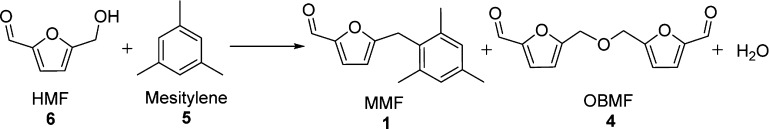
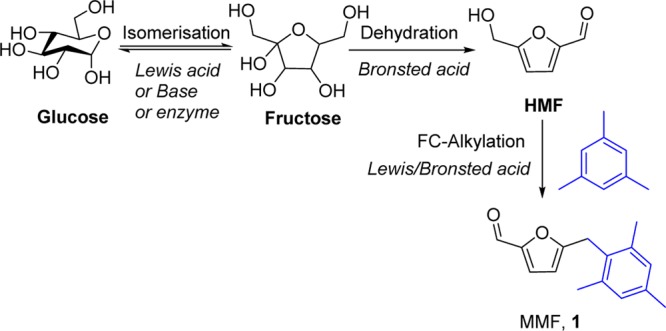
Abstract
Heterogeneous Zr-Mont catalyst prepared by a simple protocol was employed for the production of diesel fuel precursors via Friedel–Crafts (FC) alkylation of petroleum-derived arenes (e.g., mesitylene, xylene, and toluene) with biomass-derived 5-(hydroxymethyl)furfural (HMF), HMF derivatives, and carbohydrates. Initially, several acidic catalysts were screened for the FC alkylation of mesitylene with HMF in nitroethane solvent. Among all, Zr-Mont catalyst gave an exceptionally high yield (80%) of mesitylmethylfurfural (MMF). The catalytic activity of Zr-Mont was also evaluated for the alkylation of different petroleum-derived arenes with ester/halogen derivatives of HMF. Suitable acid strength and high surface area of Zr-Mont were its major attributes to make it the most efficient solid acid catalyst for this FC reaction. Even after several reuses, the catalytic activity of Zr-Mont was found to be consistent, which was also evidenced by the acidity measurements of fresh and reused Zr-Mont catalysts by temperature-programmed desorption of ammonia and pyridine Fourier transform infrared spectroscopy techniques. Direct conversion of glucose to diesel fuel precursors was also attempted over Zr-Mont catalyst in mesitylene and polar nonacidic solvents at 150 °C. However, the activity of Zr-Mont catalyst was limited for glucose dehydration to HMF and MMF did not form. When the same experiment was performed in formic acid medium, MMF was produced in 34% yield. After the addition of formic acid, the reaction becomes biphasic which contains mesitylene as an organic phase and formic acid as an aqueous phase. Formic acid worked as a solvent, reactant, and cocatalyst, whereas mesitylene worked as a reactant and product extraction phase to enable easy product isolation. With this strategy, other diesel fuel precursors were also produced in 26–30% yields from glucose and different arenes. Similar strategy was successfully extended for the conversion of sucrose to diesel fuel precursors.
Introduction
For chemicals and liquid fuels, the society is heavily relying on crude oil which has certainly a limited stock on the earth. This imminent crisis can be overcome by an alternative feedstock such as cellulosic biomass; the latter has become one of the most exploited research areas.1 5-(Hydroxymethyl)furfural (HMF) derived from cellulose is recognized as a principle raw material for the production of liquid fuels2−6 and a monomer for the polymers.7,8 To produce diesel-range C11–C23 alkanes from HMF, it needs to undergo carbon upgradation process via C–C bond formation.9 Some of the efforts made in this direction involving C–C bond-forming reactions include (i) base-catalyzed aldol condensation of HMF with acetone,10 (ii) amine-catalyzed Baylis–Hillman reactions between methylacrylate and HMF,11 (iii) coupling of HMF and isoprene over a ruthenium complex,12 and (iv) condensation of HMF with 2-methylfuran.13 These upgraded products are carbon-rich; hence, they can be converted into diesel-range liquid hydrocarbons via ring-opening–dehydration–hydrodeoxygenation processes.10,14
In 2015, Corma et al. produced alkyl naphthenic kerosene from HMF and substituted benzenes via Friedel–Crafts (FC) alkylation using zeolites followed by hydrodeoxygenation process over a platinum-based catalyst.14 However, currently there is only one industrial process for the bulk production of HMF from biomass-derived feedstocks.15 Because HMF has high solubility in aqueous reaction mixtures, its extraction is a tedious task. In addition to that HMF has low stability in acidic mediums and also produces a large amount of tarry degradation products during its recovery by distillation at high temperature. Therefore, researchers have focused their attention on technologies where the carbohydrates can be directly converted into advanced chemicals/fuel precursors without isolating the unstable intermediate (HMF). In this direction, Zhou and Rauchfuss reported an excellent work on the production of hybrid diesel fuel precursors from carbohydrates and petrochemicals using formic acid as a reactive solvent to dissolve carbohydrates as well as a reagent for making a reactive intermediate named as 5-(formyloxymethyl)furfural (FMF).16 However, in this reaction sequence, catalytic amount of concd HCl was required for the isomerization of glucose and other glucose-based carbohydrates into fructose, which limits its commercial exploitation because of its hazardous nature as well as waste formation. Recently, Nale and Jadhav reported alkylation of aromatic arenes with fructose over Glu–Fe3O4–SO3H catalyst without isolation of the HMF intermediate.17 This approach is limited to fructose only and not useful for the complex carbohydrates such as glucose and sucrose. Glucose is relatively more stable than fructose, which makes its dehydration much more difficult. Conversion of glucose into HMF proceeds in two steps: the first step is glucose isomerization to fructose in the presence of an enzyme or a Lewis acid or a base catalyst18 and the second step is the dehydration of fructose to HMF. Wang et al. reported a montmorillonite-based (Sn-Mont) catalyst for the isomerization of glucose to fructose followed by its dehydration to HMF.19 Very recently, we have converted carbohydrates to condensation products (diesel fuel precursors) via an integrated process using Sn-Mont and formic acid without isolating furfural intermediates.20 This approach avoided the tedious isolation and purification of furfural intermediates.
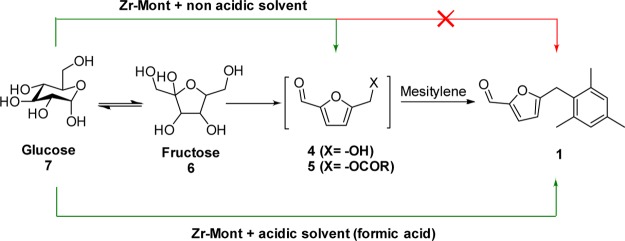
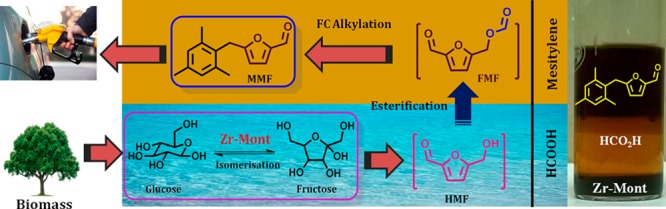
In this work, Zr-Mont catalyst easily prepared via the ion exchange of natural montmorillonite with an aqueous ZrOCl2·8H2O solution was employed for the carbon upgradation processes. The catalytic activity of Zr-Mont was initially evaluated for FC alkylation of arenes (e.g., mesitylene, xylene, and toluene) with HMF and HMF derivatives. We have continued our efforts for the conversion of carbohydrates to diesel fuel precursors. The schematic of our approach for directly producing diesel fuel precursors from glucose is elaborated in Scheme 1. This approach is a three-step reaction sequence which involves (i) glucose isomerization to fructose, (ii) dehydration–esterification of fructose to HMF derivatives, and (iii) FC alkylation of arenes with HMF derivatives. The Zr-Mont catalyst that acts as a Lewis acid because of the presence of Zr4+ ions was also found to be efficient for the isomerization of complex carbohydrates (e.g., glucose and sucrose) to fructose. In situ-formed fructose could be dehydrated to HMF/HMF-ester and could subsequently undergo FC alkylation with an arene molecule in formic acid medium. Formic acid served as a reactive solvent as well as a cocatalyst. Notably, the use of formic acid in this process is appealing as it is obtained from biomass degradation,21 regenerable,22 safe, and cheap.23 The phenomenal stability and recyclability of Zr-Mont makes it a very viable alternative to the liquid acid (e.g., HCl) usually used in this process.
Scheme 1. Production Path for Diesellike Products from Glucose.
