Abstract
T-box riboswitches are a widespread class of structured non-coding RNAs in Gram-positive bacteria that regulate the expression of amino acid-related genes. They form negative feedback loops to maintain steady supplies of aminoacyl-tRNAs to the translating ribosomes. T-box riboswitches are located in the 5’ leader regions of mRNAs that they regulate and directly bind to their cognate tRNA ligands. T-boxes further sense the aminoacylation state of the bound tRNAs and, based on this readout, regulate gene expression at the level of transcription or translation. T-box riboswitches consist of two conserved domains — a 5’ Stem I domain that is involved in specific tRNA recognition and a 3’ antiterminator/antisequestrator (or discriminator) domain that senses the amino acid on the 3’-end of the bound tRNA. Interaction of the 3’-end of an uncharged but not charged tRNA with a thermodynamically weak discriminator domain stabilizes it to promote transcription readthrough or translation initiation. Recent biochemical, biophysical and structural studies have provided high-resolution insights into the mechanism of tRNA recognition by Stem I, several structural models of full-length T-box-tRNA complexes, mechanism of amino acid sensing by the antiterminator domain, as well as kinetic details of tRNA binding to the T-box riboswitches. In addition, translation-regulating T-box riboswitches have been recently characterized which presented key differences from the canonical transcriptional T-boxes. Here, we review the recent developments in understanding the T-box riboswitch mechanism that have employed various complementary approaches. Further, the regulation of multiple essential genes by T-boxes make them very attractive drug targets to combat drug resistance. The recent progress in understanding the biochemical, structural and dynamic aspects of the T-box riboswitch mechanism will enable more precise and effective targeting with small molecules.
Introduction
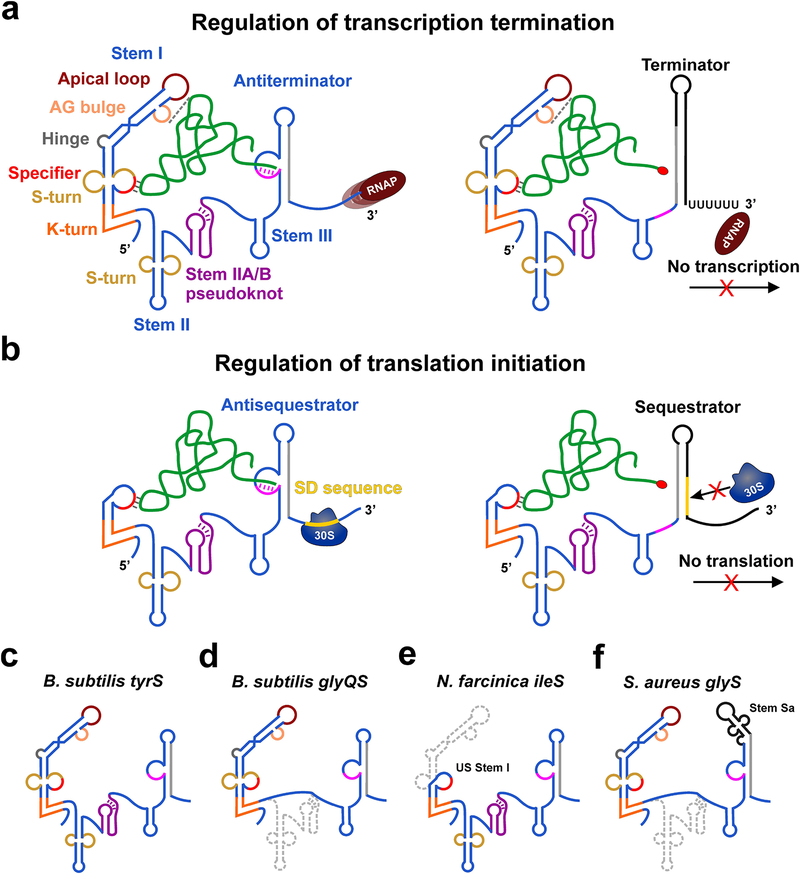
T-box riboswitches are highly structured RNA domains present in the 5’-untranslated regions of mRNAs for amino acid-related genes in many Gram-positive bacteria(13, 27). T-box riboswitches selectively bind to cognate tRNAs and directly sense their aminoacylation status to regulate the expression of mRNAs coding for aminoacyl-tRNA synthetases (aaRS) and other proteins involved in the metabolism and transport of amino acids. T-box riboswitches that recognize tRNAs bearing each of all 20 amino acids have been identified, spanning diverse phyla of bacteria with prevalence in Firmicutes(47, 27). T-box-mediated gene regulation is achieved both at the level of transcription and translation. Stable binding of a cognate uncharged tRNA to the T-box riboswitch leads to transcription readthrough by stabilizing a weak antiterminator structure, which prevents premature termination of transcription by precluding the formation of a mutually exclusive Rho-independent terminator hairpin. Similarly, for translational T-boxes, uncharged tRNA binding promotes translation initiation by formation of an antisequestrator helix that exposes the ribosome-binding site (or Shine-Dalgarno, SD sequence)(27). Aminoacylated tRNA cannot stabilize the antiterminator/antisequestrator resulting in the termination of transcription or inhibition of translation initiation by occluding the SD sequence within a sequestrator helix(43, 59) (Figure 1a,b).
Figure 1. Mechanisms of T-box riboswitch-mediated gene regulation.
(a) Cartoon showing the general architecture and mechanism of regulation of transcription termination by T-box riboswitches. The amino acid on the 3’-end of charged tRNA is shown as a red oval. (b) General mechanism of regulation of translation initiation by T-box riboswitches. (c-f) Cartoons showing the architecture of B. subtilis tyrS (c), B. subtilis glyQS (d) N. farcinica ileS (e) and S. aureus glyQ (f) T-box riboswitches. The missing structural elements in other T-boxes (d-f) compared to the canonical tyrS T-box architecture in (a) are shown in gray.
The T-box system is among the first RNA-centric, gene-regulatory mechanisms discovered in bacteria over two decades ago(17). Due to the similarity in its mechanism of gene regulation to small-molecule- sensing RNAs, the T-box system has been retrospectively referred to as a riboswitch(59). However, the T-box riboswitch differs from the metabolite-binding riboswitches in recognizing a complex, structured macromolecular ligand (tRNA), rather than a small-molecule metabolite or metal ion(22). The gene-regulatory mechanism of the T-box riboswitch is anchored by RNA-RNA interactions and does not strictly require any accessory proteins other than the RNA polymerase (RNAP) which transcribes them. Therefore, the T-box riboswitch-based gene regulation is likely an ancient system that could have evolved during a predominantly RNA world(13). By controlling the cellular availability of amino acids in the form of charged tRNAs required for protein synthesis, T-box riboswitches play a major role in bacterial survival and adaptation under nutrient stress conditions(22).
Sequence and architecture of T-box riboswitches
Bioinformatics analyses of bacterial genomes have revealed the ubiquitous nature of T-box riboswitches in controlling the expression of aminoacyl-tRNA synthetase (aaRS) genes for every amino acid(47, 21), highlighting its importance as a major gene-regulatory element in Gram-positive bacteria. Among these, the Bacillus subtilis tyrS(17, 19, 11) and glyQS(18, 20, 54) T-box riboswitches have been best studied using various genetic, biochemical, and biophysical techniques, which identified the conserved sequence and structural motifs required for tRNA recognition and gene regulation(13, 23). T-box riboswitches in general are comprised of two domains connected by a single-stranded linker region — a 5’ domain involved in selectively binding its cognate tRNA ligand and a 3’ domain that pairs with the 3’-NCCA end of the bound tRNA to sense its aminoacylation status(59, 27). The upstream tRNA recognition domain is usually composed of a Stem I followed by Stem II and Stem IIA/B (H-type) pseudoknot structures that are sometimes missing in certain systems such as the glyQS T-box riboswitches (Figure 1c–f). The tyrS T-box riboswitch contains all these structural elements which were shown to be necessary for tRNA-dependent antitermination in vivo(38). Therefore, the B. subtilis tyrS system can be considered as the canonical T-box riboswitch, being the first discovered and well-studied T-box containing all major structural elements including a long Stem I, Stem II, Stem IIA/B pseudoknot, Stem III and a common discriminator domain (Figure 1c).
