Abstract
Introduction
Silk is a promising biomaterial for controlled delivery of therapeutic molecules. Silk has a unique protein chemistry and structure that can be tuned to form different carrier formats. The protein has been studied for injectable or implantable sustained release depot systems for the targeted or localized delivery of drugs.
Areas covered
An overview of natural silk proteins for controlled delivery of therapeutics is provided, with a focus on the features of silk proteins that allow them to be useful tools for controlled delivery. Recent applications of natural silk proteins as controlled delivery systems are also summarized.
Expert opinion
The versatility of silk proteins makes them desirable biomaterials for a broad range of applications for controlled delivery of both small and large molecules. Further, the degradation profile leading to peptides and amino acids provides compatibility with pH-sensitive therapeutics like complex proteins in formulation and delivery. While silk sericin and spider silks are under study, silk fibroin extracted from silkworms (e.g., Bombyx mori) dominates pharmaceutical studies with silk. Silk fibroin can be formed into drug delivery tools for systemic or local injections, topical and transdermal applications, and implantation; depending on the target disease and therapeutic molecule. In vitro to in vivo correlations and scale-up needs are the next steps towards clinical applications.
Keywords: Silk, controlled release, drug delivery, nanoparticles, hydrogels, implants, microneedles
1. Introduction
The controlled delivery of therapeutics aims to extend the duration between doses and maintain constant therapeutic levels in plasma, tumors or local injection sites. Such systems also offer additional benefits, including reduced side effects, improved patient compliance for frequent or difficult applications and reduced cost of treatment with well designed controlled delivery systems 1. The biomaterials utilized for controlled delivery need to be cost effective, non-toxic and relatively simple to process with mild techniques in order to meet biocompatibility and regulatory demands. Organic solvents should be minimized, and release profiles should be adjustable in order to achieve clinically relevant therapeutic levels of the delivered therapeutics. Various polymers have been investigated for controlled therapeutic delivery; Including synthetic macromolecules like polyesters, polyorthoesters, polyphosphoesters, and polyanhydrides 2. Available controlled release formulations currently on the market are mostly based on Food and Drug Administration (FDA) approved synthetic polymers such as polylactide-co-glycolide acid (PLGA) and polycaprolactone (PCL), while FDA approved natural polymers like albumin, alginate, gelatin, collagen, and silk fibroin are being investigated as alternatives, in part to avoid undesirable degradation products and formulation challenges associated with polyesters such as activity loss of peptide-protein structures3–5.
Silk proteins are produced in fiber form by silkworms (e.g., B. mori, mulberry silk) or orb-weaving spiders (non-mulberry silk) and have been explored to understand their properties and potential as biomaterials. The first documentation of biomedical applications with silk are from 150 AD, where it was described as a suture material. In the 1500s, there are reports of sterilizing silk sutures in boiling oil, while the first sterile silk suture was officially introduced into clinics in 1869 6. The first attempt to reverse engineer silk cocoons and generate reprocessed silk was at the beginning of 20th century, while the first patent for the biomedical use of a regenerated silk appeared in the 1960s 7. Lyophilized silk powders, silk films and gels have been patented as wound dressings, corneal coatings or blood vessels in the 1990s, while research and commercialization activities have increased in 2000s, especially in the USA 7.
Silk proteins, particularly the fibroin has been employed for drug delivery due to the biocompatible, biodegradable, self-assembling properties, mechanical strength and controllable structure 8, 9. The source of silk is important due to considerations of supply, purity, physicochemical characteristics and biological responses. Spider silks are more diverse in composition; however they are difficult to obtain in reasonable quantities, therefore silkworm silks have been the focus of most studies10. Biosynthetic silk variants and copolymers have also been pursued for drug delivery 11, 12. Silk proteins have desirable properties for controlled delivery due to chemistry, structure and biological impact. For example, silk fibroin is a high molecular weight amphiphilic protein that self-assembles into mechanically robust structures, can be processed in aqueous or solvent systems, provides a low water content environment, and is resistant to temperature, pH and organic solvents 13. Further, these silks are considered biocompatible, can be tuned structurally (crystalline content) to control degradation rate based on enzymatic (not hydrolytic) digestion 14, offers stability to small drugs and complex proteins15–17, and can be sterilized by different modes (e.g., gamma irradiation, ethylene oxide (EtO), hydrogen peroxide, autoclave)18. In addition, the silk can be formed into various delivery platforms including but not limited to nanoparticles, microparticles, macroparticles, hydrogels, implantable rods, foams, wafers and reservoirs 19–25. Silk was first approved by FDA as a biomedical suture 26, has been approved for soft tissue reconstruction (Seri-scaffold −2008, Serica Technologies ) and most recently received 510k clearance (Silk Voice - K180631 – 2018, Sofragen) as an injectable filler for vocal fold insufficiency 27.
In this review, we discuss applications of silk proteins for the controlled delivery of therapeutics. The details of the chemistry and structure of silk have been previously reported and will not be recapitulated here 8, 10, 28. Thus, we will focus on the advantages of the unique features of silk proteins and how silk is being utilized for the controlled delivery of therapeutics.
2. Advantages of silk proteins for controlled delivery
Silk is a useful matrix for controlled delivery as the processing can be tailored for drug loading, release kinetics and stability by changing the process used in the formation and treatment of the material.
Silk consists of a fibroin protein heavy chain (~325 kDa) and light chain (~25 kDa) held together by a disulfide bone and encased in sericin proteins (20 kDa to 310 kDa) during fiber spinning by the silkworm 11. The sericins have been implicated with inflammatory responses 29, they can be removed by boiling the silk fibers in alkaline solution 30. In the absence of the sericin, silk fibroin causes minimal inflammatory reactions and essentially no immune response 10, 31. Interestingly, sericin has also been reported to be minimally inflammatory in the absence of fibroin, suggesting the interaction of sericin with fibroin may related to inflammatory outcomes 32, 33.
The amphiphilic structure of the fibroin heavy chain consists of 12 hydrophobic “crystallizable” and 11 hydrophilic “amorphous” domains. The crystallizable domains provide control over drug release kinetics and the degradation profile of the silk formulations by manipulation of the crystalline content 1. These same domains are also responsible for the self-assembly of the fibroin that leads to strong physical interactions and robust mechanical structures with the associated slow rate of degradation. The self-assembly of the heavy chain in aqueous solution without chemical additives is a key control point in the formulation of silk proteins 34, 35. Modulating the degree of crystallinity, such as by water vapor annealing or exposure to methanol can also control the rate of silk degradation. The presence of crystalline domains interspersed with less crystalline domains is also responsible for the high mechanical strength and toughness of silk materials. Furthermore, the GAGAGS amino acid sequence in silkworm silk dominants the primary sequence as a key hydrophobic block, to enhance hydrophobic drug interactions to control loading and release kinetics 36, 37. Due to the dominant hydrophobic nature of silk as mentioned above, hydrophobic therapeutic compounds usually interact better with silk and thus perform better in terms of sustained release 38. Although the primary structures of silkworm and spider silk can be different (poly(GA) and poly(A) sequences tend to dominant for the mechanically robust silks as crystalline-forming regions), while providing similar hierarchical structures 39. Chemical modification strategies of silk have also been reported, exploiting the non-crystalline domains and side chains of amino acids such as tyrosine, glutamic acid and others 10, 40.
Another important feature of silk as a biomaterial for controlled delivery is the versatility of options for sterilization. The most widely used synthetic (PLGA) 41 or natural polymers (collagen) 42 in drug delivery are more limited in terms of options for common sterilization methods due to their low thermal stability or degradation with gamma irradiation. Silk can be sterilized by autoclaving, gamma radiation and ethylene oxide 43. In most cases it is also possible to filter sterilize silk solutions during preparation under aseptic conditions 19.
In Table 1, we compare silk proteins with commonly used synthetic and natural polymers in terms of critical features for drug delivery.
Table 1.
Natural and Synthetic Polymers for Controlled Delivery of Therapeutics
| Polymer | Synthetic/Natural | FDA approved | Biodegradability Biocompatible | Stimuli responsive | Sterilization | Challenges |
|---|---|---|---|---|---|---|
| Albumin44 | Natural | Yes | Yes/Yes | Thermoresponsive | -Sterile filtration | Various side effects (allergic reactions, immunological response) |
| Alginate 45 | Natural | Yes | Yes/Yes | Thermoresponsive pH responsive |
-Sterile filtration -Heat treatment |
Limited long-term stability in physiological conditions |
| Chitosan 46 | Natural | No | Yes/Yes | Thermoresponsive pH responsive |
-Ozonation -Ultraviolet |
Poor solubility at physiological pH Batch to batch variation |
| Collagen 42 | Natural | Yes | Yes/Yes | No | - Chloroform - Ethylene oxide - Gamma rad. |
Poor mechanical strength |
| Dextrins 47 | Natural | Yes | Yes/Yes | No | - Ethylene oxide - Gamma rad. |
Microbial contamination, Uncontrolled rates of hydration Decrease in viscosity during storage |
| Gelatin 48, 49 | Natural | Yes | Yes/Yes | Thermoresponsive pH responsive |
-Steam heat -Sterile filtration - Gamma rad. |
Poor mechanical properties |
| HA 50 | Natural | Yes | Yes/Yes | No | -Heat treatment -Sterile filtration |
Accumulation in liver Low accumulation in tumor sites |
| HPMA 51 | Synthetic | Yes | No/Yes | No | -Heat treatment | Non biodegradable Elimination via kidney |
| PCL 52 | Synthetic | Yes | Yes/Yes | No | -Ultraviolet | Low melting point |
| PEG 51, 53 | Synthetic | Yes | No/Yes | Thermoresponsive | -Sterile filtration | Accumulation in the body Low peptide/protein conjugation |
| PEI 54 | Synthetic | No | No/No | No | -Ethylene oxide -Gamma rad. -Steam heat |
Cytotoxicity |
| PLGA 4, 41 | Synthetic | Yes | Yes/Yes | No | - Plasma - Ethylene oxide - Gamma rad. |
Low solubility High rate of degradation Acidic degradation products Challenge of biosynthesis Protein aggregation and instability Burst release of large molecules |
|
Silk Fibroin
Silk Sericin 19, 43 |
Natural | Yes | Yes/Yes | pH responsive Thermoresponsive Enzyme activity Physical stimuli |
-Steam heat -Sterile filtration -Gamma rad. |
Batch to batch variation |
HA: Hyaluronic acid, HPMA: [N-(2- hydroxypropyl)methacrylamide], PEG: polyethylene glycol, PEI: Polyethylenimine, PLGA: poly lactic-co-glycolic acid, PCL: Polycaprolactone
3. Applications of natural silk proteins for controlled delivery of therapeutics
Silk has been used as a biomaterial for a variety of applications for drug delivery via multiple formats55. Silk fibroin from silkworm cocoons (B. mori) is the most commonly used silk for controlled drug/protein delivery, while sericin and spider silk proteins have also been investigated. Injectable formats including nanoparticles, microparticles and hydrogels; implantable forms like films, wafers, foams, tubes, rods, reservoirs and transdermal systems like microneedles are among the silk-based delivery systems reported (Figure 1). Silk-based O/W/O micro-emulsions were used to encapsulate and control the delivery of oils and volatile compounds such as fragrances, also applicable to deliver hydrophobic therapeutic molecules 56. Silk fibroin has also been used as a coating material to increase residence time and cell recognition 57. Here we focus on recent developments in silk-based delivery systems and discuss the benefits for controlled delivery of therapeutics. This article does not focus on the material preparation techniques with silk, since we have previously published a protocol paper to summarize the fabrication methods for major silk carrier systems 55. As most of the studies are focused on silk from B. mori cocoons, the silk term used will refer to mulberry silk unless otherwise is stated.
