Abstract
Background
Cervical dystonia (CD) can present with head tremor. It is unclear whether ataxic features are differentially associated with this phenotype at onset of CD.
Objectives
We sought to evaluate: (1) the demographic features of CD patients with (Tr-CD) and without head tremor (nTr-CD) at onset, and (2) the differential ataxic features between these CD subtypes.
Methods
For the first objective, we compared demographic data in Tr-CD versus nTr-CD subtypes in the entire cohort of CD subjects enrolled in the Dystonia Coalition Natural History and Biorepository studies (n=1608). For the second objective, we rated the standardized videos from consecutively enrolled Tr-CD subjects (n=50) and age-, gender-, and disease duration-matched nTr-CD subjects (n=50) for ataxia severity scoring using the Scale for the Assessment and Rating of Ataxia (SARA) and the International Cooperative Ataxia Rating Scale (ICARS); and for dystonia severity using the Toronto Western Spasmodic Torticollis Rating Scale section-I (TWSTRS) and the Global Dystonia Rating Scale (GDRS).
Results
Of 1,608 subjects, 18.1% (n=291) were classified as Tr-CD and 81.9% (n=1317) as nTr-CD. The Tr-CD cohort was older, predominantly female, and had longer disease duration than the nTr-CD cohort (p= 0.01). Compared to nTr-CD, Tr-CD subjects had worse generalized ataxia, speech, and gait and posture scores. High ataxia severity with low dystonia severity distinguished Tr-CD from nTr-CD with high accuracy (area under the curve, 0.91 (95%CI: 0.85–0.97).
Conclusions
Head tremor at disease onset represents a clinically distinguishable subtype of cervical dystonia affecting predominantly older women, with worse ataxia and milder dystonia than the non-tremulous dystonic phenotype.
Keywords: Dystonia, Tremor, Ataxia, Cerebellum, Head tremor
INTRODUCTION
Head tremor has long been recognized as one of the early presentations of cervical dystonia (CD) [1–3]. The classic tonic phenotype is sustained turning (torticollis), tilting toward a shoulder (laterocollis), head flexion (anterocollis), extension (retrocollis), or combinations thereof, without tremor (NTr-CD). Tremor-dominant CD (Tr-CD) can manifest as “no-no”, “yes-yes” or mixed jerky head oscillations [4–6]. These patients seek attention because of the tremor rather than the posturing.
Head tremor can also be a manifestation of acute or chronic cerebellar dysfunction [7, 8]. The cerebellum is a critical pathophysiologic node in the generation and expression of tremor and dystonia, and CD in particular [9–12]. Neuroimaging functional studies documented increased activation of the anterior cerebellar regions ipsilateral to the direction of head rotation and reduced activation in the posterior cerebellar regions [13, 14]. Symptomatic CD can occur after cerebellar stroke or hemorrhage [15, 16], and dystonic features improve after deep brain stimulation of the anterior lobe of the cerebellum [17]. Finally, post-mortem pathological studies have shown patchy loss of cerebellar Purkinje cells, as well as areas of focal gliosis and torpedo bodies (fusiform swelling of Purkinje cell axons) in patients with CD [18]. The extent to which cerebellar dysfunction might underlie the tremor and tremorless clinical phenotypes of CD remains to be clarified.
We sought to fulfill two research objectives: (1) to compare the demographic features of patients with and without head tremor at onset in a large CD cohort, and (2) to determine whether ataxic features are differentially associated with Tr-CD and NTr-CD.
MATERIALS AND METHODS
Patients
Objective 1: Full-cohort analysis
We reviewed all CD patients from the Dystonia Coalition Natural History and Biorepository studies [19, 20] from over 2,000 patients with dystonia recruited from 37 Centers in the United States, Canada, Europe, and Australia. Initial analysis evaluated the clinical and demographic features (age, disease duration, gender) for the entire cohort classified into Tr-CD or NTr-CD as per this item of the Dystonia Coalition data collection form: “Is this patient’s dystonia dominated by tremor more than tonic or twisting movements?” In addition to tremor “dominance” from the Biorepository dataset we also captured whether tremor was the “initial” feature from the Natural History dataset. Exclusion criteria were generalized, multifocal, or segmental dystonia and use of medications known to be associated with tremor, such as neuroleptics, antidepressants, and mood stabilizers.
Objective 2: Video rating
Fifty consecutive Tr-CD subjects (n=15 from the Natural History and n= 35 from the Biorepository study, proportionate to their different sample sizes) were individually gender-, age-, and disease duration-matched with consecutively selected 50 NTr-CD subjects from the same datasets. We chose consecutive patients for both groups to minimize selection bias in sampling of video segments used for ataxia and dystonia scoring. All subjects must have had focal dystonia predominantly affecting the cervical region and age ≥ 18 years. Videos were rated for ataxic and dystonic features by two blinded evaluators, naïve to the study hypothesis. Each video, lasting approximately 10–15 minutes, was collected in accordance with the Dystonia Coalition protocol and included a standardized battery of 32 tasks assessing patients while seating, standing, and walking, and including tasks that can be amenable for rating of cerebellar features using standardized scales. Given the heterogeneous presentation of dystonia, which may involve different body segments, the video protocol was specifically designed with the aim of providing a comprehensive neurological examination, including tasks required for the rating of intention and action tremor, ataxia, and speech (Online Resource 1) [19].