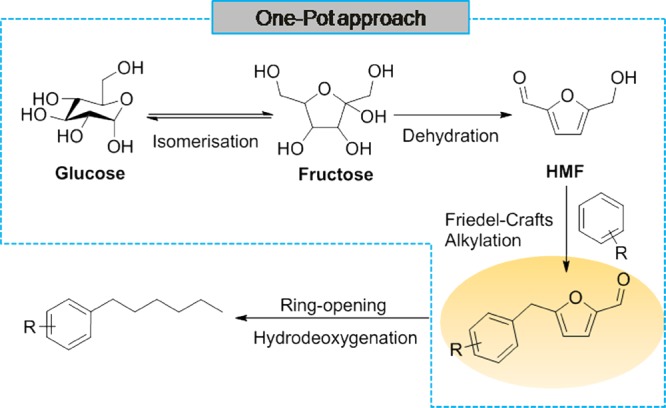
Results and Discussion
Catalyst Characterization
Catalysts such as montmorillonite, Fe-Mont, Al-Mont, Sn-Mont, and Zr-Mont are well-characterized with their physicochemical properties presented in Table 1 and Supporting Information (Figures S1 and S2).
Table 1. Physicochemical Properties of Various Montmorillonite Catalysts.
| acidity |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PyFTIRa (μmolpyridine g–1cat.) |
||||||||||||
| 100 °C |
200 °C |
300 °C |
||||||||||
| catalyst | SBET (m2 g–1) | NH3-TPD (μmolammonia desorbed g–1cat.) | B | L | B | L | B | L | total | B/L ratio | Vmicro | Vmeso |
| Mont | 19 | 90.08 | 08.23 | 09.00 | 60.00 | 21.00 | 13.00 | 8.17 | 119.40 | 2.13 | 6 | 15 |
| Fe-Mont | 131 | 190.63 | 17.76 | 11.44 | 94.00 | 49.00 | 33.00 | 17.00 | 222.20 | 1.87 | 39 | 93 |
| Al-Mont | 141 | 309.17 | 24.92 | 26.53 | 151.00 | 59.00 | 51.00 | 19.00 | 331.45 | 2.29 | 49 | 104 |
| Sn-Mont | 183 | 511.90 | 85.10 | 51.02 | 210.00 | 86.05 | 65.09 | 35.11 | 532.37 | 2.09 | 69 | 128 |
| Zr-Mont | 192 | 380.30 | 55.68 | 31.00 | 187.00 | 57.00 | 57.00 | 21.46 | 404.14 | 2.73 | 77 | 151 |
| Zr-Mont-R3Ab | 171 | 359.10 | 51.12 | 30.26 | 182.03 | 55.20 | 51.07 | 19.20 | 388.88 | 2.71 | 69 | 139 |
| Zr-Mont-R3Bc | 158 | 344.44 | 48.57 | 29.64 | 179.23 | 54.35 | 49.00 | 18.54 | 379.33 | 2.69 | 56 | 103 |
The quantification of B and L sites present in catalysts was done by using the Emeis equation.25
Temperature-Programmed Desorption of Ammonia Analysis
The temperature-programmed desorption of ammonia (NH3-TPD) analysis reveals that the total acid strength of Sn-Mont was much higher than that of montmorillonite, Fe-Mont, Al-Mont, and Zr-Mont. The trend of acid strength of all of these catalysts was as follows: montmorillonite (90.08 μmol g–1) < Fe-Mont (190.63 μmol g–1) < Al-Mont (309.17 μmol g–1) < Zr-Mont-R3B (344.44 μmol g–1) < Zr-Mont-R3A (359.10 μmol g–1) < Zr-Mont (380.30 μmol g–1) < Sn-Mont (511.90 μmol g–1). Although, Sn-Mont showed the highest acidity, Zr-Mont with the second highest acidity was found to be the most suitable catalyst for our targeted application; therefore, its stability in terms of acidity retention was evaluated by NH3-TPD analysis of the used catalyst samples (Figure 1). Almost identical NH3-TPD profiles were observed for Zr-Mont-fresh, Zr-Mont-R3A (after reusing three times for HMF to mesitylmethylfurfural (MMF) reaction; Figure 6), and Zr-Mont-R3B (after reusing three times for glucose to MMF reaction, Figure 9). A very marginal decrease in the total acidities of reused samples was noticed (Table 1).
Figure 1.
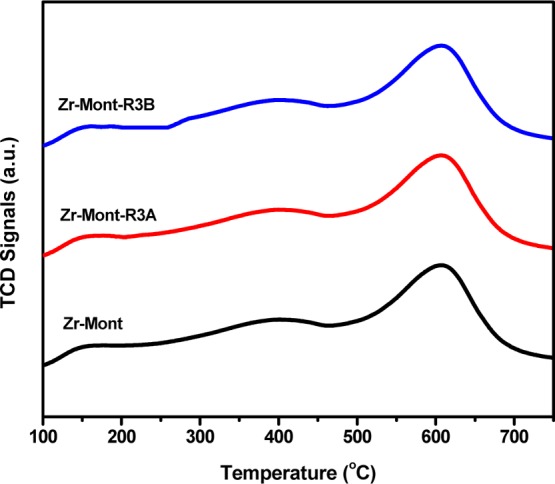
NH3-TPD of Zr-Mont, Zr-Mont-R3A, and Zr-Mont-R3B.
Figure 6.
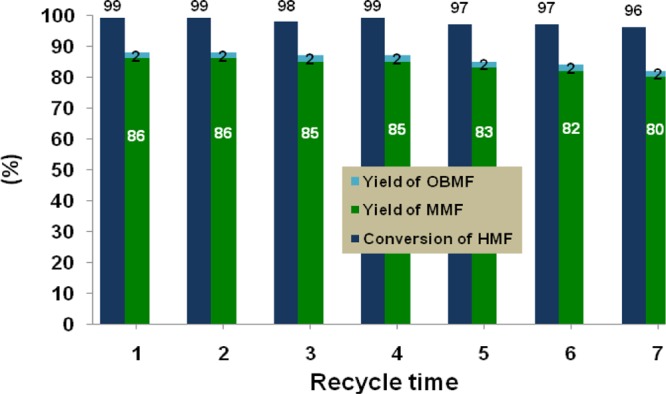
Recycle study of Zr-Mont for the FC alkylation of mesitylene with HMF. Reaction conditions: HMF (0.252 g, 2 mmol), mesitylene (5 mL), nitroethane (5 mL), and Zr-Mont (0.1 g), 110 °C, 16 h. After each cycle, the catalyst was washed with acetone and dried in the oven at 100 °C for 1 h prior to use for the next cycle. Conversion of HMF was measured using HPLC and reported yields were isolated.
Figure 9.
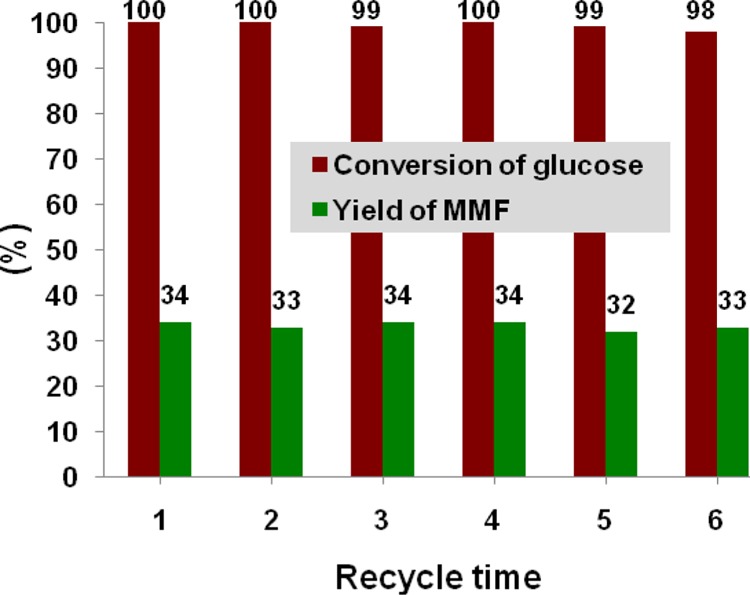
Recycle study of Zr-Mont for MMF production from glucose. Reaction conditions: glucose (0.5 g), mesitylene (10 mL), formic acid (5 mL), and Zr-Mont (0.2 g), 150 °C, 16 h. After each cycle, the catalyst was washed with acetone and dried in the oven at 100 °C for 1 h prior to use for the next cycle.