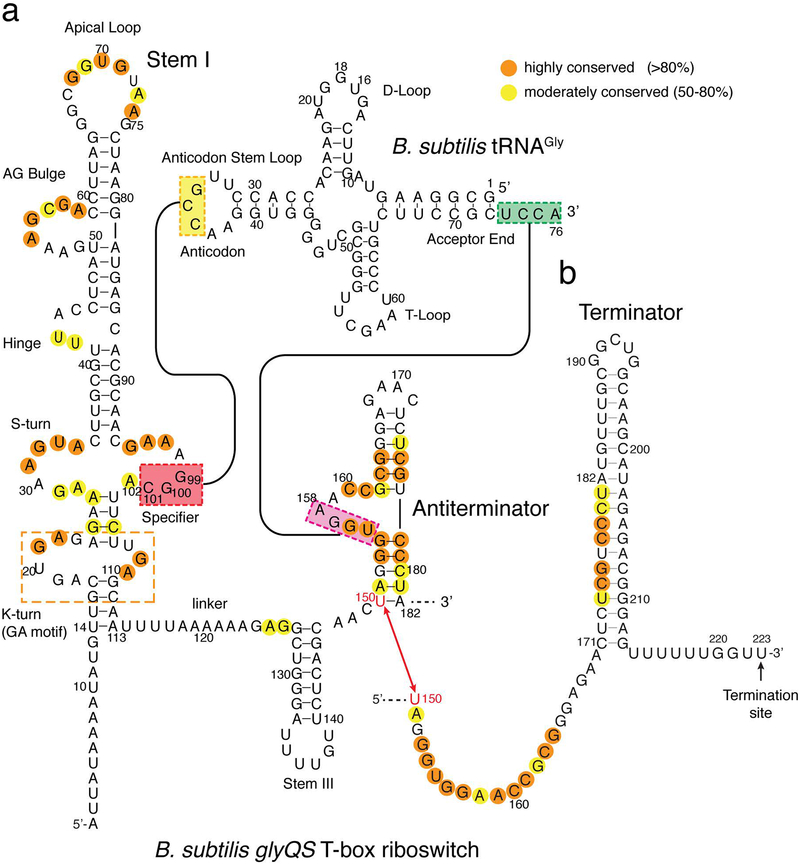
In most T-boxes, such as the tyrS and glyQS riboswitches, an elongated (~100 nucleotides, nt) Stem I domain is present consisting of multiple known RNA structural motifs, and a highly conserved ~7-nt “Specifier loop” that contains the key 3-nt codon-like sequence complementary to the tRNA anticodon (Figure 2)(59). The interaction of the Specifier trinucleotide with the tRNA anticodon is the principal determinant for cognate tRNA selection. Mismatches in this key interface invariably reduce tRNA binding affinity and tRNA-dependent transcription antitermination, to different extents that depend on the position of the mismatch within the 3-nt Specifier sequence(5). As expected, mismatches in the middle position are least tolerated compared to those in the flanking positions, including the wobble position(5). A fourth conserved purine immediately following the specifier sequence stacks below the 3-bp intermolecular helix providing additional stability(59). Recently, T-box riboswitches with longer 8-nt Specifier loops were shown to respond to two cognate tRNAs by utilizing a 4-nt Specifier sequence containing overlapping codons(40). These dual-specificity T-boxes can sense more than one tRNA that carry metabolically related amino acids, such as tRNAGlu and tRNAAsn. Apart from the anticodon-specifier interaction, other tRNA features and characteristics such as local structure, post-transcriptional modifications, and flexibility and deformability likely provide additional specificity for the cognate tRNA(30). This notion was supported by genetic findings using the tyrS system which demonstrated that the T-box riboswitch can be made to respond to non-cognate tRNAs simply by mutating the specifier sequence to pair with non-cognate tRNA anticodons. Nonetheless, the extent of gene regulation observed with the chimeric systems were consistently and significantly lower than that of the original cognate tRNA(16). This finding demonstrated that besides the anticodon, T-box riboswitches recognize additional sequence and structural features that are specific to their cognate tRNA ligand, which likely co-evolved with the T-box.
Figure 2. Sequence and secondary structure of a T-box riboswitch.
(a) Sequence and secondary structures of the B. subtilis glyQS T-box riboswitch and its cognate tRNAGly showing the conservation pattern and base-pairing contacts. (b) Sequence and secondary structure of the Rho-independent terminator hairpin, with the transcription termination site indicated.
The distal region of long Stem I domains in canonical T-boxes includes a conserved “AG bulge” and an apical loop that interact with each other to form an interdigitated double T-loop motif. This motif generates a flat platform which stacks against the base pair that makes up the tRNA elbow (Figures 1,2)(57, 59). In addition, a short 2–3 nt long pyrimidine-rich bulge located between the AG bulge and the Specifier loop acts as a flexible hinge enabling Stem I to act as a tRNA-measuring caliper(56). Additional Stem II and Stem IIA/B pseudoknot elements are present 3’ of Stem I in most T-box riboswitches, such as the tyrS system (Figure 1c)(16, 27). These conserved motifs are absent from the simpler glycyl T-box riboswitches (Fig. 1d) and their roles in T-box function are not understood. The Stem II and Stem IIA/B pseudoknot motifs were recently suggested to contact the tRNA near the D-loop and T-arm regions and therefore may act to provide tRNA binding affinity and selectivity, especially in T-boxes where Stem I is short and lacks the elbow-binding double T-loop motif(42). The tyrS and many other T-boxes contain all three elements, suggesting that these contacts are not mutually exclusive and presumably occur at different locations and may even collaborate to provide necessary tRNA avidity and specificity. No T-box examples have been found so far that lack all three elements (the Stem I double T-loop motif, Stem II, and Stem IIA/B), providing support for this notion(47, 27, 42).
In T-box riboswitches with long Stem I domains, the nucleotides on the 5’-side of the Specifier sequence and in the loop of the opposite strand form a loop E motif (also known as the S-turn or bulged-G motif). A similar S-turn motif is also present in Stem II, such as in the tyrS or ileS T-boxes(43). In addition, Stem I also includes a kink-turn motif (K-turn or GA motif) below the Specifier loop (Figure 2)(27). K-turns are conserved structural elements that introduce a sharp ~120° bend in the RNA backbone and are also observed in ribosomal RNA and other small-molecule binding riboswitches(25). In T-boxes, the K-turn at the base of Stem I was proposed to be important for orienting the 3’ antiterminator domain to facilitate its interaction with the tRNA acceptor end(13, 57). Mutations disrupting the K-turn motif have been shown to abolish tRNA-dependent transcription antitermination in vivo(53). Many bacteria, including T-box-containing Gram-positive species, have more than one K-turn-binding protein that exhibit high binding affinities to these motifs and may help maintain them in a kinked conformation(4, 24). However, some T-boxes such as the glyQS can efficiently function in vitro in the absence of K-turn-binding proteins, raising the question of the general importance of K-turn binding proteins for T-box function in vivo. It is important to note that optimal function of T-box riboswitches in vitro, as judged by tRNA-dependent antitermination, requires high (at least 5 mM, generally 10–15 mM) Mg2+(13). The reason behind the requirement for such non-physiologically high Mg2+ concentrations is not clear but may include the strong Mg2+ dependency (millimolar concentrations) of some K-turns to adopt the kinked conformation in the absence of their binding proteins. The K-turn motif is not required for tRNA recognition by Stem I, as deleting it did not appreciably affect the Kd for tRNA binding(57). Interestingly, recent single-molecule studies of the glyQS T-box riboswitch could not detect even transient tRNA binding events to Stem I under Mg2+ concentrations below 5 mM(45, 55). These observations suggest that the high Mg2+ concentration might be required for the various structural elements in Stem I to adopt their intended conformations, either through non-specific electrostatic effects of the divalent cation that mitigates charge repulsion between the tRNA and T-box or through site-specifically bound Mg2+ ions to different motifs in Stem I.
A single-stranded linker region connects the 5’ domain to the 3’ domain, which is either an anti-terminator or anti-sequestrator in transcriptional and translational T-boxes, respectively (Figure 1). The linker is purine rich and tends to be longer than 12 nt in T-boxes lacking Stem II and Stem IIA/B pseudoknot (such as glyQS) and a bit shorter in their presence (Figure 2). Nonetheless, this linker invariably harbors a Stem III of varying length towards its 3’-end, adjacent to the 3’ domain, suggesting an important yet unknown function (Figure 2). Analogous to the expression platform in small-molecule riboswitches, sequences on the 3’-side of Stem III form the discriminator domain, the conformation of which dictates the fate of gene expression(59). This region can fold into mutually exclusive terminator/antiterminator or sequestrator/antisequestrator structures in transcription- or translation-regulating T-boxes, respectively(27). This region also contains a 14-nt segment of highly conserved nucleotides, which forms the 5’-half of the antiterminator. In transcriptional T-boxes, the antiterminator contains two short helices A1 and A2 connected by a highly conserved 7-nt bulge (Figure 2). The bulge includes a 4-nt 3’-NGGU-5’ sequence that is complementary to the universal 5’-NCCA-3’ sequence at the 3’-end of tRNA, followed by a highly conserved ACC trinucleotide at the 3’ edge of the bulge (thus the 7-nt bulge sequence is 5’-UGGNACC-3’). The fourth nucleotide in the T-box bulge co-varies with the tRNA discriminator nucleotide immediately 5’ to the tRNA CCA end so that a 4-bp intermolecular helix is maintained between the T-box and its cognate tRNA. In some T-boxes, such as in the glyS T-box from S. aureus, the antiterminator domain contains an additional extended helix on top, termed Stem Sa (Figure 1f), the function of which is not clearly known(3).
