Abstract
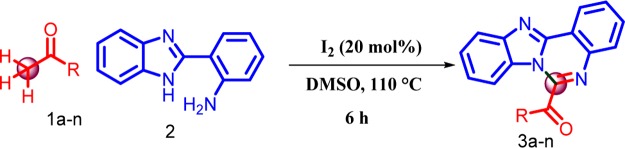
A general and efficient iodine-catalyzed metal-free oxidative cross-coupling reaction of methyl ketones with 2-(1H-benzo[d]imidazol-2-yl)aniline has been established. This is a new synthetic strategy for the synthesis of benzimidazo[1,2-c]quinazoline derivatives involving C(sp3)–H oxidation, condensation, and cyclization processes.
Introduction
The direct oxidative C–H bond functionalization has emerged as an effective tool for the construction of carbon–carbon and carbon–heteroatom bonds.1 Transition metals play vital role in oxidative C–H coupling reactions.2 Metal-free organic syntheses for the construction of C–C and C–X bonds are also popular.3 Molecular iodine is a popular catalyst in various organic transformations owing to its easy availability and inexpensive, nontoxic, eco-friendly, and nonmetallic nature.4 Additionally, molecular iodine has superfluous advantages as it has the lowest dissociation energy, no radioactivity, and a moderate redox potential.5 In 2010, Li et al. reported I2-mediated oxidative cyclization of enamines via iodide intermediates.6
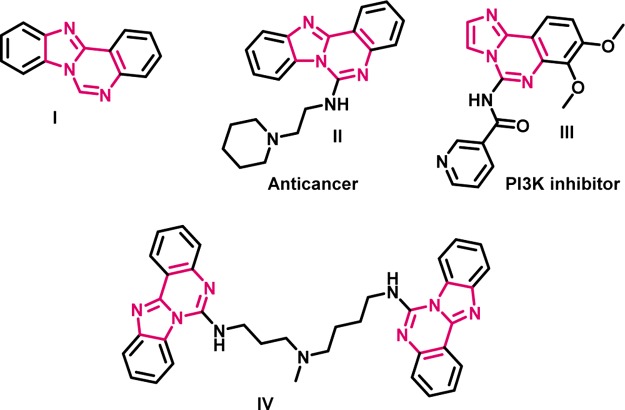
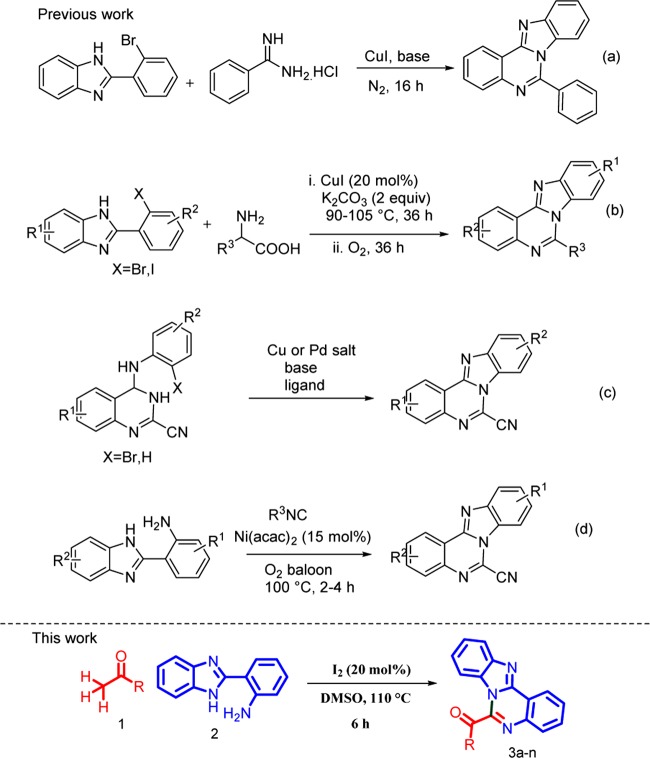
Nitrogen-containing heterocycles are ubiquitous scaffolds in numerous natural products, biomolecules, and organic materials.7 Benzo[4,5]imidazo[1,2-c]quinazoline is a trinitrogen heterocycle that hosts both the fused structure of benzimidazole and quinazoline via a shared bond.8 The combination of benzimidazole and quinazoline frameworks, benzimidazoquinazoline derivatives, are valuable substrates with different biological activities as shown in Figure 1 and benzoimidazo[1,2-c]quinazoline derivatives exhibit wide spectrum of therapeutic activities such as antimicrobial,9 antitumor,10 anticancer,11 antiviral,12 anti-inflammatory,13 and anticonvulsants.14 Due to the remarkable importance of these molecules, we planned to propose a new method for construction of benzoimidazo[1,2-c]quinazoline derivatives. A number of synthetic methodologies for construction of the compounds containing this core structure were reported. Hulme et al.15 reported a cesium carbonate-promoted three-component reaction, CuI-catalyzed Ullmann N-arylation.16 Fu et al. reported a copper-catalyzed cascade synthesis of benzimidazoquinazoline derivatives under mild conditions (Scheme 1a).17 Fu et al. developed a general method for the synthesis of benzoimidazo[1,2-c] quinazolines via copper-catalyzed reactions of substituted 2-(2-halophenyl)-1H-benzo[d]imidazoles with α-amino acids (Scheme 1b).18 Later, Koutentis and co-workers reported Cu- and Pd-catalyzed oxidative and nonoxidative C–N coupling reactions to give the corresponding products in high yields (Scheme 1c).19 Recently, Sarada et al. established a nickel-catalyzed aerobic oxidative isocyanide insertion reaction for the synthesis of a novel benzimidazoquinazolines via sequential double annulation cascade protocol (Schemes 1d and 2).20 All of the above reports suffer from drawbacks such as the use of prefunctionalized starting materials, harsh reaction conditions, longer reaction time, and metal catalyst. Therefore, the development of new approaches to the synthesis of benzimidazoquinazolines using simpler and more readily available starting materials under milder conditions is still highly desirable.
Figure 1.
Biologically important benzoimidazo[1,2-c]quinazolines.
Scheme 1. Recent Approaches for the Construction of Benzoimidazo[1, 2-c]quinazolines.
Scheme 2. Synthesis of Benzoimidazo Quinazolinones.
