The prevalence of clonal haematopoiesis of indeterminate potential (CHIP), defined as the presence of somatic mutations associated with myeloid malignancies (predominantly DNMT3A, TET2 and ASXL1) among individuals without a myeloid neoplasm, increases with age, reaching >10% after 70 years of age (Genovese et al, 2014; Jaiswal et al, 2014; Young et al, 2016). CHIP increases the risk of haematological cancers, cardiovascular events, and overall mortality. It can also be detected within tumours and blood of patients with solid cancers (Coombs et al, 2017; Severson et al, 2018; Xie et al, 2014). DNA-damaging chemotherapy carries ~1% risk of treatment-related myeloid neoplasms (t-MN), often harbouring complex karyotypes and TP53 mutations. Circulating clones with TP53 and CHIP-associated mutations have been detected in cancer patients long before t-MN diagnosis (Coombs et al, 2017; Gibson et al, 2017; Gillis et al, 2017; Takahashi et al, 2017). We conducted a prospective cross-sectional study of cancer survivors exposed to myelotoxic chemotherapy to examine CHIP prevalence among these patients compared with an age-matched general population, rate of TP53 mutations and association with time elapsed from completion of chemotherapy.
We collected blood samples from patients who had received anthracycline- or alkylator-containing chemotherapy for curative treatment of breast cancer or aggressive lymphoma. Subjects were clinically free of cancer, without any haematological disorders or unexplained cytopenias. We preferentially recruited patients aged 50–70 years, targeting a mean of 60 years, to allow comparison with the expected 5% CHIP prevalence observed in population-based studies at age 60 years (Genovese et al, 2014; Jaiswal et al, 2014). We identified CHIP by a next-generation sequencing, amplicon-based assay using Illumina TruSeq Custom Amplicon kit (MiSeq V2.2). The assay included 757 coding exons of 95 genes frequently mutated in haematological malignancies (Table SI), at mean coverage of 1500x (Kluk et al, 2016). Because recurrences of breast cancer may be delayed or clinically inconspicuous, we sequenced purified CD45+ cells to avoid potential contamination by circulating carcinoma cells (see Supplementary methods). A CHIP-associated mutation was called upon identification of a pathogenic single nucleotide variant or indel with variable allele frequency (VAF) ≥ 2%, excluding known minor germline alleles. The study had 80% power (with one-sided α=0.05) to reject the null hypothesis in a sample of 80 subjects. We used univariate generalized linear models for further analysis of association between CHIP and explanatory variables. The study was approved by the Institutional Review Board at Rhode Island Hospital.
Among 80 enrolled subjects, 46 (57%) were survivors of breast cancer and 34 (43%) had survived aggressive lymphoma. Mean age in the study cohort was 62 years (standard deviation [SD], ±7, range 47–75), with 78% women and 88% white non-Hispanic subjects. Median time from completion of chemotherapy to enrolment was 27 months (interquartile range, 11–59). All patients had received either adjuvant or primary curative myelotoxic chemotherapy containing an anthracycline and/or an alkylating agent, as listed in Table SII. Mean coverage depth for the sequenced samples was 1407x (SD, ±227), and ≥200x coverage was achieved in a mean 91.2% (SD, ±1.8%) of target amplicons.
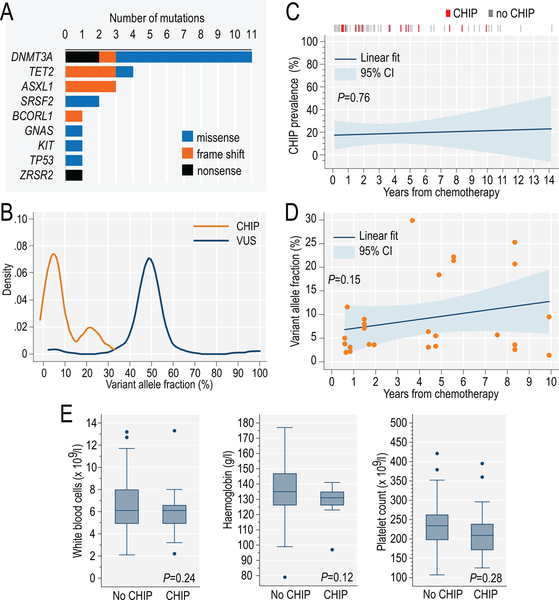
We detected CHIP in 15 subjects (prevalence 19%; binomial 95% confidence interval: 12 to 29%), ruling out the 5% prevalence expected from age-matched population data (two-sided P=9×10−6 on the binomial probability test). Table I lists clinical characteristics of patients with CHIP, and Table SIII shows the characteristics of 26 pathogenic mutations. The most common mutated genes were DNMT3A, TET2, ASXL1 and SRSF2, typical for CHIP in the general population, with only 1 case of TP53 mutation (6.7%, Fig. 1A). Mean VAF for CHIP mutations was 9.2% (SD, ±8.3, Fig. 1B). Eight out of 15 (53%) patients with CHIP had ≥2 (and up to 4) pathogenic mutations, notably higher than in population-based studies (<10%) (Jaiswal et al, 2014). We did not observe an association between CHIP and time elapsed from completion of chemotherapy (P=0.76, Fig. 1C), although average VAF of CHIP-associated mutations non-significantly increased over time (P=0.15, Fig. 1D). Furthermore, CHIP was not significantly associated with age (P=0.25, within the narrow age range in this study), sex (P=0.80), race (P=0.46) or blood counts (Fig. 1E). We observed no significant difference between survivors of breast cancer or lymphoma in the prevalence of CHIP (P=0.35) or of specific mutations (Fig. S1). The long latency makes it unlikely that mutations were derived from a clinically occult relapse of an aggressive lymphoma.
