Abstract
Lanthipeptides, which belong to the superfamily of ribosomally synthesized and posttranslationally-modified peptides (RiPPs), are associated with interesting biological activities. Lanthipeptides can be subdivided into four classes that are defined by the characteristics of the corresponding posttranslational-modification enzymes. Class IV lanthipeptide synthetases consist of an N-terminal lyase, a central kinase, and a C-terminal cyclase domain. Here, we present the first in-depth characterization of such a kinase domain from the globisporin-maturation enzyme SgbL that originates from Streptomyces globisporus sp. NRRL B-2293. Catalytic residues were identified by alignments with homologs and structure modelling. Their roles were confirmed by employing proteins with Ala substitutions in in vitro modification and fluorescence polarization binding assays. Furthermore, the protein region that is binding the leader peptide was identified by hydrogen-deuterium exchange (HDX) – mass spectrometry experiments. By fusion of this protein region to the maltose binding protein, a protein was generated that can specifically bind the SgbA leader peptide, albeit with reduced binding affinity compared to full length SgbL. Combined, the results of this study provide a firmer grasp of how lanthipeptide biosynthesis is accomplished by class IV synthetases and suggest by homology analysis that biosynthetic mechanisms are similar in class III lanthipeptide processing enzymes.
Graphical Abstract

INTRODUCTION
Lanthipeptides are members of the natural product superfamily of ribosomally synthesized and posttranslationally-modified peptides (RiPPs).1–3 These natural products can exhibit interesting biological properties including, but not limited to, antimicrobial (these are then also called lantibiotics), antifungal, or antiviral activities.1, 2 Recently, it was shown that they can also be used in phage- and yeast-display techniques to select for high affinity binders of human cell surface receptors with medical relevance.4–6 In addition, lanthipeptide libraries have been successfully used to screen for inhibitors of protein-protein interactions.7
Lanthipeptide biosynthesis starts with the ribosomal synthesis of a genetically-encoded precursor peptide (LanA) that is matured by an enzymatic processing machinery.1–3 The precursor peptide can be subdivided in an N-terminal leader region, needed for enzymatic recognition, and a C-terminal core peptide, where the modifications are introduced. The mature lanthipeptide is eventually released by proteolytic removal of the leader peptide. The defining feature of lanthipeptides is the presence of β-thioether crosslinks between two amino acids, called lanthionines and methyllanthionines, that originate from Cys and Ser/Thr residues.1, 2 The biosynthesis involves the dehydration of Ser and Thr residues to dehydroalanines (Dha) and dehydrobutyrines (Dhb), respectively (Figure 1). Next, Cys thiol groups act as nucleophiles and attack the double bonds in a 1,4-conjugate addition, which yields enolate intermediates that are converted to the final (methyl)lanthionines through protonation.
Figure 1.
a) Schematic depiction of class III and IV lanthipeptide biosynthetic enzymes. b) Class III and IV lanthipeptide biosynthesis starts with the ATP-dependent phosphorylation of Ser/Thr hydroxy groups and subsequent elimination of phosphate and continues with c) ring formation by nucleophilic attack of Cys thiol groups. Class III enzymes can also catalyze the formation of labionin moieties. d) Sequence of the SgbA precursor peptide and e) sequence of the class IV lanthipeptide globisporin. Residues undergoing dehydration are highlighted in blue, cysteines involved in thioether formation are in orange, and the previously16 reported minimal leader peptide recognition site is in red. Abu = aminobutyric acid. Dha = dehydroalanine. Dhb = dehydrobutyrine.
The four known classes of lanthipeptides are differentiated by their corresponding lanthipeptide synthetases.2 A single enzyme catalyzes both the dehydration and cyclization reactions in class II (LanM),2, 8–10 class III (LanKC),2, 11–13 and class IV (LanL).2, 14–16 For these three enzyme classes, the formation of Dha and Dhb residues is accomplished by ATP-dependent phosphorylation of Ser/Thr side chains and subsequent phosphate elimination.2 In contrast, two separate enzymes are used in class I systems; a dehydratase (LanB) and a cyclase (LanC).2, 17–21 In this case, the LanB transfers a glutamate from glutamyl-tRNA to the Ser/Thr side chain to activate the hydroxyl group for subsequent glutamate elimination.19, 20
Whereas class I and class II lanthipeptide biosynthetic enzymes have been thoroughly studied and structurally characterized,10, 17, 19, 20 enzymes of classes III and IV are not understood as well. In general, class III and IV proteins share a three-domain architecture, featuring an N-terminal lyase, a central kinase, and a C-terminal cyclase domain (Figure 1a).2, 11, 13–16, 22, 23 Although the kinase and lyase domains are similar in both classes, the differences of their cyclase domains differentiate them. The cyclase domains of class IV enzymes contain conserved zinc binding residues that are also present in enzymes of classes I and II. However, these amino acids are not conserved in class III enzymes. Another interesting difference is that class III enzymes can also form labionin moieties,11–13, 23–27 whereas these have not been observed thus far in class IV lanthipeptides. These labionins are made when the enolate intermediate generated during (methyl)lanthionine formation performs another nucleophilic attack on a second Dha residue instead of being protonated, yielding a spiro bicyclic structure (Figure 1c).
In a recent study,16 we investigated the gene cluster involved in the biosynthesis of the lanthipeptide globisporin and examined interactions of the precursor peptide SgbA with the class IV processing enzyme SgbL. By using a combination of mutational analysis in a heterologous production system and in vitro binding assays, we showed that the N-terminal to central part of the leader peptide is recognized by the kinase domain of the SgbL protein.16 Whereas the catalytic residues in class IV lyase domains were identified previously,15 the amino acids that are important for catalysis in the kinase domains have not been experimentally investigated.
In this study, we identified residues in the SgbL kinase domain that are important for enzyme activity by alignments with structurally characterized Ser/Thr kinases and structure modelling. The importance of these residues for catalysis was confirmed by in vitro modification and leader peptide binding assays using corresponding alanine substitution variants. In addition, hydrogen-deuterium exchange (HDX) – mass spectrometry (MS) experiments were carried out to determine the site of leader peptide binding in the kinase domain. The identified region was expressed with an N-terminal maltose binding protein (MBP) tag and the fusion protein was able to selectively bind SgbA.
RESULTS AND DISCUSSION
Prediction of the Catalytic Residues in the SgbL Kinase Domain.
