Abstract
Background.
The link between hypertensive disorders of pregnancy (HDP) and short-term adverse outcomes has long been recognized. However, there is now evidence that survivors of HDP remain at risk of adverse biological events long after giving birth, including ocular complications. We sought to determine whether HDP is associated with development of the choroidal neovascular (CNV) form of age-related macular degeneration (AMD), a leading cause of blindness.
Methods.
We identified women who experienced HDP (preeclampsia/eclampsia, HELLP syndrome, and gestational hypertension) as recorded in Utah birth certificates from 1939-2013. Exposed subjects were matched 1:2 to unexposed women on: mother’s age at delivery, and on child’s birth year, sex, and birth order. An AMD outcome from 1992-2015 was determined from electronic medical records. Risk of CNV AMD was estimated using stratified Cox regression with a competing risk of death.
Findings.
We included 31,454 HDP-exposed and 62,908 unexposed women for study. Of 681 exposed and 1,355 unexposed with subsequent AMD, 158 and 284 developed choroidal neovascularization, respectively. Women with HDP exhibited an 80% higher risk for early-onset CNV AMD before age 70 [adjusted hazard ratio (aHR) 1.8, 95%CI 1.23-2.58; p<0.002] and were 20% more likely to develop CNV AMD at any age (aHR 1.2, 95%CI 1.01-1.42; p=0.035) compared to unexposed. HDP was not associated with non-CNV AMD.
Conclusion.
Women with HDP have an increased risk for developing CNV AMD compared to unexposed women, particularly for earlier-onset disease. Our findings may have important implications for earlier screening and detection of choroidal neovascularization.
Keywords: Gestational hypertension, Preeclampsia, Age-related Macular Degeneration, Choroidal Neovascularization, Population health
Summary statement:
Do women with a history of hypertensive disorders of pregnancy have a higher incidence of neovascular age-related macular degeneration (AMD) later in life? In a population-based study of 94,362 women, those who experienced hypertensive disorders of pregnancy (HDP) were 80% more likely to develop early-onset choroidal neovascularization AMD.
Introduction
Pregnancy-related maternal deaths – primarily from the cardiovascular complication of preeclampsia and eclampsia – are unacceptably high worldwide, even in developed countries(1–3). The association between hypertensive disorders of pregnancy (HDP) and short-term adverse outcomes has long been recognized. However, there is now substantial evidence that survivors of HDP remain at risk of adverse events long after giving birth including ocular complications and the subsequent development of chronic diseases(4–7). While the majority of visual disturbances and ophthalmic exam findings associated with preeclampsia resolve completely following delivery, there are reports of persistent ophthalmic changes for several months postpartum(8–11) and recent population-based studies have established an association between preeclampsia and the development of both diabetic and non-diabetic retinopathy, as well as retinal detachment later in life(12–14). To date, an association between HDP and the subsequent development of age-related macular degeneration has not been reported, despite changes in choroidal vasculature on exam among women with HDP(15–17). Thus we sought to determine whether women with a history of HDP have increased risk for the subsequent development of choroidal neovascular (“wet”) age-related macular degeneration, a leading cause of blindness worldwide(18), in a large population-based cohort.
Methods
Study setting.
The Utah Population Database (UPDB) is a research resource housed at the University of Utah that contains computerized data records for over nine million deceased and living individuals who formerly or currently reside in the state(19). The UPDB was originally created in the 1970’s from genealogic data of 1.6 million Utah settlers and their descendants who were primarily of Northern European descent and was later expanded to incorporate statewide birth and death certificates from the early 1900’s. The UPDB was further enriched by linking of electronic medical records (EMR) beginning in 1992 of 2 million patients seen in the University of Utah Health statewide system of hospitals and clinics, including the John Moran Eye Center and its satellite facilities, as well as Medicare claims data for 275,000 Utah beneficiaries enrolled in parts A and B. These data are routinely updated and probabilistic record-linking is performed with individuals in UPDB as described(20). Approvals to conduct this human-subjects study was received from the University of Utah Institutional Review Board (IRB 00010201) and the Resource for Genetic Epidemiologic Research, the body that governs the use of UPDB data.
Study design and participants.
A retrospective cohort design was used in our investigation. Exposed participants were women born in 1900-1975 with a history of HDP recorded on a Utah birth certificate (1939-2013), including: preeclampsia/eclampsia (PE), hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome, and gestational hypertension (GHTN). Prior to 1989, the birth certificate included free-text fields for complications of pregnancy in which an HDP event could be recorded and later converted to electronically available data. In 1990 and later, the birth certificate included check boxes for complications of pregnancy including HDP. Example formats of Utah birth certificates from 1939 to current are included in an online supplement.
