level natural trends in outcomes. The examples of national-level shocks were new national guidelines about benzodiazepine prescribing and FDA warnings on benzodiazepines prescriptions. In 2016, the Centers for Disease Control and Prevention (CDC) issued new guidelines that recommended clinicians to avoid prescribing benzodiazepines with opioids [37]. In the same year, Food and Drug Administration (FDA) required both benzodiazepines and prescription opioids to carry “black box” warnings on the prescription labels to highlight the dangers of co-use [36]. These polices were assumed to influence all the states at the same time and could be captured by year and quarter indicators. We therefore did not add specific variables to control for these national-level shocks in regressions.
We further conducted a series of event studies. Event studies could test the parallel time trends assumption, the key assumption in difference-in-difference method that requires parallel time trends in outcomes prior to the policy exposure. It can also estimate lagged effects of the policy after the policy has been implemented. Two sets of events studies were conducted. The first set of event studies included 16 dummy variables indicating the 8 quarters before and the 8 quarters after the effective dates of PDMP data access. This event study model specification was the same as Model 1 except that the single indicator for PDMP data access was replaced by the 16 indicators for the quarters before and after the policy. The second set of event studies was similar to the first set, except that the 16 quarter indicators were relative to the effective dates of mandatory use of PDMP data. Any significant coefficients for quarters before the policy (PDMP data access or mandatory use of PDMP data) would indicate a violation of the parallel time trends assumption, whereas any significant coefficients for quarters after the policy would indicate policy lagged effects.
To test the robustness of the results, we conducted a series of sensitivity analyses. First, some states implemented PDMP policies for benzodiazepines and prescription opioids at the same time, and some implemented PDMP policies specific to benzodiazepines after the policies specific to prescription opioids. The main analysis included PDMP policies specific to benzodiazepines only. In sensitivity analysis, we added PDMP policies specific to prescription opioids and pill mill laws for prescription opioids to examine PDMP policies specific to benzodiazepines on top of these policies specific to prescription opioids. Second, regressions were conducted in negative binomial models instead of linear models. The outcomes were counts of drug overdose deaths instead of continuous age-adjusted drug overdose death rates. Negative binomial models were selected to account for over-dispersion of count data. As count data were not population-adjusted, state population in 100,000 was further controlled for in the negative binomial model along with other covariates in the main analysis. Third, as recommended by previous studies, [30] West Virginia was further excluded from the main analysis as its number of drug overdose deaths was an outlier in the high end. Lastly, we added 0.001 and 0.0001 instead of 0.01 to all the outcomes before log transformation to test the sensitivity of results to the small values added to zero value observations for log transformation.
This study used publicly available secondary data through a data use agreement and Institutional Review Board approval was not required.
3. Results
Table 1 presents the descriptive statistics of outcomes, PDMP policy variables, and other state covariates. States with PDMP data access had significantly higher drug overdose death rates involving benzodiazepines, prescription opioids, and both (P < 0.001), compared to states without PDMP data access. States with PDMP data access also had significantly lower level of physicians per 1,000 population, higher poverty rate, lower median household income, and higher unemployment rate (P < .001). A greater proportion of states with PDMP data access expanded Medicaid (P < 0.001). Similar patterns were observed when states with and without mandatory use of PDMP data were compared.
Table 1.
Descriptive Statistics of Variables by State PDMP Policies (Mean/Proportion and 95% Confidence Intervals).