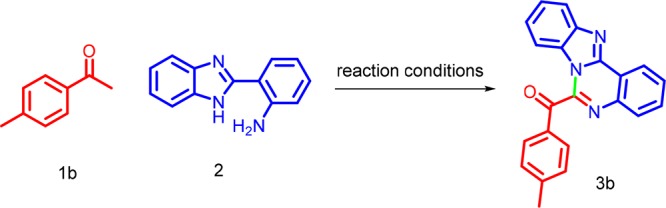
In continuation of our ongoing research to develope new routes for the construction of various heterocyclic systems,21 we investigated the reaction of 4-methylacetophenone 1b with 2-(1H-benzo[d]imidazol-2-yl) aniline 2 as model substrates for the present study. The reaction has been carried out under various reaction conditions, as depicted in Table 1. On treatment of 1b with 2 and an equivalent of iodine as the catalyst in N,N-dimethylacetamide (DMA) at 110 °C for 6 h, the desired product 3b was not formed (Table 1, entry 1). Further optimization was done by the use of other common organic solvents including N,N-dimethylformamide (DMF), toluene, N-methylpyrrolidone (NMP), acetonitrile, and DMSO (Table 1, entries 2–6), and DMSO has been proved to be the best solvent. Next, we tried the reaction in the absence of the catalyst in dimethyl sulfoxide (DMSO), but there was no product obtained even after long reaction time. Hence, additional optimization experiments were performed in the presence of various iodides such KI, NIS, TBAI, and NH4I in DMSO at 110 °C for 6 h. However, the desired product was not obtained (Table 1, entries 8–11). Further increase in the amount of iodine did not lead to significant differences in the yield (Table 1, entry 12). Further, we optimized the reaction in the presence of different stoichiometric amounts of iodine in DMSO (Table 1, entries 13–17), the corresponding product was obtained in 78, 80, 83, and 75% yield, respectively. The combination of iodine (20 mol %) and DMSO was found to be efficient for this transformation (Table 1, entry 16). So, all of the reactions were carried out under this reaction conditions.
Table 1. Optimization of Reaction Conditionsa.
| entry | catalyst (equiv) | solvent | temp | yield (%)b |
|---|---|---|---|---|
| 1 | I2 (1.0) | DMAc | 110 °C | NR |
| 2 | I2 (1.0) | DMFc | 110 °C | NR |
| 3 | I2 (1.0) | toluene | 110 °C | NR |
| 4 | I2 (1.0) | NMPc | reflux | NR |
| 5 | I2 (1.0) | CH3CN | reflux | NR |
| 6 | I2 (1.0) | DMSOc | 110 °C | 78 |
| 7 | − | DMSOc | 110 °C | NR |
| 8 | KI (1.0) | DMSO | 110 °C | NR |
| 9 | NIS (1.0) | DMSOc | 110 °C | NR |
| 10 | TBAI (1.0) | DMSOc | 110 °C | NR |
| 11 | NH4I (1.0) | DMSOc | 110 °C | NR |
| 12 | I2 (1.2) | DMSOc | 110 °C | 73 |
| 13 | I2 (0.8) | DMSOc | 110 °C | 78 |
| 14 | I2 (0.6) | DMSOc | 110 °C | 80 |
| 15 | I2 (0.4) | DMSOc | 110 °C | 80 |
| 16 | I2 (0.2) | DMSOc | 110 °C | 83 |
| 17 | I2 (0.1) | DMSOc | 110 °C | 75 |
Reaction conditions: 1b (1.0 equiv) additive and solvent (2 mL) are heated in a sealed tube at 110 °C for 6 h and then 2 (1.0 equiv) is added.
Yield of the isolated product.
DMF = N,N-dimethylformamide, DMA = N,N-dimethylacetamide, NMP = N-methylpyrrolidone, DMSO = dimethyl sulfoxide.
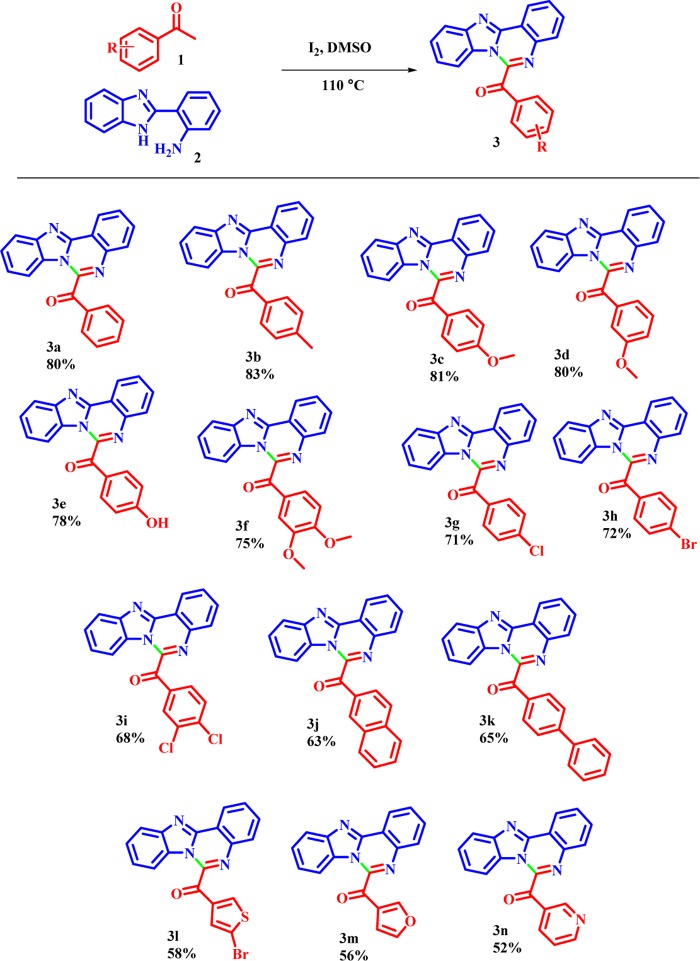
With the optimized reaction conditions in hand, we then investigated the substrate scope of the aryl methyl ketones, as shown in Scheme 3. Aryl methyl ketones bearing electron-donating and electron-withdrawing substituents were successfully converted directly into the corresponding products in moderate to good yields (73–83%; 3a–3h). Moreover, sterically hindered acetyl naphthalene has only little influence on the reaction efficiency (73–83%; 3j and 3k). Notably, heterocyclic methyl ketones also participated in the reaction affording the corresponding products 3n–3p in 59–62% yields, respectively.
Scheme 3. Substrate Scope of the Transformation,
Reaction conditions: 1 (1.0 equiv), 2 (1.0 equiv), and I2 (0.2 equiv) in DMSO (2 mL) at 110 °C.
Isolated yield.