Pyridine Fourier Transform Infrared Spectroscopy Analysis
The types of acid sites present in our catalyst samples were identified by using pyridine Fourier transform infrared (FTIR) spectroscopy. The pyridine FTIR spectra of montmorillonite and metal-exchanged montmorillonite samples (Fe-Mont, Al-Mont, and Sn-Mont) are depicted in Figure S1 (Supporting Information). To identify/evaluate the acidity of these catalysts, difference in the spectrum before pyridine adsorption from that obtained after pyridine adsorption of the respective catalyst was taken. The band at 1441 cm–1 signifies Lewis acidity; the band at 1490 cm–1 represents a combination of Lewis and Brønsted acidities, and the bands at 1548 and 1638 cm–1 signify Brønsted acidity.24 The acidity trend of montmorillonite catalysts by Py-FTIR was found to be as follows: montmorillonite (119.40 μmol g–1) < Fe-Mont (222.20 μmol g–1) < Al-Mont (331.45 μmol g–1) < Zr-Mont-R3B (379.33 μmol g–1) < Zr-Mont-R3A (388.88 μmol g–1) < Zr-Mont (404.14 μmol g–1) < Sn-Mont (532.37 μmol g–1). All metal-exchanged montmorillonite catalysts possessed significantly large amount of medium acid sites compared to their weak and strong acid sites. To investigate the different types of acidic sites and acid strengths of Zr-Mont-R3A and Zr-Mont-R3B samples, their Py-FTIR spectra (Figure 2) were recorded at different temperatures using the method proposed by Emeis.25 Brønsted acidities of Zr-Mont-R3A and Zr-Mont-R3B samples were found to be 284.22 and 276.8 μmol g–1, respectively, whereas the Lewis acidities of these samples were found to be 104.66 and 102.53 μmol g–1, respectively. Even after third reuse, the Brønsted/Lewis acid (B/L) ratios of Zr-Mont-R3A (B/L = 2.71) and Zr-Mont-R3B (B/L = 2.69) were almost similar to that of fresh Zr-Mont sample (B/L = 2.73) (Table 1).
Figure 2.
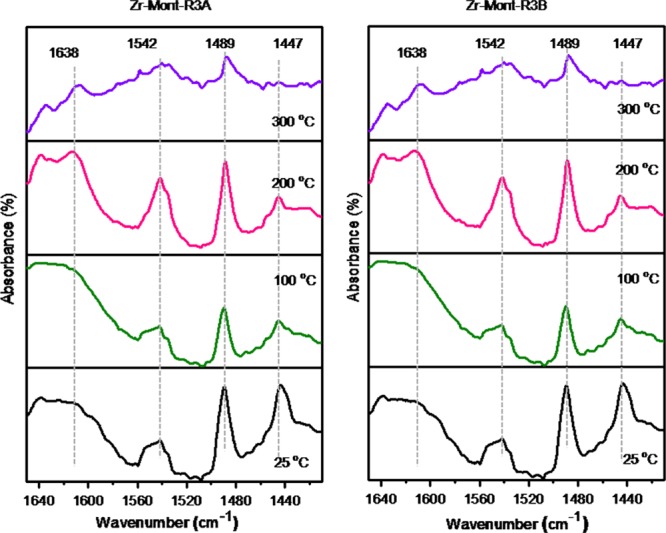
Pyridine FTIR spectra of Zr-Mont-R3A and Zr-Mont-R3B at different evacuation temperatures.
From NH3-TPD and Py-FTIR analysis, it could be confirmed that Zr-Mont did not lose its acidity in significant amount even after being reused for three times. The surface area of the spent catalysts was decreased slightly from 196 to 171 m2 g–1 for Zr-Mont-R3A and 158 m2 g–1 for Zr-Mont-R3B. The decrease in the specific surface area of Zr-Mont catalysts after their reuse was probably due to the carbon deposits on the surface. The inductively coupled plasma–optical emission spectroscopy (ICP–OES) analysis confirmed that no leaching of Zr metal from Zr-Mont catalyst took place under our reaction conditions.
Alkylation of Mesitylene with HMF over Various Catalysts
The FC alkylation of mesitylene with HMF was carried out in nitroethane over different acid catalysts, and the results are summarized in Table 2. FC alkylation of mesitylene with HMF was carried out in the presence of catalytic amount of concd H2SO4 at 80 °C for 3 h, which showed that HMF was consumed completely to give 75% yield of the coupling product (MMF, 1) along with 11% of 5,5′-oxy(bismethylene)-2-furaldehyde (OBMF) (Table 2, entry 1). Nevertheless, concd H2SO4 is hazardous in nature, nonrecyclable, and produces byproduct, which limits its further exploitation. When commercially available sulfonic acid-functionalized polystyrene (Amberlyst-15) solid acid catalyst was explored, MMF was formed in 79% yield with 7% of OBMF (Table 2, entry 2). In the presence of the Lewis acid (ZrOCl2·8H2O), MMF was produced in 30% yield after a quite longer reaction time of 16 h (Table 2, entry 3). The lower yield of MMF in the presence of ZrOCl2·8H2O was attributed to the formation of a chlorination product [5-(chloromethyl)furfural (CMF)] of HMF. To convert in-situ formed CMF into MMF, the reaction was prolonged for 16 h. Even after such a long period, the yield of MMF did not improve. The performance of naturally occurring montmorillonite clay possessing Lewis acidity was also evaluated for this reaction, but even at the elevated temperature of 110 °C, only 21% yield of product 1 was noticed (Table 2, entry 4). It is well-known that the acidity of montmorillonite could be significantly enhanced by a metal-exchange process.26,27 In this context, Zr-exchanged montmorillonite clay (Zr-Mont) was used for the FC alkylation of mesitylene with HMF. Over Zr-Mont catalyst (0.1 g) and at 110 °C, HMF was treated with mesitylene to give MMF in 86% yield with only 2% of OBMF (Table 2, entry 5). Decreasing the catalyst (Zr-Mont) amount from 0.1 to 0.05 g, affected the conversion of HMF obviously due to the shortfall of the active sites (Table 2, entry 6). On the contrary, by increasing catalyst loading to 0.2 g, the yield of OBMF was increased to 17% (Table 2, entry 7). Sn4+-incorporated montmorillonite catalyst (Sn-Mont, 0.1 g) showed 70% yield of MMF with 21% of OBMF (Table 2, entry 8). This observation well-matched with our previous reports.26b Surprisingly, even though the acidity of Sn-Mont (511.90 μmol g–1, Table 1) was higher than that of the Zr-Mont (380.3 μmol g–1, Table 1), the yield of MMF was found to be lower because of the formation of OBMF (4); the formation of OBMF was promoted because of the higher acidity of Sn-Mont along with lower B/L ratio (2.09) and lower surface area of (183 m2 g–1, Table 1) than Zr-Mont (196 m2 g–1, Table 1). Al3+- and Fe3+-exchanged montmorillonite catalysts showed 57 and 43% yield of MMF with 72 and 55% conversion of HMF, respectively (Table 2, entries 9 and 10). Thus, the suitable acid strength and high surface area of Zr-Mont contributed to its excellent activity in terms of the yield of MMF. Although, MMF was produced in high yield from HMF over a recyclable Zr-Mont catalyst, the present cost of pure HMF is a major deterrent factor for the development of downstream processes. Therefore, crude HMF obtained from fructose was reacted with mesitylene to obtain 83% yield of MMF (Table 2, entry 11). The use of crude HMF for the synthesis of desired products avoided the energy-intensive separation/purification of HMF, offering a cost-efficient process for the practical production of product 1 from fructose.
Table 2. Arylation of HMF with Mesitylene over Various Catalystsa.
| Conv.c (%) | yieldd (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| entry | catalyst | loading | T (°C) | t (h) | 6 | 1 | 4 | TOF (h–1) |
| 1 | concd H2SO4 | 10 mol % | 80 | 3 | >99 | 75 | 11 | 0.2 |
| 2 | Amberlyst-15 | 0.1 g | 80 | 4 | >99 | 79 | 07 | 13.83 |
| 3 | ZrOCl2·8H2O | 0.1 g | 80 | 16 | >99 | 30 | 11 | 0.14 |
| 4 | Mont | 0.1 g | 110 | 16 | 44 | 21 | 09 | 2.5 |
| 5 | Zr-Mont | 0.1 g | 110 | 12 | >99 | 86 | 02 | 2.63 |
| 6 | Zr-Mont | 0.05 g | 110 | 16 | 64 | 50 | 00 | 3.15 |
| 7 | Zr-Mont | 0.2 g | 110 | 16 | >99 | 75 | 17 | 1.57 |
| 8 | Sn-Mont | 0.1 g | 110 | 16 | >99 | 70 | 21 | 2.09 |
| 9 | Al-Mont | 0.1 g | 110 | 16 | 72 | 57 | trace | 2.70 |
| 10 | Fe-Mont | 0.1 g | 110 | 16 | 55 | 43 | 2.79 | |
| 11b | Zr-Mont | 0.1 g | 110 | 16 | >99 | 83 | 02 | 2.67 |
Reaction conditions: HMF (0.252 g, 2 mmol), mesitylene (5 mL), nitroethane (5 mL), and catalyst.
Crude HMF obtained from fructose.
Conversion of HMF was measured using HPLC.
Isolated yields.
Effect of Reaction Temperature on FC Alkylation of Mesitylene with HMF
FC alkylation of mesitylene with HMF was carried out over Zr-Mont catalyst for a period of 16 h under different temperatures ranging from 80 to 120 °C (Figure 3). MMF was produced in 29 and 64% yields at 80 and 100 °C, respectively. Interestingly, when the same reaction was conducted at 110 °C, MMF was obtained in significantly high yield of 86% with complete consumption of HMF. Thus, temperature lower than 110 °C was not effective to push the reaction to completion, whereas at the elevated temperature of 120 °C, 77% yield of MMF was obtained with 10% of self-etherification product (OBMF). Thus, 110 °C is the optimum reaction temperature used for further experiments.