Clinical scales for video rating
Ataxia assessment
Ataxia severity was rated using the 8-item Scale for the Assessment and Rating of Ataxia (SARA [range 0–40]) [21] and the 19-item International Cooperative Ataxia Rating Scale (ICARS [range 0–100]), which while not validated for use in appendicular tremor has been validated for the assessment of cerebellar impairments in hereditary ataxias, strokes, and tumors, among others [22]. Speech, gait, and postural ataxia were rated using the 2-items ICARS Speech Disorder (range 0–8) and the 7-item ICARS Gait and Posture (range 0–34), which are both independently validated sub-scales of the ICARS [22]. Higher scores mean worse ataxia severity in all scales. Associated appendicular tremor was rated on a scale of 0 to 32, as the composite score of items 9–14 of the ICARS, using the following tasks from the Dystonia Coalition video protocol: write “Today is a nice day” 3 times with the dominant and nondominant hand; draw Archimedes’ spiral with the dominant and non-dominant hand; hold tip of pen over dot for 10 seconds, as close as possible, with the dominant and non-dominant hand; and hold up written page with extended arms/hands supinated for 5 seconds; extended arms/hands pronated for 5 seconds; and flex elbows and hold hands/arms steady without touching in front of chest for 5 seconds; and perform the finger-to-nose test slow enough to capture accuracy five times for each hand.
Dystonia assessment
Dystonia severity was rated using the 10-item Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) section-I (Torticollis Severity Scale [range 0–35]) [23] for cervical involvement and the 14-item Global Dystonia Rating Scale (GDRS [range 0–140]) [24] for generalized involvement. Higher scores in both scales mean worse dystonia severity.
Sample size and power analysis for video rating
In the absence of prior studies assessing ataxic and dystonic differences between TrCD and NTR-CD phenotypes, a formal sample size computation could not be carried out. However, we sought to determine a moderate Cohen’s effect size (d= 0.50) for all chosen ataxia scales between Tr-CD and NTr-CD groups. Using this information, a sample size of 48 per group was found to be sufficient to detect significant moderate effect sizes with power greater than 85% at the 1% level of significance using unpaired t-tests. The level of significance was adjusted to 1% due to multiple comparisons.
Data Analysis
We assessed the demographic variables for Tr-CD and NTr-CD (age, gender distribution, and disease duration) from the entire Dystonia Coalition cohort of CD patients meeting all of the inclusion and none of the exclusion criteria (n= 1,608), and the ataxia (ICARS, SARA) and dystonia severity (TWSTRS, GDRS) for the subgroups of Tr-CD and NTr-CD (50 each). The consensus scores from the two raters were obtained and analyzed. Continuous data were expressed as mean and standard deviation (SD), while categorical data were reported as frequencies and compared using unpaired t-test or Fisher’s exact test, as appropriate. The clinical scores were compared between Tr-CD and NTr-CD groups using logistic regression analysis after accounting for clustering effect. The clustering was due to matching Tr-CD subjects with NTr-CD subjects based on age, gender, disease duration, and study type. The robust variance using the Huber-Sandwich method was used to adjust for within-cluster correlation in analysis. The results of logistic regression analysis were reported using odds ratio (OR) along with 95% confidence interval (CI) and p-value. In addition, some items of SARA scale were also compared between the two groups using Wilcoxon signed rank tests due to matched study design and validated with paired t-test analysis. Further, multiple logistic regression analysis accounting for clustering effect was used to determine independent ataxic and dystonic scores associated with Tr-CD compared to NTr-CD. Significant variables from the univariate analysis were included in the multivariable analysis. The model discriminatory performance was measured using area under the curve (AUC) along with 95% CI. A receiver operating characteristic (ROC) curve was constructed to demonstrate accuracy of the developed regression model. P-values less than 1% were considered as significant. All statistical analyses were carried out using STATA version 13 (STATA Corp., Texas, USA). This study was approved by the University of Cincinnati Institutional Review Board, and the Dystonia Coalition Executive Committee reviewed and approved the protocol and provided access to the database for analysis. All patients gave written informed consent.
RESULTS
Full cohort
Of 1,608 CD patients included in the Dystonia Coalition databases (Table 1), 18.1% (n= 291) were classified as Tr-CD and 81.9% (n= 1317) as NTr-CD. Tremor was a presenting symptom in 50% of Tr-CD cases included in the Natural History Dystonia Coalition study, from which these data were available. The Tr-CD cohort was older (p= 0.01), had longer disease duration (p < 0.001), and was predominantly female (p= 0.006) compared with the nTr-CD cohort.