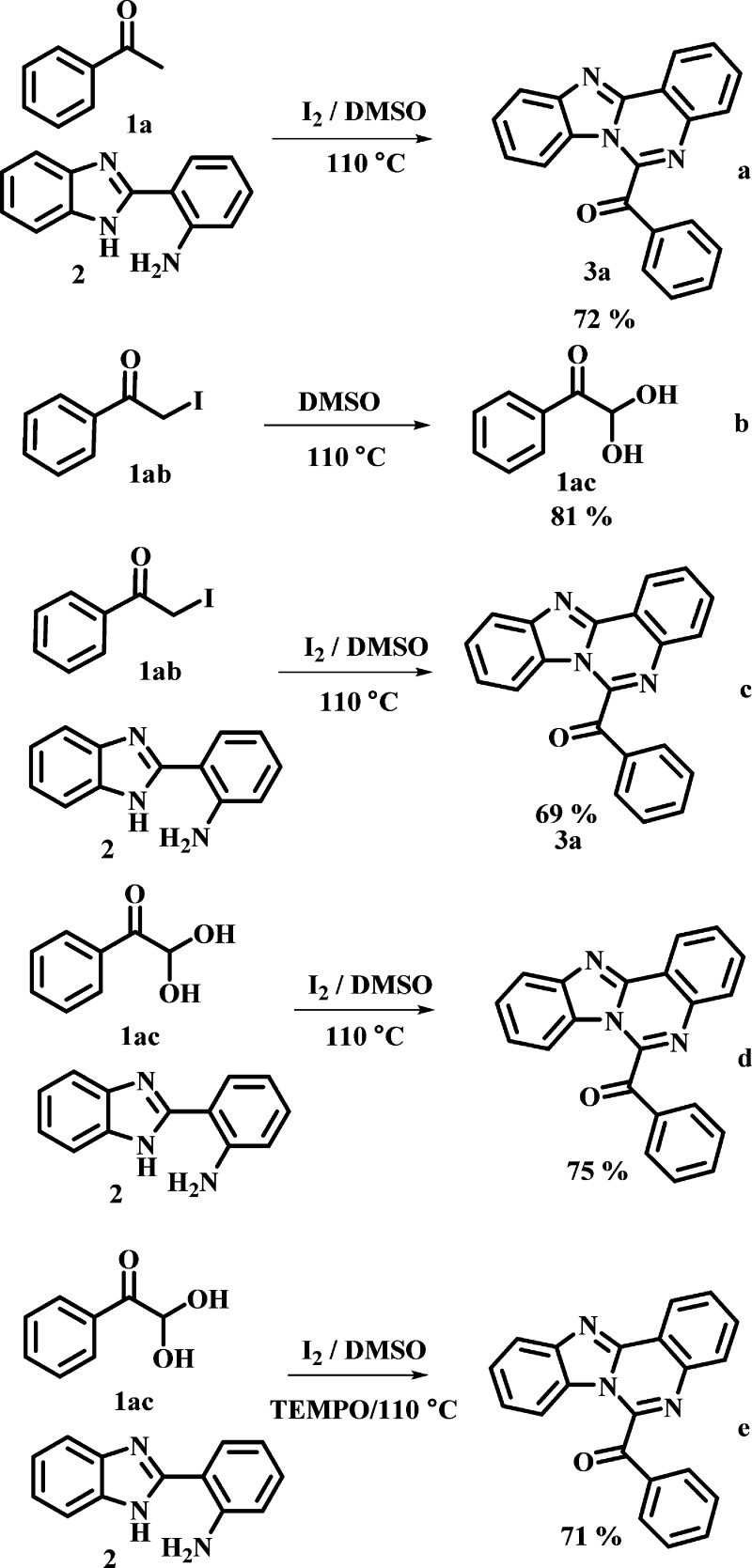
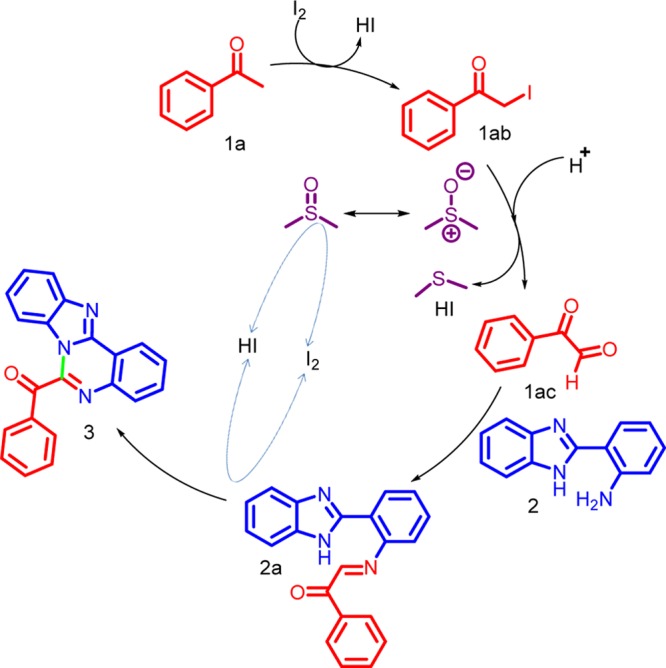
To have a better understanding of the reaction mechanism, a series of control experiments were performed (Scheme 4). The reaction of acetophenone 1a with 2 in presence of I2 affords 3a in good yields (Scheme 4a). Next, the reaction was carried out α-iodoketone 1ab with DMSO and phenylglyoxal was obtained in 81% yield (Scheme 4b). Subsequently, the reaction of α-iodoketone 1ab with 2-(1H-benzo[d]imidazol-2-yl) aniline 2 was found to be effective and the product 3a was obtained in 69% yield (Scheme 4c). When the acetophenone was replaced with phenylglyoxal 1ac, the desired product was obtained in 75% yield (Scheme 4d). These results confirmed that α-iodoketone 1ab is a possible precursor of α-ketoaldehyde 1ac. Moreover, the results also indicated that phenacyl iodide 1ab and phenylglyoxal 1ac are the key intermediates in the transformation. Finally, the radical trapping experiments were tested (Scheme 4e). 2,2,6,6-Tetramethylpiperidinyloxy (TEMPO) was used as a radical scavenger. In the presence of TEMPO, desired product was observed in good yield (71%) and this result shows that radical intermediates were not involved in this reaction.
Scheme 4. Investigation Into the Reaction Mechanism.
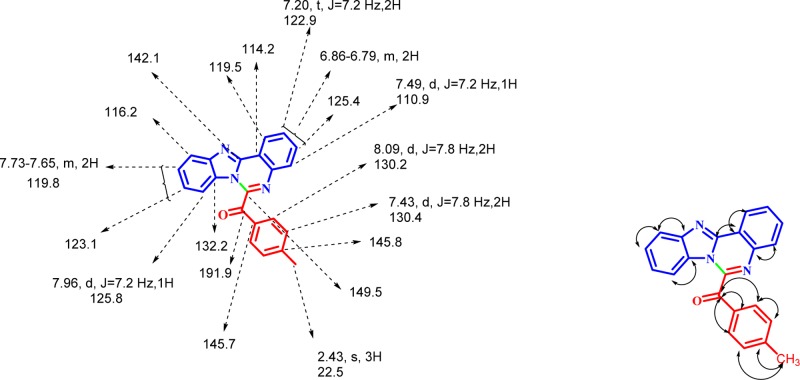
Structural elucidation of benzoimidazoquinazoline was accomplished by using one-dimensional and two-dimensional, as described for 3b. In the 1H NMR spectrum (see the SI) of 3b, the methyl protons appear as a sharp singlet at 2.43 ppm, which gives C–H COSY correlation with the carbon at 22.5 ppm and β-hydroxy β-methylbutyrate (HMB) correlation with the carbons at 130.2 and 145.7 ppm (Figure 2). The two-proton signal appears as a doublet at 8.09 ppm (J = 7.8 Hz), which gives C–H COSY correlation with the carbon at 130.2 ppm and HMB correlation with the carbons at 145.7 and 191.9 ppm.