| States with PDMP Data Access for Benzodiazepines | States without PDMP Data Access for Benzodiazepines | Between-state Difference P-value | States with Mandatory Use of PDMPs for Benzodiazepines | States without Mandatory Use of PDMPs for Benzodiazepines | Between-state Difference P-value | |
|---|---|---|---|---|---|---|
| Age-adjusted Drug Overdose Death Rate Involving Benzodiazepines per 100,000 population | 2.93 (2.76, 3.10) |
1.03 (0.98, 1.08) |
<0.001 | 3.94 (3.65, 4.24) |
1.64 (1.55, 1.72) |
<0.001 |
| Age-adjusted Drug Overdose Death Rate Involving Prescription Opioids per 100,000 population | 5.96 (5.75, 6.18) |
3.03 (2.94, 3.13) |
<0.001 | 7.33 (6.90, 7.76) |
3.98 (3.87, 4.10) |
<0.001 |
| Age-adjusted Drug Overdose Death Rate Involving Both Benzodiazepines and Prescription Opioids per 100,000 population | 1.91 (1.78, 2.03) |
0.62 (0.59, 0.66) |
<0.001 | 2.40 (2.20, 2.61) |
1.04 (0.98, 1.10) |
<0.001 |
| PDMP Data Access for Benzodiazepines | 1 | 0 | 1 | 0.36 (0.34, 0.38) |
<0.001 | |
| Mandatory Use of PDMP Data for Benzodiazepines | 0.17 (0.15, 0.19) |
0 | <0.001 | 1 | 0 | |
| Physicians per 1,000 people, n | 2.51 (2.48, 2.54) |
2.82 (2.76, 2.88) |
<0.001 | 2.56 (2.47, 2.64) |
2.70 (2.66, 2.74) |
0.05 |
| Poverty Rate, % | 13.58 (13.40, 13.76) |
12.01 (11.87, 12.15) |
<0.001 | 15.22 (14.78, 15.65) |
12.45 (12.34, 12.56) |
<0.001 |
| Median Household Income, 2016 Dollars | 55,189 (54,742, 55,636) |
57,872 (57,506, 58,238) |
<0.001 | 52,797 (51,853, 53,741) |
57,090 (56,793, 57,387) |
<0.001 |
| Unemployment Rate, % | 6.31 (6.19, 6.42) |
5.31 (5.23, 5.38) |
<0.001 | 6.19 (5.93, 6.45) |
5.67 (5.61, 5.74) |
<0.001 |
| Medicaid Expansion as Part of Affordable Care Act | 0.21 (0.18, 0.23) |
0.0084 (0.0045, 0.012) |
<0.001 | 0.47 (0.41, 0.53) |
0.059 (0.051, 0.067) |
<0.001 |
Notes: Please see Table S1 for the list of states with and without PDMP data access, and states with and without mandatory use of PDMP data. Florida was excluded from this study.
Table 2 reports main analysis results of Models 1 and 2. In Model 1, no significant associations were found between PDMP data access and changes in overdose death rates involving benzodiazepines or prescription opioids or both drugs. In Model 2, no significant associations were found between mandatory use of PDMP data and changes in any overdose death outcomes, after controlling for PDMP data access.
Table 2.
Linear Regression Results for Overdose Death Rates, 1999–2016.
| Logged Drug Overdose Death Rate involving Benzodiazepines | Logged Drug Overdose Death Rate involving Prescription Opioids | Logged Drug Overdose Death Rate involving Benzodiazepines and Prescription Opioids | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Coefficients (95% Confidence Intervals) | ||||||