Mechanisms of T-box riboswitch action
T-box riboswitches must select their cognate tRNAs among an ocean of similar intracellular tRNAs and gauge the ratio of charged vs uncharged tRNAs to either induce or repress the expression of proteins that maintain amino acid homeostasis(13). All known T-box riboswitches are ‘ON’ switches, which means that stable binding of the cognate ligand (uncharged tRNA) leads to upregulation of gene expression by favoring the antiterminator/antisequestrator formation. Under conditions of nutrient starvation or stress, the concentration of intracellular uncharged tRNA is expected to rise. For the transcriptional T-boxes, regulation occurs co-transcriptionally where Stem I, which is transcribed first, can bind the uncharged tRNA and position it so that its 3’-end can interact with the antiterminator emerging from the RNA exit channel of the RNAP. This will stabilize the thermodynamically weaker antiterminator and prevent formation of the much longer and more stable terminator helix. In this regard, the weaker stability of the antiterminator as compared to the terminator is offset in part by its kinetic advantage during transcription, and more importantly, by stabilization from tRNA 3’-end binding. The K-turn motif at the base of Stem I appears to plays an architectural role in this process to orient the emerging antiterminator close to the bound tRNA 3’-end so that the intermolecular helix can form rapidly. In a similar manner, Stem I also binds to charged tRNAs under nutrient rich conditions with comparable affinities as uncharged tRNAs. However, the presence of an amino acid on the tRNA 3’-end prevents a productive interaction with the antiterminator, leading to the formation of the thermodynamically more stable terminator that halts transcription. Therefore, charged tRNAs act as a competitive inhibitor for binding of uncharged tRNAs to the T-box riboswitch and decrease transcriptional readthrough. Through this mechanism, the tRNA-dependent antitermination depends on the ratio of charged-to-uncharged tRNAs, as shown previously(20, 13). Using in vitro kinetic transcription assays employing the glyQS T-box, it was shown that the interaction of tRNA 3’-end with the antiterminator can occur as soon as the complementary 5’-UGGN-3’ sequence in the antiterminator bulge becomes available for pairing and does not require the synthesis of the full antiterminator domain(18). Nevertheless, a full antiterminator domain helps in stabilizing the short 4-bp intermolecular stem by providing coaxial stacking interactions with helix A1. As shown for transcriptional metabolite-binding riboswitches, the kinetics of ligand binding relative to the speed of transcription by the RNAP determines the outcome of gene regulation(51). Transcriptional pausing at specific, strategically located sequences in such cases was proposed to provide appropriate time delay for ligand binding and to enable decision making before the RNAP escapes past the terminator region(51, 10). A similar mechanism likely exists for some of the transcriptional T-box riboswitches. For the B. subtilis glyQS T-box, the presence of multiple long-lived pause sites were uncovered under in vitro conditions at low concentration of NTPs(18). Three such pause sites are located near the base of Stem I, in the loop of Stem III and immediately following the antiterminator domain. Interestingly, in vivo term-seq data from B. subtilis supported the major pause site 3’ of the antiterminator in the glyQS T-box while the other long pause sites, including the pause in the loop of Stem III were not detected(28). The presence of long pauses hints that in addition to aforementioned kinetic control, thermodynamic control could also be at play, where the T-box has enough time to reach binding equilibrium with the cognate tRNAs before the transcribing RNAP must render a decision(60).
The effect of tRNA 3’ end modifications on antitermination was previously demonstrated using an “EX1C” mimic of charged tRNAGly that had an extra cytosine appended to the tRNA 3’-end(20). Recently, using different chemical modifications on the tRNA 3’-end, it was shown that the efficiency of transcriptional readthrough is inversely proportional to the molecular volume of tRNA 3’-end modification(58). This demonstrated that the T-box 3’ region can sense and discriminate even minute changes on the tRNA 3’-end such that a single phosphate group or the smallest amino acid, glycine, can effectively reduce transcription to basal levels. Based on this, the T-box riboswitch antiterminator domain was proposed to work through a steric hindrance-based mechanism for rejecting aminoacylated tRNAs, which is an effective strategy to respond to various amino acids of differing sizes and chemical compositions. This highlights the exquisite sensitivity of the T-box 3’ region to detect small modifications to the tRNA 3’-end and to modulate transcription accordingly. Further, using fluorescent nucleic acid base analogs and performing life-time measurements led to a model where the intermolecular helix stacks coaxially on top of the helix A1 of the antiterminator and forms a long continuous helical stack with the tRNA acceptor arm, the tRNA elbow and all the way through the six stacked layers of Stem I’s double T-loop platform(58). It is remarkable then that even a small chemical modification on the tRNA 3’-end would disrupt such a stable coaxial stacking interaction. The molecular details of this mechanism explaining how the T-box 3’ region acts as a molecular sieve to reject aminoacylated tRNA await high-resolution structural studies.
High-resolution structures of T-box riboswitch domains
Since their discovery nearly 3 decades ago, extensive genetic, biochemical and phylogenetic studies on T-box riboswitches have established the sequences and structural motifs that are important for specific tRNA binding(47, 27). The first structural studies on the T-box riboswitches were performed using NMR spectroscopy on the tyrS Specifier loop domain, antiterminator and Stem I domain(12, 48, 49). The NMR structure of the Specifier domain revealed a canonical, over-wound helical structure of the loop E motif and showed an overall well-defined conformation with limited dynamics(48) (Figure 3a). The Specifier sequence adopted a conformation in which the bases were stacked and oriented with their Watson-Crick edges facing towards the minor groove, which was proposed to aid in efficient recognition of tRNA anticodon. Interestingly, the NMR structure of a tyrS Specifier loop domain containing the K-turn motif showed an extended conformation in which the K-turn is not kinked, even in the presence of high Mg2+ (Figure 3b). Addition of Mg2+ resulted in a kink at the base of the Specifier loop, but not at the K-turn. The extended conformation of the K-turn motif in the absence of a K-turn binding protein may explain the general difficulty to reconstitute tRNA-mediated antitermination in vitro for many T-boxes such as the B. subtilis tyrS riboswitch(13). The NMR structure of the antiterminator domain from B. subtilis tyrS T-box showed a kinked conformation between the helices A1 and A2 containing the intervening 7-nt bulge (Figure 3c). The first 4 nt of the bulge containing the 5’-UGGN-3’ sequence complementary to the tRNA 3’-end were flexible and not pre-organized for tRNA binding(12). In contrast, the last 3 nt of the bulge containing the highly conserved ACC sequence adopted a well-ordered conformation with extensive stacking, which introduced a bend in the orientation of helix A2. Based on these observations, it was proposed that the stability of the 3’ half of the bulge facilitates tRNA acceptor end interaction with a flexible UGGN sequence leading to intermolecular helix formation via an induced-fit mechanism(12).
Figure 3. Structures of T-box riboswitches.
(a-c) NMR structures of the B. subtilis tyrS T-box riboswitch domains including the Specifier domain (a; PDB ID:2khy), Specifier and proximal domain with K-turn (b; PDB ID:2kzl), and the antiterminator domain (c; PDB ID:1n53). In (a) and (b), the specifier trinucleotide is shown in red. In (c), green and magenta segments indicate well-ordered and more flexible regions, respectively. (d) Crystal structure of the Stem I distal domain containing the double T-loop motif (PDB ID: 4jrc). (e-f) Co-crystal structures of tRNAGly bound to Stem I from O. iheyensis (e; PDB ID: 4lck) and G. kaustophilus (f: PDB ID: 4mgn). (g) tRNA elbow interaction with the interdigitated double T-loop motif in O. iheyensis Stem I. (h) Codon-anticodon like interaction between the Specifier sequence in Stem I of O. iheyensis T-box and tRNAGly.
More recently, a high-resolution crystal structure of a T-box Stem I distal region (Figure 3d) and two co-crystal structures of the glyQ(S) T-box riboswitch bound to tRNAGly (Figures 3e,f) provided a molecular basis for the selective recognition of cognate tRNA(14, 15, 57). The structure of the distal Stem I region revealed a fascinating interdigitated double T-loop motif formed by mutual intercalation of two pentanucleotide T-loops that emanate from the highly conserved AG bulge and apical loop(14) (Figure 3d). This motif is also present in the ribosome L1 stalk and in RNase P where it performs a similar task of recognizing the tRNA elbow(33, 26, 36, 56). Interestingly, bioinformatics and structural modeling analyses of T-box sequences correctly predicted the existence and unusual orientation of this motif, before being experimentally validated by the three crystal structures(29). The double T-loop motif orchestrates the presentation of a conserved base triple that uses its expansive flat surface to stack with the characteristically flat tRNA elbow to provide a platform for tRNA landing during co-transcriptional binding (Figure 3g)(56).
