Abstract
Background:
Parkinson’s disease (PD) is characterized in part by the progressive accumulation of iron within the substantia nigra (SN); however, its spatial and temporal dynamics remain relatively poorly understood.
Objectives:
To investigate spatial patterns and temporal evolution of SN iron accumulation in PD.
Methods:
Eighteen PD patients (mean disease duration = 6.2 years), receiving dopaminergic therapy, and 16 healthy controls (HC) were scanned with 3T MRI at baseline and 3 years later using quantitative susceptibility mapping (QSM), an indirect marker of iron content. Iron was assessed separately in the posterior (pSN) and anterior (aSN) at ventral and dorsal levels of the SN. Results were corrected for the false discovery rate.
Results:
A significant group effect was found for the ventral pSN (p < 0.001) and aSN (p = 0.042) QSM as well as significant group × time interaction effects (p = 0.02 and p = 0.043, respectively). In addition, a significant intra-group change over 3 years of follow-up was found only in the ventral pSN of PD (p = 0.012) but not HC. No significant effects were detected for any dorsal SN measures. No associations were identified with clinical measures.
Conclusions:
We found both cross-sectional and longitudinal SN iron changes to be confined to its more ventral location in PD. Because pathology studies also show the ventral SN to degenerate early and to the greatest extent in PD, assessment of iron levels by QSM in this area may potentially represent a disease progression biomarker in PD.
Keywords: Parkinson’s disease, MRI, quantitative susceptibility mapping, substantia nigra, iron, longitudinal
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease. The exact etiology of the disease remains elusive but the loss of dopaminergic cells in the substantia nigra (SN) is known to cause the classic motor symptoms of PD. Destruction of dopaminergic neurons begins in the SN pars compacta (1), with 30–50% already lost at the time of diagnosis (2). Histopathological studies have demonstrated that SN iron concentrations in PD are elevated by 30–35% compared to healthy control (HC) subjects.(3) Most of the SN iron tends to accumulate within the granules of neuromelanin, which act as a chelator to capture redox active iron ions (4), thereby preventing free radical-induced damage by the Fenton reaction. However, neuromelanin’s protective mechanism may eventually be exhausted after becoming fully saturated with iron. In this case, non-sequestered neuronal iron may become cytotoxic by catalyzing the production of free radicals, ultimately leading to neuronal death (5). Iron homeostasis may also be affected by reduced levels of ferritin in the SN of PD patients (6), given the role that this protein has in sequestering ferrous iron.
Several studies have demonstrated the utility of magnetic resonance imaging (MRI) techniques capable of quantifying SN iron in PD, with elevated levels frequently found compared to HCs (7–13). While it is known that SN neuronal loss tends to progress in a posterior-to-anterior fashion over time starting with cells in the ventrolateral tier (2), most studies have not assessed whether iron accumulation follows a similar pattern longitudinally. In addition, pathological studies have shown the ventral SN (more inferior location) to degenerate early in PD, corresponding to the relative lack of calcium-binding calbindin protein in this SN location, while the dorsal SN (more superior location) to not be substantially affected until more than 20 years after diagnosis (1). This ventral-dorsal gradient in SN degeneration has not been previously assessed longitudinally in terms of iron accumulation in vivo in PD. However, previous studies using diffusion tensor imaging assessments have shown the ventral posterior SN to be the most sensitive to both cross-sectional and longitudinal changes in PD (14–18). In addition, a recent study found elevated iron levels in PD within the more ventral SN, although longitudinal changes were not assessed (12).
Against this background, we aimed to investigate SN iron levels in PD in a longitudinal setting using quantitative susceptibility mapping (QSM). This MRI modality is sensitive to the presence of paramagnetic materials allowing for an indirect but accurate measurement of iron levels in subcortical gray matter structures, as shown by the strong linear correlation between QSM and post-mortem data (19). We hypothesized that we would find increased magnetic susceptibility primarily in the posterior and ventral region of the SN in PD compared to healthy controls (HC) and that iron levels would increase longitudinally in this same spatial region of the SN in PD.
Materials and Methods
Subjects
Between March 2011 and March 2013, 32 PD patients and 25 HCs, group-matched for age and sex, were enrolled. Between January and October of 2015, study participants were invited to participate in a follow-up study. At both time points, all study subjects underwent MRI and clinical investigations, which included Unified Parkinson’s Disease Rating Scale (UPDRS) Part III (20) and Modified Hoehn & Yahr (H&Y) scale. All PD subjects were on dopaminergic treatment and were evaluated clinically and by MRI in the “on” state. All PD subjects satisfied the UK Brain Bank Criteria for diagnosis (21) and were clinically evaluated by a movement disorder neurologist (TG or DL). All patients had idiopathic PD and no study participant presented with dementia. Most HCs were spouses or friends of the participants with PD. In addition, a limited number of HCs had participated in previous research studies at the University at Buffalo and had consented to be contacted for future studies.
