Abstract
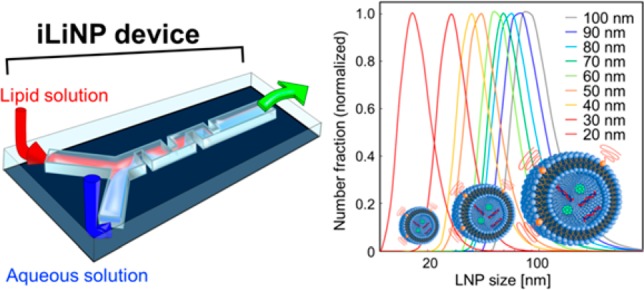
The precise size control of the lipid nanoparticle (LNP)-based nanodrug delivery system (DDS) carriers, such as 10 nm size tuning of LNPs, is one major challenge for the development of next-generation nanomedicines. Size-controlled LNPs would realize size-selective tumor targeting and deliver DNA and RNA to target tumor tissues effectively by passing through the stromal cells. Herein, we developed a baffle mixer device named the invasive lipid nanoparticle production device, or iLiNP device for short, which has a simple two-dimensional microchannel and mixer structure, and we achieved the first reported LNP size tuning at 10 nm intervals in the size range from 20 to 100 nm. In comparison with the conventional LNP preparation methods and reported micromixer devices, our iLiNP device showed better LNP size controllability, robustness of device design, and LNP productivity. Furthermore, we prepared 80 nm sized LNPs with encapsulated small interfering RNA (siRNA) using the iLiNP device; these LNPs effectively performed as nano-DDS carriers in an in vivo experiment. We expect iLiNP devices will become novel apparatuses for LNP production in nano-DDS applications.
Introduction
Development of nanometer-sized drug carriers for nanodrug delivery systems (DDSs) is expected to improve the biodistribution and delivery efficiency of drugs and mitigate side effects. Nano-DDS technology makes it possible to overcome the problems of current DDS-based medications and provides a breakthrough for next-generation chemo- and gene therapies including tailor-made medicines.1 Although several types of nano-DDS carriers2−6 have been developed and showed excellent performance for in vivo and in vitro experiments, lipid nanoparticles (LNPs) are the most widely used nanocarriers for cancer treatment.7
The size of the nanocarriers including LNPs affects the DNA and RNA delivery efficiency to target organs because large-sized nanocarriers have low permeability to stromal-rich tumors.8−12 In contrast, small-sized nanocarriers show good permeability to stromal-rich tumors. In other words, stromal cells surrounding the tumor tissues are a barrier to the delivery of nanomedicines, and the interval between the stromal cells depends on the kinds of tumors. Therefore, precisely size-controlled LNPs, such as 10 nm size tuned LNPs, could provide size-selective targeting to various tumors. The LNP size tuning at 10 nm intervals is one of the most significant challenges in the development of next-generation LNP-based nano-DDS carriers; however, it has not been achieved in any LNP preparation methods.
Microfluidic devices enable rapid LNP preparation and have the potential to satisfy requirements for both a small amount of sample consumption and control of the LNP size.13−15 In particular, the production of LNPs ranging from 20 to 100 nm, which show high penetration efficiency to the target tumors, was easily achieved by feeding the mixture of a lipid/alcohol solution and a DNA or RNA/buffer solution into a microfluidic device.11,16−24 Generally, LNP size is controlled by the flow conditions: flow rate of samples and flow rate ratio (FRR) of buffer to lipid solution. According to the literature, the chaotic mixer device is widely employed for LNP production to enhance the LNP size controllability.21−23,25 The chaotic mixer device has complicated three-dimensional grooved mixer structures that make the rapid mixing of solutions possible.26 However, LNPs easily clog the grooves of the chaotic mixers, and that leads to stagnation of sample flow. This is the major disadvantage of the chaotic mixer device for LNP production. Although the chaotic mixer device shows good LNP size controllability, its mixer design has low flexibility and robustness, especially regarding the microchannel aspect ratio, compared with two-dimensional mixer structures. This drawback affects the LNP size controllability and LNP productivity.27 Moreover, the LNP size tuning at 10 nm intervals has not been achieved, even if the chaotic mixer device was to be used for LNP preparation. For these reasons, development of the novel LNP production platform is strongly desired for production of next-generation LNP-based nano-DDS carriers, which realize the LNP size-selective tumor targeting.
In this study, we developed the two-dimensional baffle mixer device based on the LNP formation mechanism23,25,28 and a fluid dynamics simulation. We have named the baffle mixer device the invasive lipid nanoparticle production (iLiNP) device. It enabled the LNP size tuning at 10 nm intervals in the size range from 20 to 100 nm, whereas the chaotic mixer could not achieve the LNP size control. To our knowledge, this is the first report on the methodology for LNP size tuning at 10 nm intervals. Ten-nanometer tuned LNPs formed in the iLiNP device make LNP size-selective tumor targeting possible, and DNA and RNA can be delivered to target tumor tissues effectively by passing through the interval between the stromal cells. Furthermore, the iLiNP device had more flexibility and robustness of device design than the chaotic mixer devices. We also evaluated the utility of the iLiNP device for production of LNP-based nano-DDS carriers by an in vivo experiment. Eighty-nanometer-sized LNPs with encapsulated siRNA, which consisted of a pH-sensitive cationic lipid, PEGylated lipid, and cholesterol, were prepared with a narrower standard deviation of size than that of the conventional LNP production method. The siRNA-loaded LNPs were effectively delivered to hepatocytes of the extravascular region and showed good FVII gene-silencing activity.
Results and Discussion
Optimization of the Baffle Mixer Design and Its Fluid Dynamics Study
Figure 1(A) shows three-dimensional and top views of the basic structure iLiNP device. We defined the standard dimensions of the baffle mixer structure as the width (a) of 150 μm, the depth (b) of 100 μm, and the interval (c) of 100 μm. When there were 20 sets of the mixer structure, we called this the basic structure. The width and height of the microchannel were 200 and 100 μm. The baffle mixer design is similar to a zigzag-shaped microchannel29,30 and a meander microchannel.31 However, we found that the optimized baffle mixer structures (width (a), depth (b), and intervals (c)) only enabled generation of secondary flow under the high flow rate conditions (>100 μL/min), and this leads to rapid dilution of ethanol to produce the small and uniform sized LNPs. Unlike the chaotic mixer device (Figure 1B), the iLiNP device has a simple two-dimensional microchannel and mixer structure. In addition, the iLiNP device is expected to provide more effective mixing and dilution performance at the high flow rate condition compared with the chaotic mixer device from the viewpoint of fluid dynamics.30
Figure 1.