Figure 1.
Silk-based systems used for controlled delivery of therapeutics. Images were reproduced with permission from the cited articles for gels 61, fibers 62 and microneedles 63.
3.1. Silk-based particle systems
Microparticle and nanoparticle systems have been studied for controlled drug delivery due to their large surface area, enhanced permeability and targeting ability due to size and surface charges. Nanoparticles can penetrate through the physiological barriers and become incorporated into cells due to size, thus are important delivery systems for cancer treatment as they can passively target tumor sites due to enhanced permeability and retention (EPR)58. Microparticles are generally used as a subcutaneous, intramuscular or muchoadhesive drug depots, as well as passively targeted lung delivery systems due to size59, 60.
The common methods to prepare micro/nanoparticles usually require toxic organic solvents and some techniques like spray drying can result in the degradation or denaturation of the drug 64. In contrast, silk proteins can be formed into micro- or nano-particles without organic solvents, in part due to the self-assembly features of the protein, using physical methods like solution-enhanced dispersion 64, desolvation 24, self-aggregation 65 or micro injection pumps 66.
Several studies have reported encapsulated chemotherapeutics in silk nanoparticles to increase plasma retention time, increase cellular uptake, targeting to tumors via EPR, and to reduce application frequency and systemic toxicity 24, 66–71. Curcumin-loaded silk fibroin nanoparticles were prepared to treat tumors with local sustained delivery; the nanoparticles were cytotoxic to carcinogenic cells while not killing healthy cells, however in vitro release from was limited to 24 hours following burst release in the first 5 hours 68. Silk nano- and micro-particles were used for oral delivery of curcumin and larger silk particles were successful in increasing plasma circulation up to 24 hours. As a result of avoiding first-pass metabolism due to the small size of the particles, curcumin AUC0−∞ was approximately 17 times that of the curcumin alone 69. Cisplatin has also been entrapped in silk fibroin nanoparticles to achieve controlled release and enhanced cellular uptake, where the drug was released for 15 days and internalized by A549 lung cancer cells 66. To increase lysosomal accumulation, pH dependent release of doxorubicin was investigated using silk fibroin nanoparticles, and release rates were pH 4.5 > > 6.0 > 7.4 which correlated with high lysosomal uptake and potentially low plasma concentrations 24. Doxorubicin was also encapsulated in PEGylated (polyethylene glycol = PEG) silk nanoparticles to achieve pH-controlled release with a stealth design that allowed the particles to avoid the reticuloendothelial system and remain in circulation for a longer period of time 67. Folate receptor targeted release of doxorubicin was investigated via folic acid conjugated silk fibroin nanoparticles 71. Internalization of doxorubicin particles was observed in cervical cancer (HELA) cells and pH dependent drug release lasted for over 30 hours71. Doxorubicin was also formulated with spider silk from N. clavipes and pH-dependent extended release up to 15 days was achieved 72. In addition to the release profile, these nanoparticles were functionalized to bind to Her2 positive cells and the particles showed no toxicity unless they were loaded with doxorubicin 72.
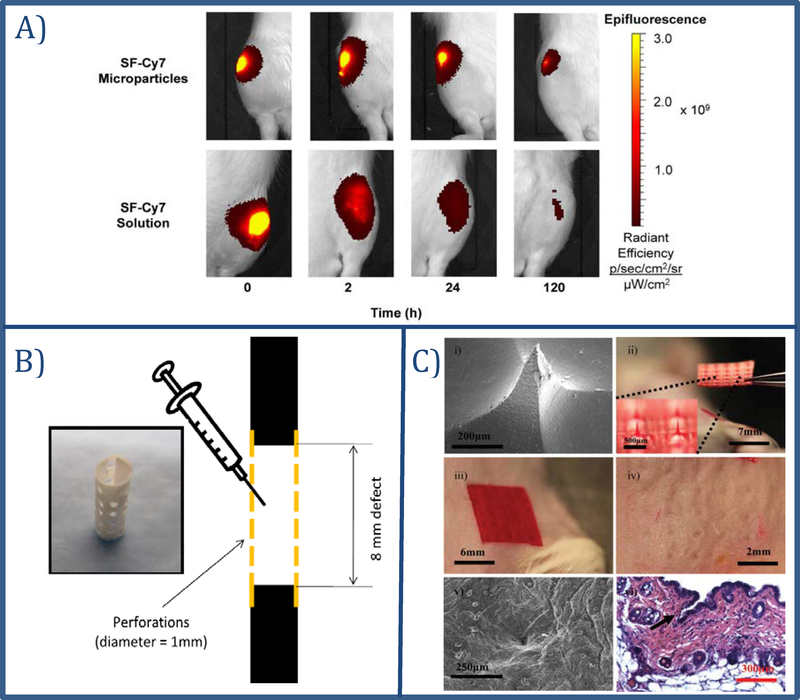
Silk microparticles have been used to increase residence time of drugs in a specific application area. The efficiency of silk fibroin microparticles for sustained release of small molecules into the articular cavity evaluated for fluorophore (Cy7) conjugated microparticles 73,. In vitro release of Cy7 lasted for over 7 days following intra-articular injection in rats; the fluorescence decay half-life increased significantly with microparticles compared to injection of silk-Cy7 solution (Figure 2A).
Figure 2.
Different applications of silk fibroin for controlled delivery of therapeutics.
A) In vivo image of rat knees following intra-articular injections of silk fibroin-Cy7 (SF-Cy7) particles or SF-Cy7 solution. Images of microparticle injected knees display stronger and more persistent fluorescence intensity 5 days after injections. Images reproduced with permission from 73. B) Perforated electrospun PCL nanofiber mesh tube placed around the defected bone and pre-gelled silk hydrogel with or without BMP-2 injected into the defect to promote bone modeling. Image reproduced with permission from 92. C) Silk microneedles for controlled drug delivery. i) SEM image of a silk microneedles, ii) picture of silk microneedle patch, iii) silk microneedles applied to mouse skin, iv) mouse skin after removal of the patch, v) SEM image of penetrated skin after removal of the patch, vi) histology showing breach of epidermis around the indentation site. Image reproduced with permission from 93.
Aside from the examples above, silk has also been used in combination with different polymers, silk sericin protein from Antheraea mylitta cocoons was blended with pluronic F-127 and F-87 74 and silk fibroin from Bombyx mori was blended with chitosan 75 for enhanced uptake of cancer therapeutics. Both studies were able to achieve sustained release, and cellular uptake of curcumin was increased with silk coating compared to uncoated curcumin and silk-chitosan coated curcumin. However, the production of these systems required addition of toxic organic solvents such as methanol, dioxane, dimethyl sulfoxide (DMSO) and N, N-dimethylformamide (DMF). A solution-enhanced dispersion method for nanoparticle preparation using supercritical CO2 has been developed and did not require solvent additions 64.
Recent advancements in silk-based particle systems for controlled delivery of therapeutics, classified based on the silk source, formulation type and the application of the systems are summarized in Table 2.
Table 2.