As previously reported (18), of the 57 study participants enrolled at baseline, 18 were not available for assessment at the 3-year time point and one was excluded due to a frontal lobe astrocytoma. Of the remaining 38 subjects, 3 cases were excluded due to excessive movement during the MRI acquisition (1 PD patient and 2 HC) and an additional HC was excluded due to corrupted MRI data that prevented QSM reconstruction. Thus, the final set of participants for our primary analyses included 18 PD patients and 16 HCs that were assessed at baseline and after 3.1 and 3.2 years of follow-up, respectively. Secondary analyses were performed using baseline data from all available participants.
Both the initial baseline and follow-up studies were approved by the Institution Review Board at the University at Buffalo and written informed consent was obtained from all participants at both time points.
MRI acquisition
All scans were acquired on the same 3T GE Signa Excite HD 12.0 MRI scanner (General Electric, Milwaukee, WI, USA) with an 8-channel head and neck coil. Data for QSM were acquired using an un-accelerated axial 3D single-echo spoiled GRE sequence with first-order flow compensation in read and slice directions, a matrix of 512×192×64 and a nominal resolution of 0.5×1×2 mm3 (field of view (FOV)=256×192×128 mm3), flip angle = 12°, echo time (TE)/repetition time (TR)=22ms/40ms. Raw k-space data of each channel were saved for off-line reconstruction of the images. The MRI scanner underwent no hardware or software upgrades during the course of the study.
MRI Assessment
Quantitative susceptibility mapping
Magnitude and phase components from the GRE acquisition were reconstructed offline using sum-of-squares and scalar phase matching (22), respectively. To achieve isotropic in-plane resolution, k-space data was zero-padded in the phase-encode direction prior to processing. Gradient non-linearity distortions were corrected (23). Phase images were unwrapped with a best-path algorithm (24), background-field corrected with V-SHARP (25, 26), and finally converted to magnetic susceptibility maps using the HEIDI algorithm (27). The zero point for the resulting maps was referenced to the average susceptibility of the brain, under the assumption that a larger reference region would reduce additional inter-subject variability, compared to a smaller reference region. In-house developed algorithms for QSM processing were written in MATLAB (2013b, The MathWorks, Natick, MA). Magnetic susceptibility values are expressed in parts per billion (ppb).
Region of interest analysis
For defining the SN, two sets of bilateral regions of interest (ROI) were manually drawn by a single rater (NB, 15 years of experience) on the native space 2mm-thick QSM images (Figure 1). First, two consecutive slices were selected in the dorsal SN, where the RN was most prominently visible. Next, two consecutive slices were chosen in the ventral SN, just inferior to where the RN was either barely or no longer visible. Posterior and anterior SN (pSN and aSN, respectively) regions were then defined in both ventral and dorsal SN areas by splitting the original SN ROI at the midway point along the anterior-posterior axis based on its length (Figure 1; red vs. green). The volume of each ROI was also measured. All ROIs were drawn blinded to clinical and demographic data as well as time point.
Figure 1. Representative regions of interest drawn on native space QSM images.
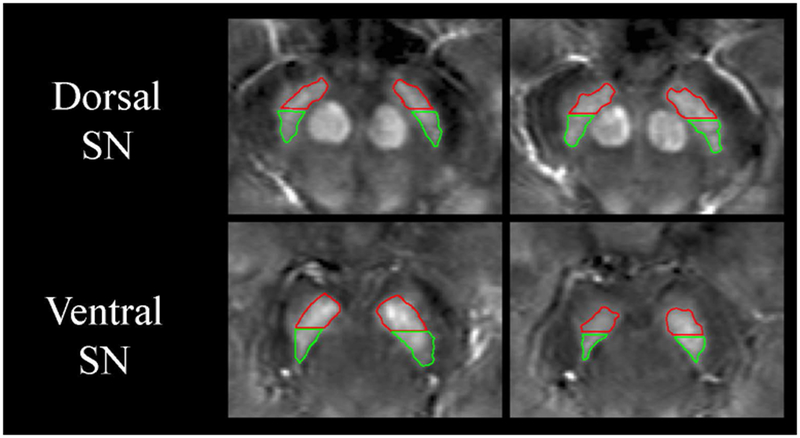
Hand-drawn regions of interest (ROIs) of the substantia nigra (SN) are shown overlaid on native space magnetic susceptibility image of a 59-year-old male with Parkinson’s disease. The red ROIs correspond to the anterior SN while the green ROIs correspond to the posterior SN. Measurements were separately made in the dorsal SN (adjacent to the red nucleus) and in the ventral SN (inferior to the red nucleus). The image contrast ranges from −100 to 200 parts per billion.