Figure 3.
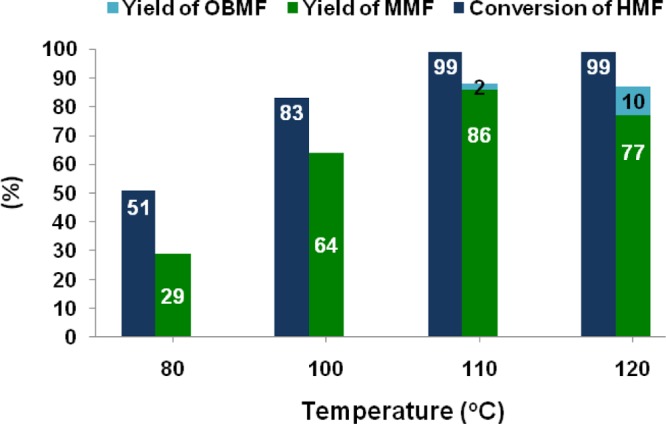
Effect of reaction temperature on FC alkylation of mesitylene with HMF. Reaction conditions: HMF (0.252 g, 2 mmol), mesitylene (5 mL), nitroethane (5 mL), and Zr-Mont (0.1 g), 80–120 °C, 16 h. Conversion of HMF was measured using HPLC and reported yields were isolated.
Effect of Reaction Time on FC Alkylation of Mesitylene with HMF
Effect of reaction time on FC alkylation of mesitylene with HMF was studied by monitoring the reaction after specific time intervals (Figure 4). In the first 2 h, HMF was consumed upto 36% with 30% yield of MMF. After 9th hour, the formation of OBMF began and reached to the maximum of 2% and remained steady at the end of 16th hour. HMF conversion and yield of MMF gradually increased to 99 and 86%, respectively, during the time span of 16 h.
Figure 4.
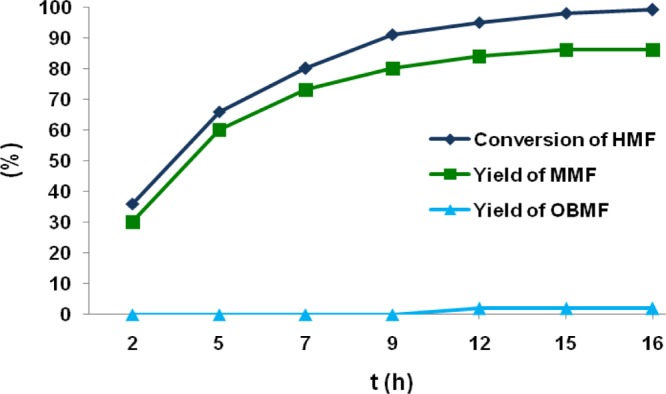
Effect of reaction time on FC alkylation of mesitylene with HMF. Reaction conditions: HMF (0.252 g, 2 mmol), mesitylene (5 mL), nitroethane (5 mL), and Zr-Mont (0.1 g), 110 °C, 2–16 h. Conversion of HMF and yield of MMF were measured using HPLC.
Influence of Solvents on the Production of MMF from Mesitylene and HMF
Influence of different solvents was studied on the FC alkylation of mesitylene with HMF, and the results are presented in Figure 5. Under the optimized experimental conditions and in the absence of solvent, MMF yield obtained was 29% along with 49% of OBMF. In the aqueous medium, the reaction did not proceed because of the equilibrium between HMF and dehydrated HMF. Solvents such as dimethylformamide, acetonitrile, and tetrahydrofuran were not suitable for this reaction. Etherification of HMF in the presence of alcohol such as methanol gave 5-(methoxymethyl)furfural in 95% yield. In dichloroethane, only 12% of MMF was formed along with 51% of OBMF. After evaluating several solvents, we found that nitroethane is the best solvent to achieve the highest yield (86%) of MMF.
Figure 5.
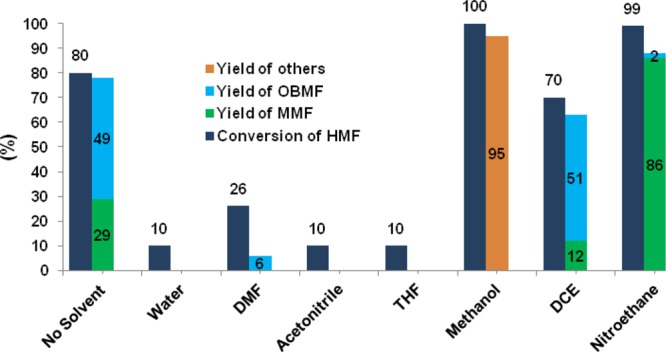
Influence of solvents on the production of MMF from HMF. Reaction conditions: HMF (0.252 g, 2 mmol), mesitylene (5 mL), solvent (5 mL), and Zr-Mont (0.1 g), 110 °C, 16 h. Conversion of HMF was measured using HPLC and reported yields were isolated.
Zr-Mont Recycling Studies
The stability and reusability of Zr-Mont catalyst were investigated for the FC alkylation of mesitylene with HMF. In a typical recycle experiment, Zr-Mont catalyst after the first run was filtered, washed with acetone (5 mL × 2), and dried in an oven for 1 h at 110 °C before using it for the next cycle. As can be seen from Figure 6, after each reuse of Zr-Mont, HMF conversion was almost >95% with slight decrease in the yield of MMF. The retention of acidity of Zr-Mont was also confirmed by NH3-TPD and Py-FTIR analysis of fresh and reused Zr-Mont sample (Figures 1 and 2).
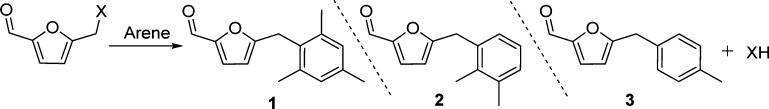
Synthesis of Arylmethylfurfural from Different HMF Derivatives
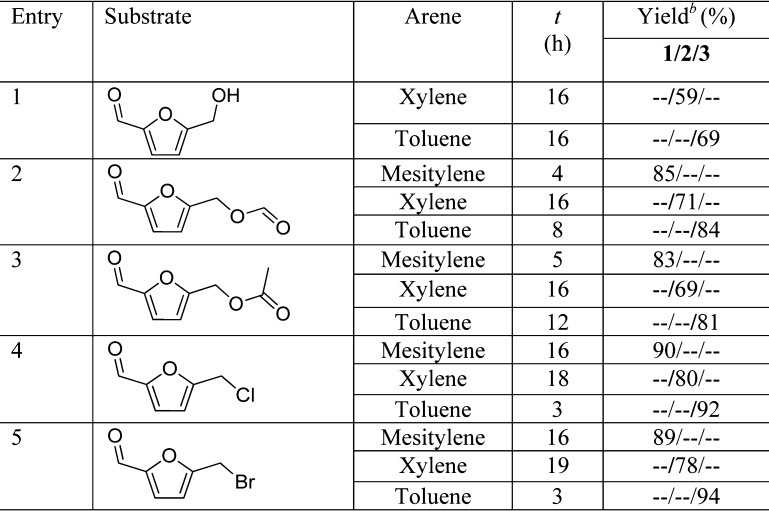
The arylation protocol using Zr-Mont catalyst was also extended to the ester and halogen derivatives of HMF, and the results are summarized in Table 3. Initially, HMF was treated with xylene to afford arylation products 2 in 59% yield. Our protocol gave higher yield of product 2 than that reported by Beller et al. using FeCl3 under a solvent-free condition providing 37% yield of the coupled product.28 When toluene was treated with HMF, coupling product 3 was produced in 69% yield (Table 3, entry 1). Ester derivatives of HMF offer some important advantages over HMF for industrial production. Compared with the hydroxymethyl functionality of HMF, the ester functionality makes them hydrophobic, stable, and more readily isolable from the aqueous reaction mixture, whereas HMF is known to be relatively unstable under acidic conditions (easily undergo polymerization) and is difficult to isolate from the aqueous reaction mixture.29 Therefore, we prepared few ester derivatives of HMF and tested them for the reaction with arenes. The formate derivative FMF of HMF seemed to be relatively more active than HMF as it provided high yields 85, 71, and 84% of arylmethylfurfurals 1, 2, and 3, respectively (Table 3, entry 2). Similarly, 5-(acetoxymethyl)furfural (AcMF) also actively reacted with mesitylene, xylene, and toluene to give high yields of arylmethylfurfurals 1, 2, and 3 (Table 3, entry 2), respectively. Electron-withdrawing ester functionality on FMF/AcMF makes them more active electrophiles to react with nucleophilic arenes. Next, we studied the reactivity of the halogen derivative of HMF such as 5-(chloro/bromomethyl)furfural. CMF was treated with mesitylene to obtain the product 1 in 90% yield, whereas after reaction with xylene, only 80% yield of 2 was obtained. On the other hand, toluene reacted relatively faster with CMF to give 92% yield of 3 (Table 3, entry 4). Under identical reaction conditions, 5-(bromomethyl)furfural (BMF) was treated with different arenes and yields of coupling products 1, 2, and 3 were comparable with the products formed from CMF (Table 3, entry 5). The starting materials such as CMF and BMF used in the above experiments were prepared from HMF (see Supporting Information).