Table 1.
Dystonia Coalition Cervical Dystonia Cohorts: Clinical and Demographic Data
| Dystonia Coalition full cohort | Video-rated cohort | ||||||
|---|---|---|---|---|---|---|---|
| NTr-CD (n= 1317) | Tr-CD (n= 291) | p-value | NTr-CD (n= 50) | Tr-CD (n= 50) | p-value | ||
| Age (years) | 59.0 (12.7) | 62.9(12.6) | 0.01 | 62.2 (11.3) | 62.2 (11.4) | N/A* | |
| Age at onset (years) | 45.1 (15.1) | 44.0 (17.3) | 0.2602 | 49.4 (14.5) | 49.4 (14.4) | N/A* | |
| Disease duration (years) | 13.9 (11.9) | 18.9 (14.5) | <0.0001 | 12.8 (10.9) | 12.8 (10.8) | N/A* | |
| Gender (women/men) | 941/376 (71.4%) | 231/60 (79.4%) | 0.006 | 43/7 (86%) | 43/7 (86%) | N/A* | |
Results are reported as average values (standard deviation), unless specified differently. p-value: statistical differences between groups (Unpaired t-test or Fisher’s Exact test). NTr-CD: Non-tremor-dominant Cervical Dystonia; Tr-CD: Tremor-dominant Cervical Dystonia; % express the ratio (women/men).
By design, the cohorts for video rating were matched for age, gender, and disease duration.
Video-based cohort
The blinded-rated cohort of 50 age-, sex-, and disease duration-matched Tr-CD and 50 NTr-CD patients consisted of 43 women and 7 men for each group (Table 1). There were no differences between cohorts in use of medications with potential influence on ataxia rating scales, including benzodiazepines (Tr-CD= 15 vs. NTr-CD= 19; p= 0.437), topiramate (Tr-CD= 1 vs. NTr-CD= 1; p= 1), primidone (Tr-CD= 3 vs. NTr-CD= 0; p= 0.242), and gabapentin (Tr-CD= 1 vs. NTr-CD= 3; p= 0.343).
Ataxia severity in video cohort
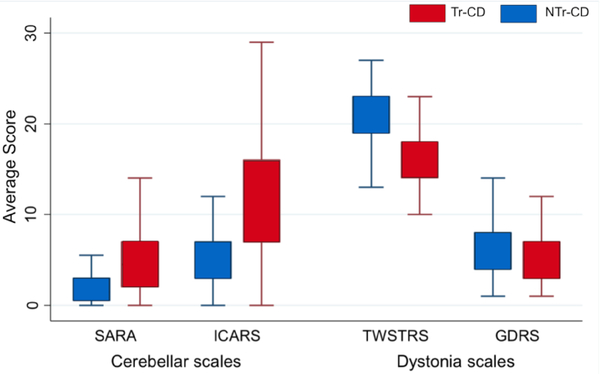
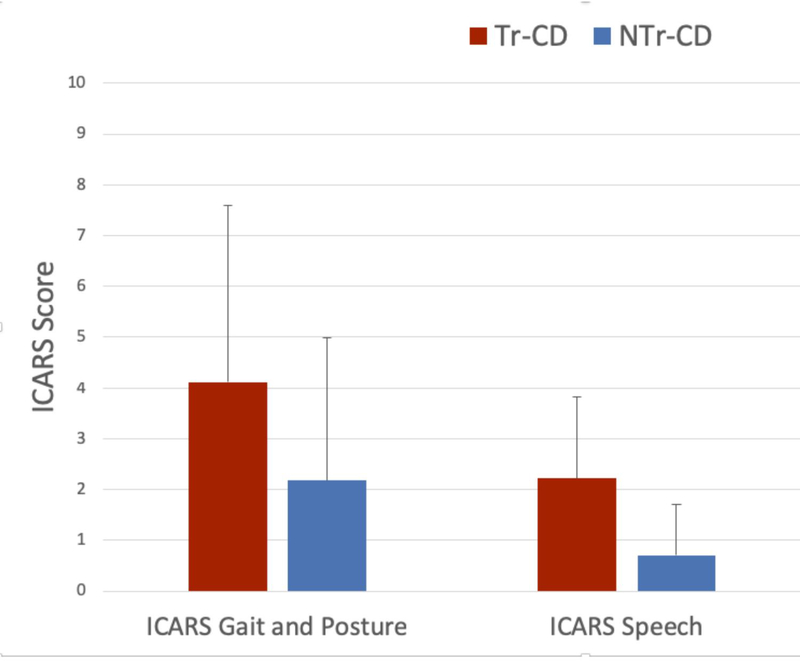
Total ICARS (12.68 ± 7.86 vs. 5.64 ± 6.08) and SARA scores (5.34 ± 4.08 vs. 2.16 ± 2.50) were greater in Tr-CD than NTr-CD (ICARS OR, 1.2; 95% CI: 1.04–1.38; p= 0.012; SARA OR, 1.45; 95% CI: 1.10–1.91; p= 0.009) (Figure 1). Speech, gait, and postural ataxia were worse in Tr-CD than NTr-CD (ICARS Speech Disorder: 2.22 ± 1.61 vs. 0.7 ± 1.02; p < 0.001; ICARS Gait and Posture: 4.12 ± 3.48 vs. 2.18 ± 2.8; p= 0.003) (Figure 2). The absolute Cohen’s effect size varied between 0.61 to 1.13, which was greater than the expected effect size used for sample size computation (0.5). Appendicular tremor was also more severe in Tr-CD than NTr-CD (Figure 2; p < 0.001).