Eight PD cases and eight HC cases were randomly selected and re-analyzed after a month to evaluate intra-rater reliability of the ROIs.
Statistical analysis
Statistical analyses were performed using SPSS (version 25; IBM Corp., Armonk, NY, USA). Demographic and clinical characteristics for PD patients and HCs were assessed using repeated measures ANOVA analyses or Fisher’s exact test, as appropriate. For the primary analyses, repeated measures ANCOVA models were used for assessing the main effects of group and time as well as the interaction effect of group × time of average magnetic susceptibility for each ROI, controlling for age and sex. In the event of a significant group × time interaction effect, post-hoc paired t-tests were used to separately assess PD and HC groups. For the PD group, associations with UPDRS-III scores were investigated using Pearson correlations while associations with H&Y stage used Spearman analyses. The same analyses were also conducted for SN volumes. Baseline magnetic susceptibility values were correlated with clinical measures at baseline as well as follow-up. In addition, associations between changes in magnetic susceptibility and changes in clinical outcomes over the course of the follow-up were investigated. To reduce the number of statistical comparisons, we considered the average magnetic susceptibility or total ROI volume across left and right hemispheres.
Secondary analyses were performed using data from all 57 cases that were available at baseline (i.e. including those that were excluded from the primary analysis due to lack of follow-up data). Baseline differences between HC and PD groups were assessed using ANCOVA models, correcting for age and sex. Pearson and Spearman correlations assessed the associations between baseline magnetic susceptibility and UPDRS-III scores and H&Y stage, respectively.
Intra-rater reliability was assessed by the intra-class correlation coefficient.
The false discovery rate for the primary analyses was controlled for using the Benjamini-Hochberg procedure (28), with corrected p-values < 0.05 considered significant. No correction for multiple comparisons was used for the secondary analyses.
Results
Demographic and clinical characteristics of the study participants are presented in Table 1. The mean disease duration of PD patients at baseline, defined as the time since diagnosis, was 6.2 years. No significant differences were detected between PD patients and HCs in terms of age or proportion of males. The group of PD patients and HCs that were included in the secondary, baseline only analyses were on average 2.3 and 2.0 years older, respectively, whereas no differences in the proportion of males was seen.
Table 1.
Baseline demographic and clinical characteristics of study participants
| HC (n = 16) | PD (n = 18) | Group effect | Time effect | Group × Time interaction effect | |||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | ||||
| Age at baseline in years, mean (SD) | 58.1 (8.7) | - | 60.1 (6.2) | - | .453* | ||
| Males, n (%) | 5 (31.3) | 11 (61.1) | - | .100# | |||
| Disease duration in years (since diagnosis), mean (SD) | - | - | 6.2 (4.2) | 9.3 (4.4) | - | < 0.001† | - |
| UPDRS-III, mean (SD) | 1.0 (1.3) | 1.4 (2.4) | 18.6 (7.6) | 19.5 (8.4) | < 0.001% | 0.346% | 0.768% |
| H&Y stage, median (IQR) | - | - | 2.0 (1.0 – 2.5) | 2.0 (2.0 – 2.6) | - | 0.007^ | - |
Legend: HC = healthy controls; PD = Parkinson’s disease; SD = standard deviation; UPDRS-III = Unified Parkinson’s disease Rating Scale-part III; IQR = interquartile range; H&Y = Hoehn & Yahr
UPDRS-III was measured in PD patients during the “on” state.
= Student’s t-test
= Fisher’s exact test
= Paired samples t-test
= Repeated measures general linear model
= Wilcoxon signed-rank test
Substantia nigra magnetic susceptibility in HC and PD patients
Table 2 shows the group, time, and group × time interaction effects of average magnetic susceptibility separately in the pSN and aSN for both the ventral and dorsal SN.
Table 2:
SN QSM outcomes over 3 years of follow-up in HC and PD
| HC (n = 16) | PD (n = 18) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Group effect | Time effect | Group × Time interaction effect | |
| Ventral substantia nigra (inferior to red nucleus) | |||||||
| pSN | 62.1 (29.1) | 58.8 (25.3) | 113.1 (36.2) | 125.4 (33.4) | < 0.001 | 0.15 | 0.02 |
| aSN | 124.0 (38.4) | 116.6 (55.2) | 160.7 (40.4) | 171.8 (55.1) | 0.042 | 0.02 | 0.043 |
| Dorsal substantia nigra (adjacent to red nucleus) | |||||||
| pSN | 66.9 (23.5) | 68.1 (13.2) | 78.0 (35.0) | 82.3 (28.5) | 0.408 | 0.716 | 0.716 |
| aSN | 100.8 (39.4) | 104.2 (33.8) | 111.3 (36.0) | 122.0 (42.2) | 0.716 | 0.655 | 0.428 |
Legend: HC=healthy controls, PD=Parkinson’s disease. Cells represent mean (standard deviation) and are expressed in parts per billion. Group, time and group × time interaction effect values are p-values after correction for the false discovery rate.