Table I.
Characteristics of patients with post-chemotherapy CHIP, and specific mutations identified.
| Age (years) | Sex | Race | Cancer | Chemo-therapy | Months from treatment | CHIP-associated mutations |
|---|---|---|---|---|---|---|
| 49 | F | W | Breast | ACT | 20 | DNMT3A |
| 50 | F | W | Breast | ACT | 7 | DNMT3A (x2) |
| 54 | F | B | Lymphoma | ABVD | 23 | DNMT3A |
| 59 | F | W | Breast | ACT | 9 | GNAS |
| 61 | F | W | Lymphoma | ABVD | 91 | DNMT3A |
| 62 | F | W | Breast | ACT | 18 | ASXL1, DNMT3A, TET2 |
| 64 | M | W | Lymphoma | RCHOP | 57 | DNMT3A, TP53 |
| 65 | F | W | Lymphoma | RCHOP | 44 | TET2 |
| 67 | M | W | Lymphoma | RCHOP | 119 | KIT, SRSF2 |
| 67 | F | W | Lymphoma | RCHOP | 8 | TET2 |
| 69 | F | W | Breast | ACT | 10 | ASXL1, DNMT3A |
| 69 | M | W | Lymphoma | RCHOP | 100 | ASXL1, BCORL1, SRSF2, ZRSR2 |
| 71 | F | W | Breast | AC | 53 | DNMT3A (x2) |
| 71 | F | W | Breast | ACT | 59 | DNMT3A |
| 75 | F | W | Lymphoma | CHOP | 67 | DNMT3A, TET2 |
ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine; AC: doxorubicin and cyclophosphamide; ACT: doxorubicin, cyclophosphamide and paclitaxel; B: black; CHIP: clonal haematopoiesis of indeterminate potential; CHOP: cyclophosphamide, doxorubicin, vincristine and prednisone; F: female; M: male; RCHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; W: white non-Hispanic.
Figure 1.
Distribution of 26 CHIP-associated pathogenic mutations identified in the study cohort (A); variant allele fractions (VAF) of mutations (N=26) classified as CHIP, or as variants of unknown significance (VUS, N=101) (B); association between presence of CHIP (C) or VAF of CHIP mutations (D) and time elapsed from completion of chemotherapy; distribution of blood counts among patients with or without detectable CHIP in the study (E); P values from generalized linear models. CHIP: clonal haematopoiesis of indeterminate potential; CI: confidence interval.
Our results suggest that CHIP among cancer survivors exposed to chemotherapy is 4 times more frequent than in the age-matched population. However, it is far too frequent to use as a sole predictor of future t-MN, which occurs in only ~1%, corresponding to the low observed prevalence of TP53 mutations. In the Cancer Genome Atlas study, CHIP was detected in 2.1% of cancer patients at diagnosis, with rare (6.8%) TP53 mutations (Xie et al, 2014). Among 8,810 patients with advanced cancers, presence of CHIP did not correlate with receipt of any chemotherapy (Coombs et al, 2017), while TP53 mutations were observed in 38% of t-MN cases with prior CHIP (Gillis et al, 2017). In contrast to these studies, we focused on patients with curable cancers treated with anthracycline/alkylator-based myelotoxic chemotherapy, and observed a typical distribution of age-related CHIP mutations, without over-representation of TP53. The notably high proportion of cases with multiple pathogenic mutations, also observed in CHIP preceding t-MN (Gibson et al, 2017), suggests that this might constitute an additional risk factor. Our results support the hypothesis that CHIP after chemotherapy is related to a competitive advantage of pre-existing (possibly multiple) clones after the stress of chemotherapy or an altered immune microenvironment, rather than a direct mutagenic effect. Our assay unfortunately did not cover PPM1D mutations, which are putatively associated with chemotherapy exposure (Coombs et al, 2017; Gibson et al, 2017). Furthermore, because of cross-sectional design and lack of a control group, we could not determine whether CHIP predated chemotherapy. A further longitudinal study may evaluate the usefulness of an affordable sequencing panel for detection of CHIP among cancer patients starting adjuvant chemotherapy, or as a surveillance tool afterwards, to predict the risk of cardiovascular toxicity or t-MN, and to optimize personalized treatment strategies.