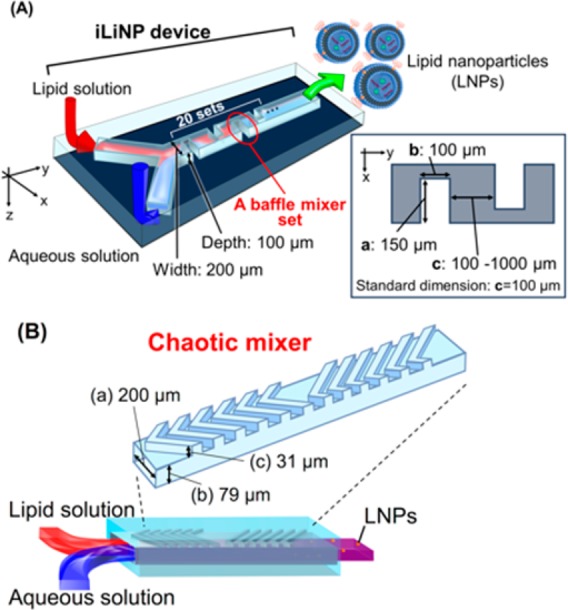
(A) Three-dimensional and top views of the iLiNP device with the basic structure of 20 baffle mixer structure sets. Standard dimensions of the baffle mixer structure were width (a) of 150 μm, depth (b) of 100 μm, and the interval (c) of 100 μm. The width and height of the microchannel were 200 and 100 μm. (B) Schematic illustration of the chaotic mixer device. The dimensions of microchannel and height of chaotic mixer structure were width (a) of 200 μm, flat channel height (b) of 79 μm, and mixer height (c) of 31 μm.
To confirm the dilution performance of the iLiNP device, we carried out computational fluid dynamics (CFD) simulation and fluid visualization experiments. Figure 2 shows the CFD simulation results of ethanol dilution in the iLiNP device at different flow rate conditions. We evaluated the dilution performance at the cross sections of the narrowed microchannel as shown in Figure 2A. Figure 2B compares CFD analysis results between the flow rates of 50 and 500 μL/min at the FRR of 3. The dilution performance was dramatically accelerated at 500 μL/min, and ethanol was completely diluted within 3 ms. The rapid ethanol dilution was enabled by the generation of the secondary flow at the baffle structure, as shown in Figure 2C. The secondary flow was only generated at the high flow rate condition, and there was no dependency on FRR. Controlling fluid characteristics is essential for the small-sized LNP production. According to the literature, small-sized LNPs were formed under the high flow rate condition and high FRR condition in microfluidic devices, regardless of the presence or absence of the chaotic mixers.25 However, we reported that the dilution performance of the chaotic mixer was deteriorated at the flow rate of 500 μL/min due to the mechanism of chaotic advection formation.23 We assumed that the iLiNP device achieved sufficient ethanol dilution within about 30 ms for producing the small-sized LNPs, regardless of the flow rate after passing through the 10th baffle mixer (Figure 2B), because rapid dilution of ethanol was more important than complete mixing of ethanol and saline.25Figure 2D–F shows the effects of dimensions of the baffle mixer structures where the width was (a), the depth was (b), and the interval was (c). At the flow rate of 500 μL/min and FRR of 3, the structures with shorter width (a) and shallower depth (b) dimensions had lower ethanol dilution efficiency than the baffle structure with the standard dimensions, even though the solutions were fed at high flow rate. Figure 2F shows the effect of baffle structure interval on the ethanol dilution performance. Increasing the baffle structure interval also deteriorated the ethanol dilution performance; however, the width and depth dimensions of baffle structures had a bigger effect on the ethanol dilution performance, more than the interval of the baffle structure.
Figure 2.
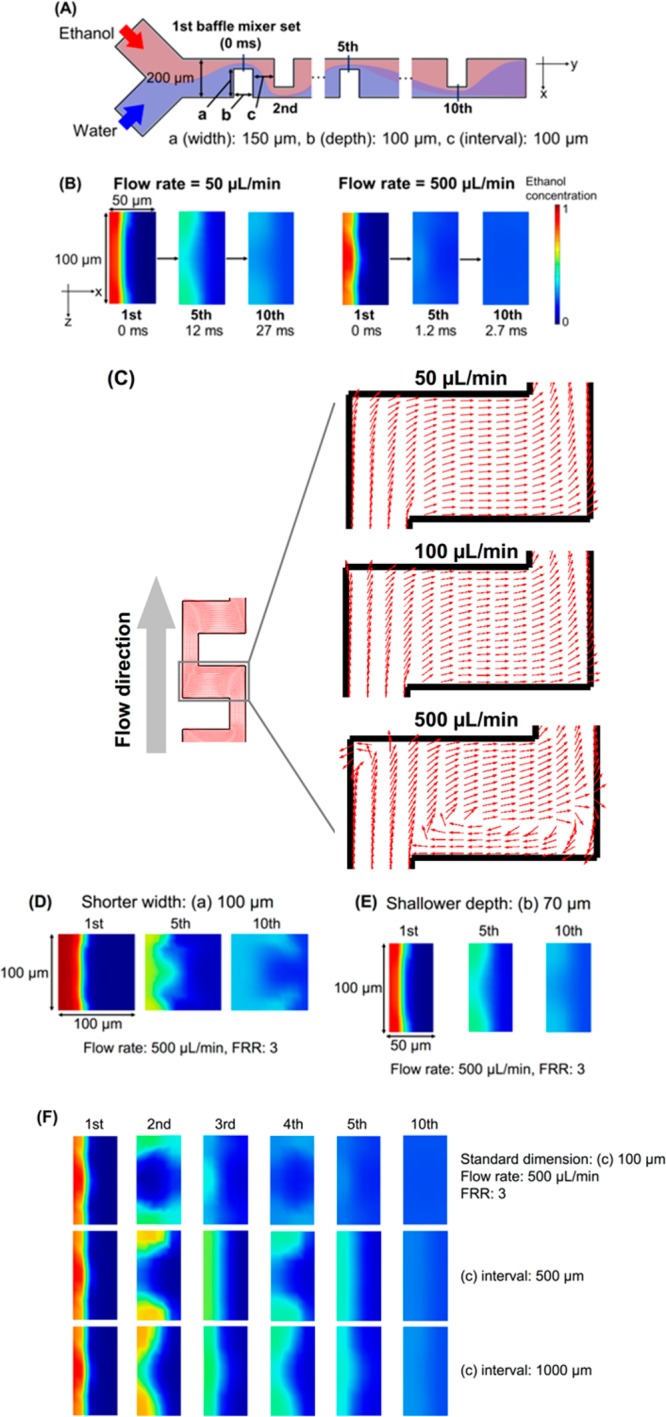
(A) Schematic illustration of the iLiNP device and part of the observation position for dilution performance. (B) Comparison of CFD simulation results for the flow rates of 50 μL/min (left) and 500 μL/min (right); flow rate ratio (FRR) of water to ethanol was 3 for both comparisons. The observation positions were the iLiNP device at the first, fifth, and tenth sets. Red and blue colors represent ethanol and water, respectively. (C) Top views of the baffle mixer region. The flow rate was varied from 50 to 500 μL/min, and FRR was 3. The arrows represent the streamlines. (D, E, F) CFD results for modified dimensions of the baffle mixer structure at 500 μL/min and FRR of 3: (D) (a) of 100 μm width, (E) (b) of 70 μm depth, and (F) (c) of 500 and 1000 μm intervals. Red and blue colors represent ethanol and water, respectively.