Silk-based nanoparticle and microparticle systems for controlled delivery of therapeutics
| Silk source | Formulation | Outcome/ Benefits | Released Agent | Ref. |
|---|---|---|---|---|
| B. mori, Fibroin | Microparticles | Sustained release over several weeks Increase in in vivo joint residence time |
Cy-7 | 73 |
| Extended release (up to 25 days) No burst release with methanol treatment Longer release with methanol treatment than NaCl treatment Enzyme activity retained during preparation |
HRP | 76 | ||
| Microparticle coating | Delayed degradation of PLGA microparticles (up to 30 days) Sustained protein release as diffusion barrier |
HRP, Rh-BSA | 77 | |
| Nanoparticles | Preparation by super critical CO2 – no organic solvent Sustained release (2 days) |
Indomethacin | 64 | |
| Stimulus responsive pH-dependent release, pH 4.5>>pH 6.0>pH 7.4 (up to 6 days) Enhanced endocytic uptake and lysosomal accumulation |
Doxorubicin | 24 | ||
| Silk- albumin conjugates Sustained release: 85% release over 12 days High drug encapsulation and loading efficiency Cytocompatible |
Methotrexate | 65 | ||
| Sustained release (up to 3 days) Increased curcumin bioavailability Cytotoxic to carcinogenic cells Not toxic to healthy cells |
Curcumin | 68 | ||
| Dual drug loading Drug release for 7 days Suppression of cancer cell growth |
Paclitaxel, Doxorubicin | 70 | ||
| Simple nanoparticle manufacturing Sustained release |
Doxorubicin | 78 | ||
| Silk fibroin (shell) and polyvinyl alcohol (PVA, core) Controlled release for 72h Control of drug release achieved by alternating PVA/silk ratios and by applying ultrasound |
Doxorubicin | 79 | ||
| Drug release over 15 days Internalization in cancer cells Low toxicity in mouse fibroblasts |
Cisplatin | 66 | ||
| Carrier-in-carrier delivery system Silk/curcumin nanoparticles loaded in extracellular vesicles Release of drug for up to 100h Improved curcumin bioavailability |
Curcumin | 80 | ||
| Improved cyto- and hemo compatibility of the drugs High encapsulation efficiency Sustained release up to 56 hours |
Celecoxib, Curcumin | 81 | ||
| Enhanced cellular uptake and prolonged retention time In vitro drug release up to 200 hours In vivo biocompatibility Accumulation and extended retention time in retina following intravitreal injection |
FITC-BSA | 82 | ||
| In vivo transdermal delivery of fluorescent dyes | Rhodamine B (fluorescent dye) | 83 | ||
| Particles | Release based on charge interactions Prolonged release with positively charged molecules (up to 14 days) |
Alcian blue, rhodamine B, crystal violet | 84 | |
| High encapsulation efficiency Release up to 7 weeks Bioactivity preserved during \ release |
Salicylic acid, propranolol hydrochloride, IGF-I | 85 | ||
| Targeted and pH-responsive, folic acid-modified particles Controlled release over 32 days Higher drug release at low pH with enhanced cell internalization |
Doxorubicin | 71 | ||
|
B. mori and A. mylitta Fibroin |
Nanoparticles | Accumulation of nanoparticles in cytoplasm of carcinoma cells Sustained release up to 3 weeks | VEGF | 86 |
| B .mori PEGylated silk | Nanoparticles | Stealth design for increased clearance time High encapsulation efficiency (>93%) pH-dependent release over 14 days Cytotoxic to breast cancer cells |
Doxorubicin | 67 |
| Nanoparticles Microparticles |
2 days release, longer plasma exposure Improved curcumin bioavailability Reduced burst release |
Curcumin | 69 | |
| B .mori Sericin | Nanoparticles | 2 days release No toxicity from nanoparticle carrier Lower doxorubicin systemic toxicity Stable nanoparticles |
Doxorubicin | 87 |
|
A. mylitta Fibroin |
Nanoparticles | Folate-conjugated nanoparticles allow targeted delivery Sustained release up to 21 days with enhanced release at acidic pH |
Doxorubicin | 88 |
|
A. pernyi Fibroin |
Nanoparticles | Sustained release up to 23 days pH-dependent release |
Doxorubicin | 89 |
| Nanoparticles | Self-assembled nanoparticles using cations (Na+, Ca2+, Ce3+) Sustained/pH-dependent release up to 11 days |
Doxorubicin | 90 | |
|
A. pernyi Sericin |
Microspheres | Microspheres are made of silk sericin and hydroxyapatite Sustained/pH dependent release of the drug up to 120 hours |
Doxorubicin | 91 |
| Spider silk N. clavipes |
Nanoparticles | Her2 targeted Enhanced targeted binding pH dependent drug release (up to 15 days) Non toxic nanoparticle carriers Higher cytotoxicity for doxorubicin-loaded nanoparticles |
Doxorubicin | 72 |
3.2. Silk-based gel systems
Silk fibroin-based hydrogels have been developed for delivery systems due to their versatility, tunable properties for injection and smart gel designs for transformation by environmental stimuli 94, 95. Vortexing, ultra-sonication, pH change, enzymes or organic solvents can be used to induce gelation of silk fibroin solution 96, 97. The in situ formation of hydrogels is especially attractive as the pre-gel solution can be mixed with therapeutics and injected prior to enzyme or temperature induced gelation in the body, preserving the bioactivity of the entrapped therapeutic 98.
Injectable silk gels are useful for localized delivery of chemotherapy drugs due to their ability to maintain high concentrations of drugs at the tumor site without the need for surgical implantation. As an example, sonication-induced silk gels were investigated for intratumoral delivery of chemotherapeutic drugs 19, 21. Vincristine loaded gels sustained drug release up to 80 days and tumor growth was suppressed following intratumoral injection in a neuroblastoma-induced mouse model 19, 21. Similarly, injectable silk nanofiber hydrogels were studied for intratumoral doxorubicin delivery 99, and doxorubicin release lasted over 8 weeks and the release kinetics were pH- and concentration dependent. Furthermore, the thixotropic structure of the gels allowed injectable formulations and significant antitumor response 99. Injectable silk hydrogels were also useful for ocular drug delivery 100. In an effort to reduce the injection frequency, bevacizumab-loaded silk fibroin hydrogels were compared to standard single injections of bevacizumab solution. Following intravitreal injection in rabbits, released bevacizumab concentrations from the hydrogels at day 90 were equivalent or greater than the released drug from the standard solution injection at day 30. Three months after the injection, hydrogel biodegradation was observed 100.
Subcutaneous injection of growth factor-loaded hyaluronic acid (HA)-based gels resulted in a localized angiogenic response 101. These types of gels were ideal for injectable formulations, however they were not mechanically strong enough for orthopedic applications. One approach to overcome these limitations was to reinforce HA-silk hydrogels with electrospun silk mats 102. In another study, acidic fibroin hydrogels (pH 3.8) for bone morphogenetic protein 2 (BMP-2) delivery was studied in rabbits for 12 weeks 92. Here, polycaprolactone nanofiber tubes filled with sonication induced silk fibroin hydrogels (Figure 2B) were placed into the defect area of rat femoral segments and delivered BMP-2 with no inflammatory reactions. The hydrogel systems promoted bone remodeling and were completely degraded by the end of the study, 12 weeks after surgery 92.
Dual delivery systems combining hydrogels with nanoparticles, fiber mats or solid polymeric support systems were explored to enhance the mechanical properties of hydrogels, to design implantable systems or to use the hydrogels as a carrier platform 103–105. Silk hydrogels loaded with silk nanoparticles were prepared to achieve dual drug delivery using fluorescein isothiocyanate (FITC) and rhodamine B 103. The system showed no significant cytotoxicity against human mesenchymal stem cells and achieved rapid rhodamine B release from hydrogels and slow FITC release (over 55 hours) from the entrapped nanoparticles 103.
As an alternative approach, silk fibroin lyogels were prepared by lyophilization of hydrogels to increase release time and stability of an antibody, IgG 104. IgG released for 38 days upon lyophilization of the hydrogels, while hydrogels without lyophilization released 10 days. Stability of the released antibody was also investigated and no significant physical or biological losses were observed 105.
Details for recent applications of silk fibroin gel systems are summarized in Table 3.
Table 3.
Silk-based gel systems for controlled delivery of therapeutics
| Silk source | Formulation | Outcome/ Benefits | Released Agents | Ref. |
|---|---|---|---|---|
|
B. mori, Fibroin |
Lyogels & Hydrogels |
Controlled release of IgG1 Longer release with lyogels compared to hydrogels (38 days) Antibody maintained activity |
IgG1 | 104 |
| Controlled release of IgG1 (26 days) Higher release with hydrated gel (lowest density of silk) Antibody oxidation levels decreased with addition of methionine |
IgG1 | 105 | ||
| Gels | Sustained release (25 days) Dual combination of vincristine and doxorubicin Intratumoral injections→ significant decrease in tumor growth Higher intratumoral drug concentration than IV administration Reduced systemic exposure |
Vincristine Doxorubicin |
19 | |
| Sustained intratumoral delivery via multiple injections (80 days) Diffusion distance halved, growth inflexion time doubled |
Vincristine | 21 | ||
| Intratumoral injections→ significant decrease in tumor growth Slower tumor growth compared to foam format |
Vincristine Doxorubicin |
106 | ||
| Hydrogels | Controlled release (30 days) Mixed with polyacrylamide to yield semi-interpenetrating gels Hydrogel with better mechanical properties Maximum release achieved with 70:30 of silk/polyacrylamide ratio |
Trypan blue FITC-inulin |
107 | |
| Controlled release and degradation (up to 42 days) In vivo degradation Improved mechanical properties by embedding silk fiber mat in hyaluronic acid network Sufficient mechanical strength for fastening to tissues in vivo |
Dexamethasone Hydrocortisone 6a-methylprednisolone Cortisone, Prednisolone Prednisone VEGF, FITC-dextran |
108 | ||
| Stabilization of antibiotics Focal delivery of antibiotics Sustained release (up to 4 days from the hydrogels) In vivo ampicillin efficacy in a murine infected-wound model |
Penicillin Ampicillin Gentamicin Cafazolin Rifampicin Eryhtromycin Tetracycline |
109 | ||
| Drug release over 8 weeks pH- and concentration-dependent release kinetics Thixotropic hydrogel structure → injectable Significant antitumor response in vivo |
Doxorubicin | 99 | ||
| Doxorubicin release over 4 weeks Intratumoral injection – Reduced tumor growth Minimized systemic side effects |
Doxorubicin | 61 | ||
| Curcumin-loaded cationic nanoparticles in silk fibroin hydrogel Deeper skin penetration in mouse with psoriasis In vitro sustained release for over 72 hours with no burst |
Curcumin | 110 | ||
| Controlled release (28 days) High crystallinity and high drug loading |
Risperidone | 111 | ||
| Sustained ocular delivery - release over 90 days Intravitreal injection – biodegradation starts after 3 months Drug available in vitreous 90 days after injection |
Bevacizumab | 100 | ||
| Extended release over 5 weeks Acid-modified silk hydrogels prevented burst release |
Chemokine CXCL12 | 112 | ||
| Acidic fibroin hydrogels (pH 3.8) – polycaprolactone nanofibers Local bone delivery – promoted bone remodeling |
Bone morphogenetic protein 2 | 92 | ||
| Silk nanoparticle loaded silk hydrogels – Dual drug delivery | FITC and Rhodamine B | 103 |
3.3. Solid silk formats
Silk fibroin based solid carrier systems have been used for local or transdermal delivery of both small molecules and proteins. A variety of carrier systems including silk films, wafers, reservoirs, discs foams and microneedles have been developed, allowing adjustments in release kinetics, mechanical strength and size of the delivery system (Table 4).
Table 4.