To identify the catalytic residues in the kinase domain of SgbL, which was previously established to span residues 208–548,16 several bioinformatic tools were utilized. Because structures of class IV lanthipeptide synthetases are not available, a structure prediction of the SgbL kinase domain was performed with Phyre228 (Figure 2a–2b). The homologous structures that were chosen by Phyre2 as basis for the modelling were human Ser/Thr kinases as well as protein kinase B (PknB) and G (PknG) from Mycobacterium tuberculosis. PknG is a virulence factor that facilitates survival during latency and is needed for survival of M. tuberculosis in host macrophages, the latter of which is accomplished by inhibition of phagolysosomal fusion.29–33 PknB is important for cell growth by playing a regulatory role during cell wall biosynthesis and triggers morphological changes in the course of cell division.32, 34, 35
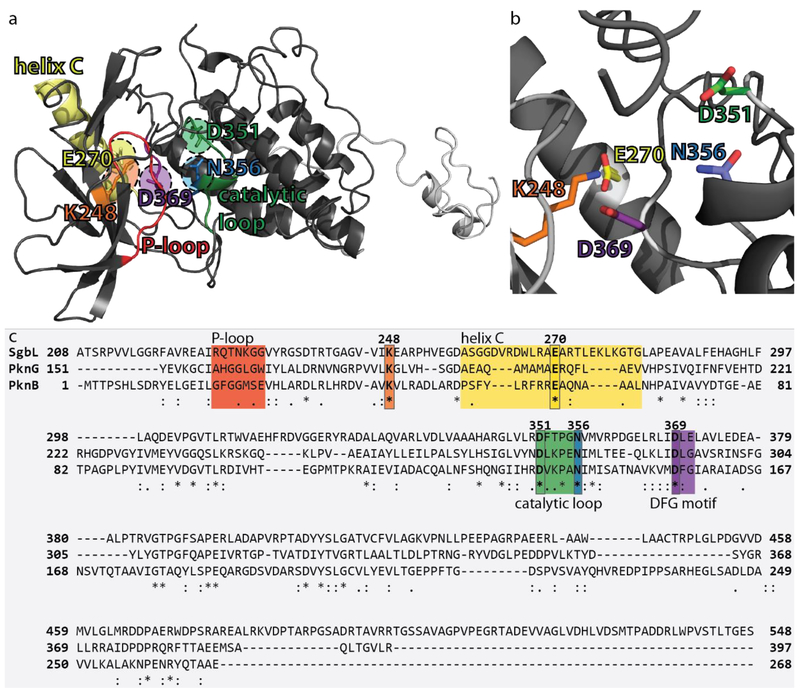
Figure 2.
a) Phyre2 model of SgbL(kinase 208–548). Regions and residues important for activity are color-coded in the same way in all three figure panels. The unstructured region following the kinase domain is depicted in light gray and is needed for solubility when expressing the kinase domain. This region does not have high sequence conservation among LanL enzymes and is predicted to serve as a linker region between the kinase and cyclase domain. b) Close-up of the active site of the SgbL(kinase 208–548) model. Residues proposed to be important for catalysis and/or ATP/Mg2+ binding are colored and labeled. c) Alignment of the kinase domains of SgbL, PknG, and PknB. Regions of interest and catalytic residues are highlighted by color. Numbering shown above the alignment refers to the number of the respective residue in SgbL(kinase 208–548). SgbL(kinase 208–548) has 21.9%/33.4% identity/similarity with PknB(1–268) and 22.2%/31.4% with PknG(151–397).
Secondary structure prediction36 of the SgbL kinase domain was also used to schematically visualize the α-helical and β-sheet regions of this protein domain (Supporting Information Figure S1). An alignment of the kinase domains of SgbL (residues 208–548), PknB (residues 1–268), and PknG (residues 151–397) was performed to identify conserved residues (Figure 2c). Kinases like PknB and PknG have several conserved structural elements that are important for their activity.29, 34, 37 These are helix C, the phosphate-binding loop (P-loop), the catalytic loop, the DFG-motif, and the activation loop. Helix C, the catalytic loop, and the DFG motif contain residues important for ATP/Mg2+ binding and catalysis. Additional contacts are formed between the P-loop backbone and phosphate groups of ATP. The activation loop is a regulatory element that controls the catalytic activity of a kinase by switching between different states, which are typically dependent on the phosphorylation states of its residues. The low homology between this region in SgbL with the corresponding regions in PknB and PknG suggests the absence of such a phosphorylation-based regulation of SgbL, which is in line with its role as a RiPP biosynthetic enzyme instead of a kinase with regulatory functions.
Based on sequence and structural comparisons, five residues in SgbL(kinase 208–548) were identified that could be important for catalysis and coordination of ATP and Mg2+ ions. We will first describe the predicted roles of these residues based on the functions of the homologous residues in PknB and PknG. Lys248 (Lys40 in PknB, Lys181 in PknG) is believed to form contacts with the α- and β-phosphates of ATP and a conserved glutamate in helix C.29, 34, 37 Although helix C is not conserved on a sequence level, the region of SgbL(kinase 208–548) that aligns with the C-helices of PknB and PknG is also predicted to adopt an α-helical conformation (residues 260–277) and contains Glu270 that aligns with the conserved Glu residues in PknB/PknG (Glu59 in PknB, Glu198 in PknG). The catalytic loops of PknB (138-DVKPAN-143) and PknG (271-DLKPEN-281) align well with residues in SgbL (351-DFTPGN-356) and contain highly conserved Asp (Asp351 in SgbL, Asp138 in PknB, Asp271 in PknG) and Asn (Asn356 in SgbL, Asn143 in PknB, Asn281 in PknG) residues. The Asp in the catalytic loop is an invariable catalytic residue that functions as acceptor in proton transfer, whereas the conserved Asn is involved in coordination of Mg2+ ions and additionally forms a canonical contact to the catalytic Asp.29, 34, 37 Another conserved Asp is also involved in the coordination of Mg2+ ions29, 34, 37 and is found in the DFG motif and is present in all three kinase domains, although the motif is not completely conserved (Asp369 in a DLE sequence in SgbL, Asp156 in a DFG sequence in PknB, Asp293 in a DLG sequence in PknG). In PknB and PknG additional interactions of the ATP phosphate groups are observed with main chain amides of Gly residues in the P-loop (18-GFGGMSE-24 in PknB, 158-AHGGLGW-164 in PknG).29, 34, 37 The corresponding sequence stretch in SgbL shows only poor homology (226-RQTNKGG-232) and is missing the central Gly residues (Figure 2c). While these residues indeed form a loop that connects two β-sheets and are oriented towards the active site in the structure model, the two glycines in this region (Gly231 and Gly232) appear to be positioned too far away for backbone interactions. Therefore, we deemed the P-loop prediction to be of insufficient quality to pursue for mutagenesis without an actual structure and decided to investigate only the five conserved predicted active site residues Lys248, Glu270, Asp351, Asn356, and Asp369.