Then, we also performed a visualization experiment23 using a laser scanning confocal microscope for the basic structure iLiNP device. We prepared the 10 mg/mL DOPC containing 0.1 mol % Rho-PE ethanol solution and saline and confirmed that both solutions were completely mixed within 3 ms at the flow rate of 500 μL/min (Figure 3, inset). Figure 3 compares the mixing performance between the iLiNP device and the chaotic mixer device. We used the reported chaotic mixer device,23 and the flow rate was 500 μL/min and FRR 9. The results suggested that the iLiNP device was able to dilute ethanol more effectively than the chaotic mixer device at the high flow rate condition. We considered that the rapid dilution of ethanol using the iLiNP device made it possible to control the LNP size precisely and produce the small-sized LNPs.
Figure 3.
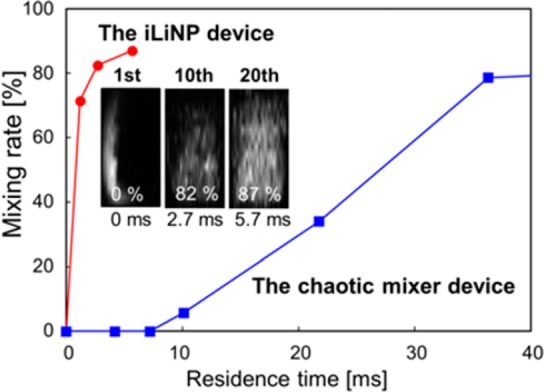
Comparison of the mixing performance for the iLiNP device and the chaotic mixer device (Figure 1B, right). These images were observed at the 1st, 10th, and 20th mixer set positions of the iLiNP device. The flow rate was 500 μL/min, and FRR was 9 (n = 1).
LNP Size Controllability of the iLiNP Devices
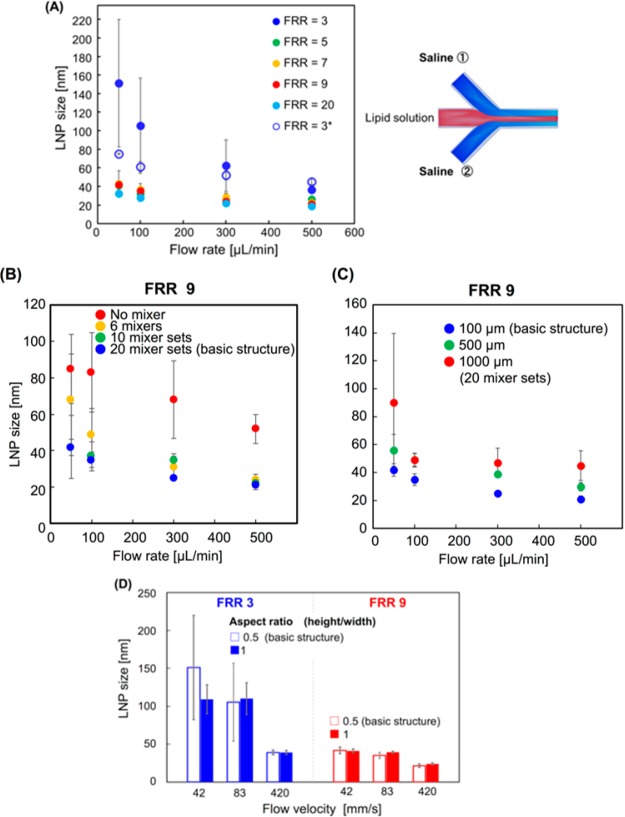
First, we attempted to demonstrate the possibility of the LNP size tuning at 10 nm intervals using the basic structure iLiNP device. We used 10 mg/mL of POPC solution and saline for the LNP production. The flow rate was varied from 50 to 500 μL/min, and FRR was from 3 to 9. Figure 4A shows the LNP size distributions, and the average LNP sizes ranged from 20 to 100 nm. The iLiNP device was able to precisely control the LNP size, and it achieved 10 nm size tuning of LNPs by changing the flow conditions and slightly modifying the microchannel design. This result indicated that the iLiNP device would offer good LNP size controllability in a wide LNP size range and very limited flow rate range. In fact, the iLiNP device produced LNPs in the size range of 20–40 nm at the flow rates of 50, 100, and 500 μL/min and FRR of 9, as shown in Figure 4B. Twenty-nanometer-sized LNPs are theoretically the smallest particle size in the lipid system, and these small sized LNPs would penetrate the target tissues and organs effectively. On the other hand, the chaotic mixer device produced the narrow range of LNPs sized from 30 to 40 nm, in spite of the same flow rate conditions.