Solid silk formats for controlled delivery of therapeutics
| Silk source | Formulation | Outcome / Benefits | Released Agents | Ref. |
|---|---|---|---|---|
|
B. mori, Fibroin |
Disk | Local delivery of HIV inhibitors (rectal & vaginal) Sustained release for over 4 weeks Higher microbicide stabilization during storage (up to 14 months) |
HIV inhibitors: Griffithsin Griffithsin-C37 5P12-RANTES 5P12-RANTES-C37 |
124 |
| Sustained released up to 31 days for both IgG and 5P12-RANTES Preserved stability of antibody HIV inhibition in both blood and human colorectal tissue |
IgG 5P12-RANTES |
125 | ||
| Fiber mat | Silk – gelatin fiber mats Controlled release for 36h |
Methylene Blue | 126 | |
| Controlled delivery (up to 3 months) Local delivery of human platelet lysate for wound healing Improved storage and handling of human platelet lysate |
Human platelet lysate FITC-albumin |
127 | ||
| Silk membrane attached on anal fistula plug For Crohn’s disease treatment Controlled dual drug release (up to 10 days) |
Curcumin 5-aminosalicylic acid |
62 | ||
| Silk electrospun nanofibers combined with silk nanoparticles Dual drug delivery Sustained release for 40 hours |
Doxorubicin HCl Curcumin |
128 | ||
| Film | Sustained release (up to 28 days) Higher efficacy with intratumoral application of films when compared to intravenous application of drugs |
Doxorubicin Crizotinib |
122 | |
| Sustained release (up to 14 days) | Vincristine, Doxorubicin | 120 | ||
| Sustained release of cytokines for macrophage polarization Release up to 10 days |
IFN-γ. IL-4 | 119 | ||
| Transdermal delivery Chitosan-silk fibroin cross-linked films pH dependent release (pH2>pH 5.5> pH 7.2) over 10h Released drug: salicylic acid>theophylline>diclofenac sodium>amoxicillin |
Theophyllin Diclofenac Sodium Amoxicillin Salicylic acid |
114 | ||
| Silk/gelatin blend film for wound healing In vitro release up to 48 hours In vivo rat model → faster healing in 7 days PEG-modified film allowed better uptake results |
Ciproflaxin | 132 | ||
| Stabilization of antibiotics Implant systems for focal delivery of antibiotics Sustained release (up to 5 days from the films) |
Penicillin Ampicillin Gentamicin Cafazolin Rifampicin Eryhtromycin Tetracycline |
109 | ||
| Designed to be used as a nerve conduit in peripheral nerve defect NGF release for over 3 weeks PC12 cells proliferation and maturation |
Nerve growth factor | 133 | ||
| Heparin releasing composite material against thrombosis Silk fibroin and polyurethane films Controlled release up to 24 hours |
Heparin | 115 | ||
| Controlled release for 29 hours Diffusion model for in vitro release |
FITC-dextran | 117 | ||
| Controlled delivery up to 30 days Effects of silk degumming time on drug release was investigated |
Azoalbumin Reactive-red 120, Rifampicin Indigo carmine |
118 | ||
| Alginate/silk fibroin blend films Controlled release for over 30h Higher release rate with higher alginate ratio |
Tetracycline | 116 | ||
| Controlled release up to 30 days Intratumoral application → slower tumor growth |
Doxorubicin | 121 | ||
| Sustained release without a burst with silk/gelatin/glycerin films Release up to 350 minutes using films Release up to 24h using spray-dried microparticles |
Naproxen | 134 | ||
| Coating | Sustained release up to 40 days with layer by layer silk coating | Rhodamine B Evans Blue Azoalbumin |
135 | |
| Multi layer silk coating for vascular stent Reduced platelet adhesion Promotion of human aortic endothelial cell proliferation |
Heparin Paclitaxel Clopidogrel |
136 | ||
| Layer by layer heparin-silk nanofilms Controlled release up to 7 days Higher efficacy against cancer cells with higher beta sheet ratio |
Epirubicin Hydrochloride | 137 | ||
| Silk used as the film coating material on a tablet 1-ethyl-3-(3-dimethyl aminopropyl)carbodiimide (EDC) and Polyethylene glycol (PEG) blends Sustained release following zero order kinetics Enhanced release with EDC blend |
Theophyllin | 113 | ||
| Foams | Intratumoral implantation → decreased tumor growth Sustained release up to 48 days release |
Vincristine | 123 | |
| Sustained release Dual combination of vincristine and doxorubicin Intratumoral injections→ significant decrease in tumor growth Reduced systemic exposure |
Vincristine Doxorubicin |
19 | ||
| Microneedles | Controlled release up to 48h Mild drug encapsulation method 10-fold reduction in bacterial density |
Horseradish peroxidase (HRP) Tetracycline |
93 | |
| Swellable silk fibroin microneedles for trandermal delivery Better release kinetics over non-swellable microneedles Release up to 95 hours/ high mechanical strength |
FITC-dextran | 129 | ||
| Microneedles using polydimethylsiloxane (PDMS) mold PEG diacrylate and sucrose as needle matrix Release kinetics controlled by sucrose content Up to 144h release |
Rhodamine Indocyanine green Doxorubicin |
138 | ||
| Vaccine coated on silk microneedles Transdermal application on mice (24hours) Booster immunization 2 weeks after initial dose In vivo immune response >28 days |
Vaccination against: Influenza C. difficile Shigella |
130 | ||
| Reservoirs | Silk rod reservoirs – implant systems for cancer therapy Sustained release up to 91 days following zero order kinetics |
Anastrozole | 22 | |
| Sustained release up to 14 days following zero order kinetics Implant system for local delivery of adenosine for epilepsy |
Adenosine | 139 | ||
| Sustained release up to 30 days Entrapment of drug powder allows high drug loading Intratumoral application → decrease in tumor growth |
Cisplatin | 140 | ||
| Wafer | Sustained release up to 45 days Intratumoral application → decrease in tumor growth Tumor cell necrosis adjacent to wafers |
Etoposide | 20 | |
| Sustained release up to 7 weeks Intratumoral application → decrease in tumor growth Survival up to 60 days |
Vincristine | 123 | ||
|
Spider Silk eADF4(C16) |
Films | Mono- and multi-layer films→ prolonged release (90 days) Release correlated with molecular weight of the drug |
Paracetamol FITC-dextran FITC-BSA |
141 |
One of the earlier applications of silk fibroin was as a coating to extend the release of pharmaceuticals. Aqueous silk fibroin solution was used to coat theophylline tablets to achieve zero order release 113. PEG/silk combinations (17/83 w/w) and 1-ethyl-3-(3-dimethyl aminopropyl)carbodiimide (EDC) cross-linked silk coatings formed film coatings and followed zero order release kinetics 113.
Silk fibroin films are commonly used as delivery systems due to their easy preparation and applications with tunable mechanical properties. In earlier studies, silk fibroin was blended with different polymers such as polyurethane, chitosan or alginate to prepare controlled release composite films. Chitosan/silk fibroin blend films were prepared by crosslinking with glutaraldehyde and several drugs (Table 4) were tested in terms of in vitro release kinetics under different pH conditions 114. The highest drug release was observed at pH 2 due to swelling of the polymers. Different ratios of silk fibroin and polyurethane were tested to optimize film thickness, drug loading and release; heparin release correlated with film thickness and silk: polyurethane ratio 115. Tetracycline hydrochloride was studied with silk/alginate-blended films to evaluate drug release and film transparency. Increased silk fibroin/alginate ratios resulted in decreased drug release and the system was suggested as a good controlled release platform for water-soluble drugs 116. The mechanism of controlled release from silk films was addressed using fluorescein-iso-thio-cyanate (FITC)-labeled dextrans117, as a function of silk molecular weight and film methanol treatment. Diffusion coefficients were smaller for higher molecular weight dextran and methanol-treated films. To understand the effects of silk processing, different silk degumming times (10 to 90 minutes) has been investigated 118. Degumming was found to be a useful control point for silk molecular weight, viscosity and degradation. These earlier studies helped with understanding how to control the interactions between silk and target molecules in order to optimize formulations.
Silk films have been studied for focal delivery of antibiotics using microspheres, hydrogels, microsphere-hydrogel dual systems and silk coatings. Bacterial inhibition by f penicillin and ampicillin loaded films were evaluated; methanol treatment of the films did not degrade antibiotic activity and approximately half of the drug load was delivered within the first 24 hours of exposure 109. Silk fibroin films have been used to entrap interferon gamma (IFN-γ) or interleukin-4 (IL-4) for macrophage polarization 119. The crystalline (β-sheet) content of the films was utilized to optimize solubility of the films and to adjust release rates. Insoluble films with high β-sheet content did not release the entrapped molecules, however they still polarized macrophages that adhered to the film surface. Soluble films with low crystalline structure released the contents in 24 hours, however the duration of release was extended up to 10 days by conjugating IFN- γ to the silk films 119.
Focal tumor therapy is also a major application for implantable silk-based delivery systems, including films, wafers and reservoirs 19, 20, 120–123. Binding and release of the chemotherapeutic drugs vincristine and doxorubicin has been modulated in silk fibroin films 120. In terms of drug binding no difference was found between low and high β-sheet (crystalline) content films. In contrast, binding was pH-dependent and optimum drug binding was observed at pH 6 120. Both drugs bound at higher loadings to carboxylated and sulfonated silk films than to unmodified silk films, however in vitro release from all films were similar and lasted about 28 days. Doxorubicin-loaded films were implanted in an orthotopic neuroblastoma mouse model and decreased tumor growth was superior to the control intravenous administration of the drug 121, 122. To increase drug loading and optimize in vitro drug release, silk hydrogels, foams and wafers were also evaluated for intratumoral delivery of doxorubicin and vincristine 19, 123. The duration of in vitro release from the silk wafers lasted longer than from the silk foams or hydrogels. Post-operative survival rates were less than 20 days following intravenous injections of vincristine or doxorubicin, while the animals treated with vincristine wafers and vincristine/doxorubicin combination foams survived for 2 and 6 months, respectively 19, 123. Silk wafers were also utilized for etoposide delivery and achieved extended release up to 45 days and decreased tumor growth in vivo 20. A reservoir system, silk rods, was designed to achieve therapeutic doses of drugs by entrapping high contents of powdered drug into the center hollow part of silk tubes, followed by sealing, the ends of the tubes via dip coating. The chemotherapeutic drug anastrozole was released from the system for 91 days with zero-order kinetics. The rods were implanted in rats for 6 months and an in vitro – in vivo pharmacokinetic correlation was found 22.
Larger foam systems, discs, were designed for vaginal or rectal delivery of HIV (human immunodeficiency virus) inhibitors 5P12-RANTES and griffithsin 124. These proteins remained functional in the silk discs over 14 months even when stored at 50°C. Sustained release of griffithsin lasted for 4 weeks and the released protein was sufficient to inhibit HIV transmission based on their activity against CAP210 and PVO4 infection of TZM-bl cells. Ex-vivo studies showed that released 5P12-RANTES levels were sufficient for HIV inhibition in both blood and human colorectal tissue 125.