Fig. 1. Comparison of Ataxia and Dystonia Scores in CD Subtypes.
NTr-CD: Non-tremor-dominant Cervical Dystonia; Tr-CD: Tremor-dominant Cervical Dystonia; SARA: Scale for the Assessment and Rating of Ataxia; ICARS: International Cooperative Ataxia Rating Scale; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale; GDRS: Global Dystonia Rating Scale
Fig. 2. Ataxia and Tremor Sub-scores in CD Subtypes.
NTr-CD: Non-tremor-dominant Cervical Dystonia; Tr-CD: Tremor-dominant Cervical Dystonia; ICARS: International Cooperative Ataxia Rating Scale; Tremor: Composite score of items 11–14 of the ICARS; * = p < 0.05
Dystonia severity in video cohort
TWSTRS score was lower in Tr-CD than NTr-CD (16.44 ± 4.20 vs. 20.30 ± 3.64; OR= 0.78; 95% CI: 0.69–0.88; p< 0.001) and GDRS score was moderately reduced in Tr-CD than NTr-CD (5.64 ± 3.59 vs. 7.42 ± 5.80; OR= 0.91; 95% CI: 0.81–1.02; p= 0.09). As measure of overall dystonia severity, GDRS was relatively low in both groups preselected for cervical involvement, but with a trend for even lower overall severity in Tr-CD (Figure 1). Neither age at onset nor disease duration correlated with dystonia severity (TWSTRS and GDRS).
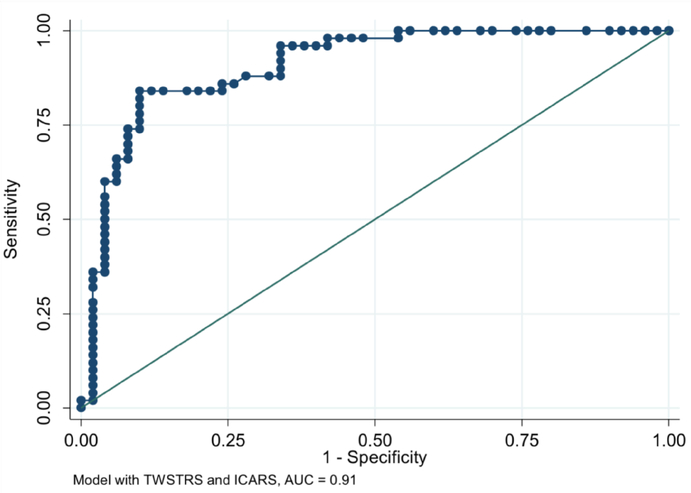
The combination of low TWSTRS (less severe CD; OR= 0.70, 95% CI: 0.60–0.81; p< 0.001) and high ICARS (more severe ataxia; OR= 1.29, 95% CI: 1.04–1.60; p= 0.021) differentiated Tr-CD from NTr-CD with an AUC of 0.91 (95%CI: 0.85–0.97) (Figure 3).
Fig. 3. Receiver Operator Curve.
Sensitivity and Specificity Values in Differentiating Tr-CD and NTr-CD using the combination of TWSTRS and ICARS. ROC: Receiver Operator Curve; AUC: Area Under the Curve; ICARS: International Cooperative Ataxia Rating Scale; TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale
DISCUSSION
We found that (1) tremor-dominant CD was more prevalent in older women in a large CD cohort and (2) was associated with more severe ataxia, milder dystonia, and longer disease duration compared to non-tremor-dominant CD. Also, Tr-CD patients were more frequently affected by concomitant appendicular tremor. The combination of minimal dystonic features and greater ataxia scores reliably distinguished Tr-CD from NTr-CD with high sensitivity and specificity, supporting the concept of Tr-CD as a unique clinical phenotype.