Figure 4.
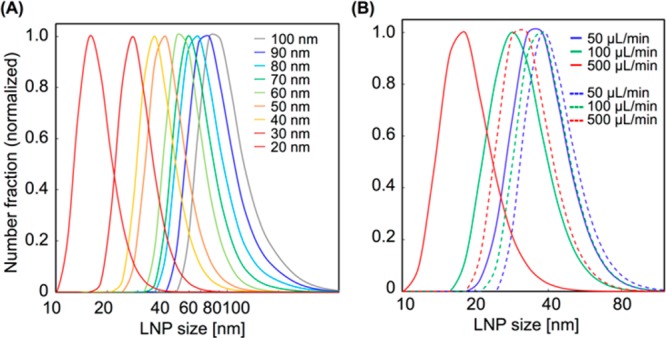
(A) Size distribution ranged from 20 to 100 nm for LNPs formed in the iLiNP device. The flow rate was varied from 50 to 500 μL/min, and FRRs were from 3 to 9. The sizes ranged from 60 to 80 nm for the LNPs formed in the sheath flow-type iLiNP device. (B) Comparison of the size distributions of LNPs formed in the iLiNP device (solid lines) and the chaotic mixer device (dashed lines). The flow rates were 50 to 500 μL/min and FRR was 9. The Y-axis was normalized with the maximum intensities of each value as 1.
Figure 5A shows the effect of the flow rate and FRR on the LNP size. Increasing flow rate and FRR led to small-sized LNP formation, the same as was observed for the chaotic mixer device.23 The basic structure iLiNP device was able to produce the LNPs except in the size range from 60 to 80 nm by simply controlling the flow rate and FRR. The standard deviations of LNP sizes produced at the low flow rate and FRR of 3 were slightly larger than that of the high flow rate and high FRR. The particle size polydispersity index (PDI) values were smaller than 0.1 without the LNPs produced at the 50 and 100 μL/min flow rates and FRR of 3. We assumed that the effect of secondary flow was deteriorated at the low flow rate condition and it reduced the dilution performance of ethanol. For this reason, the LNP size controllability was decreased at the low flow rate condition.
Figure 5.
(A) Effect of the flow rate and FRR on the LNP size using the iLiNP device. The flow rate is varied from 50 to 500 μL/min, and FRR range is from 3 to 20. For FRR = 3*, the LNP sizes formed in the sheath flow system are described on the right side. The error bars represent the standard deviation calculated from repeating each LNP formation experiment at least three times. (B) The effect of number of sets of the baffle structure on the LNP size. The numbers of the sets were 0 (no mixer), 6, 10, and 20. Flow rates were 50, 100, 300, and 500 μL/min, and FRR was 9. (C) Relationship between the baffle structure intervals and LNP size at different flow rate conditions. Twenty sets of the baffle mixers with different intervals were used for the experiments. (D) Effect of microchannel aspect ratio on LNP size controllability. FRRs were 3 (blue) and 9 (red). The error bars represent the standard deviation calculated from repeating each LNP formation experiment at least three times.
To enhance the LNP size controllability at the flow rate of 50 μL/min and FRR of 3, we modified the design of the flow system from the Y-shaped flow to sheath flow as shown in Figure 5A (right). The sheath flow system leads to the rapid consumption of lipid molecules dissolved in ethanol because of the increased liquid–liquid interface area. We previously reported the effect of lipid concentration on the LNP size.23 The LNP size was decreased with decreasing lipid concentration in ethanol. We observed similar LNP formation behavior by changing the flow system (sheath flow system) with low concentration of lipid, and we considered that increasing the liquid–liquid interface area allowed us to achieve the rapid dilution of ethanol and consumption of lipid molecules. The improved iLiNP device enabled the production of 60 to 80 nm sized LNPs with a narrow particle size distribution, and the size standard deviations also became small compared with those of the Y-shaped flow system, as shown in Figure 5A. Remarkably, the iLiNP device was able to control the LNP size range from 20 to 100 nm, whereas the chaotic mixer device produced LNPs in the size range from 30 to 80 nm at the same flow rate conditions. These results suggested that the LNP production performance, that is, the LNP size controllability and productivity of small-sized LNPs, of the iLiNP device and flexibility of the device design were higher than those of the chaotic mixer device due to the two-dimensional simple device geometries.
Effects of the Number of Baffle Structure Sets and Intervals on LNP Size
We fabricated other types of iLiNP devices: (A) the iLiNP device equipped with different numbers of the baffle mixer structure sets (0, 6, 10, 20 (designated as the basic structure)) arranged at the interval of 100 μm and (B) the iLiNP device with 20 baffle structure sets arranged at intervals of 100, 500, and 1000 μm. Figure 5B shows the effect of the number of baffle structure sets on the LNP size. The LNP size was decreased on increasing the flow rate the same as for the chaotic mixer device and microfluidic devices without the mixer structure. The iLiNP device showed good LNP size controllability at flow rates of 300–500 μL/min, regardless of the number of sets. In spite of the smaller number of sets, 20 nm sized LNPs formed at 500 μL/min. On the other hand, the microfluidic device with 6 baffle mixer sets produced the large-sized LNPs at the low flow rate condition due to the short dilution time of ethanol within the mixer region.
Figure 5C shows the relationship between the baffle structure intervals and LNP size at different flow rate conditions. Controlling the LNP size was enabled by changing arrangement intervals of 20 sets of baffle mixers. We confirmed that the iLiNP device was able to produce the LNP sizes from 20 to 60 nm with narrow standard deviations at the flow rate of 300–500 μL/min and FRR of 9. This result indicated that the LNP formation process was controllable by passing through the baffle mixer rapidly, and the baffle mixer was more suitable for rapid dilution under the high flow rate condition than the chaotic mixer device was. We considered that the baffle mixer arranged at a wide interval would also be useful for controlling the LNP size for other lipid systems including cationic lipid–PEGylated lipid mixtures at the high flow rate condition because the formation or aggregation kinetics are different from those of the simple POPC lipid system. Generally, the aggregation kinetics of the lipid system are faster than that of the POPC lipid system due to the electrostatic interaction between RNAs and cationic lipids, the small area of the lipid headgroup, and the addition of PEGylated lipids. Therefore, the iLiNP device provided the desired ethanol dilution rate for controlling the LNP size in a wide range at the constant flow rate condition by simply changing the baffle structure intervals. Consequently, we found that the minimum number of baffle mixer structure sets was 10, and they should be arranged at an interval of 100 μm to satisfy both the precise LNP size controllability and production of 20 nm sized LNPs.