For the ventral pSN, there was a significant group effect revealing greater magnetic susceptibility in PD compared to HC (p < 0.001) and a significant group × time interaction (p = 0.02). In addition, the ventral pSN was the only location demonstrating a significant intra-group increase in magnetic susceptibility in PD over the follow-up (p = 0.012) while there was a non-significant intra-group decrease in HC (p = 0.297) (Figure 2).
Figure 2. Ventral posterior substantia nigra magnetic susceptibility.
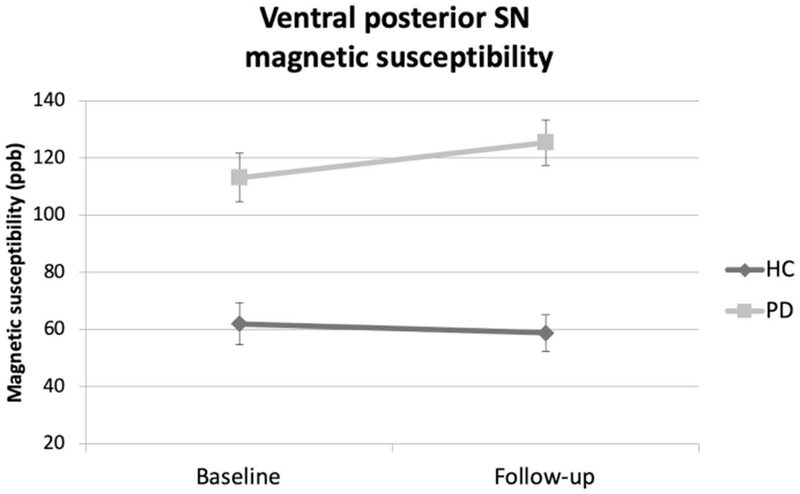
Mean magnetic susceptibility values in the ventral posterior substantia nigra are shown comparing Parkinson’s disease (PD) and healthy controls (HC). False discovery rate-corrected group, time and group × time interaction p-values were < 0.001, 0.15, and 0.02, respectively. Comparisons were made adjusting for age and sex. The error bars represent ± 1 standard error of the mean.
Legend: PD - Parkinson’s disease; HC – healthy controls; SN–substantia nigra; ppb – parts per billion
For the ventral aSN, there were a significant group effect revealing greater magnetic susceptibility in PD compared to HC (p = 0.042), a significant effect of time (p =0.02) and a significant group × time interaction (p = 0.043). However, the ventral aSN did not reveal any significant intra-group changes in magnetic susceptibility either within the PD or HC groups over the follow-up (p = 0.109 and p = 0.188, respectively) (Figure 3).
Figure 3. Ventral anterior substantia nigra magnetic susceptibility.
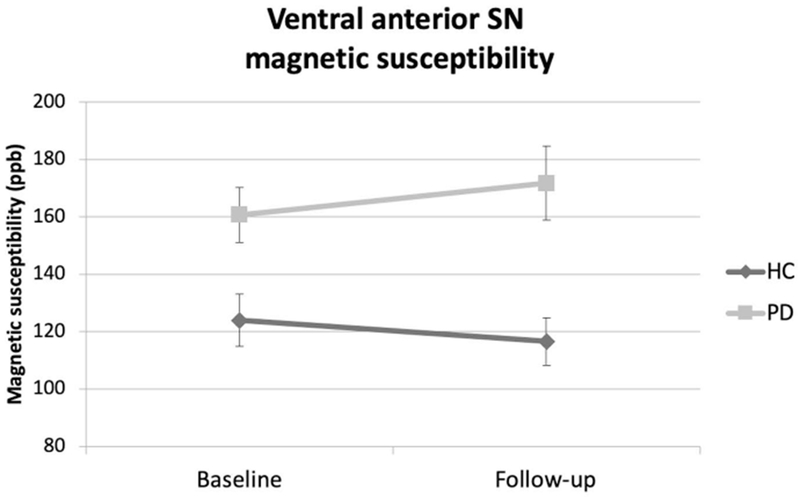
Mean magnetic susceptibility values in the ventral anterior substantia nigra are shown comparing Parkinson’s disease (PD) and healthy controls (HC). False discovery rate-corrected group, time and group × time interaction p-values were 0.042, 0.02, and 0.043, respectively. Comparisons were made adjusting for age and sex. The error bars represent ± 1 standard error of the mean.