Figure 2.
1H, 13C chemical shifts and HMB correlations of 3b.
The structure of the product 3 was confirmed by an electrospray ionization mass spectroscopy (ESI-MS). On the basis of the above results and literature reports, a plausible mechanism for the formation of 3 is shown in Scheme 5.5b First, the reaction of 1 with I2 results in the formation of α-iodoketone 1ab. Subsequently, oxidation of 1ab with DMSO at 110 °C gives arylglyoxal 1ac, with the removal of dimethyl sulfide. The next step is the reaction between arylglyoxal 1ac and 2 that generates an iminium ion intermediate 2a. The intermediate 2a is quickly converted to the desired product 3.
Scheme 5. Plausible Mechanism for the Formation of Benzoimidazoquinazoline Derivatives.
Conclusions
We have developed a simple, novel, and efficient method for the synthesis of benzoimidazoquinazoline by iodine-catalyzed oxidative C–H amination of aryl methyl ketones and 2-(1H-benzo[d]imidazol-2-yl) aniline. The initial studies of the mechanism suggest that this reaction could have occurred through a self-sequenced iodination/Kornblum oxidation/C–H amination and intramolecular cyclization reactions.
General Methods
The melting points were measured in open capillary tubes and were uncorrected. The 1H and 13C NMR spectra were recorded on a Bruker (Avance) 300 MHz NMR instrument using TMS as internal standard either CDCl3 or DMSO-d6 as solvent. Chemical shifts are given in parts per million (δ-scale) and the coupling constants are given in hertz (Hz). Silica gel-G plates (Merck) were used for thin layer chromatography (TLC) analysis with a mixture of petroleum ether (60–80 °C) and ethyl acetate as eluent. High-resolution mass spectra were recorded on a Waters Q-TOF micromass spectrometer using ESI mode.
General Experimental Procedure for 3 Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(phenyl)methanone (3a–3n)
A mixture of aryl methyl ketone 1 (100 mg, 0.83 mmol) and 2-(1H-benzo[d]imidazol-2-yl) aniline 2 (174 mg, 0.83 mmol) was taken in a 10 mL round-bottom flask in DMSO and 2 mL of I2 (42 mg, 20 mol %) was added and reaction mixture was refluxed at 110 °C for 6 h. The reaction was monitored by TLC using n-hexane/ethyl acetate mixture (3:2) as eluent. After the completion of the reaction, the mixture was poured into ice water, worked up with sodium thiosulphate, and extracted with ethyl acetate. The organic layer was collected, dried on anhydrous sodium sulfate, and the solvent was evaporated on a rotary evaporator to get the crude product. The crude product was purified by silica gel column using 30:70 n-hexane/ethyl acetate.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(phenyl)methanone (3a)
Yellow solid (215 mg, 80%); mp 222–224 °C; 1H NMR (300 MHz, DMSO-d6) δ 8.18 (d, J = 7.9 Hz, 1H), 8.03 (d, J = 7.7 Hz, 2H), 7.80 (d, J = 7.8 Hz, 1H), 7.61 (d, J = 7.1 Hz, 1H), 7.56–7.44 (m, 2H), 7.29–7.14 (m, 3H), 7.00 (s, 1H), 6.93 (t, J = 7.1 Hz, 1H), 6.76 (d, J = 8.1 Hz, 1H), 6.28 (s, 1H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 190.1, 147.4, 143.0, 140.0, 133.2, 132.1, 130.6, 128.1, 127.4, 124.1, 121.6, 118.7, 118.0, 115.0, 112.3, 108.2.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(p-tolyl)methanone (3b)
Yellow solid (209 mg, 83%); mp 226–228 °C; 1H NMR (300 MHz, DMSO-d6) δ 8.13 (d, J = 7.5 Hz, 1H), 7.91 (d, J = 8.0 Hz, 2H), 7.77 (d, J = 7.8 Hz, 1H), 7.28–7.19 (m, 3H), 7.16–7.10 (m, 2H), 6.99 (d, J = 7.7 Hz, 1H), 6.88 (dd, J = 9.8, 5.1 Hz, 1H), 6.74 (d, J = 7.9 Hz, 1H), 6.52 (d, J = 3.4 Hz, 1H), 2.39 (s, 3H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 191.9, 149.0, 145.8, 144.7, 142.1, 134.0, 132.2, 131.2, 130.4, 130.2, 125.4, 123.1, 122.9, 119.7, 119.5, 116.2, 113.8, 110.5, 22.1; HRMS (m/z) (ESI): calcd for C22H15N3O 337.1215; found 337.1219 [M + H]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(4-methoxyphenyl)methanone (3c)
Yellow solid (191 mg, 81%); mp 194–196 °C; 1H NMR (300 MHz, DMSO-d6) δ 8.11 (d, J = 8.8 Hz, 2H), 8.06 (d, J = 7.8 Hz, 1H), 7.72 (d, J = 7.8 Hz, 1H), 7.35 (d, J = 3.5 Hz, 1H), 7.29–7.12 (m, 5H), 7.00 (d, J = 8.8 Hz, 2H), 6.83 (dd, J = 15.2, 7.8 Hz, 2H), 3.87 (s, 3H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 189.3, 164.0, 148.2, 143.8, 141.1, 132.9, 131.4, 131.2, 125.6, 124.7, 122.4, 122.2, 119.1, 118.7, 115.4, 114.1, 112.9, 108.9, 55.5; HRMS (m/z) (ESI): calcd for C22H15N3O2 353.1164; found 353.1169 [M + H]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(3-methoxyphenyl)methanone (3d)
Yellow solid (188 mg, 80%); mp 183–185 °C; 1H NMR (300 MHz, DMSO-d6) δ 8.16 (d, J = 7.6 Hz, 1H), 7.89 (d, J = 3.7 Hz, 1H), 7.78 (d, J = 7.8 Hz, 2H), 7.70 (d, J = 4.9 Hz, 1H), 7.29–7.18 (m, 5H), 7.15–7.08 (m, 1H), 6.96–6.87 (m, 2H), 6.85–6.70 (m, 3H) 3.86 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 188.4, 163.1, 147.2, 142.9, 140.2, 132.0, 130.5, 130.3, 124.7, 123.8, 121.5, 121.3, 118.2, 117.8, 114.5, 113.2, 112.0, 108.0, 54.5.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(4-hydroxyphenyl)methanone (3e)