Mutational Analysis of the Predicted Catalytic Residues of the Kinase Domain of SgbL.
Instead of mutating the predicted catalytic residues in a gene encoding just the kinase domain, we instead opted for mutational analysis of SgbL(lyase-kinase 1–548), which accomplishes full dehydration of the SgbA precursor peptide in vitro, while only incomplete modification is observed when using the kinase domain on its own.16 The five Ala variants of His6-SgbL(lyase-kinase 1–548) were expressed in E. coli, isolated, and purified. The proteins were then used for both in vitro modification assays of His6-SgbA (Figure 3a) as well as for fluorescence polarization (FP) based binding assays (Figure 3b) employing SgbA(leader) whose N-terminus was tagged with fluorescein isothiocyanate (FITC). The latter experiments were performed to make sure that a lack of activity would be because of substitution of a catalytic residue and not owing to disruption of binding interactions with the substrate peptide.
Figure 3.
a) Overnight in vitro modification assays of His6-SgbA with His6-SgbL(lyase-kinase 1–548) and variants thereof. The reference shown is His6-SgbA incubated under assay conditions in the absence of any enzyme. b) Fluorescence polarization binding assays using 100 nM FITC-SgbA(leader) and varying concentrations of His6-SgbL(lyase-kinase 1–548) and variants thereof. All measurements were performed in triplicate.
For the D351A, N356A, and D369A variants, no modification of SgbA was observed (Figure 3a), even though their binding affinities with FITC-SgbA(leader) were comparable to those of the WT protein (Kd(WT) = 251 ± 20 nM, Kd(D351A) = 576 ± 42 nM, Kd(N356A) = 321 ± 18 nM, Kd(D369A) = 353 ± 18 nM; Figure 3b). These observations show that the loss of activity was indeed caused by mutation of catalytically important residues. In contrast, the K248A and E270A variants were still able to modify SgbA, although they showed reduced activity compared to the WT protein. Whereas WT His6-SgbL(lyase-kinase 1–548) accomplished full conversion of SgbA to a fourfold dehydrated species under the reaction conditions, the K248A and E270A variants yielded a mixture of peptides ranging from unmodified substrate to fourfold dehydrated SgbA, which includes all intermediate dehydration states. The FP binding studies revealed that substrate binding of these variants was also significantly impaired (Kd(K248A) = 7114 ± 557 nM, Kd(E270A) = 1717 ± 83 nM). Therefore, it is uncertain if the observed decrease in activity originates from perturbation of catalysis or the reduced substrate binding affinity. Alternatively, it could also be that the exchange of these residues affects the stability of the active conformation of the protein by disruption of the predicted Lys248-Glu270 salt bridge and thus is affecting both leader peptide binding as well as catalysis. Regardless, we clearly demonstrated that all five residues play important roles in the activity of the SgbL kinase domain. Indeed, an alignment of previously reported examples of class III11, 38–41 and class IV14, 16, 42 lanthipeptide synthetases (Supporting Information Figure S2) revealed that all five residues are completely conserved in these enzymes.
HDX-MS Experiments Identify the Leader Peptide Binding Site.
As mentioned before, it was previously shown that the kinase domain has a dual function.16 It not only phosphorylates Ser/Thr residues in the core peptide, but also binds the leader peptide. To identify those parts of the domain that interact with the leader peptide, hydrogen-deuterium exchange (HDX) experiments were carried out. The level of HDX was analyzed after 10 s, 30 s, 60 s, 360 s, and 900 s of incubation in a D2O buffer and the experiments were performed either with only His6-SgbL(kinase 208–548) or with His6-SgbL(kinase 208–548) and SgbA(leader). After quenching the HDX reaction, the protein was digested on-line with pepsin, and the resulting fragments were analyzed via liquid chromatography-mass spectrometry (LC-MS) to determine the deuterium uptake for each peptide. In this way, a sequence coverage of 97% was accomplished (Supporting Information Figure S3). The exchange dynamics of the lone kinase domain demonstrated that the sites of high and low solvent accessibility were in general agreement with the structure model (Figure 4a and Supporting Information Figure S4). Finally, a comparison between the HDX levels of His6-SgbL(kinase 208–548) in the presence or absence of SgbA(leader) yielded information about the potential site of peptide binding (Figure 4b–c and Supporting Information Figure S5–S6).
Figure 4.
a) Phyre2 model of SgbL(kinase 208–548) colored according to the general solvent accessibility as determined by HDX. b) Phyre2 model of SgbL(kinase 208–548) colored according to the observed differences in HDX levels in the absence or presence of saturating concentrations of the SgbA leader peptide. c) HDX plots of representative peptides of His6-SgbL(kinase 208–548) in the unbound (black) and bound (red) states. The peptide and charge state are labeled in each panel. Residues in the region denoted as 188–207 correspond to the His6-tag of the protein.
Whereas the level of HDX is not, or only weakly, affected by addition of SgbA(leader) for most parts of the protein, a significant decrease in HDX is observed for the first ~100 residues of the kinase domain (residues 208–310) as revealed by the corresponding proteolytic peptides. This result suggests that the SgbA(leader) peptide binds to this particular region of SgbL(kinase 208–548) and thereby shields this part of the protein against exchange of hydrogen for deuterium. Alternatively, leader peptide binding could induce conformational changes that result in altered HDX patterns. A comparison of the predicted secondary structure motifs in this region of SgbL with homologous regions in other LanL and LanKC enzymes (Supporting Information Figure S7) shows high structural conservation and suggests that leader peptide binding occurs in this part of the kinase domain for the entire family. In SgbL, this region includes residues Lys248 and Glu270, whose substitution with alanine affected both leader peptide binding affinity and catalytic activity. Especially the peptide fragment that corresponds to helix C and harbors Glu270 shows a very strong decrease in deuterium uptake in the presence of the SgbA(leader) peptide (Figure 4c and Supporting Information Figure S5–S6), which possibly correlates with the expected movement of helix C upon changing from an inactive to an active conformation known from studies on other kinases.29, 34, 37 Although sequence homology suggests a catalytic function for Lys248 and Glu270, the observed effect of their Ala exchanges on leader peptide binding in combination with the HDX results implies that disruption of the proposed Lys248-Glu270 contact destabilizes structural elements in the SgbL region that are important for leader peptide recognition.