An unexposed comparison group was comprised of women with no evidence of HDP on a Utah birth certificate, randomly selected from the Utah population and individually matched 2:1 to the exposed women based on: mother’s age at child’s birth (<21y, 21-25y, 26-30y, 31-35y, 36-40y, >40y); birth year and sex of the child; and, the child’s birth order (1st, 2nd, 3rd, 4th, 5th or higher). Women who had >1 pregnancy affected by HDP were included once for the single-most severe event to the least severe event as follows: eclampsia, HELLP, preeclampsia, and GHTN. If a woman had multiple HDP-affected births of equal severity, the earliest occurrence of a preterm birth was retained; if all births were term, the event associated with the earliest birth was retained.
Exposed and unexposed women met the following criteria for study inclusion: a) singleton live birth in Utah between 1939 and 2013; b) at least one year of follow-up in Utah based on UPDB records; c) alive on or after January 1, 1992; d) age 40y or older on December 31, 2012 or if deceased, death occurred in Utah; and, e) no prior history of age-related macular degeneration (AMD) at the time of the child’s birth. Of 1,796,341 singleton birth certificates evaluated, 94,362 women met inclusion criteria: 31,454 case subjects and 62,908 unaffected matched population controls.
Outcome.
The outcome of interest was a subsequent diagnosis of choroidal neovascular age-related macular degeneration (CNV AMD) occurring between 1992-2015 in patient electronic medical records, linked to individuals in the UPDB, from: (a) all Utah inpatient and ambulatory facilities discharges; (b) the University of Utah Healthcare’s Moran Eye Center and statewide affiliated clinics; and, (c) Utah Medicare beneficiary claims data. A diagnosis of CNV AMD was based on International Classification of Diseases, Revision 9 (ICD-9) code 362.52 (exudative senile macular degeneration) appearing in the patient record in any diagnostic position. In a large Veterans Administration database, Latkany, et al. performed a validation study by chart review of exudative AMD and reported accuracy for ICD-9 code 362.52 of 98%(21). For comparison, a non-CNV AMD outcome was defined as any diagnosis of ICD-9 code 362.51 (non-exudative senile macular degeneration, the “dry” form of AMD) with no CNV AMD diagnosis in the patient record. In a review of academic medical center records, Muir and colleagues reported 92% of non-exudative AMD encounters were coded correctly(22). In either case of CNV or non-CNV AMD, a diagnosis of unspecified AMD (ICD-9 362.50) or drusen AMD (ICD-9 362.57) may have also appeared in the diagnostic record.
Statistical analyses.
In HDP-exposed and unexposed women, time was measured from the birth date of a child until: study end, December 31, 2015; date of last follow-up or death, if prior to year-end 2015; or an index date of a CNV AMD outcome, at which time observations were right-censored. The estimated risk of CNV AMD (or non-CNV AMD, in the comparison scenario) in exposed compared with unexposed women was determined from the adjusted hazard ratio (aHR) using a Cox proportional hazards model. Analogous to a conditional logistic regression, separate hazard functions (stratified Cox regressions) were incorporated for each group: an exposed subject with HDP (one or more PE, HELLP, and/or GHTN event) and two individually-matched unexposed subjects to account for mother’s age at birth, child’s birth year and sex, and birth order of the child. As AMD is typically a later-onset disorder, a competing-risks framework was incorporated to include death as a competing risk(23).
Factors previously associated with HDP(24–26) were examined for inclusion as model covariates: mother’s race/ethnicity (as white and non-Hispanic, white and Hispanic, or non-white); body mass index (BMI) from mother’s pre-pregnancy weight categorized as underweight (<18.5), normal weight (reference; 18.5-24.9), overweight (25-29.9), obese (30 and over), or unknown; history of diabetes (type I or II); history of chronic hypertension; parity (one child, two children, 3 children, or 4 or more children born to a mother); mother’s highest level of education (less than high school, high-school graduate, post-high school education or college graduate, or not reported); child’s birthweight categorized as very-low (<1500kg), low (1500 to <2500kg), normal (reference; 2500-4500kg), high (>4500kg), or not reported; child’s gestational age as very early pre-term (<29 weeks), early pre-term (29 to <33 weeks), pre-term (33 to 36 weeks), term (reference; >36 to <42 weeks), and post-term (42 or more weeks); and county of residence at time of birth as primarily urban (reference), mixed urban/rural, primarily rural, and outside of Utah. A stepwise model (P<0.25 to enter or stay) was used to select a set of covariates that included: mother’s race/ethnicity and pre-pregnancy BMI, parity, child’s gestational age, and county of residence. An unadjusted model was also estimated. Study analyses were conducted using SAS®, version 9.4 (SAS Institute).