Yellow solid (194 mg, 78%); mp 188–190 °C; 1H NMR (300 MHz, DMSO-d6) δ 10.38 (s, 1H), 8.07–8.00 (m, 2H), 7.75–7.70 (m, 1H), 7.25–7.15 (m, 5H), 6.94 (d, J = 8.7 Hz, 2H), 6.84 (dd, J = 15.9, 7.5 Hz, 2H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 188.3, 162.4, 147.3, 143.0, 140.5, 132.2, 130.8, 130.4, 123.9, 123.6, 121.5, 121.4, 118.2, 117.9, 115.0, 114.6, 112.2, 108.1.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(3,4-methoxyphenyl)methanone (3f)
Yellow solid (159 mg, 75%); mp 189–191 °C; 1H NMR (300 MHz, CDCl3 with DMSO-d6) δ 8.03 (d, J = 7.1 Hz, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.70 (d, J = 7.9 Hz, 1H), 7.54 (s, 1H), 7.43 (broad, s, 1H), 7.35 (d, J = 7.8 Hz, 1H), 7.23–7.12 (m, 3H), 7.05 (d, J = 8.5 Hz, 1H), 6.83 (t, J = 7.4 Hz, 2H), 3.93 (s, 3H), 3.85 (s, 3H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 188.4, 152.7, 147.7, 147.0, 142.5, 140.1, 131.7, 130.0, 124.4, 123.4, 122.9, 121.1, 120.9, 117.6, 117.4, 114.1, 111.5, 109.9, 109.5, 108.0, 54.7, 54.5 HRMS (m/z) (ESI): calcd for C23H17N3O3 383.1270; found 383.1274 [M + H]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(4-chlorophenyl)methanone (3g)
Yellow solid (151 mg, 71%); mp 198–200 °C; 1H NMR (300 MHz, CDCl3) δ 8.10 (d, J = 7.5 Hz, 1H), 7.96 (d, J = 8.4 Hz, 2H), 7.75 (d, J = 7.8 Hz, 1H), 7.61 (d, J = 8.4 Hz, 2H), 7.24–7.18 (m, 5H), 6.88 (t, J = 7.5 Hz, 1H), 6.80 (d, J = 8.1 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 189.4, 147.4, 143.4, 140.0, 132.3, 131.4, 131.2, 130.8, 130.0, 124.4, 122.0, 121.9, 119.2, 118.4, 115.3, 112.7, 108.1; HRMS (m/z) (ESI): calcd for C21H12ClN3O 357.0669; found 357.0676 [M + H]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(4-bromophenyl)methanone (3h)
Yellow solid (144 mg, 72%); mp 200–202 °C; 1H NMR (300 MHz, CDCl3 with DMSO-d6) δ 8.14 (d, J = 7.8 Hz, 1H), 7.92 (d, J = 8.6 Hz, 2H), 7.77 (d, J = 7.8 Hz, 1H), 7.60 (d, J = 8.5 Hz, 2H), 7.22 (ddd, J = 17.1, 10.6, 4.2 Hz, 4H), 7.01 (d, J = 4.3 Hz, 1H), 6.90 (dd, J = 9.2, 5.8 Hz, 2H), 6.79 (d, J = 7.9 Hz, 1H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 190.5, 148.2, 144.4, 140.8, 133.3, 132.5, 132.2, 131.8, 130.9, 129.6, 125.6, 123.1, 123.0, 120.5, 119.6, 116.3, 113.8, 109.0; HRMS (m/z) (ESI): calcd for C21H12BrN3O 401.0164; found: 401.0172 [M + H]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(3,4-dichlorophenyl)methanone (3i)
Yellow solid (140 mg, 68%); mp 192–194 °C; 1H NMR (300 MHz, CDCl3 with DMSO-d6) δ 8.28 (d, J = 1.8 Hz, 1H), 8.09 (d, J = 7.8 Hz, 1H), 7.95 (dd, J = 8.4, 1.9 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 7.37 (d, J = 4.5 Hz, 1H), 7.29–7.17 (m, 4H), 6.92–6.77 (m, 2H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 194.3, 153.3, 149.2, 145.7, 143.4, 138.3, 138.1, 133.6, 130.3, 127.9, 127.8, 125.2, 124.3, 121.3, 118.8, 114.2; HRMS (m/z) (ESI): calcd for C21H11Cl2N3O 391.0279; found: 391.0279 [M+].
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(naphthalen-2-yl)methanone (3j)
Yellow solid (138 mg, 63%); mp 186–188 °C; 1H NMR (300 MHz, DMSO-d6) δ 8.25 (d, J = 8.1 Hz, 1H), 8.19–8.00 (m, 3H), 7.90 (d, J = 8.5 Hz, 2H), 7.78 (d, J = 7.5 Hz, 1H), 7.66–7.47 (m, 3H), 7.38–7.27 (m, 1H), 7.22 (dd, J = 17.7, 10.8 Hz, 2H), 7.07–6.93 (m, 1H), 6.82–6.14 (m, 1H), 6.63 (d, J = 8.4 Hz, 1H); 13C NMR (75 MHz, DMSO-d6) δ 194.6, 143.3, 141.0, 132.9, 132.6, 131.5, 131.3, 130.8, 129.8, 128.0, 127.3, 126.0, 125.3, 124.7, 124.2, 124.0, 123.4, 122.3, 121.8, 121.6, 118.9, 118.5, 118.1, 115.6, 114.8, 112.5, 109.3. HRMS (m/z) (ESI): calcd for C25H15N3O 373.1215; found: 373.1216 [M + H]+.
[1,1′-Biphenyl]-4-yl(benzo[4,5]imidazo[1,2-c]quinazolin-6-yl)methanone (3k)
Yellow solid (136 mg, 65%); mp 230–232 °C; 1H NMR (300 MHz, CDCl3) δ 8.21 (d, J = 8.1 Hz, 2H), 8.09 (d, J = 7.0 Hz, 1H), 7.75 (dd, J = 7.4, 2.9 Hz, 3H), 7.65 (d, J = 6.8 Hz, 2H), 7.54–7.38 (m, 5H), 7.35–7.28 (m, 2H), 7.27–7.15 (m, 3H), 6.86 (dd, J = 15.5, 7.8 Hz, 2H). 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 195.7, 153.5, 151.5, 149.3, 146.3, 144.4, 138.4, 137.1, 136.6, 135.0, 134.2, 133.7, 132.5, 132.3, 130.2, 127.8, 127.7, 124.7, 124.2, 121.0, 118.5, 114.3; HRMS (m/z) (ESI): calcd for C27H17N3NaO 422.1269; found: 422.1471 [M + Na]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(5-bromothiophen-2-yl)methanone (3l)
Yellow solid (116 mg, 58%); mp 218–220 °C; 1H NMR (300 MHz, DMSO-d6) δ 8.14 (d, J = 6.8 Hz, 1H), 7.78 (d, J = 7.9 Hz, 1H), 7.53 (d, J = 4.1 Hz, 1H), 7.30–7.17 (m, 4H), 7.02 (d, J = 4.1 Hz, 1H), 6.93 (t, J = 7.5 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.63 (t, J = 4.0 Hz, 2H).13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 185.1, 144.3, 140.7, 140.5, 134.5, 133.4, 132.9, 132.8, 125.6, 123.7, 120.1, 119.8, 116.3, 113.4, 109.3.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(furan-2-yl)methanone (3m)
Yellow solid (165 mg, 56%); mp 194–196 °C; 1H NMR (300 MHz, CDCl3 with DMSO-d6) δ 8.06 (d, J = 7.2 Hz, 1H), 7.85–7.83 (m, 1H), 7.73 (d, J = 7.2 Hz, 1H), 7.32–7.19 (m, 5H), 6.99 (m, 3H), 6.68 (s, 1H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 179.7, 148.3, 147.4, 146.9, 143.1, 140.4, 132.1, 130.5, 123.9, 121.7, 121.6, 119.6, 118.4, 118.1, 114.5, 112.0, 108.1; HRMS (m/z) (ESI): calcd for C19H11N3O2 313.0851; found 313.0862 [M + H]+.