Tr-CD may be in the spectrum of the emerging “dystonia plus ataxia” syndrome [12]. Multiple clinical observations have shown that cerebellar lesions can be associated with, or even cause CD [25, 26] and that hereditary cerebellar diseases, including but not limited to spinocerebellar ataxia types 1, 2, 3, 6, 14, 17, and 35 may present with prominent dystonic features [10, 27–30]. One study evaluating clinical and neuroimaging data from 188 patients with cervical and segmental dystonia documented cerebellar atrophy on neuroimaging in 9% (n= 17) [10]. Over 80% of the 17 cases with cerebellar atrophy had CD (82.4%; n= 14), of whom 71.4% (n= 10) were women and 78.6% (n= 11) presented with a Tr-CD phenotype. Together with our findings, these data argue in favour of Tr-CD representing a distinguishable nosological entity characterized by potentially greater cerebellar dysfunction compared to NTr-CD.
Head tremor has been previously suggested to represent a subtype of CD [11, 31, 32], although with the confusing caveat that the spectral frequency of head tremor resembles that of “essential tremor,” and that dystonia and pure tremor disorders might co-aggregate or cluster in families [33]. While the occurrence of isolated head tremor in familial tremor disorders has never been described [34–36], CD patients presenting with head tremor often have a family history of tremor or other movement disorders and may be misdiagnosed as essential tremor [32, 37]. The loss of Purkinje cells and torpedo bodies reported in the granule cell layer of the cerebellum in brains of both CD and pure tremor disorders have led some authors to speculate on a possible association between the two conditions [11, 18, 38]. Our data, however, suggest that head tremor appearing at disease onset might represent a clinical subtype of CD, characterized by both ataxic and dystonic features. Future assessments of the Natural History Dystonia Coalition cohort may serve to determine whether ataxia progresses among the Tr-CD subtype and to examine its neurophysiologic, genetic, and neuropathologic underpinnings.
Some limitations temper the strength of our conclusions. First, our conclusions were based on a cross-sectional observational study, which might overlook potential confounders and selection biases inherent to tertiary referral centers from which subjects were recruited. These types of studies are, however, important to create new hypotheses, investigate rare outcomes, and identify associations that can then be more rigorously studied using a prospective cohort study [39, 40]. Second, the ataxia assessments were based on video material captured with a protocol aimed at documenting dystonic features rather than ataxia. While the video protocol was not designed for SARA or ICARS rating, many of the examination tasks overlap with those recommended for evaluating ataxia, including tasks associated with a comprehensive neurological examination. Of note, every potential shortcoming of this approach to measure ataxia on video material would have influenced both groups equally, which may have attenuated the effect size of the difference but with low likelihood it affected the direction of the results. Appendicular tremor, which was more frequently observed in the Tr-CD group, might also have impacted the final score of the rating scales. However, an accurate estimate of this effect is not possible since dystonia and ataxia scales are not validated for the assessment of tremor severity. Third, neuroimaging measures of the cerebellum were not available. Thus, we could not examine whether Tr-CD patients had greater cerebellar atrophy than NTr-CD, as suggested by the clinico-neuroimaging series from Queen Square [10]. Fourth, while we confirmed that there was no effect of medications on ataxia and tremor, we cannot exclude that a small proportion of patients included in this study had atypical presentations of hereditary cerebellar pathologies, since no genetic evaluations were performed. Examiner-confirmed data related to the initial presence of tremor was available only in the Natural History database, while data from the Biorepository database (with subjects having greater than 5 years of disease duration) may have been affected by recall bias given the longer duration of symptoms at enrollment. Fifth, differences in disease duration may be dependent on variability in time to diagnosis in different CD phenotypes. Only 40% of patients with CD pursue medical care within the first 6 months and fewer than 10% receive a diagnosis [41]. Thus, the possibility exists that Tr-CD might have been diagnosed earlier than nTr-CD due to their prompt referral to specialists care. Finally, this cross-sectional analysis cannot serve to estimate the progression of cerebellar disability in the two cohorts of patients, although the longer disease duration despite mild dystonia severity in the Tr-CD cohort suggests this subtype may possibly be neurodegenerative in nature.
CONCLUSIONS
Taking into account the above limitations and pending future neurophysiological, neuroimaging, neuropathological, and genetic studies, we propose that dystonic head tremor at disease onset may represent a unique nosological subtype of combined dystonia of the cervical region with ataxic features, disproportionately affecting older women, and which may become more apparent after several years of progression. Whether this subtype represents a form of primary cerebellar degeneration will require dedicated longitudinal studies.
Supplementary Material
Online Resource 1 Standardized video protocol for the assessment of patients enrolled in the Dystonia Coalition Natural History and Biorepository studies.
ACKNOWLEDGMENT
The authors acknowledge the contribution of all members and patients of the Dystonia Coalition.
FUNDING STATEMENT
This study was supported in part by a grant to the Dystonia Coalition (U54 TR001456 and NS065701) from the Office of Rare Diseases Research (ORDR) in the National Center for Advancing Translational Sciences (NCATS) and the National Institute of Neurological Disorders and Stroke (NINDS). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
FINANCIAL DISCLOSURE AND CONFLICTS OF INTEREST
Dr. Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from Abbvie, Lundbeck, Abbott, CSL Behring, and Cynapsus Therapeutics. He has received grant support from Lundbeck, Abbvie, and Abbott.