The co-crystal structures of Stem I-tRNAGly complexes from Oceanobacillus iheyensis glyQ and Geobacillus kaustophilus glyQ revealed molecular details of tRNA recognition that is based on both sequence and shape complementarity (Figure 3e,f)(15, 57). The structures showed a bent conformation of Stem I that forms two key contacts with the tRNA(15, 57). The O. iheyensis Stem I structure adopted a ‘C’ shaped structure in which two progressive backbone bends allow the double-stranded RNA (dsRNA) trajectory to closely track the L shape of tRNA. This enables Stem I to establish the interactions between the Specifier sequence and tRNA anticodon and also the newly identified contact between the double T-loop motif and tRNA elbow. The O. iheyensis Stem I backbone bends ~60° at a dinucleotide UA bulge (hinge) located between the AG bulge and the Specifier loop (Figure 3e). This bend is crucial for Stem I to follow the geometry of the tRNA by measuring the end-to-end distance between the anticodon loop and the elbow region. A second bend, seen in the O. iheyensis Stem I was observed at the K-turn motif proximal to the Specifier loop. This structure was solved as a ternary complex that contained a B. subtilis YbxF protein, a member of the K-turn binding L7Ae superfamily, which apparently stabilized the acute ~120° bend in the backbone. This sharp kink at the K-turn in Stem I proximal region was thought to allow the 3’ antiterminator domain to fold back and interact with the tRNA 3’-end.
The interaction between the Specifier sequence and the tRNA anticodon observed in the structures closely resembled the mRNA-tRNA interaction in the ribosome (Figure 3h). Two highly conserved purines, one immediately 3’ to the Specifier sequence (A90 in O. iheyensis) and the other at position 37 in the anticodon loop immediately 3’ to the anticodon trinucleotide, reinforce the 3-bp intermolecular helix by stacking on either end of it. This stabilization by the two flanking stacked nucleotides is similar to that of the codon-anticodon duplex in the ribosome, where the invariant C1400 of the ribosomal RNA occupies the same position as the T-box A90. Although both T-box structures were solved using in vitro transcribed tRNAGly lacking all modifications, they nonetheless showed how the T-box riboswitches can accommodate bulky modifications at position 37 in the tRNA anticodon loop.
Models of full-length T-box riboswitch-tRNA complexes based on SAXS analyses
Recently, two Small-angle X-ray scattering (SAXS) studies investigated the global conformations of the full glyQS T-box riboswitches from B. subtilis bound to uncharged tRNAGly (6, 8). Both suggested planar, elongated conformations for the complex, implying that most of the helical elements of the tRNA and T-box lie in the same plane. Interestingly, the thickness of the molecular envelopes matched the approximate width of an A-form dsRNA helix (30 Å), supporting a coplanar arrangement of most secondary structures in T-box and the bound tRNA. In the study by Chetnani et. al., the K-turn binding protein L7Ae was used to locate the position of K-turn in the low-resolution molecular envelope. This study aimed to visualize the location of Stem III in the overall complex to assess its functional importance in the T-box riboswitch. By comparing the molecular envelope of the wild-type complex with a T-box construct containing an extended Stem III, both studies proposed that Stem III co-axially stacks below the antiterminator helix A1 to form an extended helix. Based on this observation, the authors proposed that Stem III does not interact with the tRNA but plays a structural role in stabilizing the thermodynamically weaker antiterminator, in line with the variable length of this conserved motif.
However, the two structural models diverge on the existence of certain key contacts and overall topologies (Figure 4a,b). In contrast to the model proposed by Chetnani et al., where all three principal contacts between the T-box and tRNA are present (Figure 4a), the T-box-tRNA complex model proposed by Fang et al. only has two contacts and lacks the tRNA elbow interaction with the double T-loop motif (Figure 4c)(6, 8). As a result, the latter model is more extended as compared to a compact ring-like topology observed by Chetnani et al. Based on this extended model, Fang et al. proposed a ‘capture-and-release’ mechanism in which the double T-loop motif plays a transient role in the initial recruitment of tRNA, and subsequent formation of 3’-end interaction with the antiterminator leads to profound conformational changes that result in the loss of the elbow contact(8). It is possible that the T-box riboswitch bound to tRNA exists in a dynamic ensemble of conformations, sampling the two proposed model structures and/or alternative structures (Figure 4c). In light of the significant divergences between the two SAXS-based models and with several other proposals, higher-resolution structural studies are required to clarify the structure of and interactions within these mRNA-tRNA complexes.
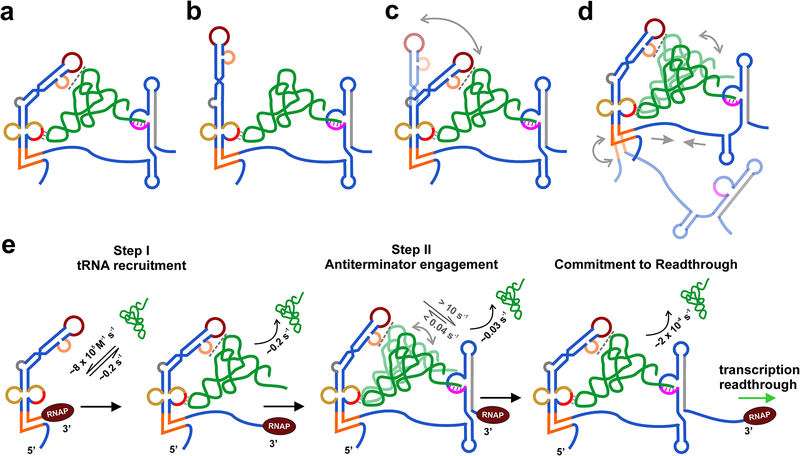
Figure 4. Structural and kinetics models of T-box riboswitch complex bound to tRNA.
(a-b) SAXS-informed model of B. subtilis glyQS T-box riboswitch bound to tRNAGly as proposed by Chetnani, et al. (a)(6) and by Fang et al.(b) (8). (c) Hypothetical model showing dynamic equilibrium between the two models shown in (a) and (b). (d) A dynamic model of the T-box riboswitch-tRNA complex based on smFRET measurements. (e) Kinetic model of the tRNA binding mediated transcription regulation by the T-box riboswitch. The rate constants for tRNA binding and dissociation for the two steps are indicated in black. The rate constants for tRNA dynamics within the complex are shown in gray.
Single-molecule studies of the T-box structural dynamics and tRNA-binding kinetics
The function of T-box riboswitches is intricately linked to tRNA binding in trans during transcription. The kinetics of tRNA (ligand) binding therefore play a major role in determining the outcome of transcription, as shown for small molecule-binding riboswitches(51). Single-molecule fluorescence techniques are well-suited for quantitative investigation of the structural dynamics and binding kinetics and can uncover multiphasic kinetics in conformationally heterogeneous systems(39, 37). Single-molecule fluorescence resonance energy transfer (smFRET) is being increasingly used to investigate the structural dynamics of various RNAs such as riboswitches that adopt multiple conformations(41, 46). Two recent single-molecule fluorescence studies investigated the conformational dynamics and the kinetics of tRNA binding by the glyQS T-box riboswitch and examined the roles of different T-box motifs in tRNA binding(45, 55).
Using two complementary single-molecule techniques, smFRET and single-molecule colocalization, both studies measured a fast and single-exponential tRNA association rate constant, kon of ~8 × 105 M−1 s−1, for the full glyQS T-box riboswitch including the antiterminator (referred to as T-box182 by Zhang et al., see Figure 2). Interestingly, the same kon value was measured by Suddala et al., for Stem I alone, suggesting a primary role for this domain in fast tRNA recruitment during transcription(45). Stem I also showed a homogeneous koff of ~0.2 – 0.3 s−1 showing that tRNAGly remains bound to this domain with a relatively short lifetime of ~4 – 5 s. Similar kon and koff values were also reported by Zhang et al., for the binding of tRNAGly to a T-box construct without the antiterminator (T-box149), and of a tRNAGly ΔNCCA construct (that cannot pair with the antiterminator) binding to the full T-box (T-box182)(55). These results support a role for the antiterminator mainly in the second step of binding, after tRNA is captured by Stem I and suggested that the linker region including Stem III does not contribute significantly to tRNA binding, in accordance with the proposed solution models for the T-box:tRNA complex(6, 8).
While the full T-box riboswitch showed a single-exponential kon, the koff was heterogeneous showing multi-exponential kinetics. Using the full T-box, both studies observed a fast koff of ~0.2 s−1 that corresponded to tRNAGly binding events to Stem I where the 3’-end interaction did not form, and a second, intermediate koff ~0.03 s−1, with a lifetime of ~35 s. A third unusually slow koff value of < 2.4 × 10−4 s−1 corresponding to a tRNA-bound lifetime of ~70 min was observed by Suddala et al. using single-molecule colocalization(45). This lifetime was interpreted as the formation of an “ultra-stable” complex with all three tRNA contacts established, likely corresponding to a structural model where a long coaxially stacked helix forms spanning the helix A1 of antiterminator and the double T-loop motif of Stem I, as proposed in a previous study(58). Since the medium koff for the full T-box riboswitch was very close to the koff of ~0.03 s−1 for a T-box construct with deletion of the double T-loop motif, this rate constant was interpreted by the authors as corresponding to complexes where the tRNA elbow interaction with the double T-loop motif is lost, as proposed in one of the SAXS models(8). Interestingly, this construct had ~2-fold lower kon compared to the wild-type T-box and did not form the ultra-stable complexes. Similar conclusions were reached by Zhang et al., using a mutant T-box182 construct with a single C56U substitution that weakens T-box contact with the tRNA elbow (Figure 2 and C44 in Figure 3g)(55). This point mutation significantly decreased kon by ~20-fold and increased koff by ~2.5-fold producing a combined drop in tRNA-binding affinity by ~50-fold, as previously measured using ITC(55). Taken together, these results support a prominent role for the double T-loop motif in tRNA recruitment to Stem I during transcription, and later in the formation of the ultra-stable, readthrough-committed complexes.