A Fusion Protein of the Binding Domain and MBP Binds SgbA(leader).
To corroborate the HDX results experimentally, we tried to express and isolate the proposed binding domain for in vitro studies. The HDX data suggest that the region involved in leader peptide binding ends in a predicted α-helix (308-LRTWVAEH-315, Supporting Information Figure S1). Therefore, gene constructs were generated to express the residues either up to (SgbL(208–305)) or including this α-helix (SgbL(208–318)). Whereas expression of these small protein segments with N-terminal His6-tags did not yield soluble protein, tagging with maltose binding protein (MBP) allowed isolation of both proteins in soluble form. MBP-tagged proteins (His6-MBP-SgbL(208–305) and His6-MBP-SgbL(208–318)) were used for FP binding assays with FITC-SgbA(leader) with His6-MBP serving as negative control (Figure 5). As a significant change in FP is only observed when there is a sufficiently large mass difference between the fluorescent probe and the complex of fluorescent probe and protein, the MBP-tag was left attached to the otherwise too small SgbL fragments.
Figure 5.
a) Precursor peptide sequences of SgbA and AciA. b) FP binding studies using 100 nM FITC-SgbA(leader) or 100 nM fluorescein-AciA(Leader) with His6-MBP-SgbL(208–305), His6-MBP-SgbL(208–318), and His6-MBP. All measurements were performed in triplicate. c) FP binding studies using 100 nM FITC-SgbA(leader) or 100 nM fluorescein-AciA(Leader) with His6-MBP-SgbL(lyase-kinase 1–548). All measurements were performed in triplicate.
While saturation was not accomplished with either His6-MBP-SgbL(208–305) or His6-MBP-SgbL(208–318) at the concentrations used (~0.8–1 mM), non-specific binding to the negative control occurred at much higher concentrations (Figure 5b). The FP change caused by the interaction of FITC-SgbA(leader) with the SgbL binding domain was estimated by subtracting the non-specific binding to His6-MBP from the data obtained with the MBP fusions (Supporting Information Figure S8). The resulting curve is in line with typical FP binding behavior and allows determination of an approximate Kd in the range of ~31 μM for the interaction between FITC-SgbA(leader) and His6-MBP-SgbL(208–318). Thus, the SgbL fragment clearly has affinity for the leader peptide of SgbA, although the binding affinity of SgbA(leader) for the MBP-fusions is lower than for the full length SgbL protein. The decrease of binding affinity might be due to the lack of additional stabilizing contacts compared to when the binding domain is embedded in the full length SgbL protein but could potentially also arise from the fact that only a certain percentage of the MBP fusion protein carries a properly folded binding domain.
Another control was carried out by evaluating the binding of the N-terminally fluorescein-labeled leader peptide of the class III lanthipeptide precursor AciA to His6-MBP-SgbL(208–305), His6-MBP-SgbL(208–318), and His6-SgbL(lyase-kinase 1–548) (Figure 5). AciA is matured into catenulipeptin by action of AciKC.41 As expected by the sequence differences of AciA(leader) and SgbA(leader) (Figure 5a), fluorescein-labeled AciA(leader) did not show a significant change in FP when assayed with His6-SgbL(lyase-kinase 1–548) (Figure 5c). Indeed, when His6-MBP-SgbL(208–305) and His6-MBP-SgbL(208–318) were assayed with fluorescein-labeled AciA(leader), we observed behavior that is similar to that of FITC-SgbA(leader) tested with His6-MBP (Figure 5a). These observations further demonstrate that the observed FP increase of FITC-SgbA(leader) with the MBP-fusions is due to specific interactions of these leader peptide/protein pairs.
CONCLUSIONS
The importance of the bioinformatically identified residues (Lys248, Glu270, Asp351, Asn356, and Asp369) was demonstrated experimentally by in vitro modification and FP binding assays with mutant enzymes. Furthermore, a better understanding of the interactions between the SgbL kinase domain and the SgbA leader peptide was obtained by HDX measurements. These experiments identified a region of ~100 residues for which hydrogen-deuterium exchange was altered considerably upon leader peptide recognition, and we substantiated these findings by binding assays between FITC-SgbA(leader) and an MBP-fusion with the identified binding region.
These results afford a firmer grasp of the biosynthetic mechanisms behind class IV and potentially also class III lanthipeptide formation. Combined with previously published studies identifying the catalytic residues in the lyase domain of the class IV lanthipeptide processing enzyme VenL,15 many of the catalytic residues enabling Ser/Thr dehydration in class IV systems have now been identified. Notably, these residues are also conserved in a number of uncharacterized, more distantly related SgbL homologs identified by genome mining (Supporting Information Figure S9). Considering that these residues are also completely conserved in class III lanthipeptide synthetases (Supporting Information Figure S9) and considering the strong overall sequence homology between the lyase and kinase domains in class III and IV enzymes, our findings may also hold true for class III systems.
METHODS
Bacterial Strains and Materials.
For cloning and mutagenesis, E. coli DH10B cells were employed, and expression was carried out in E. coli BL21(DE3) cells. DNA sequences of newly cloned or mutated plasmids were confirmed by dideoxy sequencing (ACGT, Inc.). Gibson-Assembly master mix, DpnI and Phusion DNA polymerase were bought from New England Biolabs. Oligonucleotide primers were purchased from Integrated DNA Technologies. Synthetic peptides used in this study were ordered with >95% purity from Genscript. D2O was purchased from Cambridge Isotopic Laboratories. Lysozyme, benzonase (25–29 U/μL), Millipore C18 Ziptips, NHS-fluorescein and fluorescein isothiocyanate (FITC) were obtained from Thermo Fisher Scientific. Other chemicals were procured from Sigma-Aldrich.
Bioinformatic Prediction of Catalytic Residues in the SgbL Kinase Domain.
Structure modelling was performed using the SgbL(kinase 208–548) sequence as input for Phyre2 employing the Intensive Modelling Mode.28 Secondary structure predictions were performed with the PSIPRED Protein Sequence Analysis Workbench.36 The kinase domains of PknB and PknG were aligned with the sequence of SgbL(kinase 208–548) to pinpoint catalytic residues. The residues identified through alignments were cross-checked with the structure model and the predicted secondary structures to test if the predicted catalytic residues would occur in the right parts of the structure model and if they were in regions with the same secondary structures as the respective residues in PknB and PknG.29, 34 Based on this analysis, Lys248, Glu270, Asp351, Asn356, and Asp369 were proposed to be important for the activity of the SgbL kinase domain.