Table 3. FC Alkylation of Arenes with HMF and Its Derivatives over Zr-Mont Catalystsa.
Reaction conditions: HMF derivative (2 mmol), arene (5 mL), nitroethane (5 mL), and Zr-Mont (0.1 g), 110 °C.
Isolated yields.
Direct, One-Pot Synthesis of MMF from Glucose and Mesitylene
Although, the crude HMF could be utilized for the FC alkylation with mesitylene, two separate operations must be carried out. Hence, without isolating HMF, direct conversion of its precursors (e.g., glucose, fructose, and sucrose) to diesel fuel precursors is possible as this cascade process is acid-catalyzed. Therefore, initially we selected glucose as a potential HMF precursor, which is more abundant and relatively much cheaper than HMF. The production of MMF from glucose requires three steps: (i) isomerization of glucose to fructose, (ii) dehydration of fructose to HMF, and (iii) coupling of HMF with mesitylene. As shown in the Scheme 2, this process requires two types of catalysts such as a Lewis acid (for glucose isomerization) and Brønsted acid (dehydration of fructose and FC alkylation reaction).
Scheme 2. Process for the Production of MMF (1) from Glucose.
For this purpose, we employed Zr-Mont catalyst which has a unique combination of Lewis + Brønsted acid sites for the purpose of glucose conversion to MMF. At first, glucose dehydration–FC alkylation sequence was carried out in dimethyl sulfoxide (DMSO) at 150 °C over Zr-Mont catalyst. However, even after 24 h of reaction, only HMF was formed in 34% yield without the formation of MMF (Table 4, entry 1). As we found out nitroethane was effective for FC alkylation of arenes with HMF, next experiment with nitroethane + DMSO was carried out to promote the FC alkylation reaction. However, in this experiment also, only HMF was observed without MMF (Table 4, entry 2). After unsuccessful attempt in DMSO, we tested an ionic liquid, C4mimBr (1-butyl-3-methyl-1H-imidazol-3-ium bromide) as a reaction medium, which was also found to be ineffective for the promotion of FC alkylation of in situ-formed HMF and mesitylene (Table 4, entry 3). In the above cases, MMF was not formed, which could be attributed to the limited or no activity of Zr-Mont after glucose dehydration to HMF. This loss in activity of Zr-Mont could be due to the blocking of active centers by the deposition of polymeric products generated in the glucose dehydration step.
Table 4. Direct Conversion of Glucose into MMF in Different Solvents over Zr-Monta.
| yield
(%) |
|||||
|---|---|---|---|---|---|
| entry | solvent | t (h) | Conv.b (%) | HMFc | 1d |
| 1 | DMSO | 24 | 89 | 34 | 00 |
| 2 | DMSO (3 mL) + nitroethane (2 mL) | 16 | 87 | 30 | 00 |
| 3 | BmimBr (1 mL) | 16 | 80 | 21 | trace |
Reaction conditions: glucose (0.5 g), solvent (5 mL), mesitylene (10 mL), and Zr-Mont (0.2 g), 150 °C.
Conversion of glucose.
Yield of HMF was measured using HPLC.
Isolated yields.
After the above attempts, we used acidic solvents which could dissolve glucose as well as help to promote the FC alkylation step. Thus, the first experiment was conducted in acetic acid at 150 °C for a period of 16 h to give 6% of MMF (Table 5, entry 1). Therefore, the medium with higher acidity such as formic acid was evaluated, and surprisingly, complete consumption of glucose was observed with 34% yield of MMF (Table 5, entry 2). Thus, the formation of MMF (1) from glucose was successfully achieved because of the cooperative catalysis between Zr-Mont and formic acid. Lewis acid sites present on Zr-Mont played a crucial role for glucose isomerization to fructose and further reactions could be promoted by formic acid.
Table 5. Direct Conversion of Glucose into MMF in Acidic Solvents over Montmorillonite Catalystsa.
| yield (%) |
||||||
|---|---|---|---|---|---|---|
| entry | solvent | catalyst | t (h) | Conv.b (%) | HMFc | 1d |
| 1 | CH3CO2H | Zr-Mont | 16 | 95 | 04 | 06 |
| 2 | HCO2H | Zr-Mont | 16 | 100 | 00 | 34 |
| 3 | HCO2H | Sn-Mont | 16 | 100 | 00 | 34 |
| 4 | HCO2H | Al-Mont | 16 | 100 | 00 | 32 |
| 5 | HCO2H | Fe-Mont | 16 | 98 | 00 | 31 |
| 6 | HCO2H | 16 | 27 | 00 | ||
Reaction conditions: glucose (0.5 g), solvent (5 mL), mesitylene (10 mL), and catalyst (0.2 g), 150 °C.
Conversion of glucose.
Yield of HMF was measured using HPLC.
Isolated yields.
For the comparison purpose, several other montmorillonite-based solid acids were screened in place of Zr-Mont for this reaction. In the presence of Sn-Mont catalyst also MMF was produced with the same yield (34%) of that obtained over Zr-Mont (Table 5, entry 3). Al-Mont and Fe-Mont gave 32 and 31% yield of MMF, respectively (Table 5, entries 4 and 5). From these results (Table 5, entry 6), it is clear that glucose isomerization to fructose is a key step which is not possible without using a Lewis acid catalyst (Scheme 3). However, 27% conversion of glucose seen may be due the esterification of hydroxyl groups of glucose. A very important process benefit presented in Figure 7 is that mesitylene acting as a reactant as well as a product extraction phase, formic acid acting as a reactive solvent, and Zr-Mont acting as a solid Lewis acid which settled down and to be separated by simple filtration.
Scheme 3. Glucose to MMF over Zr-Mont in Nonacidic vs Acidic Solvents.
Figure 7.
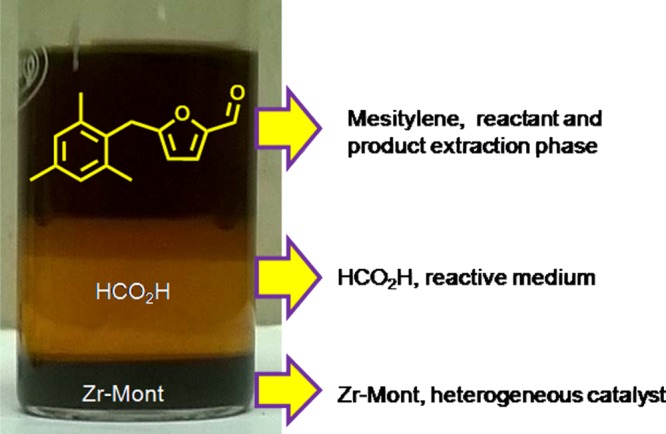
Formation of MMF in mesitylene phase from glucose.
Effect of Reaction Time on the Production of MMF from Glucose
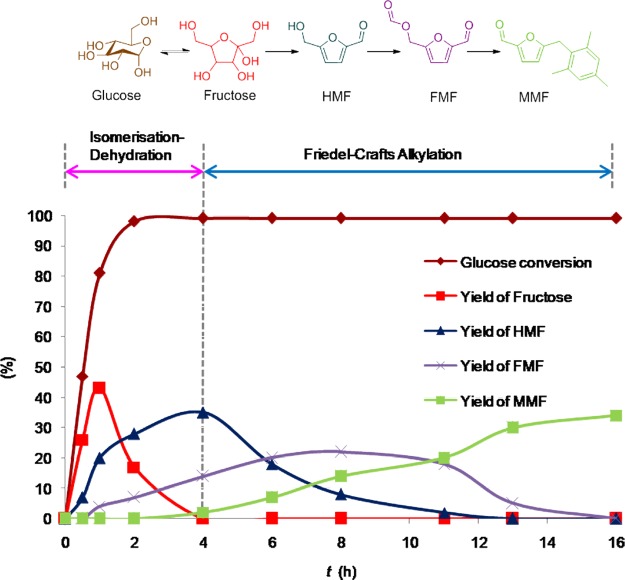
Progress of dehydration–FC alkylation of glucose with mesitylene was monitored by withdrawing samples after specific time intervals and analyzed using high-performance liquid chromatography (HPLC) (Figure 8). Under experimental conditions, in just 1 h, 81% of glucose was consumed with 43, 20, and 4% yields of fructose, HMF, and FMF, respectively. After 2 h of reaction, glucose was consumed completely with 28, 17, and 7% yields of fructose, HMF, and FMF, respectively. Dehydration of glucose appeared to be a rapid process. At the end of 4th hour, fructose was completely consumed with the maximum yield of 35% of HMF along with 14% of FMF and 2% of MMF. Then, HMF was slowly converted into FMF and was subsequently converted into MMF later. The yield of MMF was gradually increased to 34% after 16 h of reaction.