Legend: PD - Parkinson’s disease; HC – healthy controls; SN–substantia nigra; ppb – parts per billion
In the dorsal SN there were no significant group, time, or group × time interaction effects for either the pSN or aSN (Table 2).
The results from the secondary analyses that considered all available baseline data were generally in line with the primary findings with magnetic susceptibility being greater in PD patients than HCs (ventral pSN and aSN: p < 0.001 and p = 0.063, respectively; dorsal pSN and aAN: p = 0.090, 0.588, respectively).
Substantia nigra volume
No significant group, time or group × time interaction effects were found for any of the SN ROIs (Supplemental Table). Similar results were obtained when analyzing the baseline data from all available subjects with respect to group effects (minimum p-value = 0.375).
Associations between substantia nigra magnetic susceptibility and clinical outcomes
No significant associations were detected between any baseline SN magnetic susceptibility assessment and baseline UPDRS-III (minimum p-value = 0.319) or H&Y scores (minimum p-value = 0.305). Similarly, no significant relationships were detected between changes in magnetic susceptibility and changes in clinical outcomes over the 3 years of follow-up (minimum p-value = 0.179). Also, when considering the baseline data from all available subjects, no significant associations were detected between any baseline SN magnetic susceptibility assessment and baseline UPDRS-III (minimum p-value = 0.442) or H&Y scores (minimum p-value = 0.351).
Reliability of manually drawn ROIs
The agreement for all ROIs was high with intraclass correlation coefficients > 0.88, all p < 0.001.
Discussion
We found robust cross-sectional and longitudinal differences in SN iron in PD compared to HC that were completely restricted to the ventral SN. To our knowledge, this is the first report demonstrating a marked ventral-dorsal disparity in SN iron imaging assessments from the same PD cohort in a longitudinal setting. Although these data support ventral SN iron assessed by QSM to be a potential PD progression biomarker, our results also highlight the importance of the location of SN iron assessment for reflecting cross-sectional and longitudinal changes in PD. Our in vivo imaging findings reflect the PD pathological findings of early and strong degeneration occurring in the more ventral SN with the most dorsal SN, adjacent to the midpoint of the RN, not showing much degeneration until >20 years of disease duration (1). This imaging/pathology correlation supports the ventral SN as the location most likely to reflect pathology in early and mid-stage PD.
A similar ventral/dorsal spatial disparity was reported in a study using diffusion tensor imaging (DTI) to investigate SN microstructure in PD (29). This study did not detect any significant differences between PD and HC in SN fractional anisotropy (FA) when using ROIs drawn adjacent to the RN but found significantly decreased SN FA in PD when the SN was assessed inferior to the RN. However, previous PD imaging studies using QSM or other iron-sensitive MRI measures have not specifically assessed the ventral and dorsal SN separately but have included the entire SN as a single structure (7, 11, 30, 31). This may have negatively affected these studies’ results by effectively washing out ventral SN differences by having included data from the much less affected dorsal SN. Indeed, one small study using 7T MRI did show a gradient of increasing iron from the dorsal to the ventral portion of the SN among 9 PD subjects. (10) Similarly, a recent study found evidence of increased iron levels in the pars compacta, corresponding to the more ventral area of the SN (12).
In addition to the disparate ventral/dorsal spatial pattern of iron content in PD SN, we also found differences along the SN anterior-posterior axis with only ventral pSN iron significantly increasing from baseline to year 3 in the PD group. However, when comparing PD to HC, we found both significant baseline and longitudinal differences for both the ventral pSN and aSN. Previous SN imaging studies in PD have also found larger effects to be localized to the pSN, as reported in several different modalities including DTI (14–17), neuromelanin-sensitive (32) and QSM (33). Another recent study suggested that the in vivo assessment of neuromelanin with magnetization transfer contrast, particularly in the pSN pars compacta, might be a promising biomarker of the disease (34). Similar to the ventral SN, PD pathology studies have also shown the pSN to be involved early and to a greater extent compared to the aSN (2). Using calbindin D28K immunostaining techniques, it was demonstrated that the greatest cell loss was found in the so-called nigrosome 1 region of the SN (35). Although some authors have suggested that MRI signal in the posterior region of the ventral SN may reflect nigrosome 1 pathology, it must be stressed that such findings have yet to be corroborated with histological data.