Scale-Up Performance of the iLiNP Device for Mass Production
We demonstrated the scale-up performance of the iLiNP device for LNP-based nanomedicine mass production (Figure 5D). Differing from the chaotic mixer device, the iLiNP device has the two-dimensional simple microchannel geometry. We confirmed that the dimensions of the baffle structure, such as width, depth, and interval, played important roles for the LNP size control. We assumed the height of the microchannel and baffle mixer structure had good robustness for ethanol dilution performance. Then, we fabricated the iLiNP device with a 200 μm height microchannel and baffle mixers. The aspect ratio (height/width of the microchannel) of the device was calculated to be 1. As we expected from the viewpoint of fluid dynamics, the LNP size controllability did not change from the basic structure iLiNP device (aspect ratio: 0.5), and 20 nm sized LNPs formed at the flow velocity of 417 mm/s. Hood et al.27 reported the effect of the aspect ratio of the microchannel on the LNP size using the microfluidic device without mixer structures. Although the high aspect ratio microchannel showed similar LNP formation behavior to that of the low aspect ratio microchannel, the microchannels required a high flow rate of the aqueous phase, namely, FRR of 50 to 100, to produce 80 nm sized LNPs. For the DDS applications of the LNPs, the content of lipids to DNA or RNA must be adjusted to the optimal molar ratio to get the best therapeutic performance. Moreover, DNA and RNA, which are expensive materials for encapsulation into the LNPs, are dissolved into an aqueous phase. Thus, the LNP production should be performed at the low and constant FRR conditions to control the LNP composition and size range from 20 to 100 nm. In contrast, the iLiNP devices can produce a wide size range of LNPs at low FRR by changing the flow rate.
In the case of the chaotic mixer device, the deeper grooved mixer structure can accelerate the mixing performance.32 However, the LNPs clog the grooves of the chaotic mixers, regardless of the height of the grooved mixer structure. This is the major disadvantage of the chaotic mixer device for LNP production. Conversely, we did not observe any clogging of the LNPs, and there was no deterioration of ethanol dilution performance in the iLiNP device because of the two-dimensional microchannel, mixer dimensions, and effective generation of secondary flow. From these results, we believed that the iLiNP device would be an ideal apparatus for LNP-based nanomedicine production.
Applicability of the iLiNP Device for DDS
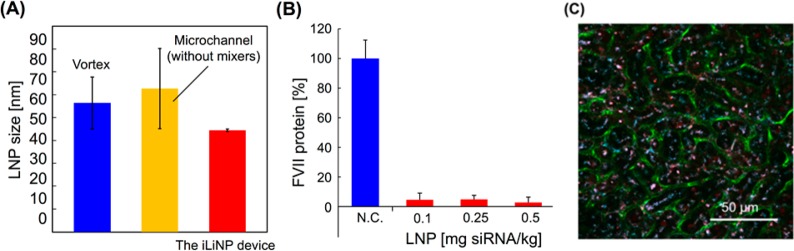
Finally, we demonstrated the applicability of the iLiNP device for DDS uses. We used a pH-sensitive cationic lipid (YSK05), cholesterol (chol), 1,2-dimirystoyl-sn-glycero, and methoxylene glycol 2000 ether (PEG-DMG) lipid system for production of LNPs encapsulating siRNA (siFVII).11 The YSK-LNP performance was evaluated by FVII gene silencing activity and intrahepatic distribution of the siRNA via an in vivo experiment. We produced the YSK-LNPs by introducing the solution of ethanol containing YSK05, chol, and PEG-DMG and the solution of 25 mM acetate buffer (pH 4.0) containing siRNA, into the iLiNP device. The concentration of total lipids was 8 mM, and the molar ratios of lipids were 50, 50, and 1 for YSK05, chol, and PEG-DMG, respectively. Figure 6A compares LNP sizes produced by the conventional vortex method, the microfluidic device without mixer structures, and the iLiNP device. In this experiment, we used the acetate buffer solution without siRNA as the aqueous phase. The iLiNP device was able to produce the 40 nm sized LNPs with smaller standard deviation of size compared with the other production methods. We previously reported the 40 nm sized LNPs formed in the chaotic mixer device at the flow rate of 1.5 mL/min and FRR of 3. However, 40 nm sized LNPs were produced at the flow rate of 500 μL/min and FRR of 3 using the iLiNP device. Thus, the iLiNP device achieved one-third sample consumption compared to the chaotic mixer device.11 This result indicated that the iLiNP device had sufficient performance to produce the small-sized LNPs, which can deliver siRNA to hepatocytes.
Figure 6.
Results of YSK-LNPs formed in the iLiNP device and in vivo experiments. (A) Comparison of the YSK-LNP sizes produced by the conventional vortex method, the microfluidic device without mixer structures, and the iLiNP device. The error bars represent the standard deviation calculated from repeating each YSK-LNP formation experiment at least three times. (B) FVII gene-silencing activity of siFVII-loaded YSK-LNPs at different doses to mice. N.C.: negative control. (C) Confocal microscopic images of the mice liver tissues. Blood vessels, LNPs, and siRNAs were visualized as green, cyan, and red, respectively.