Mutagenesis and Cloning.
Mutagenesis of the His6-sgbL(lyase-kinase 1–548) pET28a expression plasmid16 was carried out by using site-directed ligase-independent mutagenesis (SLIM) according to published protocols.43, 44 In short, four primers were used for mutagenesis: two base primers (FP and RP) that anneal to the region flanking the site of mutagenesis and two tail primers (FPtail and RPtail) that have a 5’ sequence overhang with the mutated sequence attached to the base primer sequence. For every mutation, two 50 μL PCRs were carried out using Phusion DNA polymerase. One PCR was performed using the FP and RPtail primer pair, the other employing FPtail and RP as primers. After using 10 μL of each PCR to check if amplification of the target DNA was successful via agarose gel electrophoresis, a DpnI digest was performed to digest the template DNA (40 μL PCR + 4.6 μL NEB CutSmart Buffer + 1.0 μL DpnI, incubated for 2 h at 37 °C; then inactivation of DpnI by incubation at 80 °C for 20 min). The hybridization reaction was carried out by mixing 10 μL of each DpnI-treated PCR with 10 μL 5X hybridization buffer (750 mM NaCl, 125 mM Tris, 100 mM EDTA, pH 8.0) and 20 μL of ddH2O. Then, the hybridization mixture was incubated in a thermocycler for 3 min at 99 °C, followed by three cycles of incubation at 65 °C for 5 min and 30 °C for 40 min. The hybridized, circular DNA was used to transform E. coli cells, and colonies carrying the target plasmids were isolated by using kanamycin as selection marker. After plasmid preparation, plasmids featuring the correct mutations were identified via sequencing. The primers used for the SLIM PCRs are listed in Supporting Information Table S1.
Cloning was performed by Gibson-Assembly. The mbp gene was amplified by PCR using Phusion DNA polymerase and the primers listed in Supporting Information Table S2. Then, the pET28a plasmid carrying the His6-sgbL(kinase 208–548) gene16 was linearized by PCR with Phusion DNA polymerase and primers also listed in Table S2. These primers were designed with 5’ overhang sequences that would allow Gibson-Assembly with the amplified mbp gene. The target DNAs of both PCRs were isolated after agarose gel electrophoresis using the Qiagen Gel Extraction Kit and employed for a Gibson-Assembly reaction for 1 h at 50 °C with NEB Gibson-Assembly master mix according to the manufacturer’s protocol. After transformation, kanamycin was used as selection marker to screen for cells carrying the target plasmid. Plasmid DNA was isolated and sequences of correct expression plasmids were confirmed by sequencing. In this way, His6-mbp-sgbL(kinase 208–548) pET28a was generated. Next, this plasmid was used as template to generate His6-mbp-sgbL(208–305) pET28a and His6-mbp-sgbL(208–318) pET28a via SLIM-mediated deletion of unwanted sequences. SLIM was carried out as described above using the primers listed in Supporting Information Table S3.
Protein Expression, Isolation, and Purification.
All proteins were expressed in lysogeny broth (LB) medium containing 50 μg/mL kanamycin as selection marker. For expression, LB cultures (2 L aliquots in 4 L baffled flasks) were prewarmed to 37 °C for 2 h and inoculated 1:100 with 37 °C LB overnight cultures. After inoculation, cultures were grown at 37 °C until reaching an optical density at 600 nm (OD600) of 0.2–0.25. At this point, the temperature of the incubation shaker was reduced to 18 °C and cells were induced 1 h later at OD ~0.5–0.7 by addition of 400 μL of an isopropyl β-D-1-thiogalactopyranoside (IPTG) stock solution (0.5 M) to accomplish a final concentration of 0.1 mM of IPTG. Expression was carried out overnight at 18 °C after induction.
Expression cultures were harvested by centrifugation and the cell pellets were resuspended in 20 mL of lysis buffer (300 mM NaCl, 50 mM HEPES, 10% glycerol, pH 7.5) per 1 L of original culture volume. The cell suspension was then treated for 1 h on ice with 5 μL of benzonase per 20 mL suspension and a 1 mg/mL final concentration of lysozyme. Next, cells were lysed by sonication on ice using a Vibra Cell sonicator (Sonics & Material) set to an amplitude of 40% and alternating between 2 s on- and 5 s off-pulse for a total sonication time of 5 min. Lysates were cleared by centrifugation at 75000×g and 4 °C for 30 min and the resulting supernatant was filtered through a syringe filter (0.45 μm).
For protein isolation, Nickel nitrilotriacetic acid (NiNTA) affinity chromatography using a 5 mL NiNTA HisTrap column (GE Healthcare) and a peristaltic pump was performed. The NiNTA column was first equilibrated with 25 mL of wash buffer (25 mM imidazole, 300 mM NaCl, 50 mM HEPES, 10% glycerol, pH 7.5) and then loaded with the cleared lysate. After loading, the column was first washed with another 25 mL of wash buffer and then the target protein was eluted with 15 mL of elution buffer (500 mM imidazole, 300 mM NaCl, 50 mM HEPES, 10% glycerol, pH 7.5). To accomplish higher purity, the elution fraction was concentrated to 3–4 mL utilizing an Amicon centrifugal filter unit with a suitable MW cut-off and then applied to size exclusion chromatography (SEC) using a Superdex200 column on an Äkta FPLC (GE Healthcare) set to a flow rate of 1 mL/min of SEC buffer (300 mM NaCl, 50 mM HEPES, 10% glycerol, pH 7.5). SEC fractions were analyzed by SDS-PAGE and the purest fractions were pooled, concentrated and stored at −80 °C after flash-freezing for later use.
Expression, Isolation, and Purification of His6-SgbA.
For production and purification of His6-SgbA, a previously established protocol was adapted.16 The detailed protocol can be found in the Supporting Information.
In Vitro Modification Assays.