One promising iron-sensitive MRI measure that has been studied somewhat more than QSM as a PD biomarker is R2*. While both modalities are sensitive to iron, QSM has been shown to be more specific as R2* captures other PD-pathological processes such as alpha-synuclein accumulation (36). Several studies have found SN R2* to increase longitudinally in PD compared to HC (11, 37, 38). SN R2* was also shown to significantly increase in mice treated with the neurotoxin MPTP that caused significant SN dopaminergic neuronal loss (37), which implies R2* may reflect SN neuronal degeneration. A similar association might be expected with QSM-derived measurements due to increased iron concentrations following neuronal death. However, we did not find any significant effects with respect to SN ROI volumes, which suggests that our results are not driven by secondary effects of increased iron concentrations following SN tissue atrophy. Moreover, one recent study utilized both QSM and R2* assessments in the same cohort and found R2*, but not magnetic susceptibility, to increase longitudinally over 1.5 years in the SN pars compacta but only in later-stage PD subjects with a mean disease duration of 9.8 years at baseline (11). This study evaluated the entire SN as a single structure, which may explain the lack of R2* and QSM longitudinal changes in the early and mid-stage PD subgroups due to the relative lack of dorsal SN involvement compared to later-stage PD (1). In our present study, PD subjects had a mean disease duration of 6.2 years at baseline and we found significant, albeit relatively small, longitudinal QSM increases over 3 years, corresponding to an average annualized increase (i.e. accounting for the length of follow-up) of 3.5% in ventral pSN magnetic susceptibility. This rate of change is in line with the previously mentioned study where both SN pars compacta magnetic susceptibility and R2* increased at an annualized rate of 2.1% and 5.0%, respectively (11). In comparison, it was previously shown that DTI-derived free water in the ventral posterior SN increased by an average annualized rate of 17.2% in a cohort of PD patients with a mean disease duration of 3 years (17) and by 11.6% from baseline to 1-year of follow-up in de novo PD (14). There is also some evidence that the rate of PD-related free-water increases in this region deaccelerate over time, as evidenced by annualized rates of 12.3%, 9.7%, and 6.9% in a cohort that was evaluated after 1 year, 2 years and 4 years of follow-up, respectively (14). As of now however, there are no studies in the literature that have directly compared diffusion and iron-sensitive imaging measures in the same PD cohort.
Considering the promising longitudinal results reported here utilizing QSM and previously utilizing R2*, future PD imaging studies would greatly benefit from using both modalities when assessing the SN as well as assessing the SN separately in the ventral/dorsal and anterior/posterior axes. As R2* and QSM measures can both be derived from the same multi-echo GRE acquisition, prospective studies offer the potential to shed additional light on SN pathology in this regard.
Reports in the literature regarding the association between SN iron content and clinical outcomes are somewhat mixed. For example, two recent studies found a positive correlation between magnetic susceptibility within the SN and H&Y stage (9, 30) but were discrepant in terms of associations with UPDRS-III scores. Both of these reports examined patients in the “off” state and about 21 percent of the patients in the study by Langkammer et al. were drug naïve (9). On the other hand, all of the PD patients in our study were on dopaminergic treatments and examined in the “on” state, which would mask much of their motor symptoms as reflected in the UPDRS. Moreover, the patients remained clinically stable over the course of the 3 years of follow-up in terms of their UPDRS-III scores, which likely prevented us from detecting any longitudinal associations. Nevertheless, one of the largest longitudinal studies recently found SN changes in both R2* and QSM to correlate with changes in clinical outcomes over 19 months among 72 PD subjects assessed in the “on” state (11). Future longitudinal PD imaging studies may benefit from assessing subjects both in the “on” and “practically-defined off-state”, which may be more likely to correlate with SN imaging assessments. In sum, more studies are needed to further validate the potential of SN QSM-derived iron content as a progression marker of PD.
Our study is not without its limitations. First, the sample sizes in both groups were quite small and with a relatively high percentage of missing data at the follow-up time point. Despite this, however, we were able to find robust effects within the ventral pSN. Second, we did not separately consider the pars compacta and pars reticulata regions of the SN, which are affected by Parkinson’s related pathology in disparate manners. Given that the pars compacta is characterized by neuromelanin containing dopaminergic neurons, future studies utilizing neuromelanin-sensitive imaging may aid in disentangling a potential role of iron-related neurodegeneration in this region (12, 29, 39). Third, at the time of the study, we only had access to a single echo acquisition for QSM reconstruction, preventing us from simultaneously evaluating SN R2* measurements. Fourth, we did not separately assess data from the left and right hemispheres. It might be the case that magnetic susceptibility increases in a preferential manner related to the laterality of symptom onset. Future studies are warranted to investigate this possibility. Finally, all PD patients were also receiving dopaminergic therapy throughout this study, which may have influenced our results. A potential effect of dopaminergic therapy on SN iron levels has not previously been assessed, to our knowledge.