Then, we prepared 80 nm sized siRNA-loaded YSK-LNPs using the iLiNP device and confirmed the encapsulation efficiency of siRNA was higher than 90%. We selected the LNP size as 80 nm because 80 nm sized LNPs can show both high gene-silencing activity and penetration efficiency.15Figures 6B and C show FVII gene-silencing activity of the 80 nm sized YSK-LNPs in vivo and the intrahepatic distribution of siRNA delivered by 80 nm sized YSK-LNPs. YSK-LNPs showed high FVII gene-silencing activity with no dependency on dose. Furthermore, we confirmed the 80 nm sized YSK-LNPs showed good siRNA delivery efficiency to hepatocytes by a confocal laser scanning microscopic observation of ICR mouse liver tissues. The confocal images indicated that the YSK-LNPs showed low accumulation in blood vessels, and siRNAs were specifically localized to the extravascular region where the hepatocytes were present. From these results, we demonstrated the utility of the iLiNP device for production of LNP-based nano-DDS applications.
Conclusion
In summary, we developed the iLiNP device for producing LNPs in the size range from 20 to 100 nm based on the LNP formation mechanism and the CFD simulation. The iLiNP device gave precise LNP size control at 10 nm intervals by changing not only the flow conditions but also the baffle mixer dimensions. We found that generation of the secondary flow in the iLiNP device was indispensable for controlling the LNP size and producing the small-sized LNPs. Twenty-nanometer-sized POPC LNPs, which are considered as the theoretically smallest particle size, were formed in the iLiNP device at 500 μL/min flow rate and FRR of 9, whereas the chaotic mixer device could not produce the 20 nm sized POPC LNPs at the same flow conditions. We demonstrated the scale-up performance and robustness of the baffle mixer and microchannel designs. We also evaluated the utility of the iLiNP device for production of the LNP-based nano-DDS carriers via the in vivo experiment. The siRNA-loaded LNPs prepared by the iLiNP device effectively delivered the siRNA to hepatocytes and showed good FVII gene-silencing activity. Differing from the chaotic mixer device, the iLiNP device showed high flexibility for the LNP production apparatus, because of the two-dimensional structure, and we could optimize the mixer dimensions depending on the lipid system. We believe that the iLiNP device is more useful than the chaotic mixer device to produce small-sized and size-controlled nanocarriers, not only LNPs but also polymeric micelles, nanogels, and dendrimers, and we expect the iLiNP device can be applied as a novel nano-DDS carrier production apparatus.
Methods
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1,2-diolleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dimirystoyl-sn-glycero, and methoxylene glycol 2000 ether (PEG-DMG) were purchased from the NOF Corporation (Tokyo, Japan). Cholesterol (Chol) was purchased from Sigma-Aldrich (St. Louis, MO, USA). 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rho-PE) was purchased from Avanti Polar Lipids, Inc. (810150C, Alabaster, AL, USA). Ethanol, sodium chloride, chloroform, acetic acid, 2-morpholinoethanesulfonic acid, monohydrate (MES), potassium chloride, and disodium hydrogen phosphate 12-water were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Sodium acetate and potassium dihydrogen phosphate were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). A pH-sensitive cationic lipid, YSK05, was synthesized as described previously.33 RiboGreen and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlate (DiI) were purchased from Molecular Probes (Eugene, OR, USA). FITC-conjugated Isolectin B4 was purchased from Vector Laboratories (Burlingame, CA, USA). All siRNA samples were purchased from Hokkaido System Science Co., Ltd. (Sapporo, Japan). The siFVII sense and antisense strand sequences are 5′-GGAucAucucAAGucuuAcTsT-3′ and 5′-GuAAGAcuuGAGAuGAuccTsT-3′, respectively. The Alexa Fluor-647 (AF647)-labeled siGL4 sense and antisense strand sequences are 5′-AF647-CCGUCGUAUUCGUGAGCAATsT-3′ and 5′-UUGCUCACGAAUACGACGGTsT-3′, respectively. 2′-Fluoro-modified nucleotides are represented in lower case, and the phosphorothioate linkage is represented as s.
Experimental Conditions for the CFD Simulation
Computational fluid dynamics (CFD) simulation was used to study dilution performance of ethanol in the iLiNP device. Concentration profiles of ethanol were numerically simulated with a three-dimensional model using COMSOL Multiphysics 5.2 (COMSOL, Inc., Burlington, MA). Physical properties of both ethanol and water were selected from the material library (database) in COMSOL. The dimensions of the geometric model for the basic structure iLiNP device were: 3 mm length, 0.2 mm width, and 0.1 mm height. The device design in the geometric model had ten sets of the baffle mixer structures. Mesh elements were created by Free-Quad 2D followed by sweeping the mesh elements (2D plane) to create the 3D model, and the size was selected as “Fine” from the “Predefined list”. The flow model selected incompressible flow Navier–Stokes equations. The laminar-flow model (no slip condition) and transport of the diluted species (ethanol) were used for simulation of the ethanol dilution process in the iLiNP device. The flow rate ranged from 50 to 500 μL/min, and the flow rate ratio (FRR; water flow rate to ethanol flow rate) was from 3 to 9.
For the fluid visualization experiment, we prepared 10 mg/mL of DOPC containing 0.1 mol % Rho-PE ethanol solution and saline. The solutions were introduced into the iLiNP device at the flow rate of 500 μL/min and FRR of 3. A laser scanning confocal microscope (A1R, Nikon, Tokyo, Japan) was used for the microscopic observation. The mixing efficiency was calculated by image analysis using ImageJ (NIH). Fluid visualization in the iLiNP device was carried out at the same experimental conditions as previously studied.23
iLiNP Device Fabrication
The master molds of the iLiNP devices were fabricated by the standard photolithography.34 The master molds were made from SU-8 3050 (Nippon Kayaku Co., Ltd., Tokyo, Japan). In brief, SU-8 was spin-coated onto a 3 in. silicon wafer using a spin coater (MS-A100, Mikasa Shoji, Co., Ltd., Tokyo, Japan) to obtain a 100 μm thick SU-8 layer on the wafer. This was followed by baking at 95 °C to evaporate the solvent for SU-8. The silicon wafer was aligned with a photomask and exposed to UV light using a mask aligner (M-1S, Mikasa Shoji). After baking to get a cross-linking reaction of SU-8, the wafer was soaked in an SU-8 developer. The replica and molding process for making the PDMS microfluidic devices (iLiNP devices) was described previously.23
Synthesis of LNPs
We employed two types of LNP systems, POPC LNPs and YSK-LNPs. For preparation of POPC LNPs, we prepared a 10 mg/mL POPC/ethanol solution and saline. For preparation of YSK-LNPs, a YSK05-based lipid/ethanol solution and 25 mM acetate buffer (pH 4.0) containing siRNA of 0.071 mg/mL were introduced into the iLiNP device. YSK05, chol, and PEG-DMG were dissolved in ethanol at a molar ratio of 50/50/1, and the total lipid concentration was adjusted to 8 mM. To make a comparison of the sizes of YSK-LNPs (Figure 6(A)) formed by the conventional vortex method and two microfluidic methods (one using a flat microchannel and the other using the iLiNP device), we used 25 mM acetate buffer not containing siRNA as the aqueous phase. These solutions were injected from separate syringes (GASTIGHT 1002, Hamilton Inc., Reno, NV, USA) into the iLiNP device by using syringe pumps (model 100, BAS Inc., Tokyo, Japan). The LNP-containing solutions were collected from the outlet in the microtubes. In the case of the YSK-LNPs, the collected YSK-LNPs were dialyzed for 2 h against 20 mM MES buffer (pH 6.0) followed by an overnight dialysis against phosphate-buffered saline (PBS) (pH 7.4) using a Spectra/Por 2 dialysis membrane standard RC tubing (molecular weight cutoff 12000–14000 Da; Spectrum Laboratories, Rancho Dominguez, CA). After dialysis, LNP solutions were stored in a refrigerator at 4 °C until the size measurement was carried out by dynamic light scattering (DLS) using a Zetasizer Nano ZS ZEN3600 instrument (Malvern Instruments, Worcestershire, UK).