For testing the ability of His6-SgbL(lyase-kinase 1–548) and variants thereof to dehydrate His6-SgbA, assays were run at room temperature for 16 h. For preparing these assays, an 8X master mix was set up consisting of 80 μL of 2X assay buffer (50 mM HEPES, 300 mM NaCl, pH 7.5), 8 μL of TCEP stock solution (20 mM), 8 μL of MgCl2 stock solution (200 mM), 8 μL of ATP stock solution (50 mM), 8 μL of His6-SgbA stock solution (10 mg/mL = 1.34 mM in DMSO), and 24 μL of ddH2O. Then, 3 μL of protein stock solution (1 mg/mL = 16.4 μM) were mixed with 17 μL of the assay master mix solution yielding a reaction mixture containing 67 μM His6-SgbA, 2.5 μM His6-SgbL(lyase-kinase 1–548) (WT or protein variant), 1 mM TCEP, 10 mM MgCl2, 2.5 mM ATP, 25 mM HEPES, and 150 mM NaCl at pH 7.5. As reference, 3 μL of water was mixed with 17 μL of the master mix solution and incubated in the same manner. After incubation overnight at RT, the samples were desalted and eluted into 3 μL of 0.1% trifluoroacetic acid/80% MeCN using C18 Ziptips following the manufacturer’s protocol. For MALDI-TOF-MS sample preparation, 1 μL of the samples were mixed with 1 μL of a saturated sinapinic acid solution in 60% MeCN and then crystallized at RT. MALDI-TOF-MS measurements were performed with a Bruker UltrafleXtreme MALDI TOF/TOF mass spectrometer.
Fluorescein-Labeling.
FITC-labeling of SgbA(leader) was accomplished by mixing 0.4 mL of peptide stock solution (5 mg/mL in water) with 7 mL of phosphate buffer (100 mM phosphate, pH 8.4) and 0.125 mL of a FITC stock solution (10 mg/mL in DMSO). The reaction mixture was protected from light and slowly shaken overnight at RT. Afterwards, the solution was cleared by centrifugation and the supernatant was applied to preparative HPLC using a C18 column (Phenomenex, Luna 10 μm C18(2) 100 Å, 250×10 mm) connected to a Nexera HPLC system (Shimadzu) with solvents A (0.1% trifluoroacetic acid in H2O) and B (0.1% trifluoroacetic acid in MeCN) at a flow rate of 8 mL/min. The gradient used was as follows: Linear increase from 16% to 72% B in 30 min, followed by another linear increase from 72% to 98% B over 1 min and then keeping 98% B for another 5 min. The peak with the target mass was collected, freeze-dried and applied to a second round of HPLC using the same conditions to obtain pure compound.
For the fluorescein-labeling of AciA(leader), 13.3 mg of NHS-fluorescein was dissolved in 5.4 mL of phosphate buffer (100 mM phosphate, pH 8.4) and then 0.6 mL of peptide stock solution (5 mg/mL in DMSO) were added. The reaction mixture was protected from light and slowly shaken at RT for 2.5 h. After clearing the solution by centrifugation, the supernatant was applied to HPLC using the same gradient and conditions as for FITC-SgbA(leader). Again, the peak with the target mass was collected, freeze-dried and applied to a second round of HPLC using the same conditions to obtain pure compound.
Fluorescence Polarization (FP) Binding Assays.
Protein aliquots were thawed and mixed 1:1 with glycerol free buffer (300 mM NaCl, 50 mM HEPES, pH 7.5) to obtain stock solutions at 5% glycerol. The proteins were then concentrated using Amicon centrifugal filter units with suitable MW cut-offs to the concentrations needed for the experiments. A 1:1 dilution series spanning a total of 16 different concentrations was set up for each protein in assay buffer (300 mM NaCl, 50 mM HEPES, 5% glycerol, pH 7.5). In wells of a 384-well solid black polystyrene microplate (Corning), 5 μL of a 1 μM FITC-SgbA(leader) or fluorescein-AciA(leader) stock solution was mixed with 45 μL of the protein solutions. For each dilution series, a reference containing 5 μL of fluorescein-labeled peptide mixed with 45 μL assay buffer was also set up, to ensure that the highest protein dilutions have the same FP values as the buffer control. All assays were set up in triplicates.
After mixing, samples were equilibrated for 30 min at RT until parallel and perpendicular fluorescence intensities were measured at an excitation wavelength of 485 nm and an emission wavelength of 528 nm with a bandwidth of 20 nm using a Synergy H4 Hybrid Reader (BioTek). To calculate the FP, the perpendicular fluorescence intensity was first subtracted from the parallel one and the result was then divided through the sum of both fluorescence intensities (all collected data is shown in Supporting Information Tables 4a–m). For completed binding curves, the Kd values were determined in OriginPro2017 (OriginLab) by using a non-linear dose-response fit after plotting the normalized FP (i.e. the measured FP minus the mean FP of the buffer references) against the log of the protein concentrations.
Hydrogen-Deuterium Exchange-Mass Spectrometry (HDX-MS) Experiments.
For each protein state that was studied by HDX, a protein stock solution of 30 μM in 1X PBS (137 mM NaCl, 2.7 mM KCl, 10 mM phosphate, pH 7.4) was prepared. For the bound state of His6-SgbL(kinase 208–548), a 1:2 molar ratio of His6-SgbL(kinase 208–548):SgbA(leader) was used to ensure essentially complete binding. The concentration of His6-SgbL(kinase 208–548) in the protein complex solution was 30 μM. The solution of the protein complex was incubated at 25 °C for 1 h before conducting the initiating HDX. A quench solution consisting of 8 M guanidine chloride and 200 mM tris(2-carboxyethyl)phosphine (TCEP) was adjusted to pH 2.5 and was kept on ice to be used to quench the HDX.45
To initiate HDX, 2 μL of protein stock solution was diluted into 18 μL of D2O in 1X PBS buffer. The extent of HDX was measured at various time intervals (10 s, 30 s, 60 s, 360 s, 900 s, and 3600 s) on ice. To stop the HDX reaction, 30 μL of the quench solution was added, and the mixture was immediately introduced to a custom-packed pepsin column (2 mm × 20 mm) for on-line digestion at a flow rate of 100 μL/min. The resulting peptides were then captured on a C8 cartridge (2.1 mm × 15 mm, Agilent) at a flow rate of 100 μL/min of H2O with 0.1% trifluoracetic acid (TFA). Digestion and desalting were carried out in an ice-water bath for 3 min. The resulting peptides were then separated by liquid chromatography (LC) consisting of a reversed-phase C18 column (1.9 μm Hypersil Gold, Thermo Fisher Scientific) at a flow rate of 100 μL/min by using a linear gradient of 4% to 40% MeCN with 0.1% formic acid (FA) over 4 min and holding at 40% MeCN with 0.1% FA for another 5.5 min. To minimize back exchange, the valves, trap and analytical columns, and tubing for protein digestion and separation were submerged in an ice-water bath. MS analysis of the peptides was conducted on an LTQ-FT mass spectrometer (Thermo Fisher Scientific) equipped with a positive-ion electrospray ionization source. All experiments were conducted in duplicate.