Electrospun silk fibers are usually designed for topical applications, including drug delivery. Silk/gelatin blend fibers were optimized using methylene blue. Bead formation on the fibers was induced to provide a depot and reduce burst release. A silk fibroin/gelatin ratio of 70/30 (w/w) resulted in homogeneous bead formation on the fibers and methylene blue release lasted for 36 hours from the fiber system 126. Electrospun silk fibroin patches were also used to simplify storage and application of human platelet lysate for wound healing 127. Release studies were evaluated by quantifying FITC-albumin release from fibers in the presence of protease XIV, and silk crystalline content was manipulated to control release kinetics. Silk fibroin/FITC-albumin/ human platelet lysate fibers with >40% crystallinity released the dye for over 140 days 127. Silk fibroin electrospun nanofibers were combined with silk nanoparticles for dual delivery of doxorubicin hydrochloride (in the fibers) and curcumin (in the nanoparticles) and the system was able to release the drugs for 40 hours 128. An anal fistula plug for Crohn’s disease treatment was studied with curcumin and 5-aminosalicylic-acid loaded into silk electrospun fibers on the surface of a silk plug 62. The system showed no cytotoxicity with fibroblasts and both drugs released for about 10 days with a higher burst release for 5-aminosalicylic-acid than the curcumin 62.
As minimally invasive transdermal delivery systems, microneedles have been explored and silk showed significant success as a microneedle material with relatively simple fabrication methods like 3D printing or mold casting. Silk microneedles were prepared by casting silk solutions in polydimethylsiloxane (PDMS) molds 93. Tetracycline and horseradish peroxidase (HRP) were loaded in the casting process as a small and large molecule. An in vitro gelatin hydrogel skin model was used to study the release kinetics and 48 hours of release was achieved and the released molecules had preserved bioactivity. The mechanical functions were also tested with mice to confirm skin penetration of the microneedles (Figure 2C) 93. Swellable microneedles were also designed using 2-ethoxyethanol modified silk fibroin to enhance transdermal drug release 129. These microneedles transformed into semi-solid hydrogels upon application to the skin. Transdermal delivery of FITC-dextran showed that higher swelling ratios correlated with higher transdermal release kinetics due to the larger pore sizes 129. Silk microneedles have also been a focus for transdermal vaccine delivery. Vaccine coated silk microneedles were tested against influenza, C. difficile and Shigella on mice 130. Microneedles were applied on mouse skin for 24 hours for initial dosing and a booster dose followed 2 weeks later, and successful vaccination was achieved against all three antigens 130. Another approach was to design silk/poly(acrylic acid) (PAA) microneedles, where the PAA base rapidly dissolved following a brief application to deliver the initial vaccine dose, then methanol treated silk tips serve as vaccine depots in the skin for 2 weeks 63. The immune response to the microneedles was significantly higher than when a single intradermal injection of the vaccine was used. The pharmaceutical industry has started investing in these types of silk-based microneedle systems; Vaxess, Inc., developed a silk microneedle platform called MIMIX™ for vaccine delivery that has successfully completed Phase II clinical studies 131.
4. Conclusion
Silk proteins are useful biomaterials for drug delivery as they are easily accessible, available in large quantities via the textile industry, relatively inexpensive as a biomaterial, biologically inert yet degradable via proteases, mechanically robust and versatile in fabrication. Control over concentration, molecular weight and crystallinity of the silk protein allows tunable mechanical properties and release kinetics with the delivery systems. Moreover simple fabrication methods under mild conditions (e.g., water, room temperature) provide further versatility related to retention of bioactive features of the therapeutics being delivered in the silk devices. Despite these favorable features of silk-based materials, there remain many challenges to address such as batch-to-batch variability, scale-up and achieving therapeutic dosing levels.
In this review, we discussed the use of silk proteins for the controlled delivery of therapeutic molecules with a focus on the advantages and versatility of silk-based delivery systems. In the upcoming Expert Opinion section, we offer an outlook on the potential challenges these systems might encounter and discuss the issues that require focus in terms of research in order to achieve clinical success with the silk-based delivery systems.
5. Expert opinion
Controlled delivery is important for the treatment of chronic diseases, to reduce bolus or burst toxicity, reduce dosing frequency and to minimize undesirable side effects, while providing therapeutic levels of the therapeutic in the target area. The requirements of the system depend on the physicochemical properties of the drug, duration of the treatment needed and the target area in the body. As a result, controlled delivery systems need to be optimized on a case by case basis. Silk is a protein biomaterial that can be tuned to form various carrier platforms depending on the needs of the drug and application route, while also allowing controlled release of the therapeutic and degradation rate of the delivery system. In the past two decades there has been increased research on the fundamentals of the relationship between silk structure and function. Various fabrication methods have been developed to meet different pharmaceutical needs and in the process, fine-tuning strategies or processes have been explored to achieve the desired release kinetics and mechanical properties.
Stability and bioactivity of the entrapped molecules were also investigated to ensure the released molecules retained therapeutic efficacy. Numerous studies showed that silk carriers have a stabilizing effect on both small molecules and proteins, allowing them to preserve bioactivity and structural integrity even at more extreme conditions like higher temperatures and humidity.
Although the silk structure is established, the interactions between silk and each therapeutic molecule should be considered individually in terms of binding, loading and release kinetics. Physicochemical properties like molecular weight and hydrophobicity play an important role in the release mechanisms. Furthermore, solubility of the therapeutic molecule has a significant influence on the process, where in general low water solubility reduces drug loading and high water solubility results in burst release. Achieving therapeutic levels of drug loading is a major challenge in the formulation of hydrophobic drugs, especially for systems that require dissolving the molecule in the silk matrix. Organic solvent incorporation and modification of silk proteins are among the approaches to improve drug loading. Another challenge in silk formulation is batch-to-batch differences in silk properties due to differences in silk source or slight changes in degumming or other processing procedures. Genetically engineered silk proteins eliminate these inconsistencies, in addition to the functional benefits that they possess by designing into the primary sequence. However, limitations of scale, costs, and regulatory issues remain as challenges for such designer proteins.
Silk is known to be biodegradable due to protease enzymes, but the biodegradation of silk in the body depends on many variables such as the degree of crystallinity, formulation type and the application site in the body. As part of the drug development process, in vivo studies are essential to evaluate the application of a specific silk delivery system and the pharmacokinetics of the therapeutic molecule. Considering enzymatic degradation, blood flow or pH conditions of the application site, establishing in vitro-in vivo correlations is critical. In vivo release kinetics, pharmacokinetic profiles, efficacy of the systems as well as toxicity should be evaluated in order to support the clinical relevance of the systems. As the formulations get well-defined, the focus of the research shifts to animal studies and most of the formulation ideas highlighted in this review have already been supported with in vivo studies. Silk can induce a mild inflammatory response in vivo over 1 to 3 weeks, which is beneficial to increase the degradation of delivery systems with longer clearance time. Based on reports of successful in vivo studies regarding silk-based delivery systems, an increase in clinical studies is expected. One of the first successful silk delivery platforms, MIMIX™ microneedles, has completed Phase II clinical studies successfully as a transdermal vaccine delivery system.
In the coming years silk-based delivery systems are anticipated in clinical use with the increased industrial interest and investments. This is especially the case for implant systems such as reservoirs, films and wafers due to their promise for focal treatment of tumors, as well as microneedle systems for controlled transdermal delivery of therapeutics.
Article highlights.
Silk is a suitable candidate for controlled delivery of therapeutics due to controllable degradation and release kinetics, biocompatibility, all aqueous processing to maintain bioactive features of the therapeutics, and compatibility with sterilization.
Silk has the ability to form various delivery systems, which can be applied via injections, implantation or transdermal routes.
There is a focus on silk nanoparticles for the delivery of chemotherapeutics in order to reduce application frequency and systemic toxicity by increasing release duration, plasma circulation time and accumulation in the target area.
Silk hydrogels are being used as injectable sustained release depots as well as implant systems in combination with a solid support material.
Implantable solid silk platforms such as films, wafers, foams and reservoirs have been studied for focal delivery of chemotherapeutic molecules.
Silk microneedles are promising transdermal delivery systems with easy fabrication techniques, controllable release, mechanical strength and successful skin penetration.