Benzo[4,5]imidazo[1,2-c]quinazolin-6-yl(pyridin-3-yl)methanone (3n)
Yellow solid (139 mg, 52%); mp 224–226 °C; 1H NMR (300 MHz, CDCl3 with DMSO-d6) δ 8.61 (d, J = 27.0 Hz, 2H), 8.13 (s, 1H), 7.67 (d, J = 51.6 Hz, 3H), 7.24 (s, 4 H), 6.87 (s, 5H); 13C NMR (75 MHz, CDCl3 with DMSO-d6) δ 185.8, 155.7, 153.0, 152.6, 149.3, 147.5, 140.5, 139.2, 137.9, 136.9, 130.4, 129.0, 127.8, 127.7, 124.5, 124.4, 120.4, 117.8, 115.0. HRMS (m/z) (ESI): calcd for C20H12N4O 324.1011; found 324.1016 [M + H]+.
Acknowledgments
We thank DST, New Delhi, for assistance under the IRHPA program for the NMR facility. S.A. gratefully acknowledges UGC, New Delhi, for the award of RGNF Fellowship.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00067.
Typical procedure and characterization data for benzimidazo[1,2-c]quinazoline and NMR and HRMS spectra of all synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Shi W.; Liu C.; Lei A. Transition-metal catalyzed oxidative cross-coupling reactions to form C-C bonds involving organometallic reagents as nucleophiles. Chem. Soc. Rev. 2011, 40, 2761–2776. 10.1039/c0cs00125b. [DOI] [PubMed] [Google Scholar]; b He C.; Guo S.; Ke J.; Hao J.; Xu H.; Chen H. Y.; Lei A. Silver-Mediated Oxidative C–H/C–H Functionalization: A Strategy To Construct Polysubstituted Furans. J. Am. Chem. Soc. 2012, 134, 5766–5769. 10.1021/ja301153k. [DOI] [PubMed] [Google Scholar]; c Fiori K. W.; DuBois J. Catalytic Intermolecular Amination of C–H Bonds: Method Development and Mechanistic Insights. J. Am. Chem. Soc. 2007, 129, 562–568. 10.1021/ja0650450. [DOI] [PubMed] [Google Scholar]; d Kim M.; Mulcahy J. V.; Espino C. G.; DuBois J. Expanding the substrate scope for C-H amination reactions: oxidative cyclization of urea and guanidine derivatives. Org. Lett. 2006, 8, 1073–1076. 10.1021/ol052920y. [DOI] [PubMed] [Google Scholar]; e Liang C. G.; Robert-Peillard F.; Fruit C.; Müller P.; Dodd R. H.; Dauban P. You have full text access to this content Efficient Diastereoselective Intermolecular Rhodium-Catalyzed C[BOND]H Amination. Angew. Chem., Int. Ed. 2006, 45, 4641–4644. 10.1002/anie.200601248. [DOI] [PubMed] [Google Scholar]
- a Park Y.; Kim Y.; Chang S. Transition Metal-Catalyzed C–H Amination: Scope, Mechanism, and Applications. Chem. Rev. 2017, 117, 9247–9301. 10.1021/acs.chemrev.6b00644. [DOI] [PubMed] [Google Scholar]; b Meng D.; Tang Y.; Wei J.; Shia X. Y.; Yang M. Copper-catalyzed remote (δ) C(sp3)–H bond amination: a practical strategy to construct pyrrolidine derivatives. Chem. Commun. 2017, 53, 5744–5747. 10.1039/C7CC02624B. [DOI] [PubMed] [Google Scholar]; c Evans R. W.; Zbieg J. R.; Zhu S. L.; Li W.; MacMillan D. W. C. Simple Catalytic Mechanism for the Direct Coupling of α-Carbonyls with Functionalized Amines: A One-Step Synthesis of Plavix. J. Am. Chem. Soc. 2013, 135, 16074–16077. 10.1021/ja4096472. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Rong G.; Mao J.; Yan H.; Zheng Y.; Zhang G. Iron/Copper Co-Catalyzed Synthesis of Vinyl Sulfones from Sulfonyl Hydrazides and Alkyne Derivatives. J. Org. Chem. 2015, 80, 4697–4703. 10.1021/acs.joc.5b00558. [DOI] [PubMed] [Google Scholar]; e Nadres E. T.; Daugulis O. Heterocycle Synthesis via Direct C–H/N–H Coupling. J. Am. Chem. Soc. 2012, 134, 7–10. 10.1021/ja210959p. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yang M.; Su B.; Wang Y.; Chen K.; Jiang X.; Zhang Y. F.; Zhang X. S.; Chen G.; Cheng Y.; Cao Z.; Guo Q.; Wang L.; Shi Z. J. Silver-catalysed direct amination of unactivated C-H bonds of functionalized molecules. Nat. Commun. 2014, 5, 4707 10.1038/ncomms5707. [DOI] [PubMed] [Google Scholar]; g Sengoden M.; Bhowmick A.; Punniyamurthy T. Stereospecific Copper-Catalyzed Domino Ring Opening and sp3 C–H Functionalization of Activated Aziridines with N-Alkylanilines. Org. Lett. 2017, 19, 158–161. 10.1021/acs.orglett.6b03458. [DOI] [PubMed] [Google Scholar]
- a Yan R.; Li X.; Yang X.; Kang X.; Xiang L.; Huang G. A novel one-pot method for the synthesis of substituted furopyridines: iodine-mediated oxidation of enaminones by tandem metal-free cyclization. Chem. Commun. 2015, 51, 2573–2576. 10.1039/C4CC08834D. [DOI] [PubMed] [Google Scholar]; b Finkbeiner P.; Nachtsheim B. J. Iodine in Modern Oxidation Catalysis. Synthesis 2013, 979–999. 10.1055/s-0032-1318330. [DOI] [Google Scholar]; c Bagdi A. K.; Mitra S.; Ghosh M.; Hajra A. Iodine-catalyzed regioselective thiolation of imidazo[1,2-a]pyridines using sulfonyl hydrazides as a thiol surrogate. Org. Biomol. Chem. 2015, 13, 3314–3320. 10.1039/C5OB00033E. [DOI] [PubMed] [Google Scholar]; d Liu S.; Xi H.; Zhang J.; Wu X.; Gao Q.; Wu A. Organopromoted direct synthesis of 6-iodo-3-methylthioimidazo[1,2-a]pyridines via convergent integration of three self-sorting domino sequences. Org. Biomol. Chem. 2015, 13, 8807–8811. 10.1039/C5OB01313E. [DOI] [PubMed] [Google Scholar]; e Wu Y. D.; Geng X.; Gao Q.; Zhang J. J.; Wu X.; Wu A. Iodine-promoted sequential dual oxidative Csp3–H amination/Csp3–H iodination reactions: efficient synthesis of 1-iodoimidazo[1,5-a]pyridines. Org. Chem. Front. 2016, 3, 1430–1434. 10.1039/C6QO00313C. [DOI] [Google Scholar]