Results
Characteristics of the study population and the distribution of HDP and AMD outcomes among 94,362 women (31,454 exposed to HDP and 62,908 matched unexposed) are shown in Tables 1 and 2, respectively. Mothers exposed to HDP were more likely to be overweight or obese prior to pregnancy, have a history of diabetes or hypertension, and to have fewer children over their reproductive lives than were mothers with no HDP exposure (Table 1). Study follow-up times were lengthy (generally >20y). Unexposed women were less likely to be right censored due to death than HDP exposed (8.7% and 7.8%, respectively). Although women with HDP may be predisposed to earlier death, our study required subjects to be living at the time electronic medical records became available in 1992. Therefore, the most severe cases of HDP resulting in early mortality were not included. Children of exposed and unexposed mothers did not differ on matching variables of child’s sex, birth year, and birth order in an unmatched comparison (Table 1). Children born to mothers with HDP were more likely to be early pre-term (<33 weeks gestation) and low birth weight (<2500 kg) compared to their respective matched children born to unexposed mothers.
Table 1.
Characteristics of the study population for hypertensive disorders of pregnancy (HDP) exposed and unexposed women.
| Characteristic | HDP exposed | 2:1 unexposed | |||
|---|---|---|---|---|---|
| N | % | N | % | P* | |
| Total births | 31,454 | 100.0 | 62,908 | 100.0 | – |
| Characteristics of mother: | |||||
| Age at child’s birth (mean, ±SD) | 27.6 | ±6.38 | 27.4 | ±6.36 | <0.001 |
| 12 - 21y | 6,012 | 19.1 | 12,716 | 20.2 | |
| 22 - 26y | 9,005 | 28.6 | 17,860 | 28.4 | |
| 27 - 33y | 10,123 | 32.2 | 20,288 | 32.3 | |
| 34 - 55y | 6,314 | 20.1 | 12,044 | 19.1 | 0.22 |
| Birth year of mother | |||||
| 1900-1949 | 5,027 | 16.0 | 10,030 | 15.9 | |
| 1950-1965 | 11,452 | 36.4 | 22,711 | 36.1 | |
| 1966-1975 | 14,975 | 47.6 | 30,167 | 48.0 | 0.45 |
| Race/ethnicity of mother | |||||
| White and non-Hispanic | 26,906 | 85.5 | 54,054 | 85.9 | |
| White and Hispanic | 2,644 | 8.4 | 5,217 | 8.3 | |
| Non-white | 1,904 | 6.1 | 3,637 | 5.8 | 0.07 |
| Pre-pregnancy body mass index† | |||||
| Underweight, <18.5 | 760 | 2.4 | 3,251 | 5.2 | |
| Normal, 18.5-24.9 | 12,568 | 39.9 | 35,960 | 57.1 | |
| Overweight, 25.0-29.9 | 8,333 | 26.5 | 13,494 | 21.5 | |
| Obese, ≥30.0 | 8,830 | 28.1 | 8,560 | 13.6 | |
| Unavailable | 963 | 3.1 | 1,643 | 2.6 | <0.001 |
| History of type I or II diabetes | |||||
| No | 29,453 | 93.6 | 60,331 | 95.9 | |
| Yes | 2,001 | 6.4 | 2,577 | 4.1 | <0.001 |
| History of hypertension | |||||
| No | 30,732 | 97.7 | 62,688 | 99.7 | |
| Yes | 722 | 2.3 | 220 | 0.3 | <0.001 |
| Maximum number of children | |||||
| 1 child | 3,290 | 10.5 | 5,512 | 8.8 | |
| 2 children | 7,671 | 24.4 | 13,929 | 22.1 | |
| 3 children | 7,597 | 24.2 | 14,706 | 23.4 | |
| 4 or more children | 12,896 | 41.0 | 28,761 | 45.7 | <0.001 |
| Highest level of education | |||||
| Less than high school graduate | 494 | 1.6 | 799 | 1.3 | |
| High school graduate | 2,541 | 8.1 | 5,035 | 8.0 | |
| More than high school graduate | 24,746 | 78.7 | 49,714 | 79.0 | |
| Not reported | 3,673 | 11.7 | 7,360 | 11.7 | 0.56 |
| County of residence | |||||
| Urban counties | 26,521 | 84.3 | 54,236 | 86.2 | |
| Mixed urban/rural | 1,245 | 4.0 | 2,415 | 3.8 | |
| Rural | 3,435 | 10.9 | 5,769 | 9.2 | |
| Outside of Utah | 253 | 0.8 | 488 | 0.8 | 0.28 |