In conclusion, we have shown ventral SN iron levels, particularly in the pSN, to be significantly higher in PD than HC and to increase longitudinally, characteristics that support a disease progression biomarker. We have also shown that these changes are confined to the ventral SN in line with PD SN pathological findings in early and mid-stage disease (1). Additional longitudinal studies with larger sample sizes and multimodal magnetic resonance imaging protocols including QSM, R2* and DTI-based free-water are warranted. Finally, use of these imaging biomarkers in PD clinical trials should be considered to help identify disease-modifying therapies.
Supplementary Material
Acknowledgments
Funding
This work was supported by an investigator-initiated award from Teva Neurosciences and by the National Center for Advancing Translational Sciences of the National Institutes of Health, under award Number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures
N Bergsland has nothing to disclose.
R Zivadinov has received personal compensation from EMD Serono, Genzyme-Sanofi, Novartis, Claret-Medical, Celgene for speaking and consultant fees. He received financial support for research activities from Claret Medical, Genzyme-Sanofi, QuintilesIMS Health, Novartis and Protembis.
F Schweser has received travel support from General Electric, speaking fees from Toshiba Medical Canada, and consultant fees from Goodwin Procter LLC. He received financial support for research activities and travel from SynchroPET, Inc.
J Hagemeier has nothing to disclose.
D Lichter has received speaking and consulting fees from Teva Neuroscience and US WorldMeds.
T Guttuso has received speaking and consulting fees from Teva Neuroscience and US WorldMeds and is also President of e3 Pharmaceuticals, Inc.
References
- 1.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122 (Pt 8):1437–48. [DOI] [PubMed] [Google Scholar]
- 2.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136(Pt 8):2419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MB. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J Neurochem. 1991;56(3):978–82. [DOI] [PubMed] [Google Scholar]
- 4.Good PF, Olanow CW, Perl DP. Neuromelanin-containing neurons of the substantia nigra accumulate iron and aluminum in Parkinson’s disease: a LAMMA study. Brain Res. 1992;593(2):343–6. [DOI] [PubMed] [Google Scholar]
- 5.Kumar H, Lim HW, More SV, Kim BW, Koppula S, Kim IS, et al. The role of free radicals in the aging brain and Parkinson’s Disease: convergence and parallelism. Int J Mol Sci. 2012;13(8):10478–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dexter DT, Carayon A, Vidailhet M, Ruberg M, Agid F, Agid Y, et al. Decreased ferritin levels in brain in Parkinson’s disease. J Neurochem. 1990;55(1):16–20. [DOI] [PubMed] [Google Scholar]
- 7.Barbosa JH, Santos AC, Tumas V, Liu M, Zheng W, Haacke EM, et al. Quantifying brain iron deposition in patients with Parkinson’s disease using quantitative susceptibility mapping, R2 and R2. Magn Reson Imaging. 2015;33(5):559–65. [DOI] [PubMed] [Google Scholar]
- 8.Du G, Liu T, Lewis MM, Kong L, Wang Y, Connor J, et al. Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Mov Disord. 2016;31(3):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langkammer C, Pirpamer L, Seiler S, Deistung A, Schweser F, Franthal S, et al. Quantitative Susceptibility Mapping in Parkinson’s Disease. PLoS One. 2016;11(9):e0162460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotfipour AK, Wharton S, Schwarz ST, Gontu V, Schafer A, Peters AM, et al. High resolution magnetic susceptibility mapping of the substantia nigra in Parkinson’s disease. J Magn Reson Imaging. 2012;35(1):48–55. [DOI] [PubMed] [Google Scholar]
- 11.Du G, Lewis MM, Sica C, He L, Connor JR, Kong L, et al. Distinct progression pattern of susceptibility MRI in the substantia nigra of Parkinson’s patients. Mov Disord. 2018;33(9):1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langley J, He N, Huddleston DE, Chen S, Yan F, Crosson B, et al. Reproducible detection of nigral iron deposition in 2 Parkinson’s disease cohorts. Mov Disord. 2018;34(3):416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta-Cabronero J, Cardenas-Blanco A, Betts MJ, Butryn M, Valdes-Herrera JP, Galazky I, et al. The whole-brain pattern of magnetic susceptibility perturbations in Parkinson’s disease. Brain. 2017;140(1):118–31. [DOI] [PubMed] [Google Scholar]