Acknowledgments
Funding
This work was supported by grants from NIH (R01NS094218, U01EB014976).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
LIST OF ABBREVIATIONS
- BMP-2
Bone Morphogenetic Protein 2
- BSA
Bovine Serum Albumin
- Cy-7
Cyanine 7
- DMF
N, N-dimethylformamide
- DMSO
Dimethyl Sulfoxide
- EDC
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
- EPR
Enhanced Permeability and Retention
- EtO
Ethylene Oxide
- FDA
Food and Drug Administration
- FITC
Fluorescein Isothiocyanate
- HA
Hyaluronic acid
- HER-2
Human Epidermal Growth Factor Receptor 2
- HIV
Human Immunodeficiency Virus
- HPMA
[N-(2- hydroxypropyl)methacrylamide]
- HRP
Horseradish Peroxidase
- IFN-γ
Interferon Gamma
- IGF-I
Insulin-like Growth Factor
- IgG-1
Immunoglobin G-1
- IL-4
Interleukin 4
- NGF
Nerve Growth Factor
- PAA
Poly(acrylic acid)
- PCL
Polycaprolactone
- PDMS
Polydimethylsiloxane
- PEG
Polyethylene Glycol
- PEI
Polyethylenimine
- PLGA
Poly(lactic-co-glycolic acid)
- PVA
Polyvinyl Alcohol
- Rh-BSA
Rhodamine-labeled Bovine Serum Albumin
- SEM
Scanning Electron Microscope
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Yucel T, Lovett ML, Kaplan DL. Silk-based biomaterials for sustained drug delivery. J Control Release 2014. September 28;190:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair LS, Laurencin CT. Polymers as biomaterials for tissue engineering and controlled drug delivery In: Lee K, Kaplan D, eds. Tissue Engineering I Advances in Biochemical Engineering/Biotechnology. Berlin: Springer; 2005:47–90. [DOI] [PubMed] [Google Scholar]
- 3.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev 2016. February 24;116(4):2602–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammadi-Samani S, Taghipour B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm Dev Technol 2015. June;20(4):385–93. [DOI] [PubMed] [Google Scholar]
- 5.Crotts G, Park TG. Protein delivery from poly(lactic-co-glycolic acid) biodegradable microspheres: release kinetics and stability issues. J Microencapsul 1998. Nov-Dec;15(6):699–713. [DOI] [PubMed] [Google Scholar]
- 6.Muffly TM, Tizzano AP, Walters MD. The history and evolution of sutures in pelvic surgery. J R Soc Med 2011. March;104(3):107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The Biomedical Use of Silk: Past, Present, Future. Adv Healthc Mater 2019. January 10;8(1). [DOI] [PubMed] [Google Scholar]
- 8.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci 2007. Aug-Sep;32(8–9):991–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutta Dishari, Hossain CM, Biswas A. Silk Proteins in Drug Delivery: An Overview. RPHS 2018;4(4):514–18. [Google Scholar]
- 10.Kundu B, Kurland NE, Bano S, Patra C, Engel FB, Yadavalli VK, et al. Silk proteins for biomedical applications: Bioengineering perspectives. Prog Polym Sci 2014. February;39(2):251–67. [Google Scholar]
- 11.Numata K, Kaplan DL. Silk-based delivery systems of bioactive molecules. Adv Drug Deliv Rev 2010. December 30;62(15):1497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang WW, Rollett A, Kaplan DL. Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin Drug Del 2015. May;12(5):779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, Tee SY, et al. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci 2015. July;46:86–110. [Google Scholar]
- 14.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 2008. Aug-Sep;29(24–25):3415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluge JA, Li AB, Kahn BT, Michaud DS, Omenetto FG, Kaplan DL. Silk-based blood stabilization for diagnostics. Proc Natl Acad Sci U S A 2016. May 24;113(21):5892–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li AB, Kluge JA, Zhi M, Cicerone MT, Omenetto FG, Kaplan DL. Enhanced Stabilization in Dried Silk Fibroin Matrices. Biomacromolecules 2017. September 11;18(9):2900–05. [DOI] [PubMed] [Google Scholar]
- 17.He JY, Yavuz B, Kluge JA, Li AB, Omenetto FG, Kaplan DL. Stabilization of RNA Encapsulated in Silk. ACS Biomater Sci Eng 2018. May;4(5):1708–15. [DOI] [PubMed] [Google Scholar]
- 18.Gil ES, Park SH, Hu X, Cebe P, Kaplan DL. Impact of Sterilization on the Enzymatic Degradation and Mechanical Properties of Silk Biomaterials. Macromol Biosci 2014. February;14(2):257–69. [DOI] [PubMed] [Google Scholar]
- 19.Coburn J, Harris J, Zakharov AD, Poirier J, Ikegaki N, Kajdacsy-Balla A, et al. Implantable chemotherapy-loaded silk protein materials for neuroblastoma treatment. Int J Cancer 2017. February 1;140(3):726–35.** This is of considerable importance and focuses on focal delivery of chemotherapeutics into neuroblastoma tumors using both injectable and implantable silk formulations.
- 20.Yavuz B, Zeki J, Coburn JM, Ikegaki N, Levitin D, Kaplan DL, et al. In vitro and in vivo evaluation of etoposide - silk wafers for neuroblastoma treatment. J Control Release 2018. September 10;285:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeki J, Taylor JS, Yavuz B, Coburn J, Ikegaki N, Kaplan DL, et al. Disseminated injection of vincristine-loaded silk gel improves the suppression of neuroblastoma tumor growth. Surgery 2018. July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yucel T, Lovett ML, Giangregorio R, Coonahan E, Kaplan DL. Silk fibroin rods for sustained delivery of breast cancer therapeutics. Biomaterials 2014. October;35(30):8613–20. [DOI] [PubMed] [Google Scholar]
- 23.Srisuwan Y, Baimark Y, Srihanam P. Preparation of regenerated silk sericin/silk fibroin blend microparticles by emulsification-diffusion method for controlled release drug delivery. Part Sci Technol 2017;35(4):387–92. [Google Scholar]
- 24.Seib FP, Jones GT, Rnjak-Kovacina J, Lin YN, Kaplan DL. pH-Dependent Anticancer Drug Release from Silk Nanoparticles. Adv Healthc Mater 2013. December;2(12):1606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crivelli B, Perteghella S, Bari E, Sorrenti M, Tripodo G, Chlapanidas T, et al. Silk nanoparticles: from inert supports to bioactive natural carriers for drug delivery. Soft Matter 2018. January 24;14(4):546–57. [DOI] [PubMed] [Google Scholar]
- 26.Numata K, Subramanian B, Currie HA, Kaplan DL. Bioengineered silk protein-based gene delivery systems. Biomaterials 2009. October;30(29):5775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA. November 2018 510(k) Clearances. 2019. [cited 2019 January 25, 2019]; Available from: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/510kClearances/ucm627939.htm
- 28.Sashina ES, Bochek AM, Novoselov NP, Kirichenko DA. Structure and solubility of natural silk fibroin. Russ J Appl Chem 2006. June;79(6):869–76. [Google Scholar]
- 29.Aramwit P, Kanokpanont S, De-Eknamkul W, Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng 2009. May;107(5):556–61. [DOI] [PubMed] [Google Scholar]
- 30.Santin M, Motta A, Freddi G, Cannas M. In vitro evaluation of the inflammatory potential of the silk fibroin. J Biomed Mater Res 1999. September 5;46(3):382–89. [DOI] [PubMed] [Google Scholar]
- 31.Shen Y, Redmond SL, Papadimitriou JM, Teh BM, Yan S, Wang Y, et al. The biocompatibility of silk fibroin and acellular collagen scaffolds for tissue engineering in the ear. Biomedical Materials 2014. February;9(1). [DOI] [PubMed] [Google Scholar]
- 32.Mandal BB, Priya AS, Kundu SC. Novel silk sericin/gelatin 3-D scaffolds and 2-D films: fabrication and characterization for potential tissue engineering applications. Acta Biomater 2009. October;5(8):3007–20. [DOI] [PubMed] [Google Scholar]
- 33.Kunz RI, Brancalhao RM, Ribeiro LF, Natali MR. Silkworm Sericin: Properties and Biomedical Applications. Biomed Res Int 2016;2016:8175701.** This is of considerable importance and summarizes the properties and biomedical applications of silkworm sericin.
- 34.Zhou CZ, Confalonieri F, Jacquet M, Perasso R, Li ZG, Janin J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins-Structure Function and Genetics 2001. August 1;44(2):119–22. [DOI] [PubMed] [Google Scholar]
- 35.Dubey P, Murab S, Karmakar S, Chowdhury PK, Ghosh S. Modulation of Self-Assembly Process of Fibroin: An Insight for Regulating the Conformation of Silk Biomaterials. Biomacromolecules 2015. December;16(12):3936–44. [DOI] [PubMed] [Google Scholar]
- 36.Wenk E, Merkle HP, Meinel L. Silk fibroin as a vehicle for drug delivery applications. J Control Release 2011. March 10;150(2):128–41. [DOI] [PubMed] [Google Scholar]
- 37.Wenk E, Murphy AR, Kaplan DL, Meinel L, Merkle HP, Uebersax L. The use of sulfonated silk fibroin derivatives to control binding, delivery and potency of FGF-2 in tissue regeneration. Biomaterials 2010. February;31(6):1403–13. [DOI] [PubMed] [Google Scholar]
- 38.Hines DJ, Kaplan DL. Characterization of Small Molecule Controlled Release From Silk Films. Macromol Chem Phys 2013. January 25;214(2):280–94. [Google Scholar]
- 39.Nova A, Keten S, Pugno NM, Redaelli A, Buehler MJ. Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils. Nano Lett 2010. July 14;10(7):2626–34. [DOI] [PubMed] [Google Scholar]
- 40.Murphy AR, Kaplan DL. Biomedical applications of chemically-modified silk fibroin. J Mater Chem 2009;19(36):6443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid polyglycolic acid copolymers. Biomaterials 1996. January;17(2):93–102. [DOI] [PubMed] [Google Scholar]
- 42.Wiegand C, Abel M, Ruth P, Wilhelms T, Schulze D, Norgauer J, et al. Effect of the Sterilization Method on the Performance of Collagen Type I on Chronic Wound Parameters In Vitro. Journal of Biomedical Materials Research Part B-Applied Biomaterials 2009. August;90b(2):710–19. [DOI] [PubMed] [Google Scholar]
- 43.Rnjak-Kovacina J, DesRochers TM, Burke KA, Kaplan DL. The Effect of Sterilization on Silk Fibroin Biomaterial Properties. Macromol Biosci 2015. June;15(6):861–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sleep D Albumin and its application in drug delivery. Expert Opin Drug Deliv 2015. May;12(5):793–812. [DOI] [PubMed] [Google Scholar]
- 45.Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm 2002. July;28(6):621–30. [DOI] [PubMed] [Google Scholar]
- 46.Elgadir MA, Uddin MS, Ferdosh S, Adam A, Chowdhury AJK, Sarker MZI. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J Food Drug Anal 2015. December;23(4):619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laza-Knoerr AL, Gref R, Couvreur P. Cyclodextrins for drug delivery. J Drug Target 2010. November;18(9):645–56. [DOI] [PubMed] [Google Scholar]
- 48.Coelho JF, Ferreira PC, Alves P, Cordeiro R, Fonseca AC, Gois JR, et al. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J 2010. March;1(1):164–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foox M, Zilberman M. Drug delivery from gelatin-based systems. Expert Opin Drug Deliv 2015;12(9):1547–63. [DOI] [PubMed] [Google Scholar]
- 50.Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv 2018. November;25(1):766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larson N, Ghandehari H. Polymeric conjugates for drug delivery. Chem Mater 2012. March 13;24(5):840–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dash TK, Konkimalla VB. Poly-small je, Ukrainian-caprolactone based formulations for drug delivery and tissue engineering: A review. J Control Release 2012. February 28;158(1):15–33. [DOI] [PubMed] [Google Scholar]
- 53.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of Therapeutic Proteins. J Pharm Sci 2010. June;99(6):2557–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuberg P, Kichler A. Recent developments in nucleic acid delivery with polyethylenimines. Adv Genet 2014;88:263–88. [DOI] [PubMed] [Google Scholar]
- 55.Rockwood DN, Preda RC, Yucel T, Wang XQ, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc 2011. October;6(10):1612–31.** This is of considerable importance and summarizes fabrication methods for the most commonly used silk materials.