Supplementary Material
Acknowledgements
Presented in part at the 60th American Society of Hematology Annual Meeting & Exposition, December 1–4, 2018, San Diego, CA. This work was supported by the IDeA-CTR grant from the National Institute of General Medical Sciences (grant number U54GM115677).
Funding / competing interests: AJO is supported by the IDeA-CTR grant from the National Institute of General Medical Sciences (grant number U54GM115677) and a Research Scholar Grant from the American Cancer Society Grant (grant number 128608-RSGI-15-211-01-CPHPS). The authors declare no conflict of interest.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
References:
- Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, Hyman DM, Solit DB, Robson ME, Baselga J, Arcila ME, Ladanyi M, Tallman MS, Levine RL & Berger MF (2017) Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell, 21, 374–382 e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, Chambert K, Mick E, Neale BM, Fromer M, Purcell SM, Svantesson O, Landen M, Hoglund M, Lehmann S, Gabriel SB, Moran JL, Lander ES, Sullivan PF, Sklar P, Gronberg H, Hultman CM & McCarroll SA (2014) Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England Journal of Medicine, 371, 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CJ, Lindsley RC, Tchekmedyian V, Mar BG, Shi J, Jaiswal S, Bosworth A, Francisco L, He J, Bansal A, Morgan EA, Lacasce AS, Freedman AS, Fisher DC, Jacobsen E, Armand P, Alyea EP, Koreth J, Ho V, Soiffer RJ, Antin JH, Ritz J, Nikiforow S, Forman SJ, Michor F, Neuberg D, Bhatia R, Bhatia S & Ebert BL (2017) Clonal Hematopoiesis Associated With Adverse Outcomes After Autologous Stem-Cell Transplantation for Lymphoma. Journal of Clinical Oncology, 35, 1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis NK, Ball M, Zhang Q, Ma Z, Zhao Y, Yoder SJ, Balasis ME, Mesa TE, Sallman DA, Lancet JE, Komrokji RS, List AF, McLeod HL, Alsina M, Baz R, Shain KH, Rollison DE & Padron E (2017) Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncology, 18, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D & Ebert BL (2014) Age-related clonal hematopoiesis associated with adverse outcomes. The New England Journal of Medicine, 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluk MJ, Lindsley RC, Aster JC, Lindeman NI, Szeto D, Hall D & Kuo FC (2016) Validation and Implementation of a Custom Next-Generation Sequencing Clinical Assay for Hematologic Malignancies. Journal of Molecular Diagnostics, 18, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Riedlinger GM, Connelly CF, Vergilio JA, Goldfinger M, Ramkissoon S, Frampton GM, Ross JS, Fratella-Calabrese A, Gay L, Ali S, Miller V, Elvin J, Hadigol M, Hirshfield KM, Rodriguez-Rodriguez L, Ganesan S & Khiabanian H (2018) Detection of clonal hematopoiesis of indeterminate potential in clinical sequencing of solid tumor specimens. Blood, 131, 2501–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wang F, Kantarjian H, Doss D, Khanna K, Thompson E, Zhao L, Patel K, Neelapu S, Gumbs C, Bueso-Ramos C, DiNardo CD, Colla S, Ravandi F, Zhang J, Huang X, Wu X, Samaniego F, Garcia-Manero G & Futreal PA (2017) Preleukaemic clonal haemopoiesis and risk of therapy-related myeloid neoplasms: a case-control study. Lancet Oncology, 18, 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, McMichael JF, Schmidt HK, Yellapantula V, Miller CA, Ozenberger BA, Welch JS, Link DC, Walter MJ, Mardis ER, Dipersio JF, Chen F, Wilson RK, Ley TJ & Ding L (2014) Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature Medicine, 20, 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AL, Challen GA, Birmann BM & Druley TE (2016) Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nature Communications, 7, 12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.