- 14.Burciu RG, Ofori E, Archer DB, Wu SS, Pasternak O, McFarland NR, et al. Progression marker of Parkinson’s disease: a 4-year multi-site imaging study. Brain. 2017;140(8):2183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofori E, Krismer F, Burciu RG, Pasternak O, McCracken JL, Lewis MM, et al. Free water improves detection of changes in the substantia nigra in parkinsonism: A multisite study. Mov Disord. 2017;32(10):1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Planetta PJ, Ofori E, Pasternak O, Burciu RG, Shukla P, DeSimone JC, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain. 2016;139(Pt 2):495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, et al. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain. 2015;138(Pt 8):2322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guttuso T Jr., Bergsland N, Hagemeier J, Lichter DG, Pasternak O, Zivadinov R Substantia Nigra Free Water Increases Longitudinally in Parkinson Disease. AJNR Am J Neuroradiol. 2018;39(3):479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langkammer C, Schweser F, Krebs N, Deistung A, Goessler W, Scheurer E, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. NeuroImage. 2012;62(3):1593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movement Disorder Society Task Force on Rating Scales for Parkinson’s D. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738–50. [DOI] [PubMed] [Google Scholar]
- 21.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammond KE, Lupo JM, Xu D, Metcalf M, Kelley DA, Pelletier D, et al. Development of a robust method for generating 7.0 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases. NeuroImage. 2008;39(4):1682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polak P, Zivadinov R, Schweser F. Gradient unwarping for phase imaging reconstruction. In Proc Intl Soc Mag Reson Med 2015;2015:p3736. [Google Scholar]
- 24.Abdul-Rahman HS, Gdeisat MA, Burton DR, Lalor MJ, Lilley F, Moore CJ. Fast and robust three-dimensional best path phase unwrapping algorithm. Applied optics. 2007;46(26):6623–35. [DOI] [PubMed] [Google Scholar]
- 25.Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage. 2011;54(4):2789–807. [DOI] [PubMed] [Google Scholar]
- 26.Wu B, Li W, Guidon A, Liu C. Whole brain susceptibility mapping using compressed sensing. Magnetic resonance in medicine. 2012;67(1):137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweser F, Sommer K, Deistung A, Reichenbach JR. Quantitative susceptibility mapping for investigating subtle susceptibility variations in the human brain. NeuroImage. 2012;62(3):2083–100. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 29.Langley J, Huddleston DE, Merritt M, Chen X, McMurray R, Silver M, et al. Diffusion tensor imaging of the substantia nigra in Parkinson’s disease revisited. Hum Brain Mapp. 2016;37(7):2547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An H, Zeng X, Niu T, Li G, Yang J, Zheng L, et al. Quantifying iron deposition within the substantia nigra of Parkinson’s disease by quantitative susceptibility mapping. Journal of the Neurological Sciences. 2018;386:46–52. [DOI] [PubMed] [Google Scholar]
- 31.Murakami Y, Kakeda S, Watanabe K, Ueda I, Ogasawara A, Moriya J, et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. AJNR Am J Neuroradiol. 2015;36(6):1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimao S, Ferreira S, Nunes RG, Pita Lobo P, Neutel D, Abreu D, et al. Magnetic resonance correlation of iron content with neuromelanin in the substantia nigra of early-stage Parkinson’s disease. Eur J Neurol. 2016;23(2):368–74. [DOI] [PubMed] [Google Scholar]
- 33.Azuma M, Hirai T, Yamada K, Yamashita S, Ando Y, Tateishi M, et al. Lateral Asymmetry and Spatial Difference of Iron Deposition in the Substantia Nigra of Patients with Parkinson Disease Measured with Quantitative Susceptibility Mapping. AJNR Am J Neuroradiol. 2016;37(5):782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huddleston DE, Langley J, Sedlacik J, Boelmans K, Factor SA, Hu XP. In vivo detection of lateral-ventral tier nigral degeneration in Parkinson’s disease. Hum Brain Mapp. 2017;38(5):2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122 (Pt 8):1421–36. [DOI] [PubMed] [Google Scholar]
- 36.Lewis MM, Du G, Baccon J, Snyder AM, Murie B, Cooper F, et al. Susceptibility MRI captures nigral pathology in patients with parkinsonian syndromes. Mov Disord. 2018;33(9):1432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopes L, Grolez G, Moreau C, Lopes R, Ryckewaert G, Carriere N, et al. Magnetic Resonance Imaging Features of the Nigrostriatal System: Biomarkers of Parkinson’s Disease Stages? PLoS One. 2016;11(4):e0147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulla M, Bonny JM, Ouchchane L, Rieu I, Claise B, Durif F. Is R2* a new MRI biomarker for the progression of Parkinson’s disease? A longitudinal follow-up. PLoS One. 2013;8(3):e57904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langley J, Huddleston DE, Sedlacik J, Boelmans K, Hu XP. Parkinson’s disease–related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Movement Disorders. 2017;32(3):441–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