Figure 8.
Reaction progress of glucose conversion to MMF with time. Reaction conditions: glucose (0.5 g), mesitylene (10 mL), formic acid (5 mL), and Zr-Mont (0.2 g), 150 °C, 16 h.
Recycling of Zr-Mont Catalysts for the Production of MMF from Glucose
The stability and reusability of Zr-Mont was investigated for the production of MMF from glucose in formic acid medium. Because of the heterogeneous nature of the Zr-Mont catalyst, it could be easily recovered from the reaction mixture by filtration, and its reusability was studied for MMF production from glucose at 150 °C. It was revealed that the catalyst could be readily recycled six times without any reduction in catalyst activity (Figure 9). After each reuse of Zr-Mont, glucose conversion was almost >95% with a slight drop in the yield of MMF.
Direct, One-Pot Synthesis of Arylmethylfurfural from Glucose and Arenes
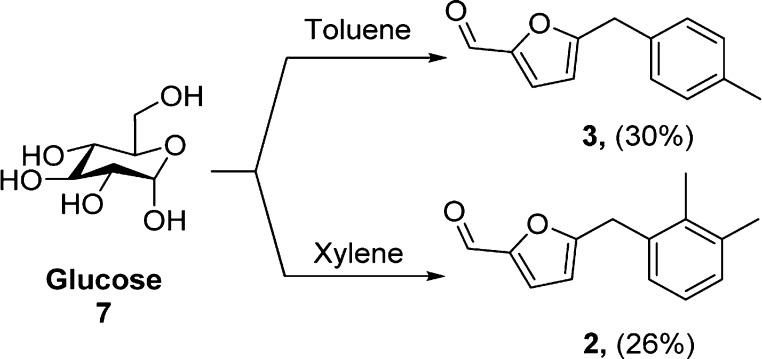
Instead of mesitylene, we further conducted the reaction of xylene and toluene with glucose in formic acid medium. With toluene, product 2 was formed in 30% yield directly from glucose, whereas with xylene, product 3 was formed in 26% yield (Scheme 4).
Scheme 4. Production of Arylmethylfurfural from Glucose and Toluene/Xylene.
One-Pot Synthesis of Coupling Products from Sucrose and Arenes
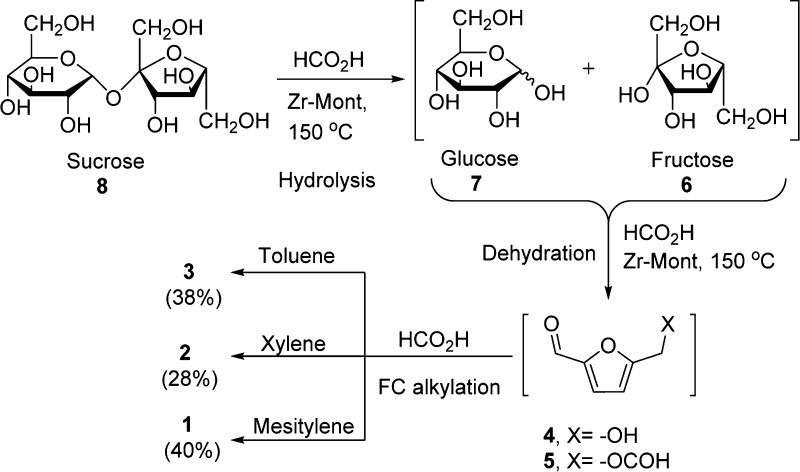
After successful conversion of glucose, the conversion of more complex carbohydrates such as sucrose (8) was also explored. Sucrose is a disaccharide containing one glucose and one fructose unit and its acid hydrolysis breaks down the glycosidic bond between glucose and fructose unit in an acidic medium. The glucose unit of sucrose was intended to isomerize to fructose over Zr-Mont catalyst. Under similar experimental conditions, sucrose was dehydrated to FMF and the generated FMF was coupled with mesitylene to afford the product 1 in 40% yield. In the next experiment, we used xylene for the FC alkylation with in situ-formed HMF/FMF to obtain product 2 in 28% yield. Similarly, product 3 was formed in 38% yield from FC alkylation of toluene with in situ-generated HMF/FMF (Scheme 5).
Scheme 5. Production of Arylmethylfurfural from Sucrose.
Conclusions
An efficient approach for the production of diesel fuel precursors is demonstrated using a combination of biomass and petroleum-derived feedstocks. FC alkylation of arenes (e.g., mesitylene, xylene, and toluene) with HMF or its derivatives was successfully achieved over Zr-Mont catalyst. Various solvents were screened for this reaction, among which nitroethane showed an excellent suitability. Appropriate acid strength and high surface area of Zr-Mont catalyst resulted in the highest yield of arylmethylfurfural. The stability of Zr-Mont was successfully confirmed by its recycle runs. Further, in a one-pot strategy to synthesize MMF from glucose, Zr-Mont promoted the sluggish isomerization of glucose to fructose and formic acid effectively converted in situ-formed fructose into MMF (1) in 34% yield. Using this protocol, arylmethylfurfural was successfully produced by treating glucose with xylene and toluene. Sucrose was also efficiently converted to arylmethylfurfural such as 1, 2, and 3 with 40, 28, and 38% yields, respectively. The phenomenal stability and recyclability of solid acid (Zr-Mont) would replace the hazardous mineral acids (e.g., concd HCl) to make the process green. Importantly, the use of formic acid was found to be attractive as it acted as a solvent to dissolve carbohydrates as well as the cocatalyst to promote FC alkylation.
Experimental Section
Materials
HMF, C4mimBr, inorganic metal precursors such as ZrOCl2·8H2O, SnCl4·5H2O, FeCl3·6H2O, AlCl3·6H2O, and montmorillonite clay were purchased from Sigma-Aldrich, India. Acetic acid (100%), formic acid (98–100%), and carbohydrates such as fructose, sucrose, and glucose were procured from Thomas Baker, India. All solvents and inorganic solids such as NaHCO3 and Na2SO4 were purchased from ChemLabs, India. The starting materials such as 5-(formyl/acetyloxymethyl)furfural and 5-(chloro/bromomethyl)furfural were prepared according to the procedure reported in the literature (see Supporting Information).
Catalyst Preparation
The catalysts were prepared as per previous reports.26,27 Montmorillonite (5 g) (CAS no. 1302-78-9) was slowly dispersed into the aqueous solution of ZrOCl2·8H2O (0.3 M, 80 mL) under stirring. Then, the mixture was further stirred for 4 h at room temperature. After the suspension was filtered for the desired time, the residue was washed with distilled water until neutral (pH = 7) washing. The filtrate was treated with AgNO3 to detect the presence of chloride ion. Finally, the residue of Zr-Mont was dried at 100 °C overnight. The dried catalyst was ground to powder in a mortar with a pestle and stored in a glass bottle. Sn-Mont, Al-Mont, and Fe-Mont were also prepared using the above method from their respective metal precursors, SnCl4·5H2O, AlCl3·6H2O, and FeCl3·6H2O.
Catalyst Characterization
NH3-TPD experiment was performed on a Micromeritics ChemiSorb 2720 instrument. In this experiment, 0.05 g of the catalyst was placed into a U-shaped, flowthrough quartz tube. Then, the catalyst was pretreated with He with a flow rate of 30 cm3/min at 200 °C for a period of 2 h. Then, a mixture of NH3 in He (10%) was passed over the catalyst with a flow rate of 30 cm3/min at 50 °C for a period of 30 min. Subsequently, the sample was flushed with He with a flow rate of 30 cm3/min at 50 °C for a period of 1 h. The TPD measurements were carried out in the range of 50–700 °C with a heating rate of 10 °C min–1. The desorbed ammonia concentration in the effluent was monitored with a thermal conductivity detector. The nitrogen adsorption experiment was also carried out on the same instrument. A PerkinElmer 2000 FTIR spectrometer (4000–400 cm–1) attached with a Harricks diffuse reflectance praying mantis assembly was used for the recording of pyridine FTIR spectra of the prepared catalysts. Before analysis, the self-supporting disk was dehydrated by heating at 400 °C for a period of 1 h under N2 flow to remove physisorbed water. The cell was cooled to room temperature, the self-supporting disk (0.25 cm, radius) was filled with KBr, and the spectrum was recorded as a background. After that KBr was replaced with the catalyst sample (50 mg, weight) and placed in an IR cell attached to a closed glass circulation system. Then, pyridine vapor was introduced into the cell at room temperature until equilibrium was reached, and then, a second spectrum was recorded. Evacuation was performed at different temperatures for the period of 10 min and spectrum was recorded. Then, the obtained spectrum was subtracted from the spectra recorded before pyridine adsorption. The quantitative analysis of Brønsted acid and Lewis acid was determined by using Emeis equations.25 Metal content in the catalyst was determined by using ICP–OES analysis. For that purpose, desired weight of the catalyst was treated with aqua regia (HNO3/HCl = 1:3) at 60 °C on a sand bath for 2 h and then diluted up to 25 mL with distilled water. The resultant sample was then analyzed by using an ICP–OES instrument.