| PDMP Data Access for Benzodiazepines | −0.17 (−0.42, 0.080) |
−0.17 (−0.42, 0.076) |
−0.070 (−0.20, 0.064) |
−0.067 (−0.20, 0.066) |
−0.23 (−0.52, 0.054) |
−0.24 (−0.52, 0.046) |
| Mandatory Use of PDMP Data for Benzodiazepines | −0.12 (−0.50, 0.26) |
0.11 (−0.10, 0.32) |
−0.17 (−0.49, 0.15) |
|||
| PDMP Enactment | 0.17 (−0.21, 0.55) |
0.17 (−0.21, 0.54) |
−0.11 (−0.28, 0.064) |
−0.11 (−0.28, 0.064) |
0.14 (−0.28, 0.57) |
0.14 (−0.28, 0.57) |
| Medicaid Expansion as Part of Affordable Care Act | 0.16 (−0.23, 0.56) |
0.17 (−0.22, 0.56) |
0.16 (−0.055, 0.37) |
0.15 (−0.056, 0.36) |
0.012 (−0.41, 0.44) |
0.024 (−0.40, 0.45) |
| Number of Active Physicians per 1,000 Population | 0.012 (−0.016, 0.040) |
0.011 (−0.017, 0.039) |
0.0035 (−0.011, 0.018) |
0.0036 (−0.011, 0.019) |
0.016 (−0.012, 0.043) |
0.015 (−0.012, 0.043) |
| Poverty Rate | −0.012 (−0.045, 0.022) |
−0.011 (−0.044, 0.022) |
0.0058 (−0.021, 0.033) |
0.0051 (−0.022, 0.032) |
0.0049 (−0.041, 0.051) |
0.0059 (−0.040, 0.052) |
| Median Household Income in Thousand Dollars | 0.010 (−0.017, 0.038) |
0.010 (−0.017, 0.037) |
−0.0054 (−0.022, 0.011) |
−0.0052 (−0.022, 0.011) |
0.023 (−0.0034, 0.050) |
0.023 (−0.0035, 0.050) |
| Unemployment Rate | 0.070 (−0.060, 0.20) |
0.071 (−0.060, 0.20) |
−0.00094 (−0.066, 0.064) |
−0.0022 (−0.064, 0.060) |
0.052 (−0.080, 0.18) |
0.054 (−0.081, 0.19) |
| Constant | −2.18* (−3.84, −0.52) |
−2.18* (−3.83, −0.52) |
−0.54 (−1.66, 0.58) |
−0.55 (−1.66, 0.57) |
−3.52*** (−5.24, −1.81) |
−3.52*** (−5.23, −1.81) |
| Number of State-Quarter Observations | 3600 | 3600 | 3600 | 3600 | 3600 | 3600 |
| Overall R2 | 0.60 | 0.60 | 0.67 | 0.67 | 0.65 | 0.65 |
P < .05,
P < .001
Notes: Data were analyzed at state-quarter level. All regressions were also controlled for state indicators, year indicators, quarter indicators, and state-specific time trends. Standard errors were clustered at state level. Florida was excluded from this study.
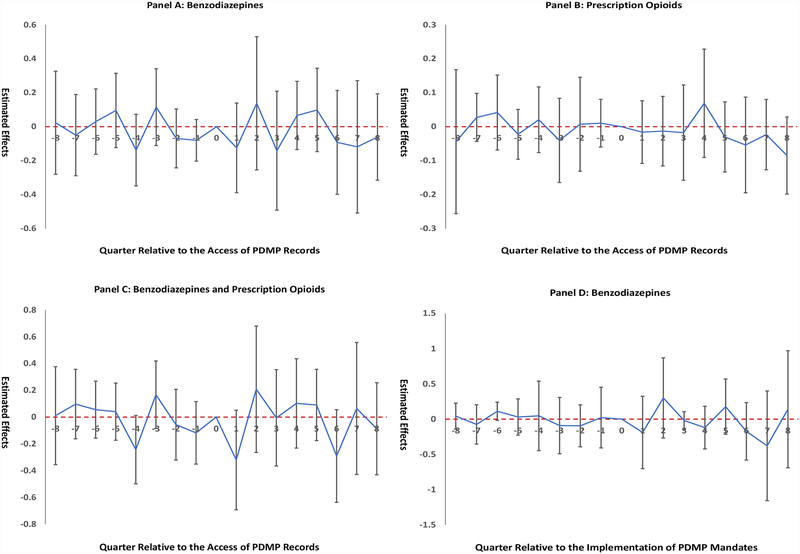
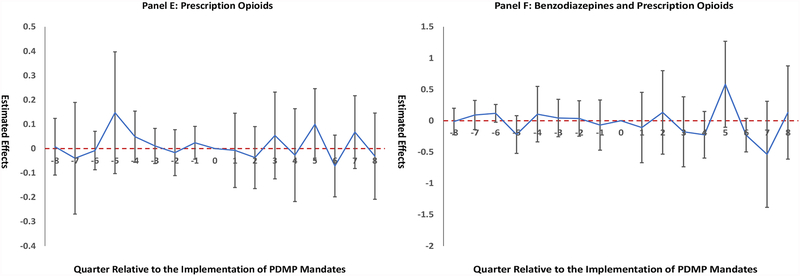
Results for event studies are illustrated in Figure 1. We did not observe significant differences in time trends before PDMP data access (Panels A-C) or the mandatory use of PDMP data (Panels D-F). The lack of significant pre-policy trends did not reject the parallel trends assumption. Figure 1 also indicates null relationships between PDMP policies and changes in drug overdose death rates in the post-policy period, consistent with findings in the main analysis.
Figure 1. Event Study Results.