The structural dynamics of the full T-box and its tRNA complexes were also investigated in both studies, by measuring FRET between the different ends of the T-box labeled with Cy3, and the Cy5-labeled tRNA. The different Cy5 labeling positions on the tRNA in the two studies gave interesting and complementary perspectives into the solution dynamics of the complexes (Figure 4d). By measuring intramolecular FRET between the two termini, both studies found that the full T-box riboswitch alone showed largely stable FRET values. Addition of unlabeled tRNAGly resulted in a small increase in the FRET ratio suggesting that the T-box is largely pre-organized and tRNA binding only leads to slight compaction. No FRET change was observed by Zhang et al., in the presence of tRNAPhe or tRNAGly ΔNCCA, showing a specific interaction dependent on the binding of uncharged wild-type tRNAGly. Interestingly, Suddala et al., observed multiple brief transitions (Fig. 4d) into high-(~0.9) FRET states in a small fraction of molecules, which were lost upon the addition of tRNAGly (45). The significance of these high-FRET states and their underlying structural configurations are unknown.
Suddala et al. observed a steady low-FRET (~0.3) value between the base of antiterminator and the tRNA, suggesting a stable complex. In contrast, by labeling the 5’-end of tRNA which positions the Cy5 fluorophore closer to the antiterminator, Zhang et al., revealed an interesting view of the tRNA dynamics in the complex(55). Two distinct FRET states of 0.4 and 0.7 were seen when tRNAGly binds to the full T-box. The 0.4 FRET state was shown to correspond to first step of binding, where the tRNA interacts with Stem I via the two contacts, while the formation of antiterminator interaction gives rise to the 0.7 FRET state. While in most traces, tRNA binding directly gave rise to stable 0.7 FRET state (type I), a small fraction (~10 %) of traces showed multiple transient (lifetime of ~0.3 s) excursions into the lower 0.4 FRET states (type II)(55). These results showed that the tRNA can shuttle between the two (partial and full) binding modes, caused by 3’-end disengagement from the antiterminator before its reformation (Fig. 4d). The direct observation of 0.7 FRET state upon tRNA binding in majority (~80 %) of molecules suggested that the kinetics of the second step binding are fast, after the initial binding to Stem I(55). The rate constant for the formation of tRNA 3’-end interaction with antiterminator following binding to Stem I was estimated to be >10 s−1, faster than the time resolution (100 ms) of imaging.
FRET measurement between the base of Stem I and tRNA-Cy5 showed slow Mg2+-dependent two-state exchange, which was interpreted by Suddala et al., as kinking or extension of the K-turn motif (Fig. 4d). Zhang et al. showed that deleting the K-turn surprisingly decreased kon by ~9-fold and increased medium koff >15-fold, and most smFRET traces showed tRNA shuttling between the 0.4 – 0.7 FRET states, suggesting weakened tRNA 3’-end interaction with the antiterminator(55). Zhang et al., showed that shortening of Stem-III in T-box182 did not significantly change the kinetics of tRNA binding in the first and second steps but resulted in ~2-fold decrease in the lifetime of the fully bound (~0.7 FRET) state. Based on these results, Stem III was suggested to play a role in stabilizing the complexes via coaxially stacking below the weak antiterminator, as proposed in the SAXS studies(6, 8).
To address how the presence of a small amino acid glycine on the tRNA 3’-end affects binding, Suddala et al., probed the kinetics of aminoacylated Gly-tRNAGly binding to the T-box(45). Charged tRNAGly was shown to exhibit the same binding kinetics to Stem I domain as its uncharged counterpart, as expected. Moreover, a same kon value and very similar fast and medium koff values were observed for binding to the full-length T-box riboswitch. Remarkably, the presence of glycine on the tRNA 3’-end resulted in the loss of the ultra-stable complexes with slow koff (lifetime ~70 min), presumably by steric hindrance-based mechanism as proposed previously(58). The same conclusions were also reached using EF-Tu:Gly-tRNAGly:GTP ternary complexes, which showed similar kinetics of binding to and dissociation from the T-box. Surprisingly, the ternary complexes with EF-Tu still retained a similar medium koff but did not show the slow koff as seen for the uncharged tRNA alone. This medium koff may correspond to events where the tRNA 3’-end forms base pairing with the complementary 4 nt sequence in the antiterminator. How the tRNA 3’-end that is likely buried deep within EF-Tu interacts with the complementary T-box bulge sequence needs to be further investigated(34).
Both studies proposed a hierarchical or two-step tRNA binding model by the T-box riboswitch (Figure 4e)(45, 55). In this model, uncharged tRNA is captured quickly by the Stem I domain that is transcribed first. This initial capture requires a specific codon-anticodon base-pairing interaction and also a platform-stacking interaction with the double T-loop motif, which was shown to be important for a fast tRNA association. Once tRNA is captured by Stem I, the second step of tRNA 3’-end interaction with the antiterminator is fast (>10 s−1) and does not require large-scale conformational changes in the T-box. The fast rate of second step is expected as it is essentially an intra-molecular interaction in which tRNA is already docked to Stem I and may simply await the emergence of the complementary T-box bulge sequence from the RNA exit channel. Further, the tRNA in these complexes may be dynamic and shuttling between the two bound states, as suggested by Zhang et al. (Figure 4d). Formation of all three principal contacts results in antitermination complexes that presumably drive transcriptional readthrough in vivo. Notably, these studies are performed without the competing terminator sequence, which may explain the unusually high stability of such unchallenged complexes. In cells, it is expected that the highly stabilized, transitory antiterminator configuration will gradually isomerize into the thermodynamically more favorable terminator conformation due to the lapse of its kinetic advantage, ultimately releasing bound tRNAs. Whether this isomerization indeed occurs await further investigation.
Translation regulation by T-box riboswitches
Bioinformatics analyses of bacterial genomes previously predicted the existence of both transcription- and translation-regulating T-boxes(47). However, all the T-box systems that have been studied over the past two decades, including the tyrS and glyQS systems, are transcriptional riboswitches. Therefore, our knowledge of the structure and function of translational T-boxes is limited. A recent study highlighted the existence of different classes of putative translation-regulating ileS T-boxes in Actinobacteria that differed in the length and architecture of Stem I(43) (Figure 1e). Some translational T-boxes contain non-canonical Stem I domains that vary in length and lack the key motifs present in the canonical transcriptional T-box systems. Comparative genomic analyses of the 5’ leader sequences of the ileS genes encoding isoleucyl-tRNA synthetases from Actinobacteria revealed a class of unusually structured Stem I regions (USSRs) found in these T-boxes. These unusual Stem I’s contain the Specifier loop and the K-turn motif (or GA motif), but lack the S-turn (Loop E) next to the Specifier sequence thought to be important for codon presentation(43). The Specifier sequence is located in a distal loop as opposed to the internal loop location as in the transcriptional T-boxes. Sequences above the Specifier loop or bulge are present in different lengths but do not seem to contain the conserved AG bulge and apical loop sequences that can interdigitate to form the double T-loop motif involved in tRNA elbow binding. The atypical architecture of Stem I and the lack of key structural elements imply a distinct mode of tRNA recognition by these riboswitches. In addition, a new class of T-boxes that contain minimal Stem I domains (termed Ultrashort or US Stem I) were also identified. These ultrashort Stem I domains clearly lack the sequences above the Specifier sequence that form the double T-loop motif, the S-turn and the dinucleotide bulge that bends the Stem I (Figure 1e). The Specifier sequence is located in a terminal loop that is generally 6-nt long(43). A Stem II of varying length harboring an S-turn motif is present in all such T-boxes while the Stem IIA/B pseudoknot element is found in most of them. The downstream sequences include a Stem III followed by sequences that form mutually exclusive antisequestrator or sequestrator structures, which control the accessibility of the SD sequence for translation initiation by the 30S ribosome (Figure 1b)(43). Biochemical analyses of one such T-box from Nocardia farcinica regulating ileS gene showed that this RNA binds tRNAIle with high affinity (~60 nM) and specificity. Single mismatches in the AUC Specifier sequence or the tRNA anticodon GAU significantly weakened tRNA binding while compensatory mutations rescued binding, similar to previous studies of the transcriptional T-box riboswitches(27).