General Procedure for the Synthesis of Arylmethylfurfural from HMF and Its Derivatives
HMF or HMF derivatives (e.g., FMF/AcMF/CMF/BMF) (1 mmol) was dissolved in nitroethane (5 mL). Subsequently, mesitylene/xylene/toluene (10 mL) and Zr-Mont (0.1 g) were added and the reaction mixture was heated at 110 °C under stirring for the period of 16 h. After complete conversion of the starting material, the reaction mixture was filtered to separate the catalyst. The mother liquor was diluted with ethyl acetate (20 mL) and partitioned by H2O (10 mL). Subsequently, the organic phase was treated with anhydrous Na2SO4 and evaporated under reduced pressure. The residue was purified by column chromatography and eluted in petroleum ether.
5-(2,4,6-Trimethylbenzyl)furan-2-carbaldehyde (1)
1H NMR (200 MHz, CDCl3): δ 2.28–2.29 (m, 9H), 4.05 (s, 2H), 5.91–5.93 (d, J = 3.54 Hz, 1H), 6.90 (s, 2H), 7.11–7.13 (d, J = 3.54 Hz, 1H), 9.53 (s, 1H); 13C NMR (50 MHz, CDCl3): δ (ppm) 19.88, 20.83, 28.59, 109.05, 123.16, 129.08, 129.63, 136.58, 136.76, 161.86, 177.00.
5-(2,6-Dimethylbenzyl)furan-2-carbaldehyde (2)
1H NMR (200 MHz, CDCl3): δ 2.26 (s, 3H), 2.31 (s, 3H), 4.00–4.09 (m, 2H), 5.90–6.20 (m, 1H), 6.97–7.18 (m, 5H), 9.54 (s, 1H); 13C NMR (50 MHz, CDCl3): δ (ppm) 19.38, 21.00, 29.73, 32.33, 109.67, 127.02, 128.40, 129.81, 131.39, 177.27.
5-(4-Methylbenzyl)furan-2-carbaldehyde (3)
1H NMR (200 MHz, CDCl3): δ 2.30–2.34 (d, J = 8.97 Hz, 3H), 4.03–4.04 (d, J = 6.57 Hz, 2H), 6.05–6.20 (m, 1H), 7.15–7.20 (m, 4H), 9.54–9.55 (d, J = 0.88 Hz, 1H); 13C NMR (50 MHz, CDCl3): δ (ppm) 19.45, 21.08, 29.73, 32.71, 34.51, 109.67, 109.76, 126.40, 127.46, 128.80, 129.49, 129.85, 130.59, 177.23.
General Procedure for the One-Pot Synthesis of Arylmethylfurfural from Carbohydrates
Carbohydrates (e.g., sucrose/glucose) (0.5 g) were dissolved in formic acid (5 mL). Subsequently, mesitylene/xylene/toluene (10 mL) and Zr-Mont (0.2 g) was added and the reaction mixture was heated at 150 °C under stirring for the period of 16 h. After complete conversion of FMF (reaction key intermediate), the reaction mixture was filtered to separate the catalyst. The mother liquor was diluted with ethyl acetate (20 mL) and partitioned by H2O (10 mL). Subsequently, the organic phase was treated with aq NaHCO3 (6 mL) and dried over anhydrous Na2SO4. Then, the organic layer was evaporated under reduced pressure. The residue was purified by column chromatography and eluted in petroleum ether.
Analysis of the Reaction Products
Merck 5554 aluminum-backed silica plates were used for thin-layer chromatography analysis, and the compounds were visualized under UV light (254 nm). Conversion of carbohydrates was calculated by using Agilent HPLC (column: Hi-Plex H USP L17, detector: RI and mobile phase: Millipore water with 0.6 mL/min flow). Yields of dehydration product of carbohydrates and coupling products were calculated by using Agilent HPLC (column: Poroshell 120 EC-C18 2.7 μm, detector: UV and mobile phase: 0.1% acetic acid in Millipore water/acetonitrile (85:15; v/v) with 0.6 mL/min flow). Pure products were characterized and confirmed by 1H NMR and 13C NMR using CDCl3 (0.01%, TMS) as the solvent on 200 and 50 MHz frequency Bruker instrument.
Acknowledgments
S.H.S. is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, CSIR-National Chemical Laboratory, Pune, and Academy of Scientific and Innovative Research (AcSIR), India.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b00560.
Catalyst characterization, experimental procedures for the synthesis of HMF derivatives, and 1H and 13C NMR spectra of the obtained compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Klass D. L.Biomass for the Renewable Energy and Fuels. Encyclopedia of Energy; Cleveland C. J., Ed.; Elsevier: London, 2004. [Google Scholar]; b Tokay B. A.Biomass Chemicals. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2005. [Google Scholar]; c Huber G. W.; Iborra S.; Corma A. Synthesis of Transportation Fuels from Biomass:Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]; d Metzger J. O. Production of Liquid Hydrocarbons from Biomass. Angew. Chem., Int. Ed. 2006, 45, 696–698. 10.1002/anie.200502895. [DOI] [PubMed] [Google Scholar]; e Serrano-Ruiz J. C.; Dumesic J. A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. 10.1039/c0ee00436g. [DOI] [Google Scholar]
- Chidambaram M.; Bell A. T. A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids. Green Chem. 2010, 12, 1253–1262. 10.1039/c004343e. [DOI] [Google Scholar]
- Gürbüz E. I.; Alonso D. M.; Bond J. Q.; Dumesic J. A. Reactive Extraction of Levulinate Esters and Conversion to γ-Valerolactone for Production of Liquid Fuels. ChemSusChem 2011, 4, 357–361. 10.1002/cssc.201000396. [DOI] [PubMed] [Google Scholar]
- Yang W.; Sen A. One-Step Catalytic Transformation of Carbohydrates and Cellulosic Biomass to 2,5-Dimethyltetrahydrofuran for Liquid Fuels. ChemSusChem 2010, 3, 597–603. 10.1002/cssc.200900285. [DOI] [PubMed] [Google Scholar]
- a Mascal M.; Nikitin E. B. Direct, High-Yield Conversion of Cellulose into Biofuel. Angew. Chem., Int. Ed. 2008, 47, 7924–7926. 10.1002/anie.200801594. [DOI] [PubMed] [Google Scholar]; b Balakrishnan M.; Sacia E. R.; Bell A. T. Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem. 2012, 14, 1626–1634. 10.1039/c2gc35102a. [DOI] [Google Scholar]
- a Jae J.; Mahmoud E.; Lobo R. F.; Vlachos D. G. Cascade of Liquid-Phase Catalytic Transfer Hydrogenation and Etherification of 5-Hydroxymethylfurfural to Potential Biodiesel Components over Lewis Acid Zeolites. ChemCatChem 2014, 6, 508–513. 10.1002/cctc.201300978. [DOI] [Google Scholar]; b Lewis J. D.; de Vyver S. V.; Crisci A. J.; Gunther W. R.; Michaelis V. K.; Griffin R. G.; Román-Leshkov Y. A Continuous Flow Strategy for the Coupled Transfer Hydrogenation and Etherification of 5-(Hydroxymethyl)furfural using Lewis Acid Zeolites. ChemSusChem 2014, 7, 2255–2265. 10.1002/cssc.201402100. [DOI] [PubMed] [Google Scholar]
- Moreau C.; Belgacem M. N.; Gandini A. Recent Catalytic Advances in the Chemistry of Substituted Furans from Carbohydrates and in the Ensuing Polymers. Top. Catal. 2004, 27, 11–30. 10.1023/b:toca.0000013537.13540.0e. [DOI] [Google Scholar]
- a Pentz W. J.Polyurethanes or isocyanurates from alkoxylated hydroxymethylfuran. U.S. Patent 4,426,460, 1984.; b Gandini A.ACS Symposium Series; American Chemical Society, 1990; pp 197–208. [Google Scholar]; c Timko J. M.; Cram D. J. Furanyl unit in host compounds. J. Am. Chem. Soc. 1974, 96, 7159–7160. 10.1021/ja00829a085. [DOI] [Google Scholar]
- Dabelstein W.; Reglitzky A.; Schutze A.; Reders K.. Automotive Fuels. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2000. [Google Scholar]
- Huber G. W.; Chheda J. N.; Barrett C. J.; Dumesic J. A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 2005, 308, 1446–1450. 10.1126/science.1111166. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam A. V.; Thayumanavan S.; Huber G. W. C-C Bond Formation Reactions for Biomass-Derived Molecules. ChemSusChem 2010, 3, 1158–1161. 10.1002/cssc.201000136. [DOI] [PubMed] [Google Scholar]
- Nicklaus C. M.; Minnaard A. J.; Feringa B. L.; de Vries J. G. Synthesis of Renewable Fine-Chemical Building Blocks by Reductive Coupling between Furfural Derivatives and Terpenes. ChemSusChem 2013, 6, 1631–1635. 10.1002/cssc.201300179. [DOI] [PubMed] [Google Scholar]
- a Corma A.; de la Torre O.; Renz M.; Villandier N. Production of High-Quality Diesel from Biomass Waste Products. Angew. Chem., Int. Ed. 2011, 50, 2375–2378. 10.1002/anie.201007508. [DOI] [PubMed] [Google Scholar]; b Balakrishnan M.; Sacia E. R.; Bell A. T. Syntheses of Biodiesel Precursors: Sulfonic Acid Catalysts for Condensation of Biomass-Derived Platform Molecules. ChemSusChem 2014, 7, 1078–1085. 10.1002/cssc.201300931. [DOI] [PubMed] [Google Scholar]; c Shinde S. H.; Rode C. V. A two-phase system for the clean and high yield synthesis of furylmethane derivatives over −SO3H functionalized ionic liquids. Green Chem. 2017, 19, 4804–4810. 10.1039/c7gc01654a. [DOI] [Google Scholar]
- Arias K. S.; Climent M. J.; Corma A.; Iborra S. Synthesis of high quality alkyl naphthenic kerosene by reacting an oil refinery with a biomass refinery stream. Energy Environ. Sci. 2015, 8, 317–331. 10.1039/c4ee03194f. [DOI] [Google Scholar]
- http://www.ava-biochem.com/pages/en/home.php.