Notes: “0” on the x-axis, indicating the quarter when policy adopted, was omitted from the regression to provide a reference category. The positive numbers on the x-axis indicate the number of quarters after policy implementation, and negative numbers indicate the number of quarters before policy implementation. Panels A-C present event studies for PDMP data access to benzodiazepine records. Panels D-F present event studies for mandatory use of PDMP data for benzodiazepines. Nonsignificant coefficients on the y-axis for quarters prior to policy implementation indicate parallel time trends prior to policy implementation between states with and without the policy. Florida was excluded from this study.
Sensitivity analyses results are reported in Tables S2–S4. After adding policies specific to prescription opioids, the associations between PDMP policies specific to benzodiazepines and changes in drug overdose death outcomes remained nonsignificant (Table S2). In Table S3, when negative binomial models were applied, the results were similar to main analysis results that used linear regressions with log transformation. The associations between PDMP policies specific to benzodiazepines and changes in drug overdose death outcomes also remained nonsignificant after excluding West Virginia (Table S4) and adding different small values to outcomes for log transformation (not reported).
4. Discussion
This study examined PDMP policies specific to benzodiazepines. It provided no evidence that PDMP data access or mandatory use of PDMP data was associated with reduction in drug overdose deaths involving benzodiazepines in 1999–2016. Furthermore, no evidence was found for the associations between these policies and reduction in drug overdose deaths involving prescription opioids or those involving both benzodiazepines and prescription opioids.
This study added to the limited literature about the impacts of PDMP policies on benzodiazepine-related health outcomes. Our findings were consistent with previous studies which found null associations of the implementation of PDMPs or access to PDMP data with changes in benzodiazepine-related emergency department visits, substance abuse treatment admissions, and overdose death rates [20, 33, 34]. Regarding mandatory use of PDMP data, our results contradicted a previous study which suggested significant associations between the mandatory use of PDMP data and changes in benzodiazepine overdose death rates [20]. The discrepancies might be explained by differences in study periods, classification of policies, and analytical approaches. We examined a longer period of time and differentiated PDMP policies specific to benzodiazepines and those specific to prescription opioids. We further controlled for several state-level covariates that may confound the relationships of interest.
PDMP policies specific to benzodiazepines might not have reduced drug overdose deaths involving benzodiazepines during the study period for several reasons. PDMP data access might have little impacts if prescribers of benzodiazepines used PDMPs infrequently. Because of the top public health concern on the opioid crisis, law and clinical enforcements have largely focused on opioid prescribing. Even for opioid prescribing, physicians reported challenges to integrating PDMPs into their workflow, and there were limited regulatory mechanisms on the mandatory use of PDMP data. [46, 47] Furthermore, prescribers interpreted PDMP data with little guidance. Only some PDMPs sent proactive alerts to help identify patients with overlapping benzodiazepine and opioid prescriptions. [48] Lastly, mandates on PDMP data use were only adopted by a few states late in the study period. The impacts of these mandates might not be realized and observed in our study.
This study had several limitations. First, this study used an ecological study design, the results of which should not be interpreted as causal relationships. Although we attempted to adjust for important state-level confounders with the difference-in-difference approach, there might be residual confounding left uncontrolled for. Second, this study examined state-level associations with state aggregate data. The regressions did not control for individual-level variations and the results do not infer individual-level responses to PDMP policy changes such as physicians’ prescribing behaviors and patients’ drug use behaviors. Third, because of data constraints we were not able to control for detailed PDMP features, such as the frequency of PDMP data updates. The infrequent data updates in early years of PDMPs might lead to underutilization of PDMP data by prescribers and partially contribute to the null results detected in this study. Recent research, however, suggested that specific features of PDMPs may have stronger protective effects on prescription opioids in recent years [25]. Future research should investigate the impacts of PDMP detailed features on benzodiazepine outcomes. Fourth, the reporting of causes of death might be inconsistent and inaccurate in the Mortality Multiple Cause Files. Particularly, drug overdose deaths might be underreported due to the lack of toxicological tests or failure to record test results on the death certificates. [17] But this limitation was not expected to confound our results unless the reporting errors of causes of death were related to PDMP policies.