- a Song L.; Tian X.; Lv Z.; Li E.; Wu J.; Liu Y.; Yu W.; Chang J. I2/KI-Mediated Oxidative N–N Bond Formation for the Synthesis of 1,5-Fused 1,2,4-Triazoles from N-Aryl Amidines. J. Org. Chem. 2015, 80, 7219–7225. 10.1021/acs.joc.5b01183. [DOI] [PubMed] [Google Scholar]; b Ren Y. M.; Cai C.; Yang R. C. Molecular iodine-catalyzed multicomponent reactions: an efficient catalyst for organic synthesis. RSC Adv. 2013, 3, 7182–7204. 10.1039/c3ra23461d. [DOI] [Google Scholar]; c Togo H.; Iida S. Synthetic Use of Molecular Iodine for Organic Synthesis. Synlett 2006, 2159–2175. 10.1055/s-2006-950405. [DOI] [Google Scholar]; d Parvatkar P. T.; Parameswaran P. S.; Tilve S. G. Recent Developments in the Synthesis of Five- and Six-Membered Heterocycles Using Molecular Iodine. Chem. - Eur. J. 2012, 18, 5460–5489. 10.1002/chem.201100324. [DOI] [PubMed] [Google Scholar]
- a Gao Q.; Zhang J. J.; Wu X.; Liu S.; Wu A. X. Direct Regioselective Oxidative Cross-Coupling of Indoles with Methyl Ketones: A Novel Route to C3-Dicarbonylation of Indoles. Org. Lett. 2015, 17, 134–137. 10.1021/ol503366r. [DOI] [PubMed] [Google Scholar]; b Zhang Z.; Xie C.; Tan X.; Song G.; Wen L.; Gao H.; Ma C. I2-Catalyzed one-pot synthesis of pyrrolo[1,2-a]quinoxaline and imidazo[1,5-a]quinoxaline derivatives via sp3 and sp2 C–H cross-dehydrogenative coupling. Org. Chem. Front. 2015, 2, 942–946. 10.1039/C5QO00124B. [DOI] [Google Scholar]; c Küpper F. C.; Feiters M. C.; Olofsson B.; Kaiho T.; Yanagida S.; Zimmermann M. B.; Carpenter L. J.; Luther G. W. III; Lu Z. L.; Jonsson M.; Kloo L. Commemorating two centuries of iodine research: an interdisciplinary overview of current research. Angew. Chem., Int. Ed. 2011, 50, 11598–11620. 10.1002/anie.201100028. [DOI] [PubMed] [Google Scholar]
- a He Z. H.; Li H. R.; Li Z. P. Iodine-Mediated Synthesis of 3H-Indoles via Intramolecular Cyclization of Enamines. J. Org. Chem. 2010, 75, 4636–4639. 10.1021/jo100796s. [DOI] [PubMed] [Google Scholar]; b He Z.; Liu W. P.; Li Z. P. I2-Catalyzed Indole Formation via Oxidative Cyclization of N-Aryl Enamines. Chem. Asian J. 2011, 6, 1340–1343. 10.1002/asia.201100045. [DOI] [PubMed] [Google Scholar]
- a Kattrizky A. R.; Pozharskii A. F.. Handbook of Heterocyclic Chemistry; Pergamon, 2nd ed.; Academic Press: Amsterdam, 2000, pp 1–760. [Google Scholar]; b Li J. J.Heterocyclic Chemistry in Drug Discovery; John Wiley & Sons, Inc.: Hoboken, 2013. [Google Scholar]
- von Niementowski S. Ueber neue Arten der Anhydroverbindungen. Ber. Dtsch. Chem. Ges. 1899, 32, 1456–1493. 10.1002/cber.18990320223. [DOI] [Google Scholar]
- a Habib O. M. O.; Hassan H. M.; El-Mekabaty A. Novel quinazolinone derivatives: synthesis and antimicrobial activity. Med. Chem. Res. 2013, 22, 507–519. 10.1007/s00044-012-0079-x. [DOI] [Google Scholar]; b Kuarm B. S.; Reddy Y. T.; Madhav J. V.; Crooks P. A.; Rajitha B. 3-[Benzimidazo- and 3-[benzothiadiazoleimidazo-(1,2-c)quinazolin-5-yl]-2H-chromene-2-ones as potent antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 524–527. 10.1016/j.bmcl.2010.10.082. [DOI] [PubMed] [Google Scholar]; c Rohini R.; Shanker K.; Muralidhar R.; Ravinder V. Synthesis and antimicrobial activities of a new class of 6-arylbenzimidazo[1,2-c]quinazolines. J. Braz. Chem. Soc. 2010, 21, 49–57. 10.1590/S0103-50532010000100009. [DOI] [Google Scholar]; d Rohini R.; Shanker K.; Reddy P. M.; Ho Y. P.; Ravinder V. Mono and bis-6-arylbenzimidazo[1,2-c]quinazolines: A new class of antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 3330–3339. 10.1016/j.ejmech.2009.03.022. [DOI] [PubMed] [Google Scholar]
- a Braña M. F.; de Vega M. J. P.; Perron D.; Conlon D.; Bousquet P. F.; Robinson S. P. Benzimidazo[1,2-c]quinazoline dimers as potential antitumor agents. J. Heterocycl. Chem. 1997, 34, 807–812. 10.1002/jhet.5570340316. [DOI] [Google Scholar]; b Braña M. F.; Castellano J. M.; Keilhauer G.; Machuca A.; Martín Y.; Redondo C.; Schlick E.; Walker N. Benzimidazo[1,2-c]quinazolines: a new class of antitumor compounds. Anticancer Drug Des. 1994, 9, 527–538. [PubMed] [Google Scholar]