In Vivo Experiments Using YSK-LNPs
We determined the encapsulation efficiency of siRNA using RiboGreen fluorescence assay. For the siRNA standard curve, 2 mg/mL of siRNA was diluted with 10 mM HEPES buffer (pH 7.4) to 500 ng/mL. LNP solutions were also diluted with 10 mM HEPES buffer to appropriate concentrations lower than 500 ng/mL. The diluted LNP solutions were applied to a 96-well microplate at a volume of 100 μL/well. We prepared two types of 10 mM HEPES buffer containing RiboGreen solutions in the presence or absence of 0.1 w/v % Triton X-100. Then, the solutions were added to each microwell applied to diluted LNP solutions at a volume of 100 μL/well. Fluorescence was measured with an Enspire 2300 Multilabel Reader (PerkinElmer, Aichi, Japan) with λEx = 500 nm and λEm = 525 nm after incubation at room temperature (700 rpm, 30 s). siRNA encapsulation efficiency was calculated from the following equation.
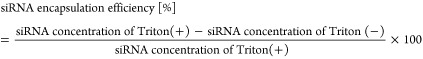 |
Triton(+) means the presence of TritonX-100, and Triton(−) means the absence of TritonX-100.
Measurement of Plasma Coagulation Factor VII (FVII) Activity
Four female ICR mice (4 weeks of age) were purchased from Japan SLC (Shizuoka, Japan) and intravenously injected with the siFVII/LNPs. Plasma FVII activity was measured using a Biophen FVII kit (Hyphen BioMed, Oise, France).
Observation of Intrahepatic siRNA Distribution
ICR mice were intravenously injected with DiI-labeled AF647-siGL4/LNPs at a dose of 0.5 mg of siRNA/kg. DiI-labeled LNPs were prepared by adding 1 mM DiI/ethanol solution to the LNP solution at a concentration of 40 μM DiI. Fifty minutes after injection of the LNPs, the mice were administered FITC-conjugated Isolectin B4 (40 μg/mouse), and liver tissues were collected after a 10 min incubation. Intrahepatic distribution of siRNA was observed using a Nikon A1 (Nikon Co. Ltd., Tokyo, Japan), and images were captured by a 40× objective lens.
Acknowledgments
This work was supported by JST CREST Grant Number JP17937657, Japan. A part of this work was also supported by the Tokyo Kasei Chemical Promotion Foundation. M. Maeki gratefully acknowledges the Nanotech CUPAL NRP program. The authors acknowledge the technical support provided by Dr. Kobayashi and the Nikon Imaging Center at Hokkaido University in the use of the confocal microscope system and carrying out the image analysis.
Author Contributions
¶ N.K. and M.M. contributed equally to this work.
The authors declare no competing financial interest.
References
- Tibbitt M. W.; Dahlman J. E.; Langer R. Emerging frontiers in drug delivery. J. Am. Chem. Soc. 2016, 138, 704–717. 10.1021/jacs.5b09974. [DOI] [PubMed] [Google Scholar]
- Peer D.; Karp J. M.; Hong S.; Farokhzad O. C.; Margalit R.; Langer R. Nanocarriers as an emerging platform foe cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- Kraft J. C.; Freeling J. P.; Wang Z.; Ho R. J. Y. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K.; Christie R. J.; Kataoka K. Polymeric micelles for nano-scale drug delivery. React. Funct. Polym. 2011, 71, 227–234. 10.1016/j.reactfunctpolym.2010.10.009. [DOI] [Google Scholar]
- Duncan R.; Vicent M. J. Polymer therapeutics-prospects for 21th century: The end of the beginning. Adv. Drug Delivery Rev. 2013, 65, 60–70. 10.1016/j.addr.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Zhai Y.; Wang J.; Zhai G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng., C 2016, 60, 560–568. 10.1016/j.msec.2015.11.041. [DOI] [PubMed] [Google Scholar]
- Pearce T. R.; Shroff K.; Kokkoli E. Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv. Mater. 2012, 24, 3803–3822. 10.1002/adma.201200832. [DOI] [PubMed] [Google Scholar]
- Lin Q.; Chen J.; Zhang Z.; Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine 2014, 9, 105–120. 10.2217/nnm.13.192. [DOI] [PubMed] [Google Scholar]
- Matsumura Y.; Maeda H. A new concept for macromolecular therapeutics in canser chemotherapy: mechanism of tumouritropic accumulation of proteins and the antitumour agent SMANCS. Cancer Res. 1986, 46, 6387–6392. [PubMed] [Google Scholar]
- Cabral H.; Matsumoto Y.; Mizuno K.; Chen Q.; Murakami M.; Kimura M.; Terada Y.; Kano M. R.; Miyazono K.; Uesaka M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- Sato Y.; Note Y.; Maeki M.; Kaji N.; Baba Y.; Tokeshi M.; Harashima H. Elucidation of the physicochemical properties and potency of siRNA-loaded small-sized lipid nanoparticles for siRNA delivery. J. Controlled Release 2016, 229, 48–57. 10.1016/j.jconrel.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Chen S.; Tam Y. Y. C.; Lin P. J. C.; Leung A. K. K.; Tam Y. K.; Cullis P. R. Development of lipid nanoparticle formulations of siRNA for hepatocyte gene silencing following subcutaneous administration. J. Controlled Release 2014, 196, 106–112. 10.1016/j.jconrel.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Tsui J. H.; Lee W.; Pun S. H.; Kim J.; Kim D. Microfluidic-assisted in vitro drug screening and carrier production. Adv. Drug Delivery Rev. 2013, 65, 1575–1588. 10.1016/j.addr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran R.; Sun Q.; Baby T.; Wibowo D.; Middelberg A. P. J.; Zhao C. Multiphase microfluidic synthesis of micro- and nanostructures for pharmaceutical applications. Chem. Eng. Sci. 2017, 169, 78–96. 10.1016/j.ces.2017.01.008. [DOI] [Google Scholar]