In conclusion, we found no evidence that PDMP data access or mandatory use of PDMP data for benzodiazepines had associations with reduction in drug overdose deaths involving benzodiazepines and/or prescription opioids. Continuous monitoring and future research on the impacts of PDMP features on benzodiazepine prescribing and related consequences, especially at the individual level, are warranted.
Supplementary Material
Acknowledgements:
The Mortality Multiple Cause Files data were obtained through a data use agreement.
Role of Funding Source: This research was supported by grant R01DA042290 (PI: Shi) from the National Institute on Drug Abuse. This article is the sole responsibility of the authors and does not reflect the views of the National Institute on Drug Abuse.
Footnotes
Conflict of Interest: The authors declared no conflict of interest.
Contributor Information
Di Liang, Department of Family Medicine and Public Health, University of California San Diego, La Jolla, CA, USA.
Yuyan Shi, Department of Family Medicine and Public Health, University of California San Diego, La Jolla, CA, USA.
References
- [1].de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ 2012;345:e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Madhusoodanan S, Bogunovic OJ. Safety of benzodiazepines in the geriatric population. Expert Opin Drug Saf 2004;3(5):485–493. [DOI] [PubMed] [Google Scholar]
- [3].Smink BE, Egberts AC, Lusthof KJ, et al. The relationship between benzodiazepine use and traffic accidents. CNS Drugs 2010;24(8):639–653. [DOI] [PubMed] [Google Scholar]
- [4].Hernandez I, He M, Brooks MM, et al. Exposure-Response Association Between Concurrent Opioid and Benzodiazepine Use and Risk of Opioid-Related Overdose in Medicare Part D Beneficiaries. JAMA Netw Open 2018;1(2):e180919–e180919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kan CC, Hilberink SR, Breteler MH. Determination of the main risk factors for benzodiazepine dependence using a multivariate and multidimensional approach. Compr Psychiat 2004;45(2):88–94. [DOI] [PubMed] [Google Scholar]
- [6].Garg RK, Fulton-Kehoe D, Franklin GM. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med Care 2017;55(7):661–668. [DOI] [PubMed] [Google Scholar]
- [7].White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction 1999;94(7):961–972. [PubMed] [Google Scholar]
- [8].Park TW, Saitz R, Ganoczy D, et al. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ 2015;350:h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health 2016;106(4):686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].NIDA. Overdose Death Rates. Available at https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed on October 11, 2018 2018.
- [11].Hirschtritt ME, Delucchi KL, Olfson M. Outpatient, combined use of opioid and benzodiazepine medications in the United States, 1993–2014. Prev Med Rep 2018;9:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McClure FL, Niles JK, Kaufman HW, et al. Concurrent use of opioids and benzodiazepines: evaluation of prescription drug monitoring by a United States Laboratory. J Addict Med 2017;11(6):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paulozzi LJ, Strickler GK, Kreiner PW, et al. Controlled substance prescribing patterns—prescription behavior surveillance system, eight states, 2013. MMWR-Morbid Mortal W 2015;64(SS09):1–14. [DOI] [PubMed] [Google Scholar]
- [14].Hwang CS, Kang EM, Kornegay CJ, et al. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002− 2014. Am J Prev Med 2016;51(2):151–160. [DOI] [PubMed] [Google Scholar]
- [15].Sun EC, Dixit A, Humphreys K, et al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017;356:j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kandel DB, Hu M-C, Griesler P, et al. Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depen 2017;178:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, united states, 2010. JAMA 2013;309(7):657–659. [DOI] [PubMed] [Google Scholar]
- [18].Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Affair 2016;35(6):1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Brady JE, Wunsch H, DiMaggio C, et al. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep 2014;129(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meinhofer A Prescription drug monitoring programs: The role of asymmetric information on drug availability and abuse. Am J Health Econ 2018;4(4):504–526. [Google Scholar]
- [21].Li G, Brady JE, Lang BH, et al. Prescription drug monitoring and drug overdose mortality. Inj Epidemiol 2014;1(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maughan BC, Bachhuber MA, Mitra N, et al. Prescription monitoring programs and emergency department visits involving opioids, 2004–2011. Drug Alcohol Depen 2015;156:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moyo P, Simoni‐Wastila L, Griffin BA, et al. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 US States. Addiction 2017;112(10):1784–1796. [DOI] [PubMed] [Google Scholar]