Hydrogen-Deuterium Exchange-Mass Spectrometry (HDX-MS) Data Analysis.
Prior to conducting HDX, experiments in the absence of D2O were performed to map the peptides generated from the digestion of His6-SgbL(kinase 208–548), generating a list of peptides that were later followed during HDX-MS data acquisition (Supporting Information Figure S3). Product-ion mass spectra were collected in a data-dependent mode, in which the six most abundant ions in each scan were selected for MS/MS analysis. The resulting MS/MS files from three parallel runs were then converted to mzXML files using MM File Conversion and submitted to MassMatrix (version 2.4.2) for peptide identification.46 Additionally, this search was carried out against a reversed sequence to discard ambiguous identifications. Data analysis of the HDX experiments was performed on HDExaminer (version 1.1.0, Sierra Analytics, Inc.), and the deuterium uptake for each peptide was manually inspected.
Supplementary Material
Funding
J. D. H. received financial support from the Deutsche Forschungsgemeinschaft (DFG Research Fellowship 309199717) and L.S. financial support from the National Institutes of Health (NIGMS grant P41 GM103422). This work was supported by the National Institutes of Health (Grant R01 GM 058822 to W.v.d.D. and P41 GM 103422 to M.L.G.)
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: XXX
Supporting figures, tables, and methods.
The authors declare no competing financial interest.
REFERENCES
- (1).Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJ, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, and van der Donk WA (2013) Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature, Nat. Prod. Rep 30, 108–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Repka LM, Chekan JR, Nair SK, and van der Donk WA (2017) Mechanistic understanding of lanthipeptide biosynthetic enzymes, Chem. Rev 117, 5457–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hetrick KJ, and van der Donk WA (2017) Ribosomally synthesized and post-translationally modified peptide natural product discovery in the genomic era, Curr. Opin. Chem. Biol 38, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Urban JH, Moosmeier MA, Aumüller T, Thein M, Bosma T, Rink R, Groth K, Zulley M, Siegers K, Tissot K, Moll GN, and Prassler J (2017) Phage display and selection of lanthipeptides on the carboxy-terminus of the gene-3 minor coat protein, Nat. Commun 8, 1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hetrick KJ, Walker MC, and van der Donk WA (2018) Development and Application of Yeast and Phage Display of Diverse Lanthipeptides, ACS Cent. Sci 4, 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bosma T, Rink R, Moosmeier MA, and Moll GN (2019) Genetically encoded libraries of constrained peptides, ChemBioChem, DOI: 10.1002/cbic.201900031. [DOI] [PubMed] [Google Scholar]
- (7).Yang X, Lennard KR, He C, Walker MC, Ball AT, Doigneaux C, Tavassoli A, and van der Donk WA (2018) A lanthipeptide library used to identify a protein-protein interaction inhibitor, Nat. Chem. Biol 14, 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Xie L, Miller LM, Chatterjee C, Averin O, Kelleher NL, and van der Donk WA (2004) Lacticin 481: in vitro reconstitution of lantibiotic synthetase activity, Science 303, 679–681. [DOI] [PubMed] [Google Scholar]
- (9).Chatterjee C, Miller LM, Leung YL, Xie L, Yi M, Kelleher NL, and van der Donk WA (2005) Lacticin 481 synthetase phosphorylates its substrate during lantibiotic production, J. Am. Chem. Soc 127, 15332–15333. [DOI] [PubMed] [Google Scholar]
- (10).Dong SH, Tang W, Lukk T, Yu Y, Nair SK, and van der Donk WA (2015) The enterococcal cytolysin synthetase has an unanticipated lipid kinase fold, eLife 4, e07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Meindl K, Schmiederer T, Schneider K, Reicke A, Butz D, Keller S, Guhring H, Vertesy L, Wink J, Hoffmann H, Bronstrup M, Sheldrick GM, and Süssmuth RD (2010) Labyrinthopeptins: a new class of carbacyclic lantibiotics, Angew. Chem. Int. Ed 49, 1151–1154. [DOI] [PubMed] [Google Scholar]
- (12).Müller WM, Ensle P, Krawczyk B, and Süssmuth RD (2011) Leader peptide-directed processing of labyrinthopeptin A2 precursor peptide by the modifying enzyme LabKC, Biochemistry 50, 8362–8373. [DOI] [PubMed] [Google Scholar]
- (13).Wiebach V, Mainz A, Siegert MJ, Jungmann NA, Lesquame G, Tirat S, Dreux-Zigha A, Aszodi J, Le Beller D, and Süssmuth RD (2018) The anti-staphylococcal lipolanthines are ribosomally synthesized lipopeptides, Nat. Chem. Biol 14, 652–654. [DOI] [PubMed] [Google Scholar]
- (14).Goto Y, Li B, Claesen J, Shi Y, Bibb MJ, and van der Donk WA (2010) Discovery of unique lanthionine synthetases reveals new mechanistic and evolutionary insights, PLoS Biol. 8, e1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Goto Y, Ökesli A, and van der Donk WA (2011) Mechanistic studies of Ser/Thr dehydration catalyzed by a member of the LanL lanthionine synthetase family, Biochemistry 50, 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Hegemann JD, and van der Donk WA (2018) Investigation of substrate recognition and biosynthesis in class IV lanthipeptide systems, J. Am. Chem. Soc 140, 5743–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, and Nair SK (2006) Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis, Science 311, 1464–1467. [DOI] [PubMed] [Google Scholar]
- (18).Li B, and van der Donk WA (2007) Identification of essential catalytic residues of the cyclase NisC involved in the biosynthesis of nisin, J. Biol. Chem 282, 21169–21175. [DOI] [PubMed] [Google Scholar]
- (19).Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, and Nair SK (2015) Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB, Nature 517, 509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ortega MA, Hao Y, Walker MC, Donadio S, Sosio M, Nair SK, and van der Donk WA (2016) Structure and tRNA specificity of MibB, a lantibiotic dehydratase from Actinobacteria involved in NAI-107 biosynthesis, Cell Chem. Biol 23, 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Abts A, Montalbán-Lopez M, Kuipers OP, Smits SH, and Schmitt L (2013) NisC binds the FxLx motif of the nisin leader peptide, Biochemistry 52, 5387–5395. [DOI] [PubMed] [Google Scholar]