- Ozcelikkale A.; Moon H.; Linnes M.; Han B. In vitro microfluidic models of tumor microenvironment to screen transport of drugs nanoparticles. Nanomed. Nanobiotechnol. 2017, 9, e1460. 10.1002/wnan.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Zhang H.; Fontana F.; Hirvonen J. T.; Santos H. A. Microfluidic-assisted fabrication of carriers for controlled drug delivery. Lab Chip 2017, 17, 1856–1883. 10.1039/C7LC00242D. [DOI] [PubMed] [Google Scholar]
- Jahn A.; Stavis S. M.; Hong J. S.; Vreeland W. N.; DeVoe D. L.; Gaitan M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 2010, 4, 2077–2087. 10.1021/nn901676x. [DOI] [PubMed] [Google Scholar]
- Jahn A.; Reiner J. E.; Vreeland W. N.; DeVoe D. L.; Locascio L. E.; Gaitan M. Preparation of nanoparticles by continuous-flow microfluidics. J. Nanopart. Res. 2008, 10, 925–934. 10.1007/s11051-007-9340-5. [DOI] [Google Scholar]
- Carugo D.; Bottaro E.; Owen J.; Stride E.; Nastruzzi C. Liposome production by microfluidics: potential and limiting factors. Sci. Rep. 2016, 6, 25876. 10.1038/srep25876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeki M.; Kimura N.; Sato Y.; Harashima H.; Tokeshi M. Recent advances in microfluidics for liposomes, lipid nanoparticles, and extracellular vesicles toward application of drug delivery systems. Adv. Drug Delivery Rev. 2018, 10.1016/j.addr.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Belliveau N. M.; Huft J.; Lin P. J. C.; Chen S.; Leung A. K. K.; Leaver T. J.; Wild A. W.; Lee J. B.; Taylor R. J.; Tam Y. K.; et al. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther.--Nucleic Acids 2012, 1, e37. 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhigaltsev I. V.; Belliveau N.; Hafez I.; Leung A. K. K.; Huft J.; Hansen C.; Cullis P. R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir 2012, 28, 3633–3640. 10.1021/la204833h. [DOI] [PubMed] [Google Scholar]
- Maeki M.; Fujishima Y.; Sato Y.; Yasui T.; Kaji N.; Ishida A.; Tani H.; Baba Y.; Harashima H.; Tokeshi M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS One 2017, 12, e0187962. 10.1371/journal.pone.0187962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.; Love K. T.; Chen Y.; Eltoukhy A. A.; Kastrup C.; Sahay G.; Jeon A.; Dong Y.; Whitehead K. A.; Anderson D. G. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 2012, 134, 6948–6951. 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- Maeki M.; Saito T.; Sato Y.; Yasui T.; Kaji N.; Ishida A.; Tani H.; Baba Y.; Harashima H.; Tokeshi M. A strategy for synthesis of lipid nanoparticles using microfluidic devices with a mixer structure. RSC Adv. 2015, 5, 46181. 10.1039/C5RA04690D. [DOI] [Google Scholar]
- Stroock A. D.; Dertinger S. K. W.; Ajdari A.; Mezić I.; Stone H. A.; Whitesides G. M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- Hood R. R.; DeVoe D. L. High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small 2015, 11, 5790–5799. 10.1002/smll.201501345. [DOI] [PubMed] [Google Scholar]
- Shinoda W.; DeVane R.; Klein M. L. Zwitterionic Lipid Assemblies: Molecular Dynamics Studies of Monolayers, Bilayers, and Vesicles Using a New Coarse Grain Force Field. J. Phys. Chem. B 2010, 114, 6836–6849. 10.1021/jp9107206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengeaud V.; Josserand J.; Girault H. H. Mixing Processes in a Zigzag Microchannel: Finite Element Simulations and Optical Study. Anal. Chem. 2002, 74, 4279–4286. 10.1021/ac025642e. [DOI] [PubMed] [Google Scholar]
- Nguyen N. T.; Wu Z. Micromixers-a review. J. Micromech. Microeng. 2005, 15, R1–R16. 10.1088/0960-1317/15/2/R01. [DOI] [Google Scholar]
- Song H.; Tice J. D.; Ismagilov R. F. A Microfluidic System for Controlling Reaction Networks in Time. Angew. Chem., Int. Ed. 2003, 42, 768–772. 10.1002/anie.200390203. [DOI] [PubMed] [Google Scholar]
- Yang J. T.; Huang K. J.; Lin Y. C. Geometric effects on fluid mixing in passive grooved micromixers. Lab Chip 2005, 5, 1140–1147. 10.1039/b500972c. [DOI] [PubMed] [Google Scholar]
- Sato Y.; Hatakeyama H.; Sakurai Y.; Hyodo M.; Akita H.; Harashima H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Controlled Release 2012, 163, 267–276. 10.1016/j.jconrel.2012.09.009. [DOI] [PubMed] [Google Scholar]
- McDonald J. C.; Whitesides G. M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]