- a Lamazzi C.; Lé once S.; Pfeiffer B.; Renard P.; Guillaumet G.; Rees C. W.; Besson T. Expeditious synthesis and cytotoxic activity of new cyanoindolo[3,2-c]quinolines and benzimidazo[1,2-c]quinazolines. Bioorg. Med. Chem. Lett. 2000, 10, 2183–2185. 10.1016/S0960-894X(00)00427-3. [DOI] [PubMed] [Google Scholar]; b Via L. D.; Gia O.; Mango S. M.; Settimo A. D.; Marini A. M.; Primofiore G.; Settimo F. D. A.; Salerno S. Synthesis, in vitro antiproliferative activity and DNA-interaction of benzimidazoquinazoline derivatives as potential anti-tumor agents. Farmaco 2001, 56, 159–167. 10.1016/S0014-827X(01)01079-5. [DOI] [PubMed] [Google Scholar]; c Lunn W. H. W.; Harper R. W.; Stone R. L. Benzimidazo[2,1-b]quinazolin-12-ones. New class of potent immunosuppressive compounds. J. Med. Chem. 1971, 14, 1069–1071. 10.1021/jm00293a012. [DOI] [PubMed] [Google Scholar]
- a Kozlowski J. A.; Rosenblum S. B.; Coburn C. A.; Shankar B. B.; Anilkumar G. N.; Chen L.; Dwyer M. P.; Jiang Y.; Keertikar K. M.; Lavey B. J.; Selyutin O. B.; Tong L.; Wong M.; Yang D. Y.; Yu W.; Zhou G.; Wu H.; Hu B.; Zhong B.; Sun F.; Ji T.; Shen C.. Tetracyclic Indole Derivatives and Methods of Use Thereof for the Treatment of Viral Diseases, WO2012040923, April 5, 2012.; b Lyakhova E. A.; Gusyeva Y. A.; Nekhoroshkova J. V.; Shafran L. M.; Lyakhov S. A. Synthesis and affinity to DNA of phenylbenzoimidazoles and benzoimidazo[1,2-c]quinazolines. Eur. J. Med. Chem. 2009, 44, 3305–3312. 10.1016/j.ejmech.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarce G. D.; Foncea R. E.; Edwards A. M.; Pessoa-mahana H.; Mahana C. D. P.; Ebensperger R. A. Biological evaluation of novel 6-Arylbenzimidazo[1,2-c]quinazoline derivatives as inhibitors of LPS-induced TNF-alpha secretion. Biol. Res. 2008, 41, 43–50. 10.4067/S0716-97602008000100006. [DOI] [PubMed] [Google Scholar]
- Wolfe J. F.; Rathman T. L.; Sleevi M. C.; Campbell J. A.; Greenwood T. D. Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J. Med. Chem. 1990, 33, 161. 10.1021/jm00163a027. [DOI] [PubMed] [Google Scholar]
- Martinez-Ariza G.; McConnell N.; Hulme C. One-Pot Two-Step Multicomponent Process of Indole and Other Nitrogenous Heterocycles or Amines toward α-Oxo-acetamidines. Org. Lett. 2016, 18, 1864–1867. 10.1021/acs.orglett.6b00634. [DOI] [PubMed] [Google Scholar]
- Pang X.; Chen C.; Li M.; Xi C. A concise and efficient synthesis of benzimidazo[1,2-c]quinazolines through CuI-catalyzed intramolecular N-arylations. Beilstein J. Org. Chem. 2015, 11, 2365–2369. 10.3762/bjoc.11.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.; Lu J.; Fu H. Copper-catalyzed cascade synthesis of benzimidazoquinazoline derivatives under mild condition. Chem. Commun. 2011, 47, 5596–5598. 10.1039/c1cc10383k. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Yang H.; Jiang Y.; Zhao Y.; Fu H. Efficient copper-catalyzed domino synthesis of tetrazoloisoquinolines. RSC Adv. 2013, 3, 15636–15644. 10.1039/c3ra41644e. [DOI] [Google Scholar]
- Mirallai S. I.; Koutentis P. A. The Conversion of 4-Anilinoquinazoline- and 3-Aryl-4-imino-3,4dihydro-quinazoline-2-carbonitriles into Benzo[4,5]imidazo[1,2-c]quinazoline-6-carbonitriles via Oxidative and Nonoxidative C–N Couplings. J. Org. Chem. 2015, 80, 8329–8340. 10.1021/acs.joc.5b01514. [DOI] [PubMed] [Google Scholar]
- Shinde A. H.; Arepally S.; Baravkar M. D.; Sharada D. S. Nickel-Catalyzed Aerobic Oxidative Isocyanide Insertion: Access to Benzimidazoquinazoline Derivatives via a Sequential Double Annulation Cascade (SDAC) Strategy. J. Org. Chem. 2017, 82, 331–342. 10.1021/acs.joc.6b02423. [DOI] [PubMed] [Google Scholar]
- a Ambethkar S.; Padmini V.; Bhuvanesh N. A green and efficient protocol for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives via a one-pot, four component reaction by grinding method. J. Adv. Res. 2015, 6, 975–985. 10.1016/j.jare.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ambethkar S.; Padmini V.; Bhuvanesh N. A one-pot sequential five-component domino reaction for the expedient synthesis of polysubstituted pyrroles. New J. Chem. 2016, 40, 4705–4709. 10.1039/C5NJ03444B. [DOI] [Google Scholar]; c Dhinakaran I.; Padmini V.; Bhuvanesh N. Chemodivergent, One-Pot, Multi-Component Synthesis of Pyrroles and Tetrahydropyridines under Solvent- and Catalyst-Free Conditions Using the Grinding Method. ACS Comb. Sci. 2016, 18, 236–242. 10.1021/acscombsci.5b00154. [DOI] [PubMed] [Google Scholar]; d Ambethkar S.; Vellimalai M.; Padmini V.; Bhuvanesh N. Iodine-mediated C–N and C–S bond formation: regioselective synthesis of benzo[4,5]imidazo[2,1-b]thiazoles. New J. Chem. 2017, 41, 75–80. 10.1039/C6NJ02102F. [DOI] [Google Scholar]; e Kumar M.; Sribalan R.; Padmini V. Er(OTf)3 Assisted Efficient Synthesis of 3-Hydroxynaphthalene-1, 4-Dione Derivatives via Pseudo Four-Component Reactions and Their Biological Evaluation. ChemistrySelect 2017, 2, 489–49. 10.1002/slct.201601340. [DOI] [Google Scholar]; f Ambethkar S.; Kalaiselvi M.; Padmini V.; Bhuvanesh N. Synthesis of 3-Substituted Quinazolinones via C-N and C–C bond Cleavage of Enaminone. ChemistrySelect 2017, 2, 5329–5332. 10.1002/slct.201700457. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.