- [24].Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med 2011;12(5):747–754. [DOI] [PubMed] [Google Scholar]
- [25].Pauly N, Slavova S, Delcher C, et al. Features of prescription drug monitoring programs associated with reduced rates of prescription opioid-related poisonings. Drug Alcohol Depen 2018;184:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yarbrough CR. Prescription drug monitoring programs produce a limited impact on painkiller prescribing in Medicare Part D. Health Serv Res 2018;53(2):671–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buchmueller TC, Carey C. The effect of prescription drug monitoring programs on opioid utilization in medicare. Am Econ J-Econ Polic 2018;10(1):77–112. [Google Scholar]
- [28].Dowell D, Zhang K, Noonan RK, et al. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Affair 2016;35(10):1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haffajee RL, Mello MM, Zhang F, et al. Four states with robust prescription drug monitoring programs reduced opioid dosages. Health Affair 2018;37(6):964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Patrick SW, Fry CE, Jones TF, et al. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Affair 2016;35(7):1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fink DS, Schleimer JP, Sarvet A, et al. Association Between Prescription Drug Monitoring Programs and Nonfatal and Fatal Drug Overdoses. Ann Intern Med 2018;168:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Finley EP, Garcia A, Rosen K, et al. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res 2017;17(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grecu AM, Dave DM, Saffer H. Mandatory Access Prescription Drug Monitoring Programs and Prescription Drug Abuse. J Policy Anal Manag 2018. [PubMed] [Google Scholar]
- [34].Bachhuber MA, Maughan BC, Mitra N, et al. Prescription monitoring programs and emergency department visits involving benzodiazepine misuse: Early evidence from 11 United States metropolitan areas. Int J Drug Policy 2016;28:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Winstanley EL, Zhang Y, Mashni R, et al. Mandatory review of a prescription drug monitoring program and impact on opioid and benzodiazepine dispensing. Drug Alcohol Depen 2018;188:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].FDA. FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use. 2016. [DOI] [PubMed]
- [37].Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rutkow L, Chang H-Y, Daubresse M, et al. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Int Med 2015;175(10):1642–1649. [DOI] [PubMed] [Google Scholar]
- [39].Delcher C, Wang Y, Wagenaar AC, et al. Prescription and Illicit Opioid Deaths and the Prescription Drug Monitoring Program in Florida. Am J Public Health 2016;106(6):e10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Delcher C, Wagenaar AC, Goldberger BA, et al. Abrupt decline in oxycodone-caused mortality after implementation of Florida’s Prescription Drug Monitoring Program. Drug Alcohol Depen 2015;150:63–68. [DOI] [PubMed] [Google Scholar]
- [41].Wing C, Simon K, Bello-Gomez RA. Designing Difference in Difference Studies: Best Practices for Public Health Policy Research. Annu Rev Public Health 2018;39:453–469. [DOI] [PubMed] [Google Scholar]
- [42].Popovici I, Maclean JC, Hijazi B, et al. The effect of state laws designed to prevent nonmedical prescription opioid use on overdose deaths and treatment. Health Econ 2018;27(2):294–305. [DOI] [PubMed] [Google Scholar]
- [43].Wen H, Schackman BR, Aden B, et al. States With Prescription Drug Monitoring Mandates Saw A Reduction In Opioids Prescribed To Medicaid Enrollees. Health Aff (Millwood) 2017;36(4):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nam YH, Shea DG, Shi Y, et al. State prescription drug monitoring programs and fatal drug overdoses. Am J Manag Care 2017;23(5):297–303. [PubMed] [Google Scholar]
- [45].Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: Evidence from the National Survey of Drug Use and Health. Addictive behaviors 2017;69:65–77. [DOI] [PubMed] [Google Scholar]
- [46].Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA 2015;313(9):891–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hildebran C, Cohen DJ, Irvine JM, et al. How clinicians use prescription drug monitoring programs: a qualitative inquiry. Pain Med 2014;15(7):1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Geissert P, Hallvik S, Van Otterloo J, et al. High-risk prescribing and opioid overdose: prospects for prescription drug monitoring program–based proactive alerts. Pain 2018;159(1):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.