| Vital status | |||||
| Alive at last follow up | 28,922 | 92.2 | 57,466 | 91.3 | |
| Died prior to study end/diagnosis | 2,462 | 7.8 | 5,442 | 8.7 | <0.001 |
| Years of follow up (mean, ±SD) | 23.2 | ±13.87 | 23.9 | ±13.54 | <0.001 |
| Characteristics of child: | |||||
| Female | 15,037 | 47.8 | 30,072 | 47.8 | |
| Male | 16,417 | 52.2 | 32,836 | 52.2 | 0.99‡ |
| Birth year of child | |||||
| 1939-1979 | 5,750 | 18.3 | 11,504 | 18.3 | |
| 1980-1999 | 18,310 | 58.2 | 36,623 | 58.2 | |
| 2000-2013 | 7,394 | 23.5 | 14,781 | 23.5 | 0.97‡ |
| Birth order of child | |||||
| 1st | 16,480 | 52.4 | 32,953 | 52.4 | |
| 2nd | 5,472 | 17.4 | 10,951 | 17.4 | |
| 3rd or higher | 9,502 | 30.2 | 19,004 | 30.2 | 0.99‡ |
| Birth weight | |||||
| Very low (<1500 gm) | 1,044 | 3.3 | 420 | 0.7 | |
| Low (1500 to <2500 gm) | 4,181 | 13.3 | 2,523 | 4.0 | |
| Normal (2500 to 4500 gm) | 25,311 | 80.5 | 58,216 | 92.5 | |
| High (>4500 gm) | 388 | 1.2 | 674 | 1.1 | |
| Not reported | 530 | 1.7 | 1,075 | 1.7 | <0.001 |
| Gestation (weeks) | |||||
| Very early preterm (<29 wk) | 1,244 | 4.0 | 744 | 1.2 | |
| Early preterm (29 to <33 wk) | 4,597 | 14.6 | 3,368 | 5.4 | |
| Preterm (33 to <37 wk) | 1,160 | 3.7 | 3,078 | 4.9 | |
| Term (37 to 41 wk) | 23,715 | 75.4 | 53,989 | 85.8 | |
| Post-term (>41 wk) | 738 | 2.3 | 1,729 | 2.7 | <0.001 |
Mantel-Haenszel chi-square test of association, categorical variables; paired t-test, continuous variables.
Weight in kilograms divided by height in meters squared.
Matching variable of exposed to unexposed subjects in the study design.
Table 2.
Distribution of hypertensive disorders of pregnancy (HDP) events and age-related macular degeneration (AMD) outcomes.
| Events and outcomes | HDP exposed women | 2:1 unexposed women | |||
|---|---|---|---|---|---|
| N | % | N | % | P* | |
| Total number of HDP events: | 36,182 | 100.0 | — | – | |
| Eclampsia | 849 | 2.3 | — | – | |
| HELLP | 369 | 1.0 | — | – | |
| Preeclampsia | 16,053 | 44.4 | — | – | |
| Pregnancy-induced hypertension (GHTN) | 18,911 | 52.3 | — | – | |
| Total number of births: | 31,454 | 100.0 | 62,908 | 100.0 | |
| Single HDP event at birth: | 26,842 | 85.3 | — | – | |
| Eclampsia | 781 | 2.5 | — | – | |
| HELLP | 144 | 0.5 | — | – | |
| Preeclampsia | 11,555 | 36.7 | — | – | |
| GHTN | 14,362 | 45.7 | — | – | |
| Multiple HDP events at birth: | 4,612 | 14.7 | — | – | |
| Eclampsia and: HELLP or preeclampsia | 32 | 0.1 | — | – | |
| Eclampsia and GHTN | 34 | 0.1 | — | – | |
| HELLP and: preeclampsia or GHTN | 104 | 0.3 | — | – | |
| Preeclampsia and GHTN | 4,326 | 13.8 | — | – | |
| Total number of AMD outcomes: | 681 | 100.0 | 1,355 | 100.0 | |
| CNV AMD | 158 | 23.2 | 284 | 21.0 | |
| Non-CNV AMD | 310 | 45.5 | 650 | 48.0 | |
| Unspecified if CNV or non-CNV | 213 | 31.3 | 421 | 31.1 | 0.44 |
| Age at index CNV AMD (mean, ±SD) | 74.7 | 8.01 | 75.8 | 7.65 | 0.28 |
| Under 70 years | 41 | 25.9 | 51 | 18.0 | |
| 70 years or older | 117 | 74.1 | 233 | 82.0 | 0.005 |
| Age at index non-CNV AMD (mean, ±SD) | 75.3 | 7.39 | 75.5 | 7.53 | 0.69 |
| Under 70 years | 70 | 22.6 | 130 | 20.0 | |
| 70 years or older | 240 | 77.4 | 520 | 80.0 | 0.40 |
| Years from HDP to CNV AMD (mean, ±SD) | 45.7 | 8.76 | 46.8 | 8.52 | 0.22 |
ABBREVIATIONS: HELLP, hemolysis, elevated liver enzymes, and low platelets. GHTN, gestational hypertension. CNV, Choroidal neovascularization.
Mantel-Haenszel chi-square test of association, categorical variables; paired t-test, continuous variables.