Dr. Dwivedi been supported by the NIH (1R01HL125016-01) as co-investigator and (R21 AI118228) as collaborator. He also serves as a statistician in 4 CPRIT grants (PP110156, PP140211, PP150031, and PP130083), Coldwell (co-investigator), MSA Coalition (collaborator), TMF and CATCH funded studies (consultant) and as a PI in TTUHSC EP mini seed grant. He serves as Director of Biostatistics & Epidemiology Consulting Lab at the TTUHSC EP.
Dr. Shaikh is supported by the Career Development Award through Dystonia Coalition (NIH U54TR001456) and Dystonia Medical Research Foundation.
Dr. Kauffman is an employee of the CONICET. He has received grant support from Ministry of Science and Technology of Argentina and Ministry of Health of Buenos Aires.
Dr. Jankovic has received research and/or training grants from: Adamas Pharmaceuticals, Inc; Allergan, Inc; Biotie Therapies; CHDI Foundation; Civitas/Acorda Therapeutics; Dystonia Coalition; Dystonia Medical Research Foundation; Hoffmann-LaRoche; Huntington Study Group; Kyowa Haako Kirin Pharma, Inc; Medtronic Neuromodulation; Merz Pharmaceuticals; Michael J Fox Foundation for Parkinson Research; National Institutes of Health; Neurocrine Biosciences; NeuroDerm Ltd; Parkinson’s Foundation; Parkinson Study Group; Pfizer; Prothena Biosciences Inc; Psyadon Pharmaceuticals, Inc; Revance Therapeutics, Inc; Sangamo BioSciences, Inc.; St. Jude Medical; Teva Pharmaceutical Industries Ltd.
Dr. Jankovic has served as a consultant or as an advisory committee member for: Adamas Pharmaceuticals, Inc; Allergan, Inc; Pfizer Inc; Prothena Biosciences, Revance Therapeutics, Inc; Teva Pharmaceutical Industries Ltd. He has received royalties or other payments from: Cambridge; Elsevier; Future Science Group; Hodder Arnold; Medlink: Neurology; Lippincott Williams and Wilkins; Wiley-Blackwell.
Dr. Comella serves on the editorial board of Clinical Neuropharmacology, Sleep Medicine and Continuum. She receives research support from the NIH R01NS074343, U54NS065701, Dystonia Medical Research Foundation, Allergan Inc. Ipsen Biopharmaceuticals, Inc, Merz Pharmaceutical and Biotie Inc. She receives compensation/honoraria for services as a consultant or an advisory committee member: Acorda Therapeutics, Allergan, Inc; Ipsen Biopharmaceuticals, Inc; Lundbeck Ltd.;Medtronic Inc.; Merz Pharmaceuticals;Acadia Pharmaceuticals; Neurocrine Biosciences Inc., Revance Therapeutic; and Ultragenyx Pharmaceuticals. She receives royalties from Cambridge, Humana Press; Wolters Kluwer. She receives research support from the Parkinson’s Disease Foundation.
Dr. Berman has received research grant support from the Dystonia Coalition, which receives the majority of its support through NIH grant NS065701 from the Office of Rare Diseases Research in the National Center for Advancing Translational Science and National Institute of Neurological Disorders and Stroke, the NIH/NCATS (Colorado CTSI Grant Number KL2 TR001080), Dana Foundation, and the Benign Essential Blepharospasm Research Foundation. He also serves on the medical advisory board for the Benign Essential Blepharospasm Research Foundation and National Spasmodic Torticollis Association.
Dr. Perlmutter serves on the Scientific Advisory Board of the American Parkinson disease association; the Scientific Advisory Committee of the Parkinson Study Group, the Medical and Scientific Advisory Committee of the Dystonia Medical Research Foundation, and the Standards Committee of the Huntington Study Group. Dr. Perlmutter is supported by NIH/NINDS/NCATS/NIA (NS41509, NS075321, NS058714, NS092865, U10NS077384), the American Parkinson Disease Association (APDA), Greater St. Louis Chapter of the APDA, Barnes Jewish Hospital Foundation (Elliot Stein Family Fund, Oertli Fund), The Fixel Foundation, Barbara & Sam Murphy Dystonia Fund, CHDI and Huntington Disease Society of America.
Dr. Jinnah is director of the Dystonia Coalition, which receives the majority of its support through National Institutes of Health (NIH) grants NS065701 and TR001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences and the National Institute of Neurological Disorders and Stroke. The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (Beat Dystonia, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The European Dystonia Federation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, The National Spasmodic Torticollis Association, Tyler’s Hope for a Dystonia Cure). Dr. Jinnah has also received research or training grants from the NIH, Pharmaceutical Companies (Ipsen Inc. and Merz Pharmaceuticals) and Private Foundations (the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, Dystonia Medical Research Foundation, and the Lesch-Nyhan Syndrome Children’s Research Foundation). He also has served on an advisory board or as a consultant for Allergan, Inc., Ipsen Pharmaceuticals, Psyadon Therapeutics, Retrophin Inc., and Saol Therapeutics.