To examine the accessibility of the SD sequence, a labelled DNA oligo complementary to the SD sequence was annealed to the T-box RNA and subsequently probed with RNase H, which cleaves the RNA strand in RNA:DNA duplexes. A cleavage product was observed only in the presence of the cognate tRNAIle but not with the non-cognate tRNAGly, tRNAVal or with tRNAIle-Ex1C, a mimic of charged tRNA with a single C extension on the 3’-end. Accessibility of the SD sequence was shown to depend specifically on the presence of uncharged tRNAIle with an intact 3’-end(43). Further supporting evidence for a cognate uncharged tRNA-triggered ribosome binding was obtained using elegant primer extension or toeprint inhibition assays, in which reverse transcriptase (RT) is roadblocked by the bound 30S ribosome. The toeprint was observed at the expected 30S subunit binding position near the SD sequence in the presence of uncharged tRNAIle and the initiator tRNAfMet, but not when non-cognate tRNAGly or tRNAIle-ΔACCA was used. These results demonstrated specific binding of the ribosome that is dependent on the tRNA 3’-end interaction with the antisequestrator. The toeprint assays also suggested that Stem II and Stem IIA/B pseudoknot elements of the T-box riboswitch form even in the absence of tRNA. Although translation regulation was not directly tested by measuring protein synthesis, these biochemical studies provided strong evidence for tRNA-mediated modulation of ribosome binding at the SD sequence by the ileS translational T-box riboswitches(43).
A subsequent study on a different translational ileS T-box riboswitch from Mycobacterium smegmatis showed similar tRNAIle binding properties with robust affinity and selectivity(42). A chimeric T-box riboswitch construct was used here where the M. smegmatis antisequestrator was replaced with the antiterminator from the tyrS T-box(16). Binding of tRNA to different length variants of the M. smegmatis ileS T-box were tested showing that the ultrashort Stem I by itself had weak affinity (> 2 μM), in contrast to the canonical longer Stem I domains, such as that of the glyQS T-box. Appending additional downstream sequences that form Stem II, Stem IIA/B pseudoknot, Stem III and the discriminator domain progressively increased tRNA binding affinity, as expected, with a ~Kd < 100 nM for the full ileS T-box riboswitch. Using SHAPE probing, tRNAIle-dependent structural changes in T-box regions implicated in tRNA recognition were observed. The AUC codon Specifier sequence in Stem I showed protection in the presence of tRNAIle. Interestingly, the S-turn motif in Stem II and the Stem IIA/B pseudoknot showed increased protection from SHAPE reagents in the presence of tRNAIle, suggesting that these regions either directly contact tRNA, or become more structured when bound to the tRNA. UV crosslinking experiments were used to identify the points of contact between the two interacting RNAs, by placing 4-thio-U at multiple locations 3’ to Stem II and Stem IIA/B pseudoknot in a bipartite T-box and also in the D-loop and T-arm of bipartite tRNAIle constructs. A slow-moving crosslinked product was seen using the 4-thio-U-containing T-box, indicating the presence of at least one contact between the labelled regions of the T-box and the tRNAIle. This band was lost upon a point mutation of the Specifier sequence. The points of contact were identified based on RT stops in the crosslinked RNAs using primer extension inhibition assays. A prominent RT stop was observed in the 3’-half of the S-turn motif in Stem II in addition to another major stop that likely corresponded to the Stem IIA/B pseudoknot element, suggesting that these regions directly contact the tRNA. Mutations that disrupt the S-turn motif or prevent the pseudoknot formation were shown to significantly decrease the crosslinked product, supporting the role of these elements in tRNA binding. Using primer extension inhibition assays on the crosslinked product containing tRNAIle, the contact points were mapped to C62 in the T-arm and G15 in the D-loop regions of tRNA. Incorporating a single 4-thio-U near these positions (at nucleotides 63 or 16) in tRNAIle showed that C62 in the T-arm likely interacts with the 3’-half of S-turn whereas G15 in the D-loop interacts with the Stem IIA/B pseudoknot. These newly discovered contacts therefore confer tRNA binding affinity and may explain the conservation of Stem II and Stem IIA/B pseudoknot motifs in many T-boxes, especially in those with short Stem I domains(47, 42). Future high-resolution structural studies of T-boxes containing these elements will provide insights into the precise nature of these novel T-box interactions with tRNAs. It is of particular interest also to ask whether local or global deformations of tRNA are required to engage these interactions and how do these new contacts compare with tRNA interactions with various protein enzymes.
T-box riboswitch as a promising drug target
Despite limited chemical diversity in its side-chain functional groups, RNA is increasingly emerging as an attractive class of macromolecular targets for small molecule-based drug design(50). The ‘druggability’ of RNA is supported by the fact that most of the commonly used antibiotics bind to the bacterial ribosomal RNA, providing inspiration for developing bioactive compounds against structured RNAs(52). Noncoding RNAs perform a multitude of important cellular functions and can adopt intricate three-dimensional structures with well-defined binding pockets — characteristics that make for promising drug targets(31). Among bacterial noncoding RNAs, riboswitches are being actively pursued as drug targets due to their highly defined structures and important roles in the regulation of essential genes(7). The complex architecture of T-box riboswitches that include various RNA structural motifs makes them attractive drug targets against Gram-positive bacteria that include a large number of pathogenic species belonging to the genera Actinomyces, Bacillus, Clostridium, Mycobacterium, Streptococci, Staphylococci, Vibrio, etc(47, 9). Since T-boxes regulate essential genes required for amino acid-metabolism and translation, targeting them with small molecules to shutdown life-sustaining gene expression presents a feasible strategy against the pathogens harboring them(27). Furthermore, most Gram-positive bacteria contain multiple operons for aaRSes or other amino acid-related genes regulated by multiple T-boxes that share similar architectures and structural motifs. This provides a unique opportunity to target the expression of many different T-box-controlled genes via a single small molecule with a low potential of developing resistance against such drugs(9).
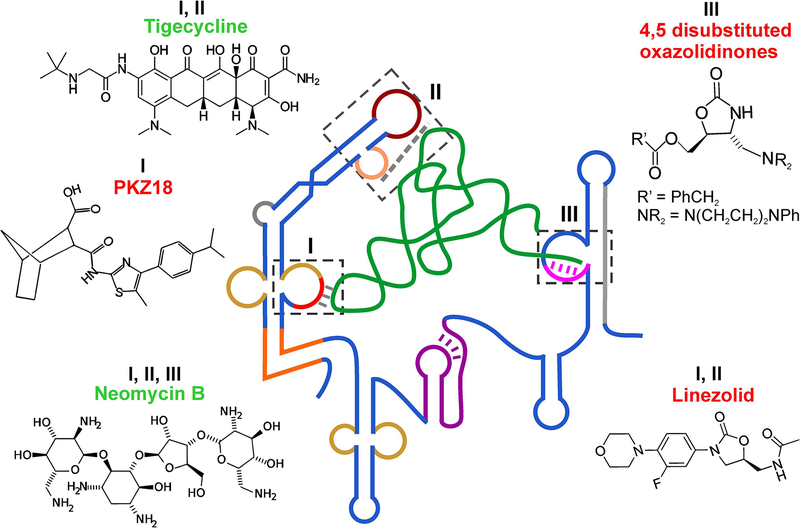
In this direction, initial efforts were made to identify compounds that bind specifically to different domains of the T-box and show activity against bacterial growth (Figure 5). One of the early studies using a model antiterminator domain AM1A from the B. subtilis tyrS T-box showed that aminoglycoside antibiotics bound to the RNA with low to mid μM affinity showing that small molecules can target T-box riboswitch motifs(32). A later study using the same AM1A RNA identified di-substituted oxazolidinones, compounds similar to the common antibiotic linezolid, that bound to the antiterminator bulge with low μM affinity (Figure 5)(2, 35). However, while one of the 4,5 di-substituted oxazolidinone compounds decreased tRNA-dependent antitermination, another similar compound was observed to stabilize the antiterminator and had the undesirable effect of promoting antitermination, even in the absence of tRNA. Similarly, the aminoglycoside antibiotic Neomycin B was shown to bind with low μM Kd to the antiterminator largely via electrostatic interactions thereby stabilizing the domain(1). Neomycin B binding site was localized to the 5’ region of the antiterminator bulge; however, tRNA binding was not affected under high concentrations of the drug.
Figure 5. Small-molecule drugs binding to T-box riboswitch.
Chemical structures of small-molecule drugs shown to bind to the T-box riboswitches. The boxes (I, II, and III) with dashed lines show the three proposed binding sites for the drugs on the T-box riboswitch. Compounds that were shown to inhibit or promote T-box riboswitch-mediated gene expression are labeled in red or green, respectively.