- (22).Jungmann NA, Krawczyk B, Tietzmann M, Ensle P, and Süssmuth RD (2014) Dissecting reactions of nonlinear precursor peptide processing of the class III lanthipeptide curvopeptin, J. Am. Chem. Soc 136, 15222–15228. [DOI] [PubMed] [Google Scholar]
- (23).Jungmann NA, van Herwerden EF, Hügelland M, and Süssmuth RD (2016) The supersized class III lanthipeptide stackepeptin displays motif multiplication in the core peptide, ACS Chem. Biol 11, 69–76. [DOI] [PubMed] [Google Scholar]
- (24).Sambeth GM, and Süssmuth RD (2011) Synthetic studies toward labionin, a new alpha,alpha-disubstituted amino acid from type III lantibiotic labyrinthopeptin A2, J. Pept. Sci 17, 581–584. [DOI] [PubMed] [Google Scholar]
- (25).Pesic A, Henkel M, and Süssmuth RD (2011) Identification of the amino acid labionin and its desulfurised derivative in the type-III lantibiotic LabA2 by means of GC/MS, Chem. Commun 47, 7401–7403. [DOI] [PubMed] [Google Scholar]
- (26).Völler GH, Krawczyk JM, Pesic A, Krawczyk B, Nachtigall J, and Süssmuth RD (2012) Characterization of new class III lantibiotics-erythreapeptin, avermipeptin and griseopeptin from Saccharopolyspora erythraea, Streptomyces avermitilis and Streptomyces griseus demonstrates stepwise N-terminal leader processing, ChemBioChem 13, 1174–1183. [DOI] [PubMed] [Google Scholar]
- (27).Krawczyk B, Völler GH, Völler J, Ensle P, and Süssmuth RD (2012) Curvopeptin: a new lanthionine-containing class III lantibiotic and its co-substrate promiscuous synthetase, ChemBioChem 13, 2065–2071. [DOI] [PubMed] [Google Scholar]
- (28).Kelley LA, Mezulis S, Yates CM, Wass MN, and Sternberg MJ (2015) The Phyre2 web portal for protein modeling, prediction and analysis, Nat. Protoc 10, 845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Scherr N, Honnappa S, Kunz G, Mueller P, Jayachandran R, Winkler F, Pieters J, and Steinmetz MO (2007) Structural basis for the specific inhibition of protein kinase G, a virulence factor of Mycobacterium tuberculosis, Proc. Natl. Acad. Sci 104, 12151–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Tiwari D, Singh RK, Goswami K, Verma SK, Prakash B, and Nandicoori VK (2009) Key residues in Mycobacterium tuberculosis protein kinase G play a role in regulating kinase activity and survival in the host, J. Biol. Chem 284, 27467–27479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Khan MZ, Bhaskar A, Upadhyay S, Kumari P, Rajmani RS, Jain P, Singh A, Kumar D, Bhavesh NS, and Nandicoori VK (2017) Protein kinase G confers survival advantage to Mycobacterium tuberculosis during latency-like conditions, J. Biol. Chem 292, 16093–16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Khan MZ, Kaur P, and Nandicoori VK (2018) Targeting the messengers: Serine/threonine protein kinases as potential targets for antimycobacterial drug development, IUBMB life 70, 889–904. [DOI] [PubMed] [Google Scholar]
- (33).Kanehiro Y, Tomioka H, Pieters J, Tatano Y, Kim H, Iizasa H, and Yoshiyama H (2018) Identification of Novel Mycobacterial Inhibitors Against Mycobacterial Protein Kinase G, Front. Microbiol 9, 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Young TA, Delagoutte B, Endrizzi JA, Falick AM, and Alber T (2003) Structure of Mycobacterium tuberculosis PknB supports a universal activation mechanism for Ser/Thr protein kinases, Nature Struct. Biol 10, 168–174. [DOI] [PubMed] [Google Scholar]
- (35).Prigozhin DM, Papavinasasundaram KG, Baer CE, Murphy KC, Moskaleva A, Chen TY, Alber T, and Sassetti CM (2016) Structural and Genetic Analyses of the Mycobacterium tuberculosis Protein Kinase B Sensor Domain Identify a Potential Ligand-binding Site, J. Biol. Chem 291, 22961–22969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Buchan DW, Minneci F, Nugent TC, Bryson K, and Jones DT (2013) Scalable web services for the PSIPRED protein analysis workbench, Nucleic Acids Res. 41, W349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Fabbro D, Cowan-Jacob SW, and Moebitz H (2015) Ten things you should know about protein kinases: IUPHAR Review 14, Br. J. Pharmacol 172, 2675–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Müller WM, Schmiederer T, Ensle P, and Süssmuth RD (2010) In vitro biosynthesis of the prepeptide of type-III lantibiotic labyrinthopeptin A2 including formation of a C-C bond as a post-translational modification, Angew. Chem., Int. Ed 49, 2436–2440. [DOI] [PubMed] [Google Scholar]
- (39).O’Connor TJ, Kanellis P, and Nodwell JR (2002) The ramC gene is required for morphogenesis in Streptomyces coelicolor and expressed in a cell type-specific manner under the direct control of RamR, Mol. Microbiol 45, 45–57. [DOI] [PubMed] [Google Scholar]
- (40).Hudson ME, Zhang D, and Nodwell JR (2002) Membrane association and kinase-like motifs of the RamC protein of Streptomyces coelicolor, J. Bacteriol 184, 4920–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wang H, and van der Donk WA (2012) Biosynthesis of the class III lantipeptide catenulipeptin, ACS Chem. Biol 7, 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Iftime D, Jasyk M, Kulik A, Imhoff JF, Stegmann E, Wohlleben W, Süssmuth RD, and Weber T (2015) Streptocollin, a type IV lanthipeptide produced by Streptomyces collinus Tü 365, ChemBioChem 16, 2615–2623. [DOI] [PubMed] [Google Scholar]
- (43).Chiu J, March PE, Lee R, and Tillett D (2004) Site-directed, Ligase-Independent Mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h, Nucleic Acids Res. 32, e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Chiu J, Tillett D, Dawes IW, and March PE (2008) Site-directed, Ligase-Independent Mutagenesis (SLIM) for highly efficient mutagenesis of plasmids greater than 8kb, J. Microbiol. Meth 73, 195–198. [DOI] [PubMed] [Google Scholar]
- (45).Chen E, Salinas ND, Huang Y, Ntumngia F, Plasencia MD, Gross ML, Adams JH, and Tolia NH (2016) Broadly neutralizing epitopes in the Plasmodium vivax vaccine candidate Duffy Binding Protein, Proc. Natl. Acad. Sci 113, 6277–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Xu H, and Freitas MA (2009) MassMatrix: a database search program for rapid characterization of proteins and peptides from tandem mass spectrometry data, Proteomics 9, 1548–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.