- 56.Pritchard EM, Normand V, Hu X, Budijono S, Benczédi D, Omenetto F, et al. Encapsulation of oil in silk fibroin biomaterials. J Appl Polym Sci 2014;131(6). [Google Scholar]
- 57.Gobin AS, Rhea R, Newman RA, Mathur AB. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int J Nanomedicine 2006;1(1):81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer-Chemotherapy - Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res 1986. December;46(12):6387–92. [PubMed] [Google Scholar]
- 59.Kutscher HL, Chao P, Deshmukh M, Singh Y, Hu P, Joseph LB, et al. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release 2010. April 2;143(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang XQ, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010. February;31(6):1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seib FP, Pritchard EM, Kaplan DL. Self-Assembling Doxorubicin Silk Hydrogels for the Focal Treatment of Primary Breast Cancer. Adv Funct Mater 2013. January 7;23(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie XS, Liu L, Zheng ZZ, Han ZF, Zhi M, Kaplan DL, et al. Silk Fibroin-Based Fibrous Anal Fistula Plug with Drug Delivery Function. Macromol Biosci 2018. April;18(4). [DOI] [PubMed] [Google Scholar]
- 63.DeMuth PC, Min Y, Irvine DJ, Hammond PT. Implantable Silk Composite Microneedles for Programmable Vaccine Release Kinetics and Enhanced Immunogenicity in Transcutaneous Immunization. Adv Healthc Mater 2014. January;3(1):47–58.* This is an important article as a key study into silk-based microneedle technology.
- 64.Zhao Z, Chen AZ, Li Y, Hu JY, Liu X, Li JS, et al. Fabrication of silk fibroin nanoparticles for controlled drug delivery. J Nanopart Res 2012. April;14(4). [Google Scholar]
- 65.Subia B, Kundu SC. Drug loading and release on tumor cells using silk fibroin-albumin nanoparticles as carriers. Nanotechnology 2013. January 25;24(3). [DOI] [PubMed] [Google Scholar]
- 66.Qu J, Liu Y, Yu YN, Li J, Luo JW, Li MZ. Silk fibroin nanoparticles prepared by electrospray as controlled release carriers of cisplatin. Mater Sci Eng C Mater Biol Appl 2014. November 1;44:166–74. [DOI] [PubMed] [Google Scholar]
- 67.Wongpinyochit T, Uhlmann P, Urquhart AJ, Seib FP. PEGylated Silk Nanoparticles for Anticancer Drug Delivery. Biomacromolecules 2015. November;16(11):3712–22. [DOI] [PubMed] [Google Scholar]
- 68.Montalban MG, Coburn JM, Lozano-Perez AA, Cenis JL, Villora G, Kaplan DL. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials (Basel) 2018. February 24;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu JB, Wang J, Zhang J, Zheng ZZ, Kaplan DL, Li G, et al. Oral Delivery of Curcumin Using Silk Nano- and Microparticles. ACS Biomater Sci Eng 2018. November;4(11):3885–94. [DOI] [PubMed] [Google Scholar]
- 70.Wu M, Yang WH, Chen S, Yao JR, Shao ZZ, Chen X. Size-controllable dual drug-loaded silk fibroin nanospheres through a facile formation process. J Mater Chem B 2018. February 28;6(8):1179–86. [DOI] [PubMed] [Google Scholar]
- 71.Sun N, Lei R, Xu J, Kundu SC, Cai Y, Yao J, et al. Fabricated porous silk fibroin particles for pHresponsive drug delivery and targeting of tumor cells. J Mater Sci 2019;54:3319–30. [Google Scholar]
- 72.Florczak A, Mackiewicz A, Dams-Kozlowska H. Functionalized Spider Silk Spheres As Drug Carriers for Targeted Cancer Therapy. Biomacromolecules 2014. August;15(8):2971–81. [DOI] [PubMed] [Google Scholar]
- 73.Mwangi TK, Bowles RD, Tainter DM, Bell RD, Kaplan DL, Setton LA. Synthesis and characterization of silk fibroin microparticles for intra-articular drug delivery. Int J Pharm 2015. May 15;485(1–2):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mandal BB, Kundu SC. Self-assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology 2009. September 2;20(35). [DOI] [PubMed] [Google Scholar]
- 75.Gupta V, Aseh A, Rios CN, Aggarwal BB, Mathur AB. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int J Nanomedicine 2009;4(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang XQ, Wenk E, Matsumoto A, Meinel L, Li CM, Kaplan DL. Silk microspheres for encapsulation and controlled release. J Control Release 2007. February 26;117(3):360–70. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, Wenk E, Hu X, Castro GR, Meinel L, Wang X, et al. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials 2007. October;28(28):4161–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wongpinyochit T, Johnston BF, Seib FP. Manufacture and Drug Delivery Applications of Silk Nanoparticles. J Vis Exp 2016. October(116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cao Y, Liu FQ, Chen YL, Yu T, Lou DS, Guo Y, et al. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci Rep 2017. September 20;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perteghella S, Crivelli B, Catenacci L, Sorrenti M, Bruni G, Necchi V, et al. Stem cell-extracellular vesicles as drug delivery systems: New frontiers for silk/curcumin nanoparticles. Int J Pharm 2017. March 30;520(1–2):86–97. [DOI] [PubMed] [Google Scholar]
- 81.Crivelli B, Bari E, Perteghella S, Catenacci L, Sorrenti M, Mocchi M, et al. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur J Pharm Biopharm 2019. April;137:37–45. [DOI] [PubMed] [Google Scholar]
- 82.Yang P, Dong Y, Huang D, Zhu C, Liu H, Pan X, et al. Silk fibroin nanoparticles for enhanced bio-macromolecule delivery to the retina. Pharm Dev Technol 2018. November 20:1–9. [DOI] [PubMed] [Google Scholar]
- 83.Takeuchi I, Shimamura Y, Kakami Y, Kameda T, Hattori K, Miura S, et al. Transdermal delivery of 40-nm silk fibroin nanoparticles. Colloids Surf B Biointerfaces 2019. March 1;175:564–68. [DOI] [PubMed] [Google Scholar]
- 84.Lammel AS, Hu X, Park SH, Kaplan DL, Scheibel TR. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010. June;31(16):4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wenk E, Wandrey AJ, Merkle HP, Meinel L. Silk fibroin spheres as a platform for controlled drug delivery. J Control Release 2008. November 24;132(1):26–34. [DOI] [PubMed] [Google Scholar]
- 86.Kundu J, Chung YI, Kim YH, Taeb G, Kundu SC. Silk fibroin nanoparticles for cellular uptake and control release. Int J Pharm 2010. March 30;388(1–2):242–50. [DOI] [PubMed] [Google Scholar]
- 87.Hu DD, Li T, Xu ZP, Liu D, Yang MY, Zhu LJ. Self-stabilized silk sericin-based nanoparticles: In vivo biocompatibility and reduced doxorubicin-induced toxicity. Acta Biomater 2018. July 1;74:385–96. [DOI] [PubMed] [Google Scholar]
- 88.Subia B, Chandra S, Talukdar S, Kundu SC. Folate conjugated silk fibroin nanocarriers for targeted drug delivery. Integr Biol (Camb) 2014. February;6(2):203–14. [DOI] [PubMed] [Google Scholar]
- 89.Lu SZ, Wang J, Mao L, Li GJ, Jin J. Antheraea Pernyi Silk Fibroin Nanoparticles for Drug Delivery. Journal of Nano Research 2014;27:75–81. [Google Scholar]
- 90.Wang J, Zhang S, Xing T, Kundu B, Li M, Kundu SC, et al. Ion-induced fabrication of silk fibroin nanoparticles from Chinese oak tasar Antheraea pernyi. Int J Biol Macromol 2015. August;79:316–25. [DOI] [PubMed] [Google Scholar]
- 91.Shuai Y, Yang S, Li C, Zhu L, Mao C, Yang M. In situ protein-templated porous protein-hydroxylapatite nanocomposite microspheres for pH-dependent sustained anticancer drug release. J Mater Chem B 2017. June 7;5(21):3945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diab T, Pritchard EM, Uhrig BA, Boerckel JD, Kaplan DL, Guldberg RE. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. J Mech Behav Biomed Mater 2012. July;11:123–31.* This is an interesting article that summarizes the use of silk hydrogels to treat bone defects with the support of electrospun silk nanofibers mesh tubes.
- 93.Tsioris K, Raja WK, Pritchard EM, Panilaitis B, Kaplan DL, and Omenetto FG.. Fabrication of silk microneedles for controlled-release drug delivery. Adv Funct Mater 2012;22:330–35. [Google Scholar]
- 94.Zhang WJ, Wang XL, Wang SY, Zhao J, Xu LY, Zhu C, et al. The use of injectable sonication-induced silk hydrogel for VEGF(165) and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials 2011. December;32(35):9415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nogueira GM, de Moraes MA, Rodas ACD, Higa OZ, Beppu MM. Hydrogels from silk fibroin metastable solution: Formation and characterization from a biomaterial perspective. Mater Sci Eng C Mater Biol Appl 2011. July 20;31(5):997–1001. [Google Scholar]
- 96.Yucel T, Cebe P, Kaplan DL. Vortex-Induced Injectable Silk Fibroin Hydrogels. Biophys J 2009. October 7;97(7):2044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang X, Ding ZZ, Wang C, Chen XD, Xu H, Lu Q, et al. Bioactive silk hydrogels with tunable mechanical properties. J Mater Chem B 2018. May 14;6(18):2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribeiro VP, Silva-Correia J, Goncalves C, Pina S, Radhouani H, Montonen T, et al. Rapidly responsive silk fibroin hydrogels as an artificial matrix for the programmed tumor cells death. PLoS One 2018. April 4;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu HC, Liu SS, Xiao LY, Dong XD, Lu Q, Kaplan DL. Injectable and pH-Responsive Silk Nanofiber Hydrogels for Sustained Anticancer Drug Delivery. ACS Appl Mater Interfaces 2016. July 13;8(27):17118–26. [DOI] [PubMed] [Google Scholar]
- 100.Lovett ML, Wang XQ, Yucel T, York L, Keirstead M, Haggerty L, et al. Silk hydrogels for sustained ocular delivery of anti-vascular endothelial growth factor (anti-VEGF) therapeutics. Eur J Pharm Biopharm 2015. September;95:271–78. [DOI] [PubMed] [Google Scholar]
- 101.Hosack LW, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials 2008. May;29(15):2336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elia R, Newhide DR, Pedevillano PD, Reiss GR, Firpo MA, Hsu EW, et al. Silk-hyaluronan-based composite hydrogels: A novel, securable vehicle for drug delivery. J Biomater Appl 2013. February;27(6):749–62. [DOI] [PubMed] [Google Scholar]
- 103.Numata K, Yamazaki S, Naga N. Biocompatible and Biodegradable Dual-Drug Release System Based on Silk Hydrogel Containing Silk Nanoparticles. Biomacromolecules 2012. May;13(5):1383–89.* This is an important article that combines silk nanoparticles with a silk hydrogel system to achieve dual drug release.