Dr. Espay has received grant support from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Adamas, Acadia, Acorda, Neuroderm, Impax, Sunovion, Lundbeck, Osmotica Pharmaceutical, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Lundbeck, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society.
Footnotes
DATA ACCESS AND RESPONSIBILITY STATEMENT
Dr Merola had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ETHICAL STANDARDS
The study has been approved by the local ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
All patients gave written informed consent prior to their inclusion in the study. Details that might disclose the identity of the subjects under study have been omitted.
AUTHORS’ ROLES
1) Research project: A. Conception; B. Organization; C. Execution
2) Statistical Analysis: A. Design; B. Execution; C. Review and Critique
3) Manuscript Preparation: A. Writing of the first draft; B. Review and Critique
Dr. Merola: 1A, 1B, 1C, 2A, 2B, 3A
Dr. Dwivedi: 1B, 2A, 2B, 3B
Dr. Shaikh: 1A, 2C, 3B
Dr. Tareen: 1B, 1C, 3B
Dr. Da Prat: 1B, 1C, 3B
Dr. Kauffman: 1A, 2C, 3B
Dr. Hampf: 1A, 2C, 3B
Dr. Mahajan: 1A, 2C, 3B
Dr. Marsili: 1A, 2C, 3B
Dr. Jankovic: 1A, 2C, 3B
Dr. Comella: 1A, 2C, 3B
Dr. Berman: 1A, 2C, 3B
Dr. Perlmutter: 1A, 2C, 3B
Dr. Jinnah: 1A, 2C, 3B
Dr. Espay: 1A, 1B, 1C, 2A, 2C, 3B
All the co-authors listed above gave their final approval of this manuscript version.
REFERENCES
- 1.Rivest J, Marsden CD (1990) Trunk and head tremor as isolated manifestations of dystonia. Mov Disord 5:60–65. [DOI] [PubMed] [Google Scholar]
- 2.Masuhr F, Wissel J, Müller J, Scholz U, Poewe W (2000) Quantification of sensory trick impact on tremor amplitude and frequency in 60 patients with head tremor. Mov Disord 15:960–964. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Kim DS, Cho JW, Park KP (2009) Disabling Head Tremor in a Patient with DYT1 Mutation. J Mov Disord 2:86–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahn S The varied clinical expressions of dystonia (1984) Neurol Clin 2:541–554. [PubMed] [Google Scholar]
- 5.Albanese A, Bhatia K, Bressman SB, et al. (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jinnah HA, Berardelli A, Comella C, et al. (2013) The focal dystonias: current views and challenges for future research. Mov Disord 28:926–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finsterer J, Muellbacher W, Mamoli B (1996) Yes/yes head tremor without appendicular tremor after bilateral cerebellar infarction. J Neurol Sci 139:242–245. [PubMed] [Google Scholar]
- 8.Chansakul C, Moguel-Cobos G, Bomprezzi R (2011) Head tremor secondary to MS resolved with rituximab. Neurol Sci 32:1157–1160. [DOI] [PubMed] [Google Scholar]
- 9.Prudente CN, Hess EJ, Jinnah HA (2014) Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience 260:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batla A, Sánchez MC, Erro R, et al. (2015) The role of cerebellum in patients with late onset cervical/segmental dystonia?--evidence from the clinic. Parkinsonism Relat Disord 21:1317–1322. [DOI] [PubMed] [Google Scholar]
- 11.Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA (2011) The functional neuroanatomy of dystonia. Neurobiol Dis 42:185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shakkottai VG, Batla A, Bhatia K, et al. (2017) Current Opinions and Areas of Consensus on the Role of the Cerebellum in Dystonia. Cerebellum 16:577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prudente CN, Stilla R, Singh S, et al. (2016) A Functional Magnetic Resonance Imaging Study of Head Movements in Cervical Dystonia. Front Neurol 7:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filip P, Gallea C, Lehéricy S, et al. (2017) Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord 32:757–768. [DOI] [PubMed] [Google Scholar]
- 15.Zadro I, Brinar VV, Barun B, Ozretić D, Habek M (2008). Cervical dystonia due to cerebellar stroke. Mov Disord 23:919–920. [DOI] [PubMed] [Google Scholar]
- 16.Wagle Shukla A, De Jesus S, Meng FG, Hu W (2017) Focal cervical dystonia presents in the setting of acute cerebellar hemorrhage. J Neurol Sci 375:307–308. [DOI] [PubMed] [Google Scholar]
- 17.Sokal P, Rudaś M, Harat M, Szylberg Ł, Zieliński P (2015) Deep anterior cerebellar stimulation reduces symptoms of secondary dystonia in patients with cerebral palsy treated due to spasticity. Clin Neurol Neurosurg 135:62–68. [DOI] [PubMed] [Google Scholar]