In HDP case births to exposed mothers, GHTN was the most common event (46%), followed by preeclampsia (37%) and having both GHTN and preeclampsia (14%; see Table 2). In most instances (85%), a single HDP was recorded in the birth certificate while 15% had multiple hypertensive diagnoses noted. Of 681 exposed and 1,355 unexposed women with subsequent AMD, 158 (23.2%) and 284 (21.0%) were diagnosed with choroidal neovascularization, respectively (Table 2). Our data suggest women with HDP were significantly more likely to be diagnosed with CNV AMD at an earlier age (under 70 years vs. 70 years or older) than their unexposed counterparts (Table 2). Given AMD is a later-onset disorder, few children of mothers with an HDP event had an AMD diagnosis themselves; 32 total in 94,362 births.
The risk of a mother developing a subsequent CNV AMD diagnosis among the HDP exposed compared to unexposed women (covariate-adjusted model) is shown in Figure 1. Women exposed to HDP were 20% more likely to develop CNV AMD compared to mothers who did not have HDP (aHR=1.20, 95%CI 1.01-1.42; p=0.035). For the non-CNV AMD phenotype, we did not observe a significant difference in risk between HDP-exposed and unexposed mothers. The risk of earlier-onset CNV AMD (diagnosed before age 70) appeared to be of greater magnitude, 80% higher in mothers exposed to HDP compared to unexposed (aHR=1.78, 95%CI 1.23-2.58; p<0.002). Results unadjusted for covariates (other than accounting for individual matching of unexposed to exposed subjects) are shown in eFigure 1 (available online).
Figure 1.
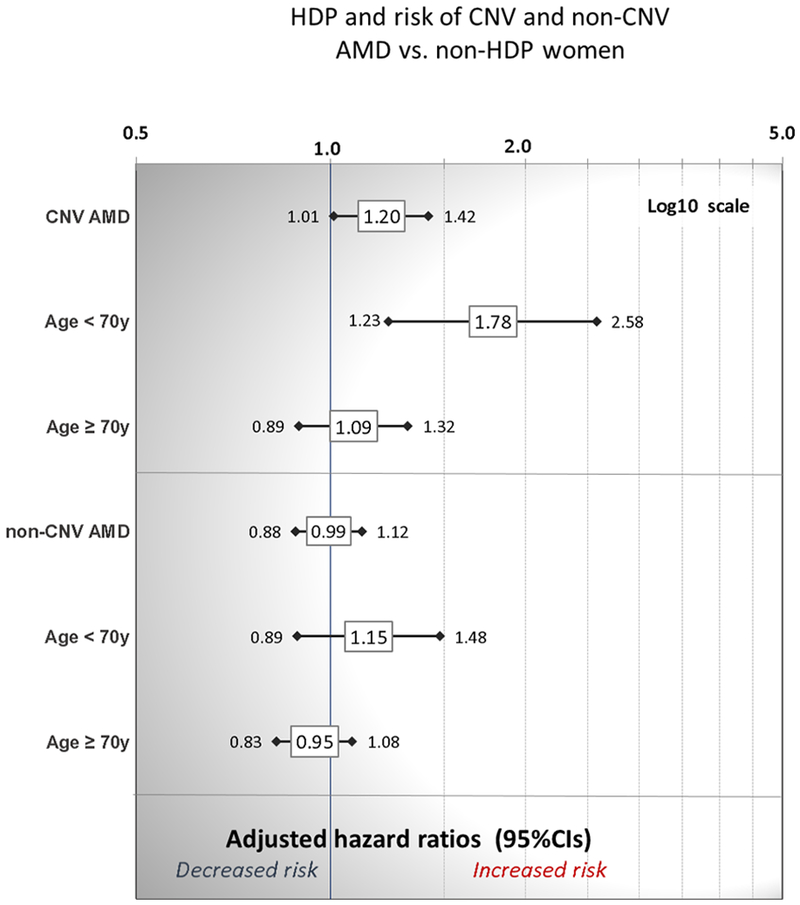
Mothers exposed to hypertensive disorders of pregnancy (preeclampsia/eclampsia, HELLP, and GHTN) and risk of choroidal neovascularization age-related macular degeneration (CNV AMD) or non-CNV AMD compared to unexposed mothers, adjusted for mother’s race/ethnicity, pre-pregnancy body mass index, parity, county of residence, and child’s gestational age.
As eclampsia, HELLP, and preeclampsia (with or without GHTN in the background) arguably represent a more severe phenotype than GHTN only, we examined risk of subsequent AMD after excluding HDP births in which the only exposure recorded on the birth certificate was GHTN. Our findings for women exposed to eclampsia, HELLP, or preeclampsia (and not GHTN exclusively) compared to respective unexposed women were consistent with HDP (Figure 1), and inclusion or exclusion of GHTN-only births did not appear to impact risk of AMD (see online Supplement, eFigure 2).