- 104.Guziewicz N, Best A, Perez-Ramirez B, Kaplan DL. Lyophilized silk fibroin hydrogels for the sustained local delivery of therapeutic monoclonal antibodies. Biomaterials 2011. April;32(10):2642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guziewicz NA, Massetti AJ, Perez-Ramirez BJ, Kaplan DL. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials 2013. October;34(31):7766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harris JC, Coburn JM, Kajdacsy-Balla A, Kaplan DL, Chiu B. Sustained delivery of vincristine inside an orthotopic mouse sarcoma model decreases tumor growth. J Pediatr Surg 2016. December;51(12):2058–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mandal BB, Kapoor S, Kundu SC. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009. May;30(14):2826–36. [DOI] [PubMed] [Google Scholar]
- 108.Elia R, Newhide DR, Pedevillano PD, Reiss GR, Firpo MA, Hsu EW, et al. Silk-hyaluronan-based composite hydrogels: a novel, securable vehicle for drug delivery. J Biomater Appl 2013. February;27(6):749–62. [DOI] [PubMed] [Google Scholar]
- 109.Pritchard EM, Valentin T, Panilaitis B, Omenetto F, Kaplan DL. Antibiotic-Releasing Silk Biomaterials for Infection Prevention and Treatment. Adv Funct Mater 2013. February 18;23(7):854–61.*This is an important article that evaluates different silk carrier systems for the delivery of a wide spectrum of antibiotics.
- 110.Mao KL, Fan ZL, Yuan JD, Chen PAPA, Yang JJ, Xu J, et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids and Surfaces B-Biointerfaces 2017. December 1;160:704–14. [DOI] [PubMed] [Google Scholar]
- 111.Ebrahimi A, Sadrjavadi K, Hajialyani M, Shokoohinia Y, Fattahi A. Preparation and characterization of silk fibroin hydrogel as injectable implants for sustained release of Risperidone. Drug Dev Ind Pharm 2018;44(2):199–205. [DOI] [PubMed] [Google Scholar]
- 112.Atterberry PN, Roark TJ, Severt SY, Schiller ML, Antos JM, Murphy AR. Sustained Delivery of Chemokine CXCL12 from Chemically Modified Silk Hydrogels. Biomacromolecules 2015. May;16(5):1582–89. [DOI] [PubMed] [Google Scholar]
- 113.Bayraktar O, Malay O, Ozgarip Y, Batigun A. Silk fibroin as a novel coating material for controlled release of theophylline. Eur J Pharm Biopharm 2005. August;60(3):373–81. [DOI] [PubMed] [Google Scholar]
- 114.Rujiravanit R, Kruaykitanon S, Jamieson AM, Tokura S. Preparation of crosslinked chitosan/silk fibroin blend films for drug delivery system. Macromol Biosci 2003. October 15;3(10):604–11. [Google Scholar]
- 115.Liu XY, Zhang CC, Xu WL, Ouyang CX. Controlled release of heparin from blended polyurethane and silk fibroin film. Mater Lett 2009. January 31;63(2):263–65. [Google Scholar]
- 116.Srisuwan Y, Baimark Y. Preparation of Biodegradable Silk Fibroin/Alginate Blend Films for Controlled Release of Antimicrobial Drugs. Adv Mater Sci Eng 2013. [Google Scholar]
- 117.Hines DJ, Kaplan DL. Mechanisms of Controlled Release from Silk Fibroin Films. Biomacromolecules 2011. March;12(3):804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pritchard EM, Hu X, Finley V, Kuo CK, Kaplan DL. Effect of Silk Protein Processing on Drug Delivery from Silk Films. Macromol Biosci 2013. March;13(3):311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reeves ARD, Spiller KL, Freytes DO, Vunjak-Novakovic G, Kaplan DL. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 2015. December;73:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coburn JM, Na E, Kaplan DL. Modulation of vincristine and doxorubicin binding and release from silk films. J Control Release 2015. December 28;220:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiu B, Coburn J, Pilichowska M, Holcroft C, Seib FP, Charest A, et al. Surgery combined with controlled-release doxorubicin silk films as a treatment strategy in an orthotopic neuroblastoma mouse model. Br J Cancer 2014. August 12;111(4):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seib FP, Coburn J, Konrad I, Klebanov N, Jones GT, Blackwood B, et al. Focal therapy of neuroblastoma using silk films to deliver kinase and chemotherapeutic agents in vivo. Acta Biomater 2015. July 1;20:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coburn JM, Harris J, Cunningham R, Zeki J, Kaplan DL, Chiu B. Manipulation of variables in local controlled release vincristine treatment in neuroblastoma. J Pediatr Surg 2017. December;52(12):2061–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang L, Herrera C, Coburn J, Olejniczak N, Ziprin P, Kaplan DL, et al. Stabilization and Sustained Release of HIV Inhibitors by Encapsulation in Silk Fibroin Disks. ACS Biomater Sci Eng 2017. August;3(8):1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yavuz B, Morgan JL, Herrera C, Harrington K, Perez-Ramirez B, LiWang PJ, et al. Sustained release silk fibroin discs: Antibody and protein delivery for HIV prevention. J Control Release 2019. March 12;301:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Somvipart S, Kanokpanont S, Rangkupan R, Ratanavaraporn J, Damrongsakkul S. Development of electrospun beaded fibers from Thai silk fibroin and gelatin for controlled release application. Int J Biol Macromol 2013. April;55:176–84. [DOI] [PubMed] [Google Scholar]
- 127.Pignatelli C, Perotto G, Nardini M, Cancedda R, Mastrogiacomo M, Athanassiou A. Electrospun silk fibroin fibers for storage and controlled release of human platelet lysate. Acta Biomater 2018. June;73:365–76. [DOI] [PubMed] [Google Scholar]
- 128.Li HJ, Zhu JX, Chen S, Jia L, Ma YL. Fabrication of aqueous-based dual drug loaded silk fibroin electrospun nanofibers embedded with curcumin-loaded RSF nanospheres for drugs controlled release. Rsc Advances 2017;7(89):56550–58. [Google Scholar]
- 129.Yin ZP, Kuang DJ, Wang SY, Zheng ZZ, Yadavalli VK, Lu SZ. Swellable silk fibroin microneedles for transdermal drug delivery. Int J Biol Macromol 2018. January;106:48–56. [DOI] [PubMed] [Google Scholar]
- 130.Stinson J, Raja WK, Lee S, Kim HB, Diwan I, Tutunjian S, Panilaitis B, Omenetto FG, Tzipori S, Kaplan DL.. Silk fibroin micronedles for transdermal vaccine delivery. ACS Biomater Sci Eng 2017;3:360–69. [DOI] [PubMed] [Google Scholar]
- 131.Vaxess. National Science Foundation Awards Follow-On Funding For Development Of Highly Effective Vaccines Via Sustained Release Microneedles. 2018. [cited 2019; Available from: http://vaxess.com/news/national-science-foundation-awards/
- 132.Alam AKMM, Shubhra QTH. Surface modified thin film from silk and gelatin for sustained drug release to heal wound. J Mater Chem B 2015;3(31):6473–79. [DOI] [PubMed] [Google Scholar]
- 133.Uebersax L, Mattotti M, Papaloizos M, Merkle HP, Gander B, Meinel L. Silk fibroin matrices for the controlled release of nerve growth factor (NGF). Biomaterials 2007. October;28(30):4449–60. [DOI] [PubMed] [Google Scholar]
- 134.Dyakonov T, Yang CH, Bush D, Gosangari S, Majuru S, Fatmi A. Design and characterization of a silk-fibroin-based drug delivery platform using naproxen as a model drug. J Drug Deliv 2012;2012:490514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang XY, Hu X, Daley A, Rabotyagova O, Cebe P, Kaplan DL. Nanolayer biomaterial coatings of silk fibroin for controlled release. J Control Release 2007. August 28;121(3):190–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X, Zhang X, Castellot J, Herman I, Iafrati M, Kaplan DL. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials 2008. March;29(7):894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Choi M, Choi D, Hong J. Multilayered Controlled Drug Release Silk Fibroin Nanofilm by Manipulating Secondary Structure. Biomacromolecules 2018. July;19(7):3096–103. [DOI] [PubMed] [Google Scholar]
- 138.Gao Y, Hou M, Yang R, Zhang L, Xu Z, Kang Y, et al. Highly Porous Silk Fibroin Scaffold Packed in PEGDA/Sucrose Microneedles for Controllable Transdermal Drug Delivery. Biomacromolecules 2019. February 11. [DOI] [PubMed] [Google Scholar]
- 139.Pritchard EM, Szybala C, Boison D, Kaplan DL. Silk fibroin encapsulated powder reservoirs for sustained release of adenosine. J Control Release 2010. June 1;144(2):159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yavuz B, Zeki J, Taylor J, Harrington K, Coburn JM, Ikegaki N, et al. Silk Reservoirs For Local Delivery Of Cisplatin For Neuroblastoma Treatment: In Vitro And In Vivo Evaluations. J Pharm Sci 2019. March 21.* This is an interesting article that describes a different approach to encapsulate drug powders in silk reservoir systems for the focal delivery of cisplatin into the tumor section.
- 141.Agostini E, Winter G, Engert J. Water-based preparation of spider silk films as drug delivery matrices. J Control Release 2015. September 10;213:134–41. [DOI] [PubMed] [Google Scholar]