- Zhou X.; Rauchfuss T. B. Production of Hybrid Diesel Fuel Precursors from Carbohydrates and Petrochemicals Using Formic Acid as a Reactive Solvent. ChemSusChem 2013, 6, 383–388. 10.1002/cssc.201200718. [DOI] [PubMed] [Google Scholar]
- Nale S. D.; Jadhav V. H. Synthesis of Fuel Intermediates from HMF/Fructose. Catal. Lett. 2016, 146, 1984–1990. 10.1007/s10562-016-1836-0. [DOI] [Google Scholar]
- a Huang R.; Qi W.; Su R.; He Z. Integrating enzymatic and acid catalysis to convert glucose into 5-hydroxymethylfurfural. Chem. Commun. 2010, 46, 1115. 10.1039/b921306f. [DOI] [PubMed] [Google Scholar]; b Takagaki A.; Ohara M.; Nishimura S.; Ebitani K. A one-pot reaction for biorefinery: combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides. Chem. Commun. 2009, 6276–6278. 10.1039/b914087e. [DOI] [PubMed] [Google Scholar]; c Watanabe M.; Aizawa Y.; Iida T.; Aida T. M.; Levy C.; Sue K.; Inomata H. Glucose reactions with acid and base catalysts in hot compressed water at 473 K. Carbohydr. Res. 2005, 340, 1925–1930. 10.1016/j.carres.2005.06.017. [DOI] [PubMed] [Google Scholar]; d Román-Leshkov Y.; Moliner M.; Labinger J. A.; Davis M. E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chem., Int. Ed. 2010, 49, 8954–8957. 10.1002/anie.201004689. [DOI] [PubMed] [Google Scholar]; e Jadhav H.; Pedersen C. M.; Sølling T.; Bols M. 3-Deoxy-glucosone is an Intermediate in the Formation of Furfurals from D-Glucose. ChemSusChem 2011, 4, 1049–1051. 10.1002/cssc.201100249. [DOI] [PubMed] [Google Scholar]; f Assary R. S.; Curtiss L. A. Theoretical Study of 1,2-Hydride Shift Associated with the Isomerization of Glyceraldehyde to Dihydroxy Acetone by Lewis Acid Active Site Models. J. Phys. Chem. A 2011, 115, 8754–8760. 10.1021/jp204371g. [DOI] [PubMed] [Google Scholar]
- Wang J.; Ren J.; Liu X.; Xi J.; Xia Q.; Zu Y.; Lu G.; Wang Y. Direct conversion of carbohydrates to 5-hydroxymethylfurfural using Sn-Mont catalyst. Green Chem. 2012, 14, 2506–2512. 10.1039/c2gc35699f. [DOI] [Google Scholar]
- Shinde S. H.; Rode C. V. An Integrated Production of Diesel Fuel Precursors from Carbohydrates and 2-Methylfuran over Sn-Mont Catalyst. ChemistrySelect 2018, 3, 4039–4046. 10.1002/slct.201800694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Srokol Z.; Bouche A.-G.; van Estrik A.; Strik R. C. J.; Maschmeyer T.; Peters J. A. Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds. Carbohydr. Res. 2004, 339, 1717–1726. 10.1016/j.carres.2004.04.018. [DOI] [PubMed] [Google Scholar]; b van Dam H. E.; Kieboom A. P. G.; van Bekkum H. The Conversion of Fructose and Glucose in Acidic Media: Formation of Hydroxymethylfurfural. Starch/Staerke 1986, 38, 95–101. 10.1002/star.19860380308. [DOI] [Google Scholar]; c Xing R.; Qi W.; Huber G. W. Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries. Energy Environ. Sci. 2011, 4, 2193–2205. 10.1039/c1ee01022k. [DOI] [Google Scholar]; d Bozell J. J. Connecting Biomass and Petroleum Processing with a Chemical Bridge. Science 2010, 329, 522–523. 10.1126/science.1191662. [DOI] [PubMed] [Google Scholar]; e Corma A.; Iborra S.; Velty A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- a Joó F. Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 2008, 1, 805–808. 10.1002/cssc.200800133. [DOI] [PubMed] [Google Scholar]; b Enthaler S. Carbon Dioxide-The Hydrogen-Storage Material of the Future?. ChemSusChem 2008, 1, 801–804. 10.1002/cssc.200800101. [DOI] [PubMed] [Google Scholar]; c Tanaka R.; Yamashita M.; Nozaki K. Catalytic Hydrogenation of Carbon Dioxide Using Ir(III)–Pincer Complexes. J. Am. Chem. Soc. 2009, 131, 14168–14169. 10.1021/ja903574e. [DOI] [PubMed] [Google Scholar]
- a Niu S.; Zhu Y.; Zheng H.; Zhang W.; Li Y. Dehydration of Glycerol to Acetol over Copper-Based Catalysts. Chin. J. Catal. 2011, 32, 345–351. 10.3724/sp.j.1088.2011.00914. [DOI] [Google Scholar]; b Sato S.; Sakai D.; Sato F.; Yamada Y. Vapor-phase Dehydration of Glycerol into Hydroxyacetone over Silver Catalyst. Chem. Lett. 2012, 41, 965–966. 10.1246/cl.2012.965. [DOI] [Google Scholar]
- Zahedi-Niaki M. H.; Zaidi S. M. J.; Kaliaguine S. Acid properties of titanium aluminophosphate molecular sieves. Microporous Mesoporous Mater. 1999, 32, 251–255. 10.1016/s1387-1811(99)00111-0. [DOI] [Google Scholar]
- Emeis C. A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. 10.1006/jcat.1993.1145. [DOI] [Google Scholar]
- a Shinde S.; Rode C. Cascade Reductive Etherification of Bioderived Aldehydes over Zr-Based Catalysts. ChemSusChem 2017, 10, 4090–4101. 10.1002/cssc.201701275. [DOI] [PubMed] [Google Scholar]; b Shinde S.; Rode C. Selective self-etherification of 5-(hydroxymethyl)furfural over Sn-Mont catalyst. Catal. Commun. 2017, 88, 77–80. 10.1016/j.catcom.2016.09.034. [DOI] [Google Scholar]
- Masui Y.; Wang J.; Teramura K.; Kogure T.; Tanaka T.; Onaka M. Unique structural characteristics of tin hydroxide nanoparticles-embedded montmorillonite (Sn-Mont) demonstrating efficient acid catalysis for various organic reactions. Microporous Mesoporous Mater. 2014, 198, 129–138. 10.1016/j.micromeso.2014.07.024. [DOI] [Google Scholar]
- Iovel I.; Mertins K.; Kischel J.; Zapf A.; Beller M. An Efficient and General Iron-Catalyzed Arylation of Benzyl Alcohols and Benzyl Carboxylates. Angew. Chem., Int. Ed. 2005, 44, 3913–3917. 10.1002/anie.200462522. [DOI] [PubMed] [Google Scholar]
- Kang E.-S.; Hong Y.-W.; Chae D. W.; Kim B.; Kim Y. J.; Cho J. K.; Kim Y. G. From Lignocellulosic Biomass to Furans via 5-Acetoxymethylfurfural as an Alternative to 5-Hydroxymethylfurfural. ChemSusChem 2015, 8, 1179–1188. 10.1002/cssc.201403252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.