Discussion
In a statewide population investigation, we observed a greater risk of CNV AMD (particularly for onset before age 70) in women who had eclampsia, HELLP, preeclampsia, GHTN, or multiple HDP events from Utah birth certificates compared to unexposed women after controlling for the birth year, mother’s age at birth, and child’s sex and birth order, and adjusting for mother’s race/ethnicity, obesity, parity, and urban/rural residence. While a recently published Canadian cohort study(12) reported a higher cumulative incidence of retinal diseases (traction and serous detachments, retinal breaks, and diabetic retinopathy) among women with a history of preeclampsia compared to women without a history of preeclampsia, the authors included AMD in a composite ‘other retinal diseases’ outcome and did not report their findings for AMD as an individual outcome. Our study is the first large, population-based report of a CNV AMD and HDP association to our knowledge.
An association between CNV AMD and HDP is plausible, given CNV is characterized by growth of abnormal vessels from the choroidal circulation into the sub-RPE or subretinal space, and HDP develops following superficial placentation in the setting of abnormal placental angiogenesis(26). Like many other complex diseases, genetics play a role in the pathogenesis of both CNV and HDP: the STOX1 gene within the 10q22 locus has been evaluated as a candidate ‘preeclampsia gene’(27, 28), and variants of the HTRA1 gene within the chromosome 10q26 locus(29) and of the CFH, CFHR1 and CFHR3 genes on chromosome 1 are associated with increased risk and/or protection for developing AMD(30, 31). The genetic basis of AMD is well-established, and our study has access to genotype information for AMD risk loci in over 12,000 patients who are linked to the UPDB. We plan to identify a subset of women in our cohort who have AMD risk genotypes and, if sufficiently powered, examine HDP and risk of chromosome 1-driven and chromosome 10-driven AMD in a background of risk or no risk alleles at chromosome 10 and 1, respectively, and in non-risk AMD genotypes to inform any shared etiology.
These findings have potential clinical relevance both for ophthalmologists and for obstetricians as AMD is the leading cause of adult-onset blindness in the United States(32), and visual impairment has significant effects on functional status and quality of life among older adults(33–35). Although there is no known effective treatment for the non-exudative form of AMD characterized by late-stage geographic atrophy, CNV AMD is amenable to intervention with intravitreous injection of a vascular endothelial growth factor inhibitor in order to limit progression of CNV AMD and stabilize or reverse vision loss(36). Early recognition and treatment of CNV AMD increases the likelihood of visual recovery(37). If the findings of this study are confirmed by others, ophthalmologists may be advised to obtain pregnancy histories on their midlife and older female patients. Likewise, pregnancy has been clearly shown to be a window to women’s future health, and obstetricians will increasingly be called on to make appropriate postpartum referrals for women at increased risk for various chronic diseases. Women whose pregnancies are complicated by HDP have increased risks for cardiovascular disease and diabetes, and should be followed by their primary care providers for screening and management. Thus, our study suggests regular ophthalmology evaluations may be added to these preventive health recommendations for women with a history of HDP.
Strengths of our approach include ascertainment of HDP events from Utah birth certificates spanning several decades and linkage to statewide AMD diagnoses. As a majority of women in Utah are Caucasians primarily of northern European descent, our results may not be generalizable to non-Caucasian populations. We acknowledge HDP recorded on the birth certificates may contain inaccurate diagnoses, particularly as the criteria for diagnosing subtypes of HDP have changed over time. Although previous validation studies of large administrative databases have demonstrated that billing codes captured a clinical diagnosis of AMD with a high degree of accuracy(21, 22), the outcomes of CNV AMD or non-CNV AMD subtypes were based on ICD-9 diagnosis codes and not a clinical diagnosis of AMD, thus misclassification error may be present. We acknowledge that some women with non-CNV AMD in 1992-2015 may have developed the exudative form of AMD after that time. Given that the Moran Eye Center’s system of hospitals and clinics provides comprehensive eye services by experienced ophthalmologists throughout Utah representing more than 325,000 patient visits, we believe any bias from misclassification is minimal.
Women have an increased risk of developing cardiovascular disease after pre-eclampsia based on systematic reviews and a meta-analysis of several studies(38, 39) and may be at risk of cataract extraction later in life(40). In a meta-analysis including several prospective studies, previous cataract surgery is strongly associated with late AMD and to a modest degree, cardiovascular disease as well(41). As data on previous cataract surgery was not available to our study and comprehensively assessing cardiovascular disease in our cohort was beyond the scope of this project, we were limited in our ability to analyze these intermediate risk factors in relation to HDP exposure and CNV outcome. However, as a competing risk of death was incorporated in our analysis, we accounted for severe cardiovascular morbidity. As our study is retrospective, it is descriptive in nature and well-designed prospective cohort studies are needed to confirm our findings; however, given the long lead times between a pregnancy complicated by HDP and onset of AMD, often 50 or more years, a prospective design is problematic.
Conclusions
Women exposed to hypertensive disorders of pregnancy (HDP) have an increased risk of developing the CNV phenotype of AMD, with highest risk for onset before age 70y compared to women without an HDP history. Because early recognition of CNV AMD increases the likelihood of visual recovery with treatment, our findings will likely have important clinical implications.