- 18.Prudente CN, Pardo CA, Xiao J, et al. (2013) Neuropathology of cervical dystonia. Exp Neurol 241:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan L, Hicks M, Winslow K, et al. (2015) Secured web-based video repository for multicenter studies. Parkinsonism Relat Disord 21:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirio Richardson S, Wegele AR, Skipper B, et al. (2017) Dystonia treatment: Patterns of medication use in an international cohort. Neurology 88:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramony SH (2007) SARA--a new clinical scale for the assessment and rating of ataxia. Nat Clin Pract Neurol 3:136–137. [DOI] [PubMed] [Google Scholar]
- 22.Trouillas P, Takayanagi T, Hallett M, et al. (1997) International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci 145:205–211. [DOI] [PubMed] [Google Scholar]
- 23.Consky ES, Basinki A, Belle L, Ranawaya R, Lang AE (1990) The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS): assessment of validity and inter-rater reliability. Neurology 40:445. [Google Scholar]
- 24.Comella CL, Leurgans S, Wuu J, et al. (2003) Rating scales for dystonia: a multicenter assessment. Mov Disord 18:303–312. [DOI] [PubMed] [Google Scholar]
- 25.LeDoux MS, Brady KA (2003) Secondary cervical dystonia associated with structural lesions of the central nervous system. Mov Disord 18:60–69. [DOI] [PubMed] [Google Scholar]
- 26.Kumandaş S, Per H, Gümüş H, et al. (2006) Torticollis secondary to posterior fossa and cervical spinal cord tumors: report of five cases and literature review. Neurosurg Rev 29:333–338. [DOI] [PubMed] [Google Scholar]
- 27.Cancel G, Dürr A, Didierjean O, et al. (1997) Molecular and clinical correlations in spinocerebellar ataxia 2: a study of 32 families. Hum Mol Genet 6:709–715. [DOI] [PubMed] [Google Scholar]
- 28.Hagenah JM, Zühlke C, Hellenbroich Y, Heide W, Klein C (2004) Focal dystonia as a presenting sign of spinocerebellar ataxia 17. Mov Disord 19:217–220. [DOI] [PubMed] [Google Scholar]
- 29.Lang AE, Rogaeva EA, Tsuda T, Hutterer J, St George-Hyslop P (1994) Homozygous inheritance of the Machado-Joseph disease gene. Ann Neurol 36:443–447. [DOI] [PubMed] [Google Scholar]
- 30.Le Ber I, Clot F, Vercueil L, et al. (2006) Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology 67:1769–1773. [DOI] [PubMed] [Google Scholar]
- 31.Jankovic J, Leder S, Warner D, Schwartz K (1991) Cervical dystonia: clinical findings and associated movement disorders. Neurology 41:1088–1091. [DOI] [PubMed] [Google Scholar]
- 32.Pal PK, Samii A, Schulzer M, Mak E, Tsui JK (2000) Head tremor in cervical dystonia. Can J Neurol Sci 27:137–142. [PubMed] [Google Scholar]
- 33.Hedera P, Phibbs FT, Fang JY, Cooper MK, Charles PD, Davis TL (2010) Clustering of dystonia in some pedigrees with autosomal dominant essential tremor suggests the existence of a distinct subtype of essential tremor. BMC Neurol 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bain PG, Findley LJ, Thompson PD, et al. (1994) A study of hereditary essential tremor. Brain 117:805–824. [DOI] [PubMed] [Google Scholar]
- 35.Louis ED (2013) When do essential tremor patients develop head tremor? Influences of age and duration and evidence of a biological clock. Neuroepidemiology 41:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albanese A, Sorbo FD (2016) Dystonia and Tremor: The Clinical Syndromes with Isolated Tremor. Tremor Other Hyperkinet Mov 6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrag A, Münchau A, Bhatia KP, Quinn NP, Marsden CD (2000) Essential tremor: an overdiagnosed condition? J Neurol 247:955–959. [DOI] [PubMed] [Google Scholar]
- 38.Louis ED, Kuo SH, Wang J, et al. (2017) Cerebellar Pathology in Familial vs. Sporadic Essential Tremor. Cerebellum 16:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W, Zilov A, Soewondo P, et al. (2010) Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract 88(suppl1):3–9. [DOI] [PubMed] [Google Scholar]
- 40.Hannan EL (2008) Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv 1:211–217. [DOI] [PubMed] [Google Scholar]
- 41.Bertram KL, Williams DR (2016) Delays to the diagnosis of cervical dystonia. J Clin Neurosci 25:62–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1 Standardized video protocol for the assessment of patients enrolled in the Dystonia Coalition Natural History and Biorepository studies.