Most of the commonly used antibiotics that inhibit protein synthesis bind to ribosomal RNA and are known to possess non-specific RNA binding properties due to their positive charge(52). Recent work probing the binding of different antibiotics to the S. aureus glyS T-box found that, surprisingly, multiple compounds bound to the T-box RNA and directly modulated tRNA-mediated transcriptional antitermination(44). Proposed binding sites by these protein synthesis inhibitors included the Specifier loop, the AG bulge and apical loops in Stem I, the T-box bulge, and the Staphylococci-specific Stem Sa of the antiterminator (Figure 5). Interestingly, some of these compounds also seem to bind to tRNAGly in the D-loop region, near the wobble position of the anticodon loop and at the stacking interface between anticodon stem and D-arm. Similar to previous observations, the antibiotics neomycin B, pactamycin, paramomycin and tigecycline increased tRNA-mediated antitermination whereas linezolid and chloramphenicol decreased it. These studies demonstrated that while it is possible to target the T-box antiterminator bulge with small molecules to decrease gene expression, identification of compounds that destabilize antiterminator and/or prevent tRNA binding to reduce gene expression is non-trivial.
Recently, a small-molecule named PKZ18 was identified that showed inhibitory properties against multiple T-box riboswitches and exhibited potent inhibitory activity against Gram-positive but not Gram-negative bacteria(9) (Figure 5). This compound was identified through in silico docking of a large (>300,000) library of compounds for binding to the tyrS T-box Specifier loop, followed by screening the top hits for antibacterial activity. PKZ18 was shown to bind to the Specifier loop of tyrS and glyQS T-boxes from B. subtilis in vitro. Activity assays at the level of transcription and translation confirmed that PKZ18 inhibited production of glyQS mRNAs and thrS protein, consistent with inhibition of multiple T-box riboswitches. Remarkably, this compound exhibited very low occurrences of drug resistance, presumably due to its general binding properties against multiple T-boxes in bacteria(9). This is an important step in the direction of developing effective small-molecule drugs against common T-box motifs and structures involved in tRNA binding and gene regulation, so that the multiple T-boxes present in the same pathogen can be simultaneously targeted with a single ‘magic bullet’ thereby greatly enhancing the drug efficacy while minimizing the chances of resistance development.
Conclusions and Future directions
The T-box riboswitch system provides a fascinating paradigm to examine how interactions between two large and structured noncoding RNAs can achieve affinity and selectivity, and be coupled to adaptive gene regulation in the absence of accessory proteins(27). Since the discovery of the T-box riboswitches in 1993, tremendous progress has been made in our understanding of the sequence and structural features that drive specific tRNA binding and gene regulation. The recent biochemical, structural and biophysical studies have further expanded our view of the conformations and dynamics of the riboswitch and its mechanism of amino acid sensing on the tRNA. However, many key questions about the T-box mechanism still remain unanswered. How exactly is the amino acid sensed by the T-box 3’ region? Is it truly independent of any proteins, including the transcribing RNAP? How is the sensing of tRNA aminoacylation coupled to the crucial RNA conformational selection? How do the Stem I, the inter-domain linker, and the antiterminator coordinate their binding to the incoming tRNA? What is the role of the Stem III and the single-stranded linker? How do post-transcriptional modifications contribute to the T-box mechanism? In addition, the structure and functions of Stem II and Stem IIA/B pseudoknot elements are unclear, although recent studies suggested that they directly contact the tRNA D-loop and T-arm regions(42). High-resolution structures of T-boxes containing these domains in complex with tRNAs will be required to reveal the exact nature of these novel contacts. From the perspective of tRNA binding, the temporal order of formation of contacts with the Specifier sequence and the double T-loop motif is also not certain. In this direction, a detailed picture of co-transcriptional folding of the riboswitch coupled to concurrent tRNA binding will illuminate how the kinetics of ligand binding, RNA folding and RNAP pausing may have been optimized to drive the function of the transcriptional T-box riboswitches. Since most of the intracellular aminoacyl-tRNAs are thought to be bound by EF-Tu, the relationship of EF-Tu and T-box regulation also needs to be further clarified.
To mechanistically understand the recently validated modality of translation regulation by T-boxes, we need to examine the presumed dynamic equilibrium between the antisequestrator/sequestrator conformations and observe how it responds to the binding by aminoacyl- and non-aminoacyl tRNAs. In addition, the bioinformatics prediction of the existence of diverse types of species-specific T-boxes with distinct architectures in the Stem I, inter-domain linker, and antiterminator domains offer more interesting candidates for expanding the core strategies used by T-box RNA for specific recognition of tRNAs. Recent identification of other more unusual T-boxes, such as the two-codon sensing T-boxes and tandem T-boxes further highlight the scarcity of our knowledge of this pervasive class of evolutionarily ancient and efficient RNA-based systems. Ongoing and future studies of the themes and variations of the T-box paradigm not only hold the potential to uncover fundamental principles of recognition between large structured RNAs, but also offers attractive targets for developing new classes of antibiotics with reduced resistance development, at a time when bacterial drug resistance against mainstream antimicrobials has been raging at an alarming pace. In addition, understanding the T-box mRNA-tRNA recognition principles will inform RNA engineering for synthetic biology and nanotechnology purposes and for designing RNA-based functional devices, such as specific tRNA modifying and aminoacylating ribozymes.
Acknowledgements
We thank Shuang Li, Charles Bou-Nader and Jackson Gordon for helpful comments on the manuscript. This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The authors declare no conflicts of interests.
Abbreviations:
- tRNA
transfer RNA
- aaRS
aminoacyl-tRNA synthetase
- nt
nucleotide
- SD
Shine-Dalgarno
- K-turn
kink-turn
- RNAP
RNA polymerase
- NTPs
nucleoside triphosphates
- NMR
nuclear magnetic resonance
- dsRNA
double-stranded RNA
- SAXS
Small-angle X-ray scattering
- smFRET
single-molecule fluorescence resonance energy transfer
- LNA
locked nucleic acid
- DTM
double T-loop motif
- ITC
isothermal titration calorimetry
- EF-Tu
elongation factor thermos unstable
- USSRs
unusually structured Stem I regions
- US
ultrashort Stem I
- RT
reverse transcriptase
- SHAPE
selective 2’-hydroxyl acylation analyzed by primer extension
References
- [1].Anupam R, Denapoli L, Muchenditsi A, and Hines JV (2008) Identification of neomycin B-binding site in T box antiterminator model RNA. Bioorg Med Chem 16, 4466–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anupam R, Nayek A, Green NJ, Grundy FJ, Henkin TM, Means JA, Bergmeier SC, and Hines JV (2008) 4,5-Disubstituted oxazolidinones: High affinity molecular effectors of RNA function. Bioorg Med Chem Lett 18, 3541–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apostolidi M, Saad NY, Drainas D, Pournaras S, Becker HD, and Stathopoulos C (2015) A glyS T-box riboswitch with species-specific structural features responding to both proteinogenic and nonproteinogenic tRNAGly isoacceptors. RNA 21, 1790–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baird NJ, Zhang J, Hamma T, and Ferre-D’Amare AR (2012) YbxF and YlxQ are bacterial homologs of L7Ae and bind K-turns but not K-loops. RNA 18, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Caserta E, Liu LC, Grundy FJ, and Henkin TM (2015) Codon-Anticodon Recognition in the Bacillus subtilis glyQS T Box Riboswitch: RNA-DEPENDENT CODON SELECTION OUTSIDE THE RIBOSOME. J Biol Chem 290, 23336–23347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chetnani B. and Mondragon A (2017) Molecular envelope and atomic model of an anti-terminated glyQS T-box regulator in complex with tRNAGly. Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deigan KE. and Ferre-D’Amare AR (2011) Riboswitches: discovery of drugs that target bacterial gene-regulatory RNAs. Acc Chem Res 44, 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang X, Michnicka M, Zhang Y, Wang YX, and Nikonowicz EP (2017) Capture and Release of tRNA by the T-Loop Receptor in the Function of the T-Box Riboswitch. Biochemistry 56, 3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frohlich KM, Weintraub SF, Bell JT, Todd GC, Vare VYP, Schneider R, Kloos ZA, Tabe ES, Cantara WA, Stark CJ, Onwuanaibe UJ, Duffy BC, Basanta-Sanchez M, Kitchen DB, McDonough KA, and Agris PF (2019) Discovery of Small-Molecule Antibiotics against a Unique tRNA-Mediated Regulation of Transcription in Gram-Positive Bacteria. ChemMedChem. [DOI] [PubMed] [Google Scholar]
- [10].Garst AD. and Batey RT (2009) A switch in time: detailing the life of a riboswitch. Biochim Biophys Acta 1789, 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gerdeman MS, Henkin TM, and Hines JV (2002) In vitro structure-function studies of the Bacillus subtilis tyrS mRNA antiterminator: evidence for factor-independent tRNA acceptor stem binding specificity. Nucleic Acids Res 30, 1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gerdeman MS, Henkin TM, and Hines JV (2003) Solution structure of the Bacillus subtilis T-box antiterminator RNA: seven nucleotide bulge characterized by stacking and flexibility. J Mol Biol 326, 189–201. [DOI] [PubMed] [Google Scholar]