Supplementary Material
Acknowledgments.
Generous support for data collection, management, extraction and analysis was provided by the Steele Center for Translational Medicine (GSH), the University of Utah Department of Ophthalmology and Visual Sciences, an unrestricted grant from Research to Prevent Blindness, Inc. and the John A. Moran Presidential Professorship (GSH). Research reuse of Utah Medicare claims data (originally obtained by KRS) was authorized by the Centers for Medicare & Medicaid (CMS) in Data Use Agreement 25883, “Genetic and Molecular Studies Of Eye Diseases” (GSH). We express our appreciation to Dr. Heidi Hanson for her invaluable assistance in extracting data from Medicare beneficiary claims. Partial support for data collection and analysis within the Utah Population Database (UPDB) was provided by the Huntsman Cancer Foundation, University of Utah, and Huntsman Cancer Institute Cancer Center Support grant, P30 CA2014 from the National Institutes of Health (NIH)/National Cancer Institute. The University of Utah’s Program in Personalized Health and Center for Clinical and Translational Science supported in part the design and conduct of the study. Support for the collection, use, and management of Medicare files linked to the UPDB was provided by NIH/National Institute on Aging R01 AG022095 Early Life Conditions, Survival, and Health: A Pedigree-Based Population Study (KRS). We thank the Utah State Department of Health for additional support for sharing of statewide health data. MWV is supported by an NIH/National Center for Advancing Translational Sciences award 1UL1TR001067 and by the HA and Edna Benning Presidential Endowment.
Funding. Support from: the University of Utah Steele Center for Translational Medicine and Department of Ophthalmology and Visual Science, Research to Prevent Blindness, National Institutes of Health (NIH) National Cancer Institute Cancer Center Support Grant P30-A2014 and an NIH National Center for Advancing Translational Sciences award 1UL1TR001067.
Footnotes
This work was completed at the University of Utah School of Medicine.
An abbreviated abstract of these findings has been accepted for a poster presentation at the 2018 meeting of the Society for Maternal-Fetal Medicine (Jan 29 – Feb 3, 2018).
Disclosures. Dr. Gregory S. Hageman is a shareholder and co-founder of Voyant Biotherapeutics, LLC. The other authors have no conflicts of interest to disclose.
References
- 1.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP, et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014;121 Suppl 1:14–24. doi: 10.1111/1471-0528.12629. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman CM, Platner MH, Spatz ES, Illuzzi JL, Xu X, Campbell KH, et al. Severe cardiovascular morbidity in women with hypertensive diseases during delivery hospitalization. Am J Obstet Gynecol. 2019. doi: 10.1016/j.ajog.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 4.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Theilen LH, Fraser A, Hollingshaus MS, Schliep KC, Varner MW, Smith KR, et al. All-Cause and Cause-Specific Mortality After Hypertensive Disease of Pregnancy. Obstet Gynecol. 2016;128(2):238–44. doi: 10.1097/AOG.0000000000001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Wang Z, Wang L, Qiu M, Wang Y, Hou X, et al. Hypertensive disorders during pregnancy and risk of type 2 diabetes in later life: a systematic review and meta-analysis. Endocrine. 2017;55(3):809–21. doi: 10.1007/s12020-016-1075-6. [DOI] [PubMed] [Google Scholar]
- 7.Pauli JM, Repke JT. Preeclampsia: Short-term and Long-term Implications. Obstet Gynecol Clin North Am. 2015;42(2):299–313. doi: 10.1016/j.ogc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Dornan KJ, Mallek DR, Wittmann BK. The sequelae of serous retinal detachment in preeclampsia. Obstet Gynecol. 1982;60(5):657–63. [PubMed] [Google Scholar]
- 9.Lara-Torre E, Lee MS, Wolf MA, Shah DM. Bilateral retinal occlusion progressing to long-lasting blindness in severe preeclampsia. Obstet Gynecol. 2002;100(5 Pt 1):940–2. [PubMed] [Google Scholar]
- 10.Murphy MA, Ayazifar M. Permanent visual deficits secondary to the HELLP syndrome. J Neuroophthalmol. 2005;25(2):122–7. [DOI] [PubMed] [Google Scholar]
- 11.Theodossiadis PG, Kollia AK, Gogas P, Panagiotidis D, Moschos M, Theodossiadis GP. Retinal disorders in preeclampsia studied with optical coherence tomography. Am J Ophthalmol. 2002;133(5):707–9. [DOI] [PubMed] [Google Scholar]
- 12.Auger N, Fraser WD, Paradis G, Healy-Profitos J, Hsieh A, Rheaume MA. Preeclampsia and Long-term Risk of Maternal Retinal Disorders. Obstet Gynecol. 2017;129(1):42–9. doi: 10.1097/AOG.0000000000001758. [DOI] [PubMed] [Google Scholar]
- 13.Beharier O, Davidson E, Sergienko R, Szaingurten-Solodkin I, Kessous R, Charach R, et al. Preeclampsia and Future Risk for Maternal Ophthalmic Complications. Am J Perinatol. 2016;33(7):703–7. doi: 10.1055/s-0036-1571321. [DOI] [PubMed] [Google Scholar]
- 14.Gordin D, Kaaja R, Forsblom C, Hiilesmaa V, Teramo K, Groop PH. Pre-eclampsia and pregnancy-induced hypertension are associated with severe diabetic retinopathy in type 1 diabetes later in life. Acta Diabetol. 2013;50(5):781–7. doi: 10.1007/s00592-012-0415-0. [DOI] [PubMed] [Google Scholar]
- 15.Bruckmann A, Seeliger C, Lehmann T, Schleussner E, Schlembach D. Altered retinal flicker response indicates microvascular dysfunction in women with preeclampsia. Hypertension. 2015;66(4):900–5. doi: 10.1161/HYPERTENSIONAHA.115.05734. [DOI] [PubMed] [Google Scholar]
- 16.Schreyer P, Tzadok J, Sherman DJ, Herman A, Bar-Itzhak R, Caspi E. Fluorescein angiography in hypertensive pregnancies. Int J Gynaecol Obstet. 1991;34(2):127–32. [DOI] [PubMed] [Google Scholar]
- 17.Wagener HP. Arterioles of the retina in toxemia of pregnancy. Journal of the American Medical Association. 1933;101(18):1380–4. doi: 10.1001/jama.1933.02740430026009. [DOI] [Google Scholar]
- 18.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–38. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 19.The University of Utah Pedigree and Population Resource: Utah Population Database, Overview. http://healthcare.utah.edu/huntsmancancerinstitute/research/updb/ Accessed March 1, 2018.
- 20.Prahalad S, O’Brien E, Fraser AM, Kerber RA, Mineau GP, Pratt D, et al. Familial aggregation of juvenile idiopathic arthritis. Arthritis Rheum. 2004;50(12):4022–7. doi: 10.1002/art.20677. [DOI] [PubMed] [Google Scholar]
- 21.Latkany P, Duggal M, Goulet J, Paek H, Rambo M, Palmisano P, et al. The need for validation of large administrative databases: Veterans Health Administration ICD-9CM coding of exudative age-related macular degeneration and ranibizumab usage. J Ocul Biol Dis Infor. 2010;3(1):30–4. doi: 10.1007/s12177-010-9052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of international classification of diseases, ninth revision, clinical modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013;131(1):119–20. doi: 10.1001/jamaophthalmol.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–32. [PubMed] [Google Scholar]
- 24.English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control. 2015;8:7–12. doi: 10.2147/IBPC.S50641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Force USPST, Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, et al. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(16):1661–7. doi: 10.1001/jama.2017.3439. [DOI] [PubMed] [Google Scholar]
- 26.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 27.Rigourd V, Chelbi S, Chauvet C, Rebourcet R, Barbaux S, Bessieres B, et al. Re-evaluation of the role of STOX1 transcription factor in placental development and preeclampsia. J Reprod Immunol. 2009;82(2):174–81. doi: 10.1016/j.jri.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk M, van Bezu J, van Abel D, Dunk C, Blankenstein MA, Oudejans CB, et al. The STOX1 genotype associated with pre-eclampsia leads to a reduction of trophoblast invasion by alpha-T-catenin upregulation. Hum Mol Genet. 2010;19(13):2658–67. doi: 10.1093/hmg/ddq152. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314(5801):992–3. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 30.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102(20):7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hageman GS, Hancox LS, Taiber AJ, Gehrs KM, Anderson DH, Johnson LV, et al. Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: Characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38(8):592–604. doi: 10.1080/07853890601097030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 33.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 34.Felson DT, Anderson JJ, Hannan MT, Milton RC, Wilson PW, Kiel DP. Impaired vision and hip fracture. The Framingham Study. J Am Geriatr Soc. 1989;37(6):495–500. [DOI] [PubMed] [Google Scholar]
- 35.Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc. 2001;49(5):508–15. [DOI] [PubMed] [Google Scholar]
- 36.Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JH, Wickremasinghe SS, Xie J, Chauhan DS, Baird PN, Robman LD, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012;153(4):678–86, 86 e1–2. doi: 10.1016/j.ajo.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22. doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 39.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Auger N, Rheaume MA, Paradis G, Healy-Profitos J, Hsieh A, Fraser WD. Preeclampsia and the risk of cataract extraction in life. Am J Obstet Gynecol. 2017;216(4):417 e1–e8. doi: 10.1016/j.ajog.2016.11.1043. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. doi: 10.1186/1471-2415-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