- [13].Green NJ, Grundy FJ, and Henkin TM (2010) The T box mechanism: tRNA as a regulatory molecule. FEBS Lett 584, 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grigg JC, Chen Y, Grundy FJ, Henkin TM, Pollack L, and Ke A (2013) T box RNA decodes both the information content and geometry of tRNA to affect gene expression. Proc Natl Acad Sci U S A 110, 7240–7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grigg JC. and Ke A (2013) Structural determinants for geometry and information decoding of tRNA by T box leader RNA. Structure 21, 2025–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grundy FJ, Collins JA, Rollins SM, and Henkin TM (2000) tRNA determinants for transcription antitermination of the Bacillus subtilis tyrS gene. RNA 6, 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Grundy FJ. and Henkin TM (1993) tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74, 475–482. [DOI] [PubMed] [Google Scholar]
- [18].Grundy FJ. and Henkin TM (2004) Kinetic analysis of tRNA-directed transcription antitermination of the Bacillus subtilis glyQS gene in vitro. J Bacteriol 186, 5392–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Grundy FJ, Hodil SE, Rollins SM, and Henkin TM (1997) Specificity of tRNA-mRNA interactions in Bacillus subtilis tyrS antitermination. J Bacteriol 179, 2587–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grundy FJ, Yousef MR, and Henkin TM (2005) Monitoring uncharged tRNA during transcription of the Bacillus subtilis glyQS gene. J Mol Biol 346, 73–81. [DOI] [PubMed] [Google Scholar]
- [21].Gutierrez-Preciado A, Henkin TM, Grundy FJ, Yanofsky C, and Merino E (2009) Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol Mol Biol Rev 73, 36–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Henkin TM (2008) Riboswitch RNAs: using RNA to sense cellular metabolism. Genes Dev 22, 3383–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Henkin TM (2014) The T box riboswitch: A novel regulatory RNA that utilizes tRNA as its ligand. Biochim Biophys Acta 1839, 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Huang L. and Lilley DM (2013) The molecular recognition of kink-turn structure by the L7Ae class of proteins. RNA 19, 1703–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang L. and Lilley DM (2016) The Kink Turn, a Key Architectural Element in RNA Structure. J Mol Biol 428, 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Korostelev A, Trakhanov S, Laurberg M, and Noller HF (2006) Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell 126, 1065–1077. [DOI] [PubMed] [Google Scholar]
- [27].Kreuzer KD. and Henkin TM (2018) The T-Box Riboswitch: tRNA as an Effector to Modulate Gene Regulation. Microbiol Spectr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Larson MH, Mooney RA, Peters JM, Windgassen T, Nayak D, Gross CA, Block SM, Greenleaf WJ, Landick R, and Weissman JS (2014) A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science 344, 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lehmann J, Jossinet F, and Gautheret D (2013) A universal RNA structural motif docking the elbow of tRNA in the ribosome, RNAse P and T-box leaders. Nucleic Acids Res 41, 5494–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lorenz C, Lunse CE, and Morl M (2017) tRNA Modifications: Impact on Structure and Thermal Adaptation. Biomolecules 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Matsui M. and Corey DR (2017) Non-coding RNAs as drug targets. Nat Rev Drug Discov 16, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Means JA. and Hines JV (2005) Fluorescence resonance energy transfer studies of aminoglycoside binding to a T box antiterminator RNA. Bioorg Med Chem Lett 15, 2169–2172. [DOI] [PubMed] [Google Scholar]
- [33].Nikulin A, Eliseikina I, Tishchenko S, Nevskaya N, Davydova N, Platonova O, Piendl W, Selmer M, Liljas A, Drygin D, Zimmermann R, Garber M, and Nikonov S (2003) Structure of the L1 protuberance in the ribosome. Nat Struct Biol 10, 104–108. [DOI] [PubMed] [Google Scholar]
- [34].Nissen P, Kjeldgaard M, Thirup S, Polekhina G, Reshetnikova L, Clark BF, and Nyborg J (1995) Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 270, 1464–1472. [DOI] [PubMed] [Google Scholar]
- [35].Orac CM, Zhou S, Means JA, Boehm D, Bergmeier SC, and Hines JV (2011) Synthesis and stereospecificity of 4,5-disubstituted oxazolidinone ligands binding to T-box riboswitch RNA. J Med Chem 54, 6786–6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Reiter NJ, Osterman A, Torres-Larios A, Swinger KK, Pan T, and Mondragon A (2010) Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA. Nature 468, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rinaldi AJ, Lund PE, Blanco MR, and Walter NG (2016) The Shine-Dalgarno sequence of riboswitch-regulated single mRNAs shows ligand-dependent accessibility bursts. Nat Commun 7, 8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rollins SM, Grundy FJ, and Henkin TM (1997) Analysis of cis-acting sequence and structural elements required for antitermination of the Bacillus subtilis tyrS gene. Mol Microbiol 25, 411–421. [DOI] [PubMed] [Google Scholar]
- [39].Roy R, Hohng S, and Ha T (2008) A practical guide to single-molecule FRET. Nat Methods 5, 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Saad NY, Stamatopoulou V, Braye M, Drainas D, Stathopoulos C, and Becker HD (2013) Two-codon T-box riboswitch binding two tRNAs. Proc Natl Acad Sci U S A 110, 12756–12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Savinov A, Perez CF, and Block SM (2014) Single-molecule studies of riboswitch folding. Biochim Biophys Acta 1839, 1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sherwood AV, Frandsen JK, Grundy FJ, and Henkin TM (2018) New tRNA contacts facilitate ligand binding in a Mycobacterium smegmatis T box riboswitch. Proc Natl Acad Sci U S A 115, 3894–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sherwood AV, Grundy FJ, and Henkin TM (2015) T box riboswitches in Actinobacteria: translational regulation via novel tRNA interactions. Proc Natl Acad Sci U S A 112, 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stamatopoulou V, Apostolidi M, Li S, Lamprinou K, Papakyriakou A, Zhang J, and Stathopoulos C (2017) Direct modulation of T-box riboswitch-controlled transcription by protein synthesis inhibitors. Nucleic Acids Res 45, 10242–10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Suddala KC, Cabello-Villegas J, Michnicka M, Marshall C, Nikonowicz EP, and Walter NG (2018) Hierarchical mechanism of amino acid sensing by the T-box riboswitch. Nat Commun 9, 1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Suddala KC. and Walter NG (2014) Riboswitch structure and dynamics by smFRET microscopy. Methods Enzymol 549, 343–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vitreschak AG, Mironov AA, Lyubetsky VA, and Gelfand MS (2008) Comparative genomic analysis of T-box regulatory systems in bacteria. RNA 14, 717–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang J, Henkin TM, and Nikonowicz EP (2010) NMR structure and dynamics of the Specifier Loop domain from the Bacillus subtilis tyrS T box leader RNA. Nucleic Acids Res 38, 3388–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang J. and Nikonowicz EP (2011) Solution structure of the K-turn and Specifier Loop domains from the Bacillus subtilis tyrS T-box leader RNA. J Mol Biol 408, 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Warner KD, Hajdin CE, and Weeks KM (2018) Principles for targeting RNA with drug-like small molecules. Nat Rev Drug Discov 17, 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wickiser JK, Winkler WC, Breaker RR, and Crothers DM (2005) The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol Cell 18, 49–60. [DOI] [PubMed] [Google Scholar]
- [52].Wilson DN (2014) Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat Rev Microbiol 12, 35–48. [DOI] [PubMed] [Google Scholar]
- [53].Winkler WC, Grundy FJ, Murphy BA, and Henkin TM (2001) The GA motif: an RNA element common to bacterial antitermination systems, rRNA, and eukaryotic RNAs. RNA 7, 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yousef MR, Grundy FJ, and Henkin TM (2005) Structural transitions induced by the interaction between tRNA(Gly) and the Bacillus subtilis glyQS T box leader RNA. J Mol Biol 349, 273–287. [DOI] [PubMed] [Google Scholar]
- [55].Zhang J, Chetnani B, Cormack ED, Alonso D, Liu W, Mondragon A, and Fei J (2018) Specific structural elements of the T-box riboswitch drive the two-step binding of the tRNA ligand. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang J. and Ferre DAR (2016) Trying on tRNA for Size: RNase P and the T-box Riboswitch as Molecular Rulers. Biomolecules 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhang J. and Ferre-D’Amare AR (2013) Co-crystal structure of a T-box riboswitch stem I domain in complex with its cognate tRNA. Nature 500, 363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang J. and Ferre-D’Amare AR (2014) Direct evaluation of tRNA aminoacylation status by the T-box riboswitch using tRNA-mRNA stacking and steric readout. Mol Cell 55, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang J. and Ferre-D’Amare AR (2015) Structure and mechanism of the T-box riboswitches. Wiley Interdiscip Rev RNA 6, 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang J, Lau MW, and Ferre-D’Amare AR (2010) Ribozymes and riboswitches: modulation of RNA function by small molecules. Biochemistry 49, 9123–9131. [DOI] [PMC free article] [PubMed] [Google Scholar]